Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
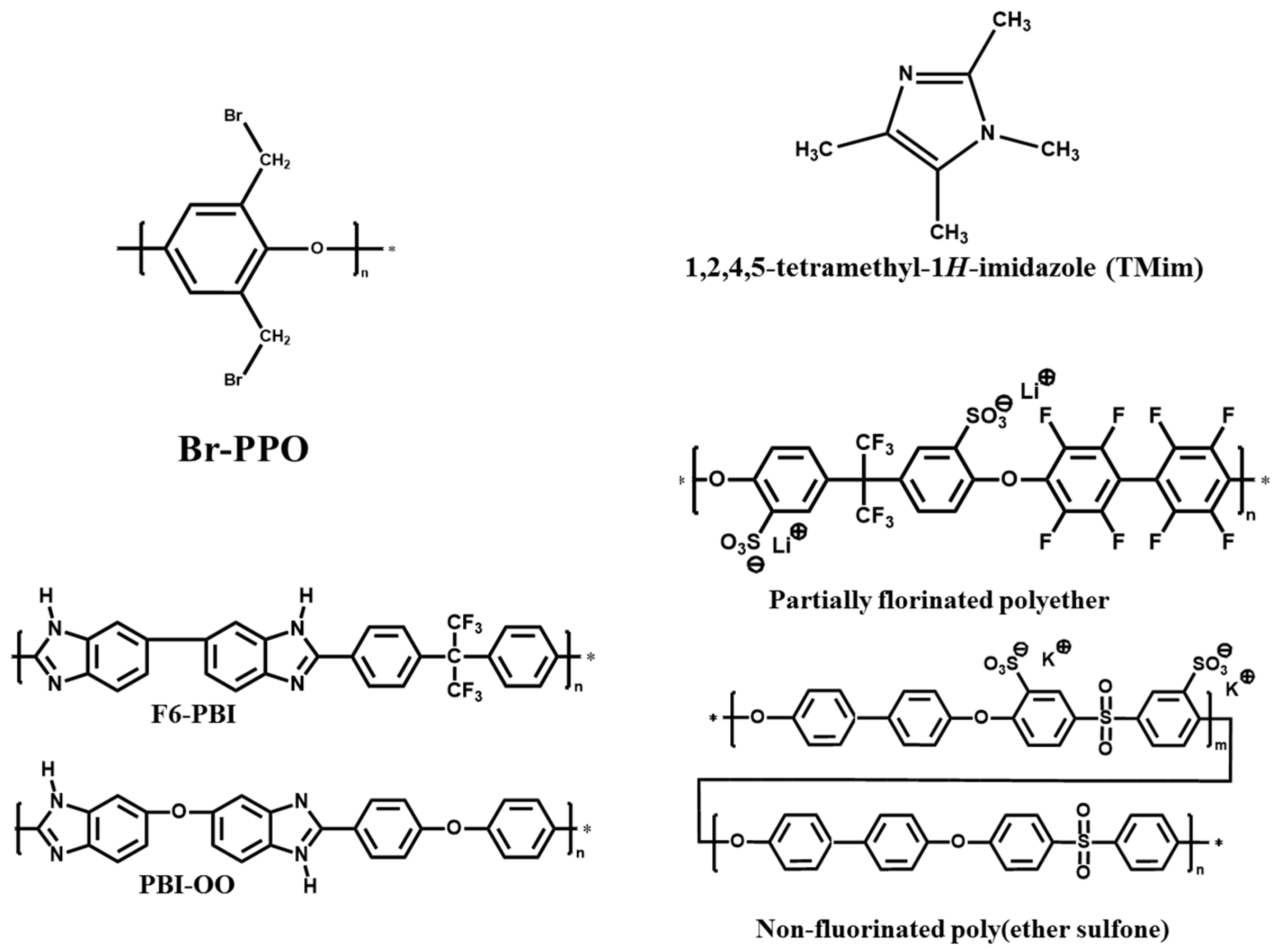
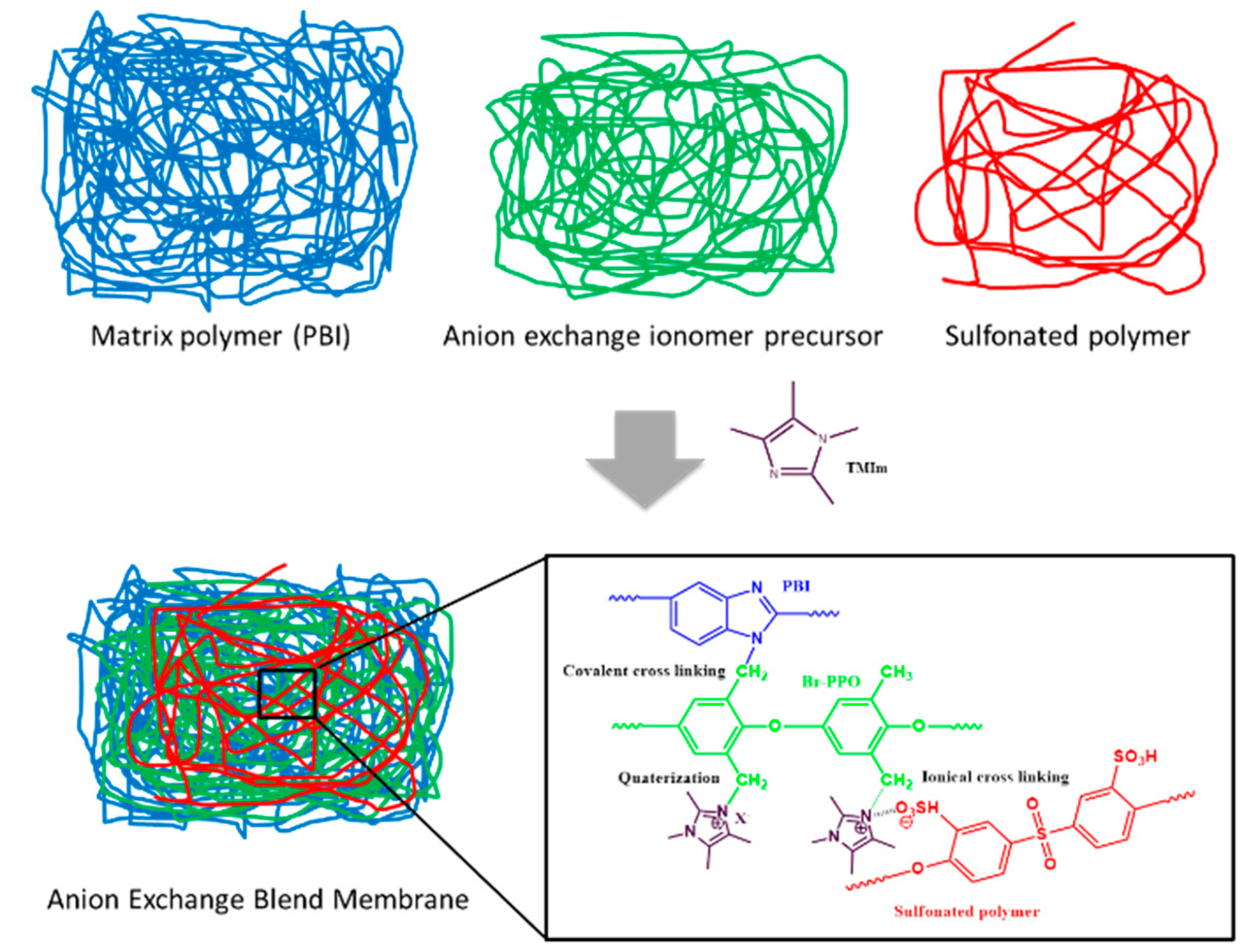
2.2. Membranes Preparation
2.3. Membranes Characterization
2.3.1. Ion Exchange Capacity (IEC)
2.3.2. Conductivity
2.3.3. Water Uptake (WU) and Swelling Ratio (SR)
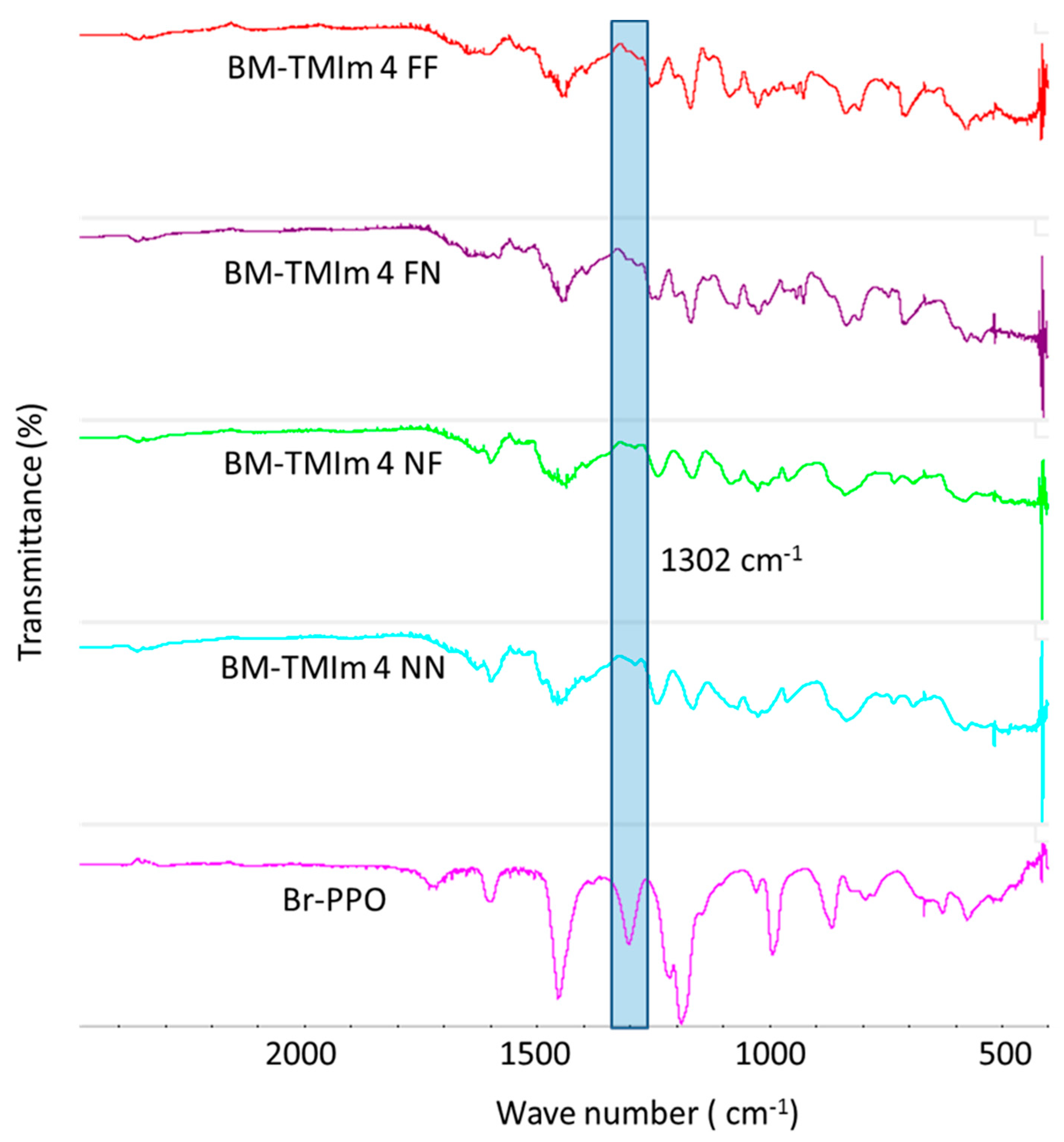
2.3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.5. Gel Content by Extraction
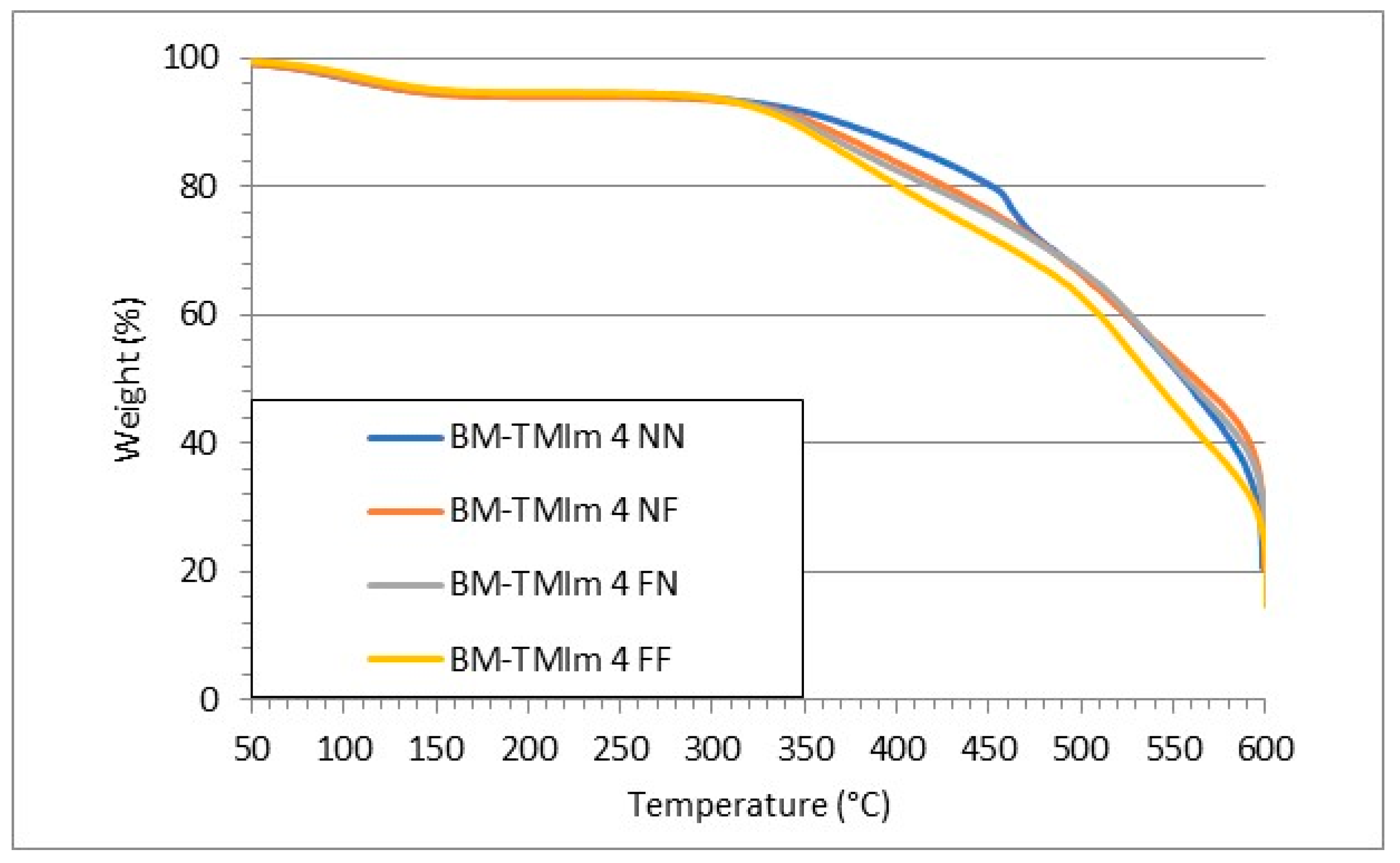
2.3.6. Thermal Stability
2.3.7. Weight Gain Measured in 30% Sulfuric Acid
2.4. Vanadium Redox Flow Battery (VRFB) Single Cell Test
3. Results and Discussion
3.1. Membranes Preparation
3.2. Membranes Properties
3.3. Vanadium Redox Flow Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Sum, M.S.-K. A study of the V(II)/V(III) redox couple for redox flow cell applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Yang, J.H.; Kim, H.T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Ashraf Gandomi, Y.; Aaron, D.S.; Mench, M.M. Coupled Membrane Transport Parameters for Ionic Species in All-Vanadium Redox Flow Batteries. Electrochim. Acta 2016, 218, 174–190. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, T.; Skyllas Kazacos, M. Modification of anion-exchange membranes for vanadium redox flow battery applications. J. Power Sources 1996, 63, 179–186. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Sun, C.N.; Tang, Z.; Belcher, C.; Zawodzinski, T.A.; Fujimoto, C. Evaluation of Diels-Alder poly(phenylene) anion exchange membranes in all-vanadium redox flow batteries. Electrochem. Commun. 2014, 43, 63–66. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimized Anion Exchange Membranes for Vanadium Redox Flow Batteries. ACS Appl. Mater. Interfaces 2013, 5, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S.; Xing, D.; Han, R.; Yin, C.; Jian, X. Quaternized poly(phthalazinone ether ketone ketone) anion exchange membrane with low permeability of vanadium ions for vanadium redox flow battery application. J. Power Sources 2012, 217, 296–302. [Google Scholar] [CrossRef]
- Cha, M.S.; Jeong, H.Y.; Shin, H.Y.; Hong, S.H.; Kim, T.H.; Oh, S.G.; Lee, J.Y.; Hong, Y.T. Crosslinked anion exchange membranes with primary diamine-based crosslinkers for vanadium redox flow battery application. J. Power Sources 2017, 363, 78–86. [Google Scholar] [CrossRef]
- Chromik, A.; dos Santos, A.R.; Turek, T.; Kunz, U.; Häring, T.; Kerres, J. Stability of acid-excess acid-base blend membranes in all-vanadium redox-flow batteries. J. Membr. Sci. 2015, 476, 148–155. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, D.; Liu, B.; Wang, J.; Song, Y. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone)/quaternized poly(ether imide) for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 17590–17597. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, C.; Dai, Y.; Zheng, W.; Ruan, X.; He, G. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery. J. Membr. Sci. 2017, 544, 98–107. [Google Scholar] [CrossRef]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Application of Novel Anion-Exchange Blend Membranes (AEBMs) to Vanadium Redox Flow Batteries. Membranes 2018, 8, 33. [Google Scholar] [CrossRef]
- Gorman, C.B.; Vest, R.W.; Palovich, T.U.; Serron, S. Preparation of poly(cyanoacetylene) using late-transition-metal catalysts. Macromolecules 1999, 32, 4157–4165. [Google Scholar] [CrossRef]
- Katzfuß, A.; Gogel, V.; Jörissen, L.; Kerres, J. The application of covalently cross-linked BrPPO as AEM in alkaline DMFC. J. Membr. Sci. 2013, 425–426, 131–140. [Google Scholar]
- Chromik, A.; Kerres, J.A. Degradation studies on acid-base blends for both LT and intermediate T fuel cells. Solid State Ion. 2013, 252, 140–151. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. (Oxf.) 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel imidazolium-functionalized anion-exchange polymer PBI blend membranes. J. Membr. Sci. 2015, 476, 256–263. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel morpholinium-functionalized anion-exchange PBI-polymer blends. J. Mater. Chem. A 2015, 3, 1110–1120. [Google Scholar] [CrossRef]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage 2017, 15, 010801. [Google Scholar] [CrossRef]
- Kerres, J.; Ullrich, A.; Hein, M.; Gogel, V.; Friedrich, K.A.; Jörissen, L. Cross-Linked Polyaryl Blend Membranes for Polymer Electrolyte Fuel Cells. Fuel Cells 2004, 4, 105–112. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. V5+ degradation of sulfonated Radel membranes for vanadium redox flow batteries. Phys. Chem. Chem. Phys. 2013, 15, 11299–11305. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Zeng, Y.K.; Zhang, Z.H. Highly stable pyridinium-functionalized cross-linked anion exchange membranes for all vanadium redox flow batteries. J. Power Sources 2016, 331, 452–461. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Wei, L.; Zhang, C. The use of polybenzimidazole membranes in vanadium redox flow batteries leading to increased coulombic efficiency and cycling performance. Electrochim. Acta 2015, 153, 492–498. [Google Scholar] [CrossRef]
- Strużyńska-Piron, I.; Jung, M.; Maljusch, A.; Conradi, O.; Kim, S.; Jang, J.H.; Kim, H.J.; Kwon, Y.; Nam, S.W.; Henkensmeier, D. Imidazole based ionenes, their blends with PBI-OO and applicability as membrane in a vanadium Redox flow battery. Eur. Polym. J. 2017, 96, 383–392. [Google Scholar] [CrossRef]
- Peron, J.; Ruiz, E.; Jones, D.J.; Rozière, J. Solution sulfonation of a novel polybenzimidazole. A proton electrolyte for fuel cell application. J. Membr. Sci. 2008, 314, 247–256. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Yan, C.; Qiao, Y. Corrosion behavior of a positive graphite electrode in vanadium redox flow battery. Electrochim. Acta 2011, 56, 8783–8790. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Optimizing membrane thickness for vanadium redox flow batteries. J. Membr. Sci. 2013, 437, 108–113. [Google Scholar] [CrossRef]
- Noh, C.; Jung, M.; Henkensmeier, D.; Nam, S.W.; Kwon, Y. Vanadium Redox Flow Batteries Using meta -Polybenzimidazole-Based Membranes of Different Thicknesses. Acs Appl. Mater. Interfaces 2017, 9, 36799–36809. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
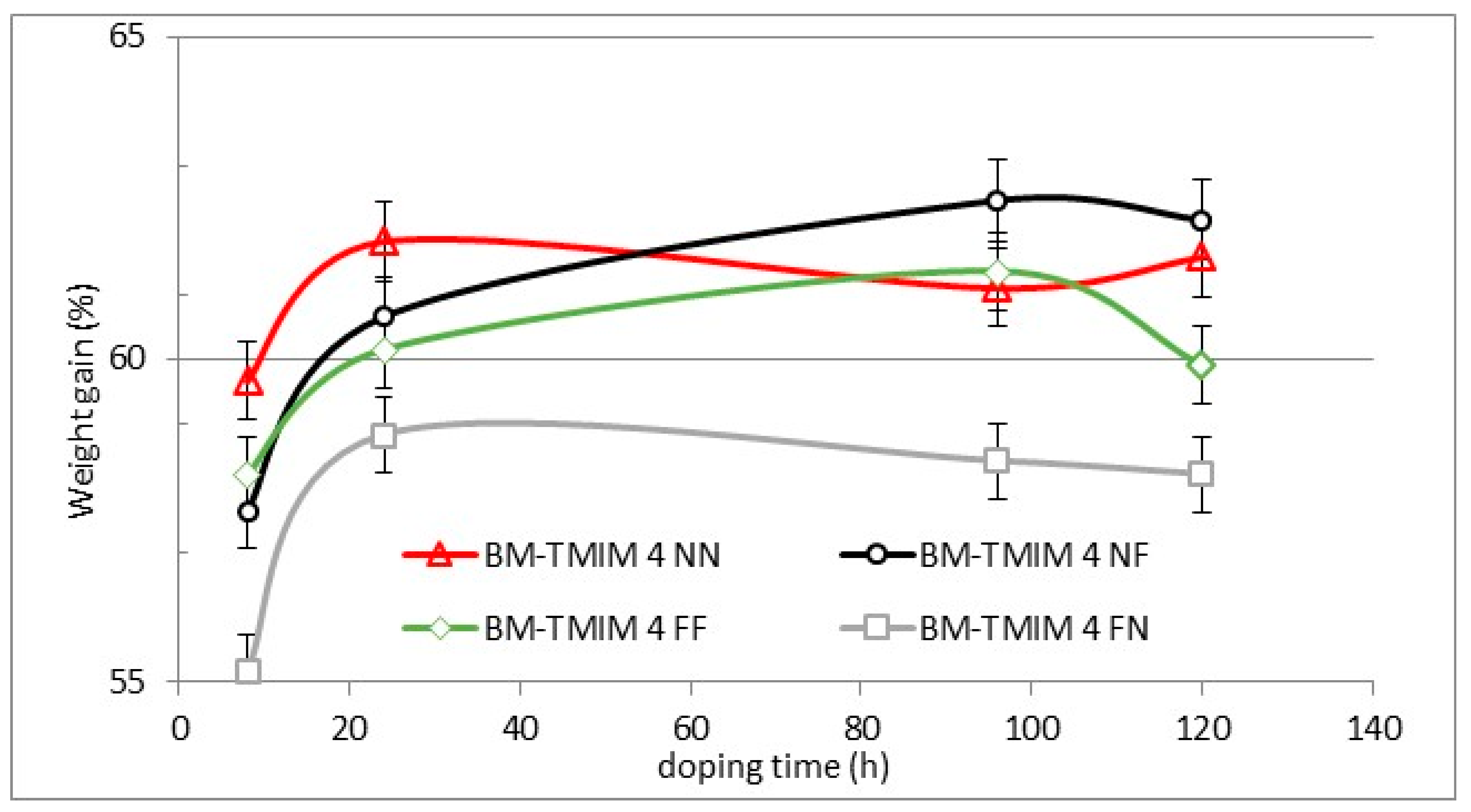
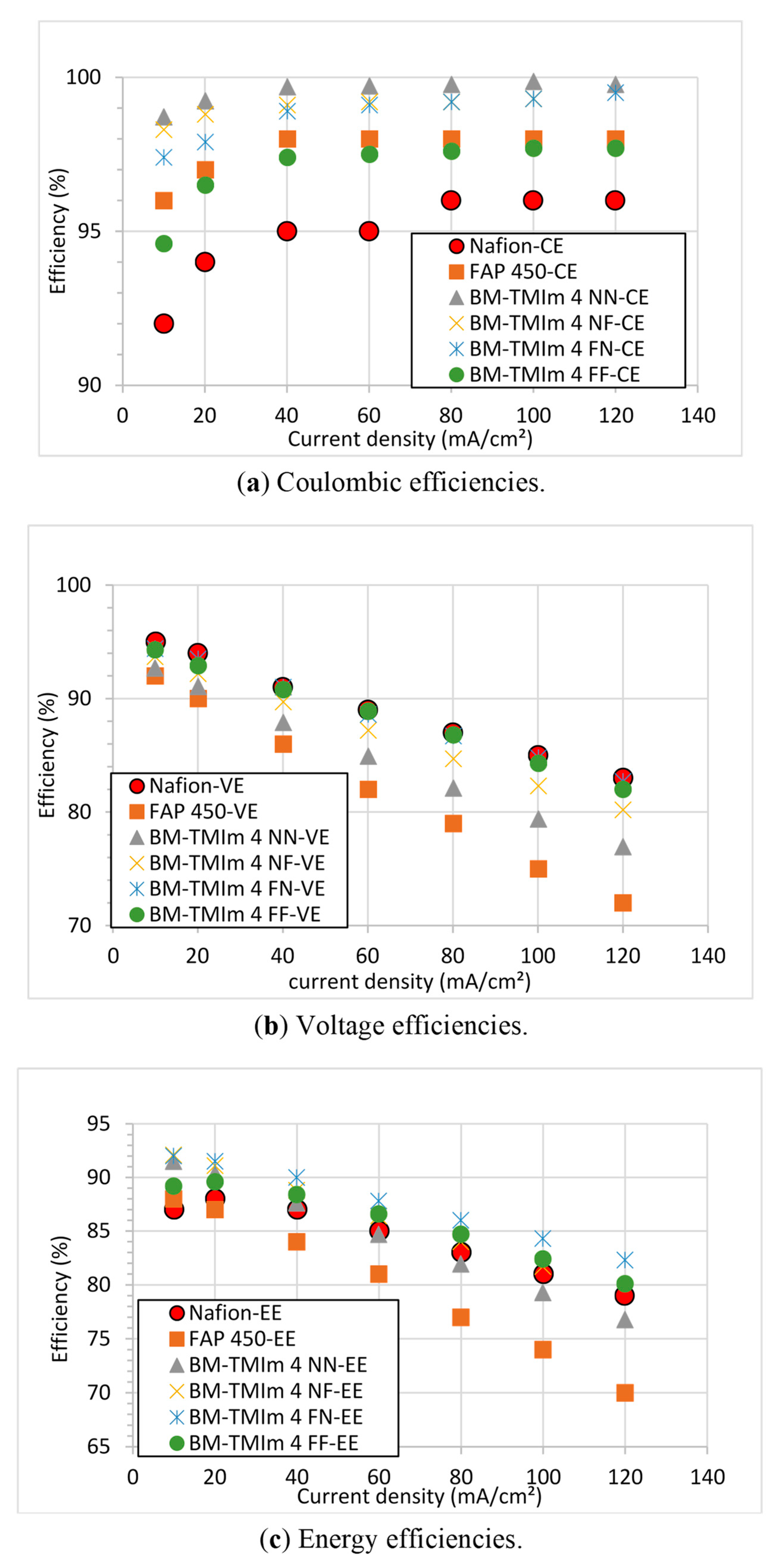
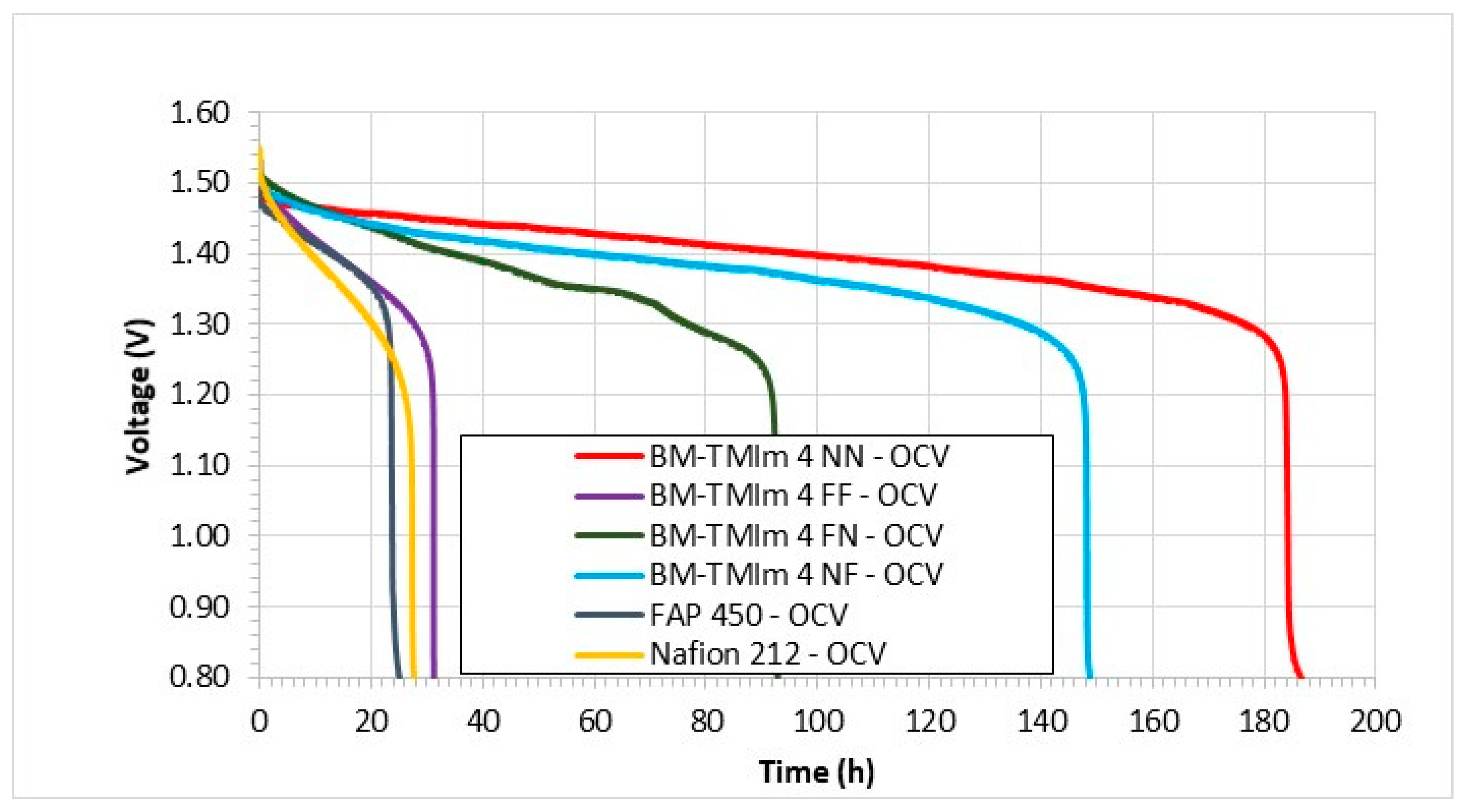
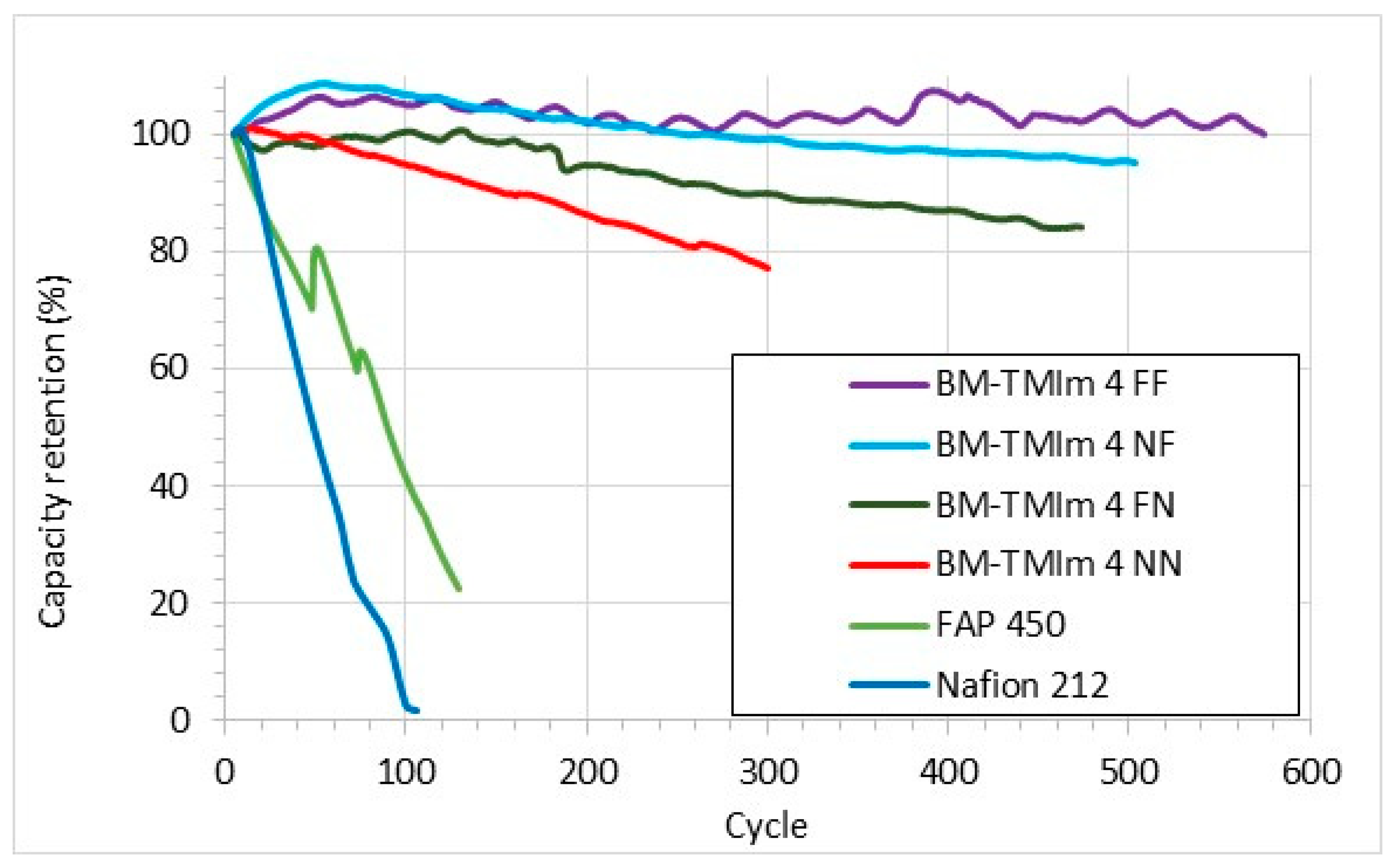
| Membrane | Mass of Br-PPO (g) | Mass (g) and Type of PBI | Mass (g) and Type of S-Polymer | Wet Thickness (μm) |
|---|---|---|---|---|
| BM-TMIm 4 NN | 1 | 1 (PBI-OO) | 0.24 (Non-fluorinated polymer) | 56 ± 0.96 |
| BM-TMIm 4 NF | 1 | 1 (PBI-OO) | 0.24 (Fluorinated polymer) | 43 ± 5.25 |
| BM-TMIm 4 FN | 1 | 1 (F6-PBI) | 0.24 (Non-fluorinated polymer) | 37 ± 0.82 |
| BM-TMIm 4 FF | 1 | 1 (F6-PBI) | 0.24 (Fluorinated polymer) | 34 ± 0.58 |
| FAP 450 | - | - | - | 58 |
| Nafion 212 | - | - | - | 53 |
| Membrane | IECs (mmol/g) | Conductivity (mS/cm) | WU (%) | SRL (%) | SRT (%) | SRW (%) | Extraction (%) | T onset (oC) |
|---|---|---|---|---|---|---|---|---|
| BM-TMIm 4 NN | 2.75 | 21.3 ± 0.8 | 22 ± 2.0 | 11 ± 0.3 | 6 ± 2 | 11 ± 0.3 | 93 | 280 |
| BM-TMIm 4 NF | 2.55 | 25.4 ± 0.8 | 14 ± 0.9 | 9 ± 0.7 | 8 ± 0.7 | 10 ± 1.0 | 93 | 284 |
| BM-TMIm 4 FN | 2.58 | 24.9 ± 0.7 | 17 ± 1.1 | 9 ± 0.8 | 7 ± 0.7 | 9 ± 1.1 | 92 | 278 |
| BM-TMIm 4 FF | 2.37 | 26.6 ± 1.3 | 13 ± 0.8 | 8 ± 0.8 | 4 ± 1.1 | 7 ± 1.2 | 92 | 265 |
| FAP 450 | 2.18 | 35.2 ± 7.3 | 14 ± 3.2 | 8 ± 0.7 | 3 ± 1.4 | 8 ± 1.5 | - | 305 |
| Nafion 212 | 0.88 (H+) | 98.5 ± 5.0 | 13 ± 1.2 | 11 ± 0.6 | 8 ± 1.5 | 13 ± 2.2 | - | 300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes 2019, 9, 31. https://doi.org/10.3390/membranes9020031
Cho H, Krieg HM, Kerres JA. Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes. 2019; 9(2):31. https://doi.org/10.3390/membranes9020031
Chicago/Turabian StyleCho, Hyeongrae, Henning M. Krieg, and Jochen A. Kerres. 2019. "Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries" Membranes 9, no. 2: 31. https://doi.org/10.3390/membranes9020031
APA StyleCho, H., Krieg, H. M., & Kerres, J. A. (2019). Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes, 9(2), 31. https://doi.org/10.3390/membranes9020031




