Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Samples
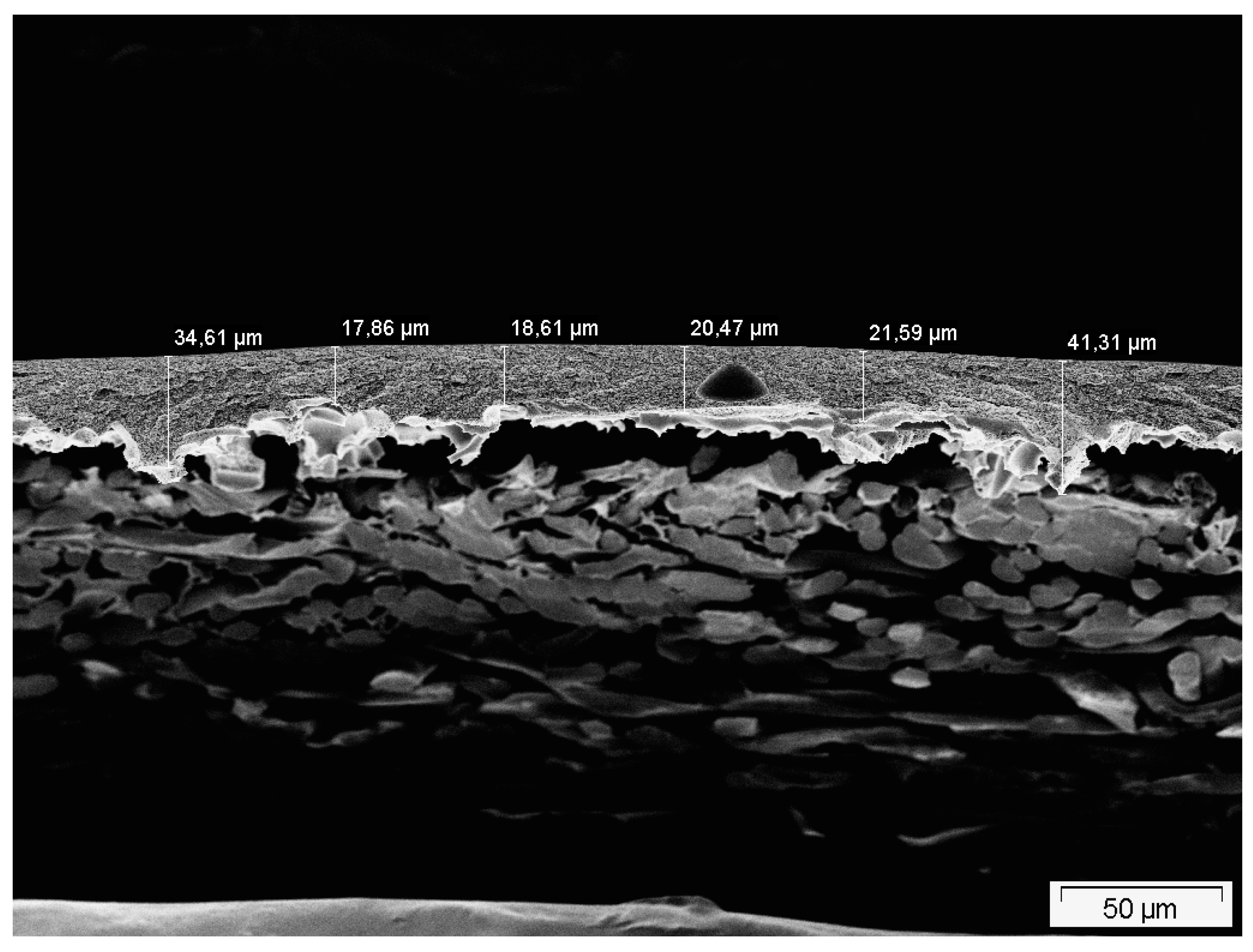
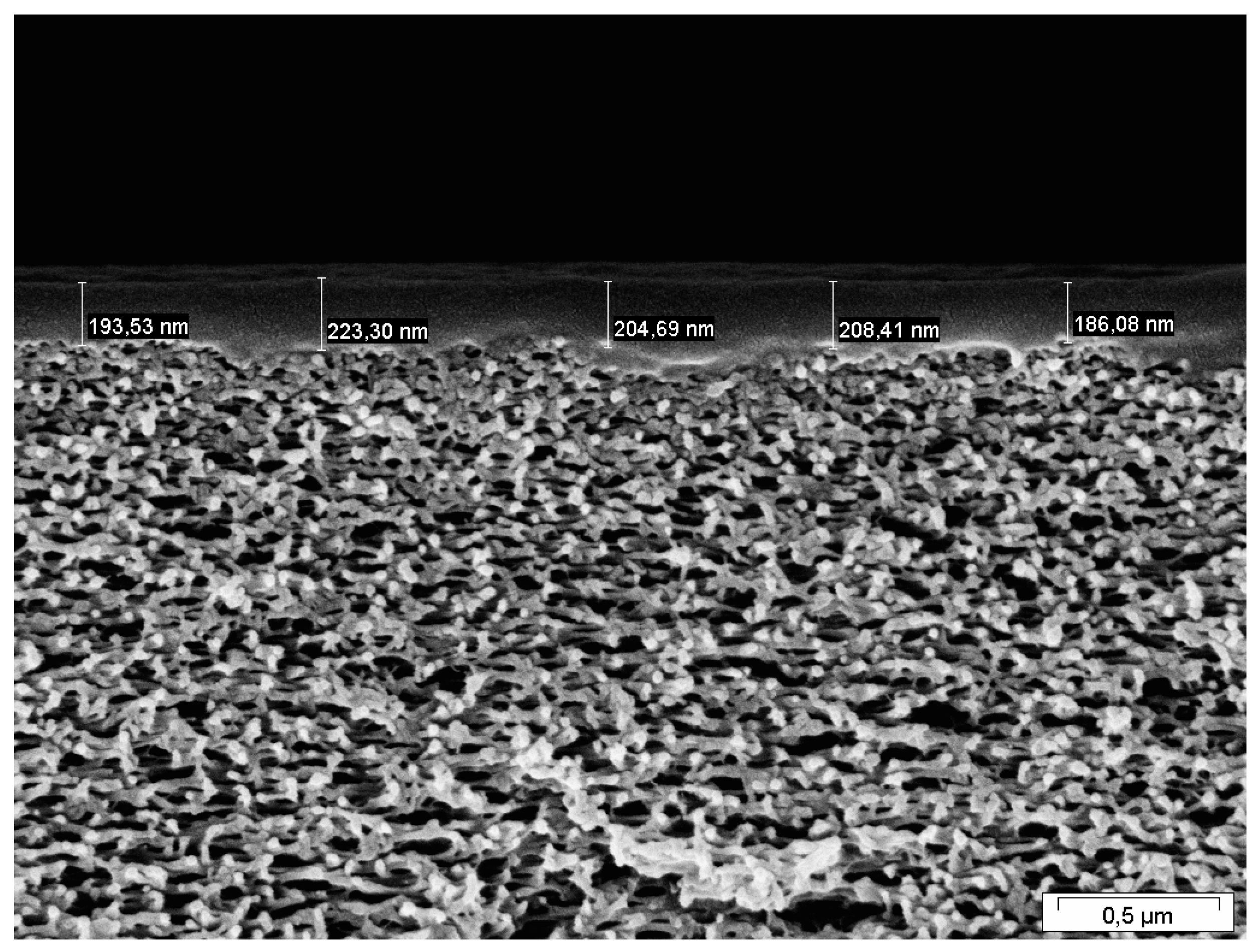
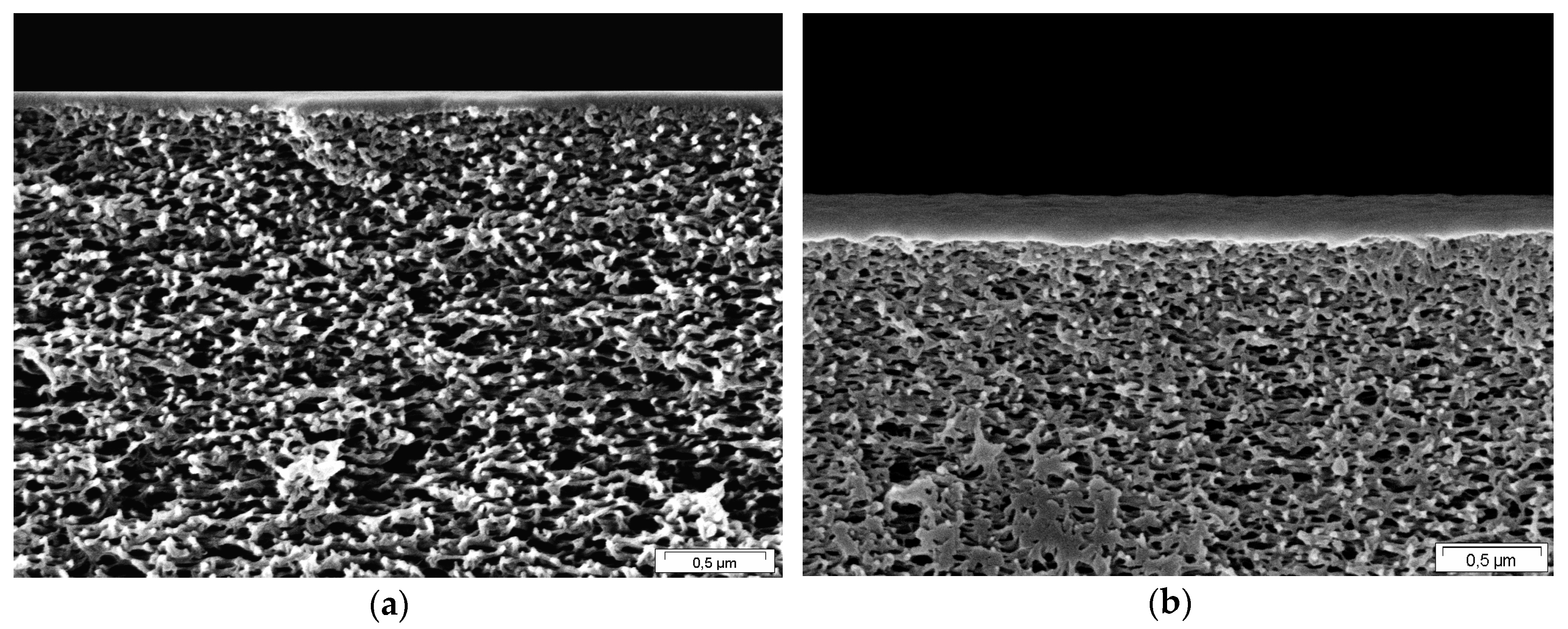
2.2. Membrane Characterization
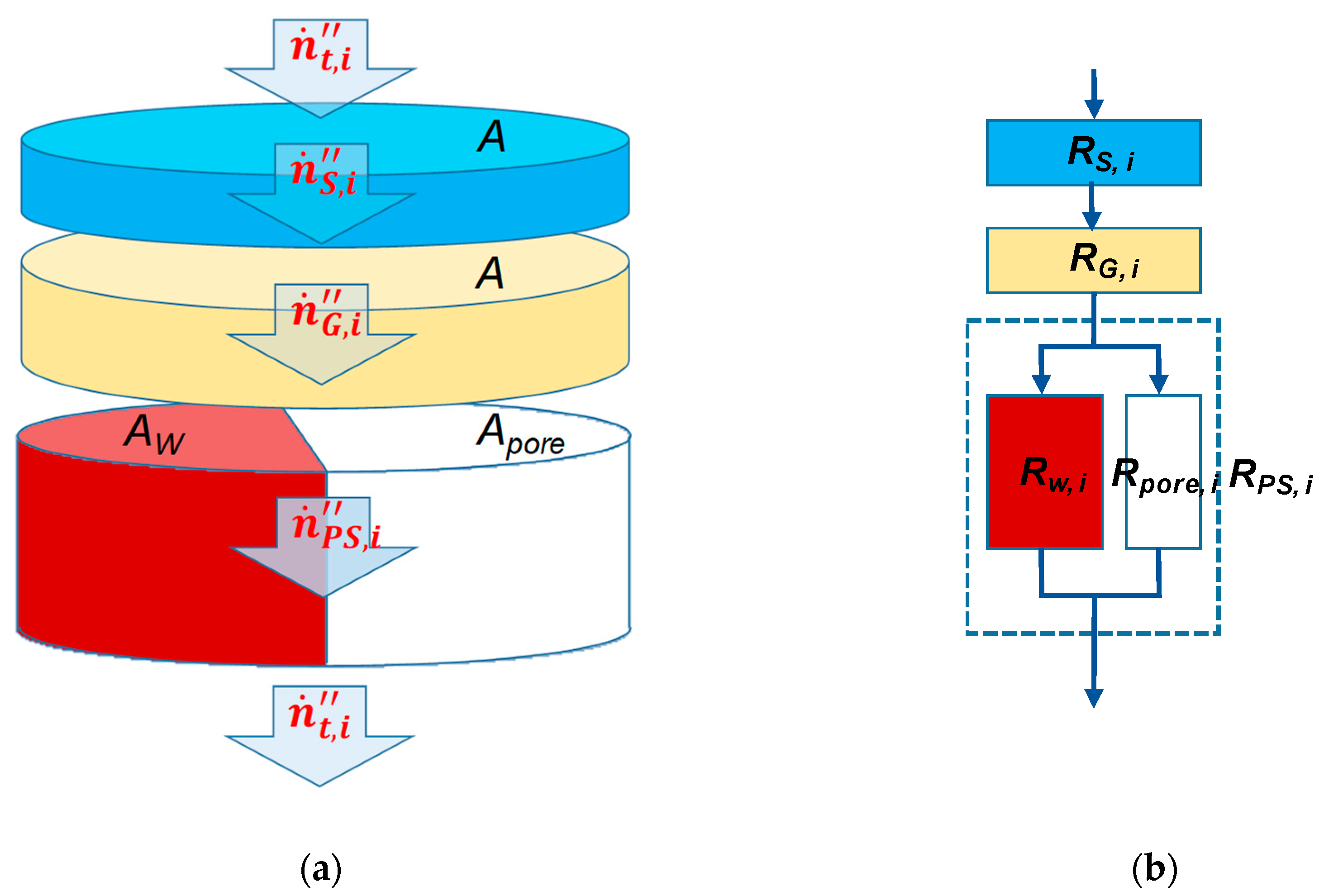
3. Model Development
3.1. Gas Transport in Porous Layers
3.2. Gas Permeation in Dense Layers
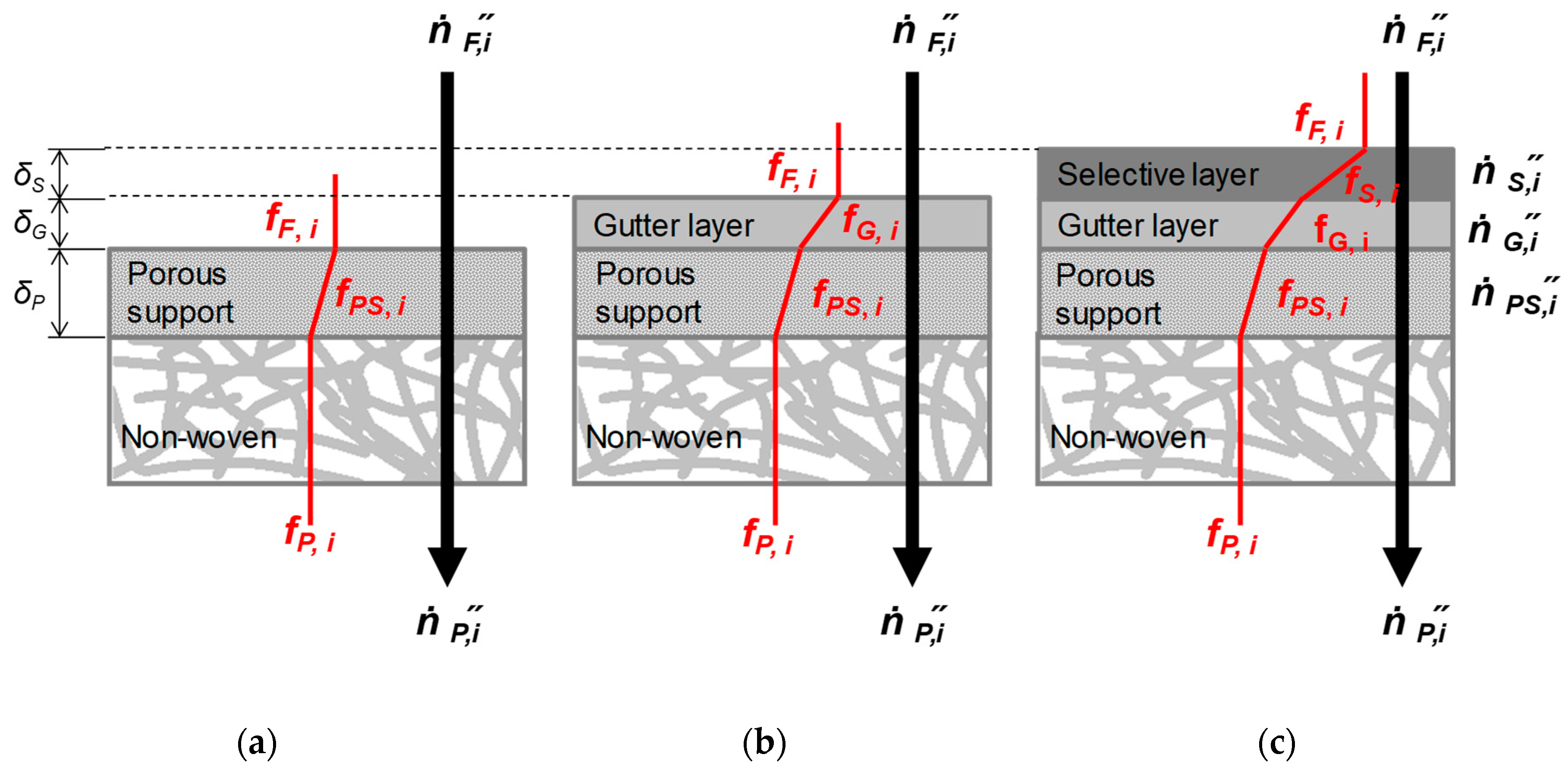
3.3. Resistance Model for TFCM
4. Results and Discussion
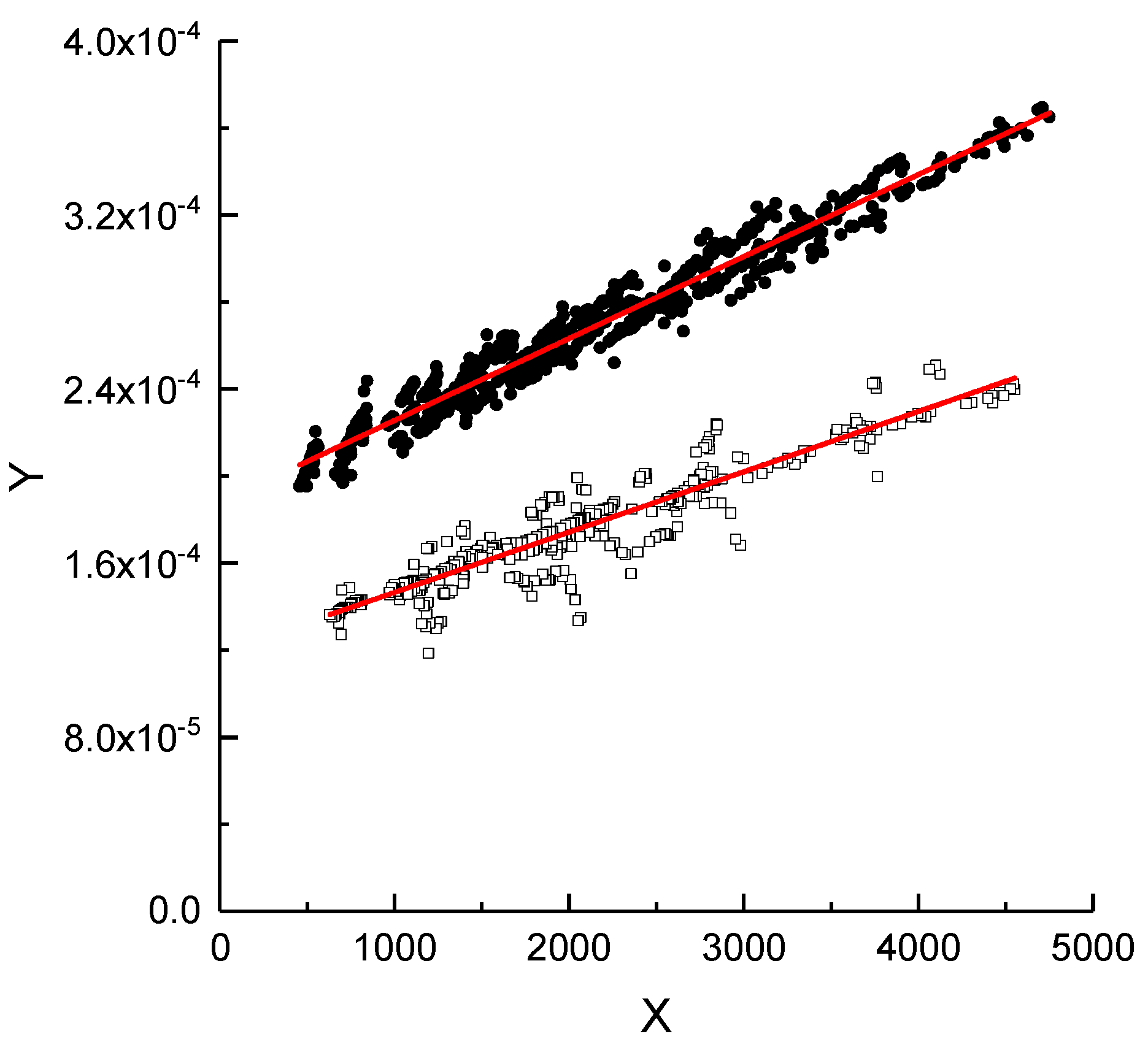
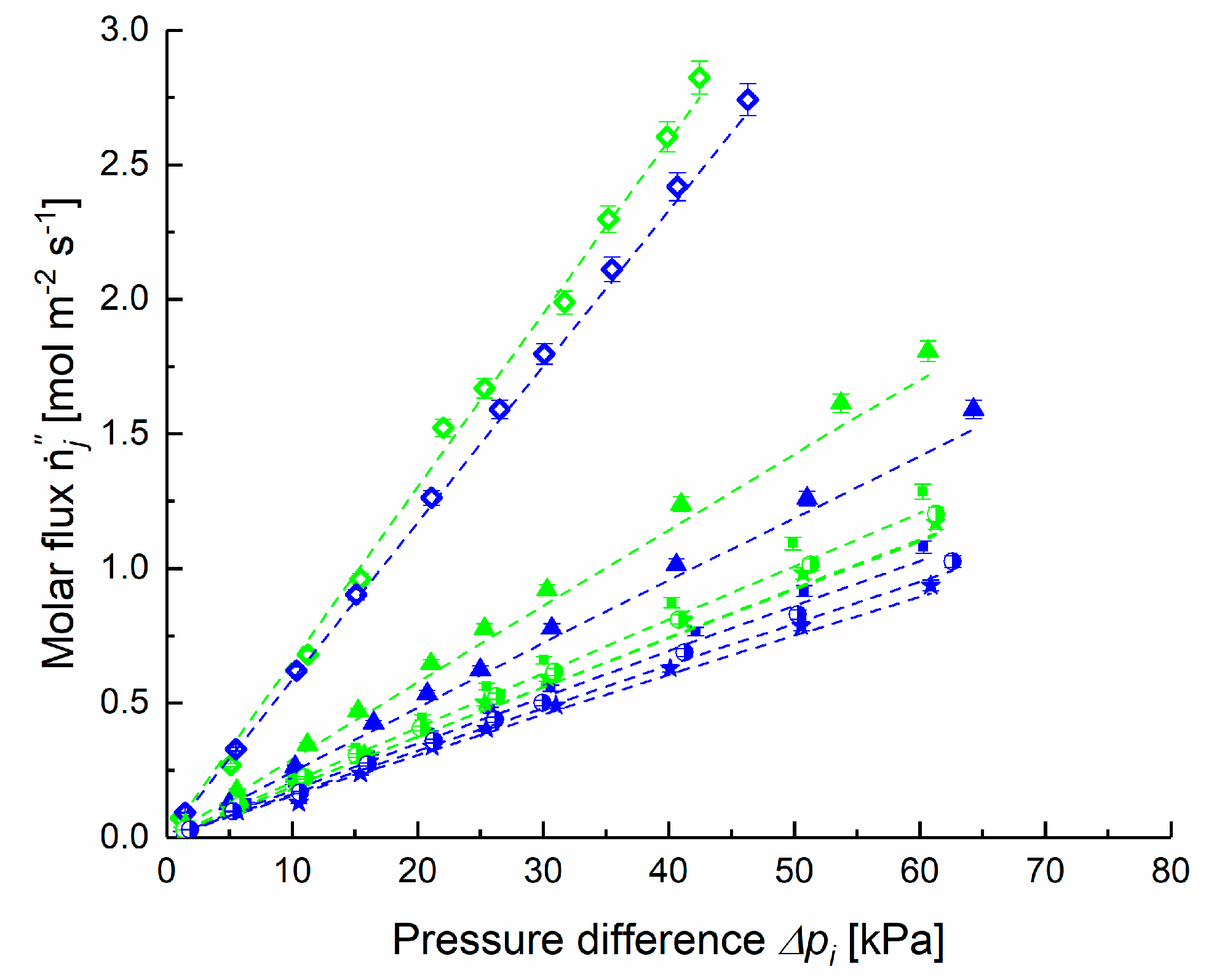
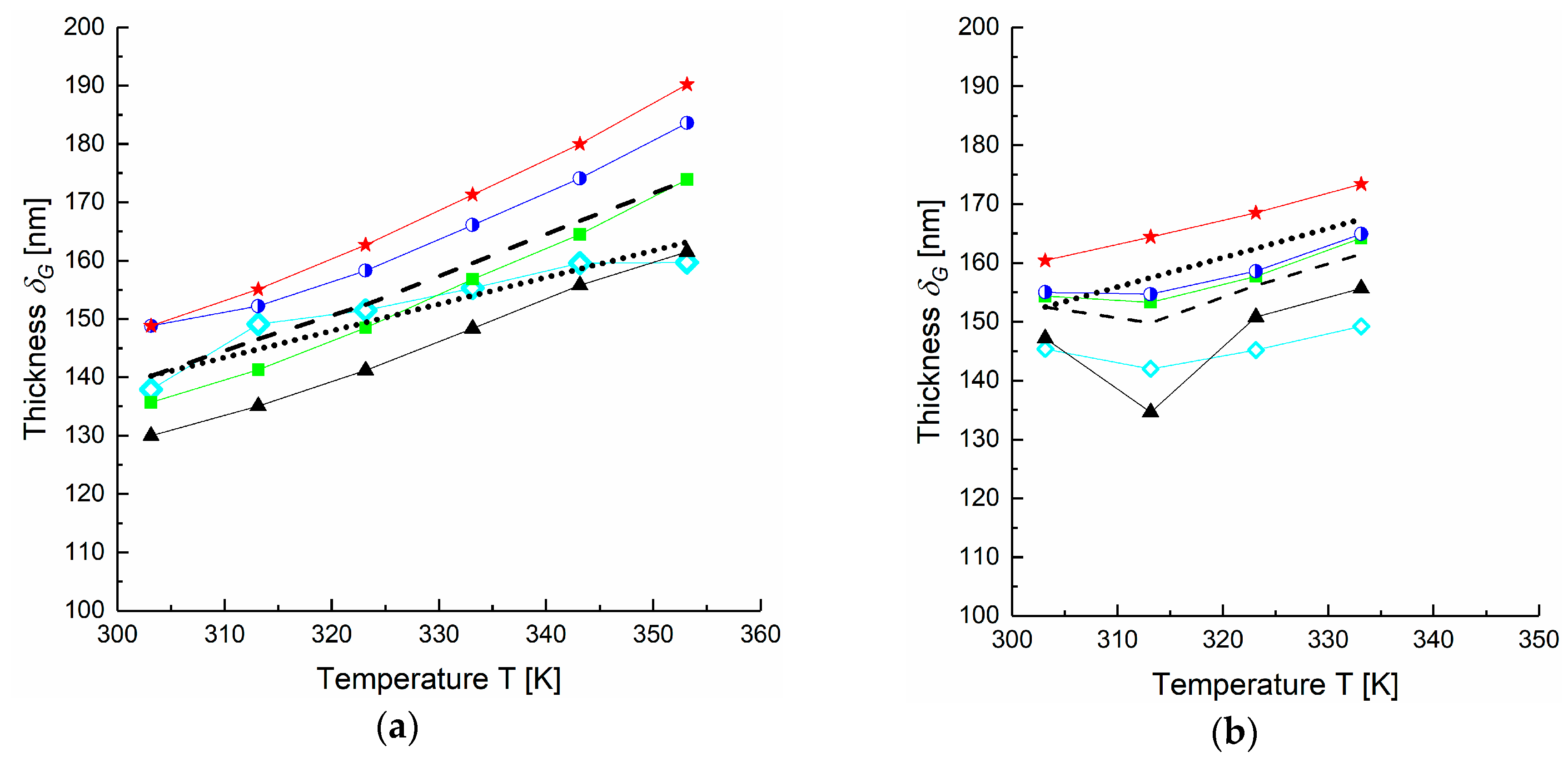
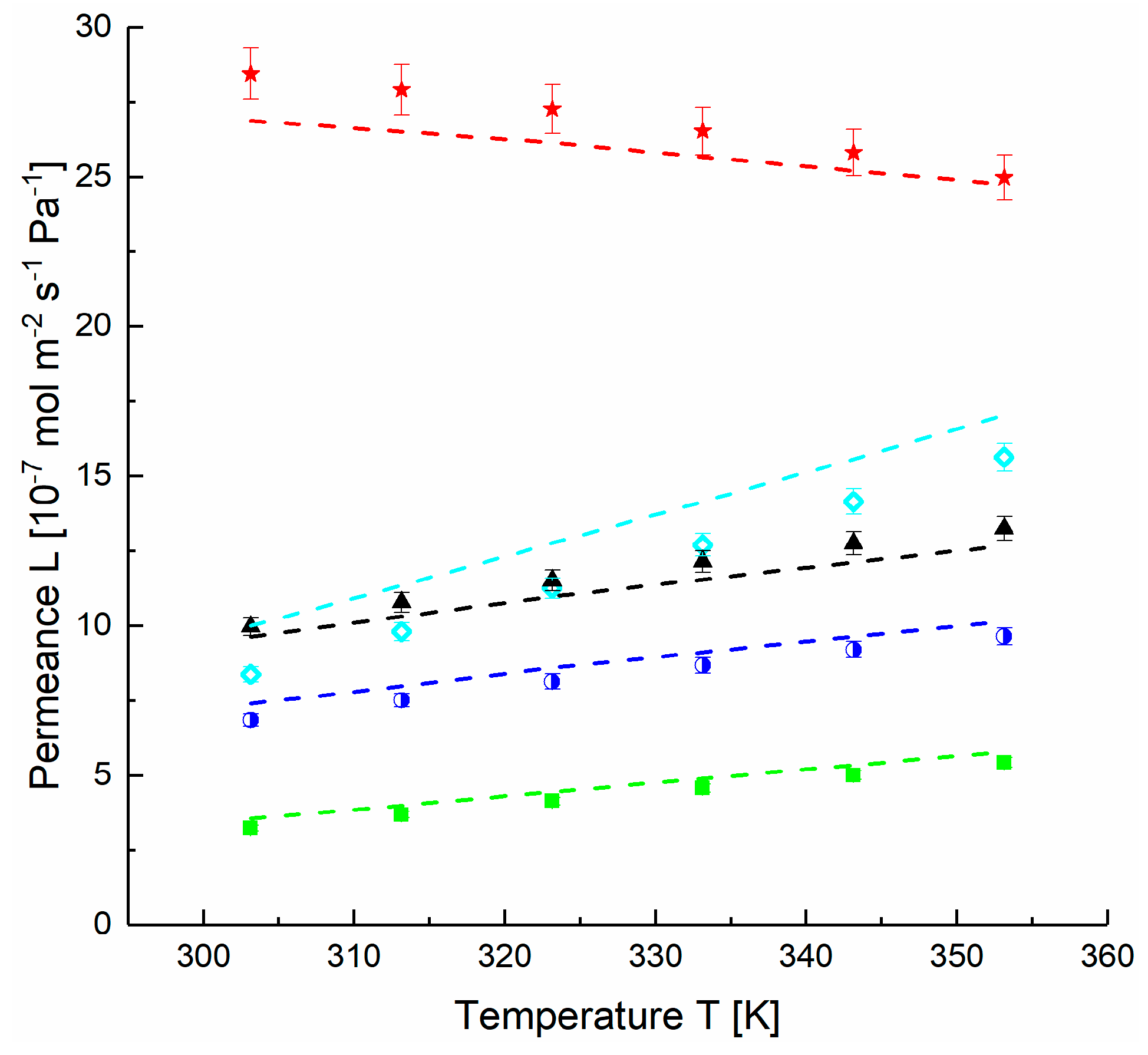
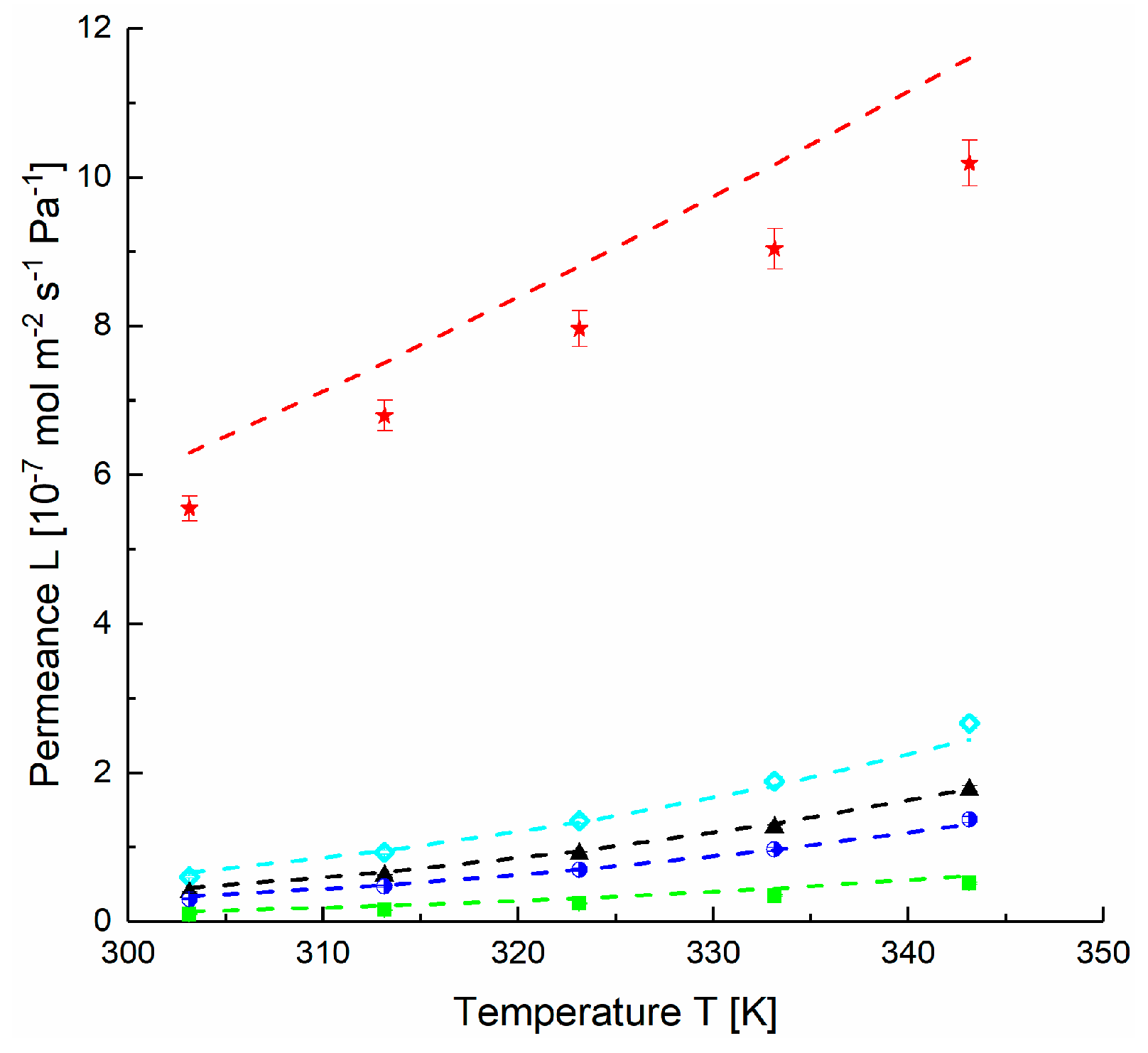
4.1. Application of DGM to Experimental Data
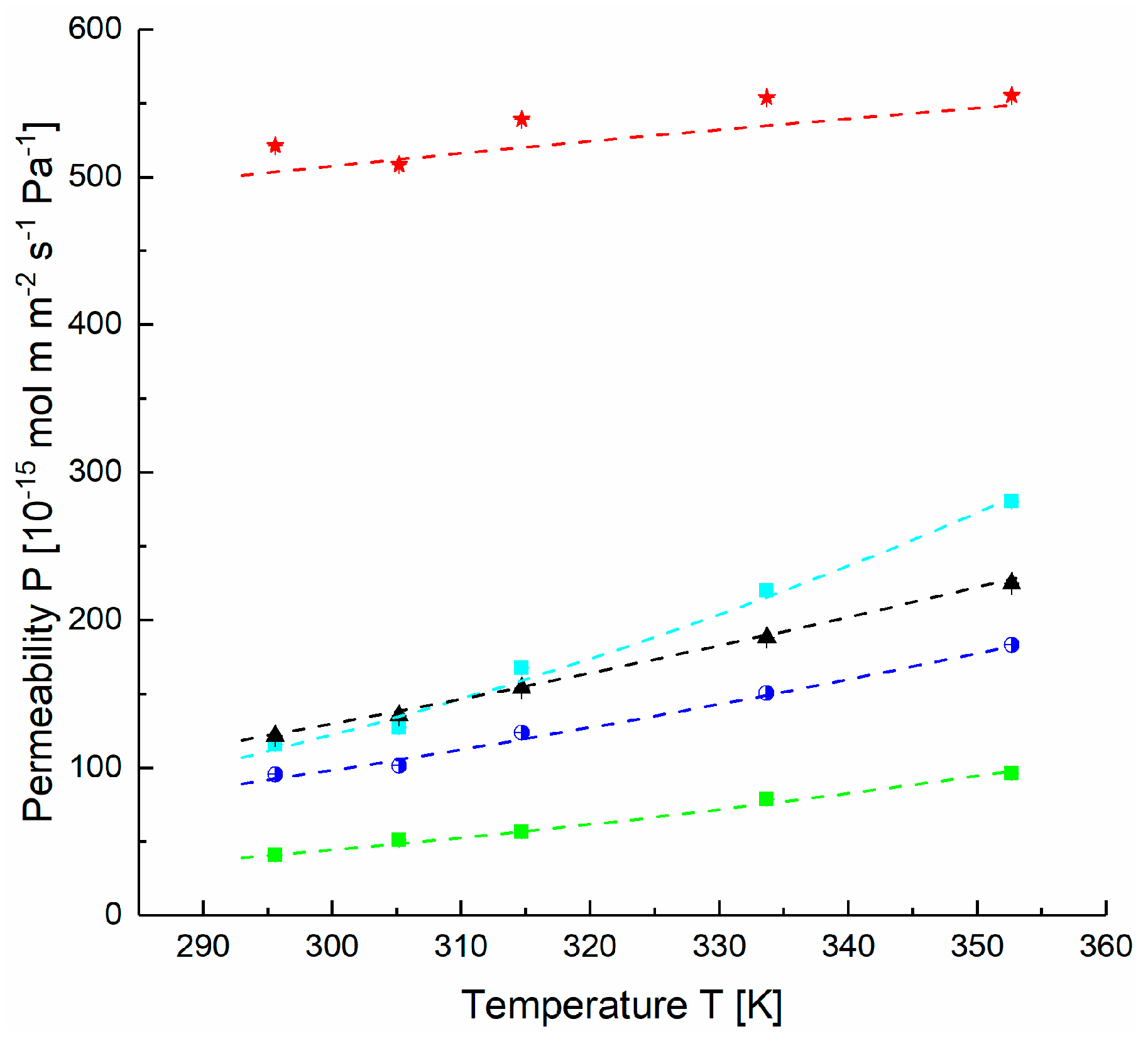
4.2. Estimation of FVM Parameters for Dense Layers
- Scenario 1: using gas transport data of Stamp 1-2 and estimated pore diameter dpore = 133 nm and structure parameter ε/τ=0.056. Thickness of porous support δPS = 30 µm.
- Scenario 2: using gas transport data of Stamp 1-2 with dpore = 133 nm and ε/τ = 0.056. The thickness of the porous support δPS was decreased from 30 µm to 23 µm in order to examine the influence of the roughness of the porous support.
- Scenario 3: using gas transport data of Stamp 1-2 with dpore = 133 nm and ε/τ = 0.056. The thickness of the porous support δPS was increased from 30 µm to 40 µm.
- Scenario 4: using gas transport data of Stamp 2-2 with dpore = 119 nm and ε/τ = 0.099 in order to compare the results of modeling with the results for Stamp 1.
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| A | Cross sectional membrane area, m2 |
| dpore | Pore diameter, m |
| Eact | Activation energy of permeability, J/mol |
| f | Fugacity, Pa |
| L | Permeance, mol∙m/(m2∙s∙Pa) |
| m0 | Swelling factor at zero temperature, Pa−1 |
| mT | Factor of temperature dependency of the swelling, K−1 |
| M | Molecular weight, kg/mol |
| Flow rate, mol/s | |
| Molar flux through the membrane divided by area, mol/(m2∙s) | |
| p | Pressure, Pa |
| P | Permeability coefficient, mol∙m/(m2∙s∙Pa) |
| R | Gas constant, 8.314 Pa∙m3/(mol∙K) |
| t | Time, s |
| T | Temperature, K |
| V | Volume, m3 |
| Volumetric flow rate, m3/s | |
| Greek symbols | |
| ∆α | Thermal expansion coefficient, K−1 |
| Thickness of layer j, m | |
| ε | Fractional void volume of porous medium |
| η | Dynamic viscosity of the gas, Pa∙s |
| τ | Tortuosity factor |
| Subscripts | |
| a | Average |
| act | Activation |
| F | Feed |
| G | Gutter layer |
| i | Component i |
| j | Layer j |
| P | Permeate |
| pore | Pore |
| PS | Porous support |
| S | Selective layer |
| t | Total |
| T | Temperature |
| W | Pore wall |
| ∞ | Temperature → ∞ |
| Superscripts | |
| ∙ | Time derivative |
| ´´ | Area derivative |
| 0 | Pressure → 0 |
Appendix A
References
- Henis, J.M.; Tripodi, M.K. Multicomponent Membranes for Gas Separations. U.S. Patent US4230463A, 28 October 1980. [Google Scholar]
- Fouda, A.; Chen, Y.; Bai, J.; Matsuura, T. Wheatstone Bridge Model for the Laminated Polydimethylsiloxane Polyethersulfone Membrane for Gas Separation. J. Membr. Sci. 1991, 64, 263–271. [Google Scholar] [CrossRef]
- Shilton, S.J.; Bell, G.; Ferguson, J. The deduction of fine structural details of gas separation hollow fibre membranes using resistance modelling of gas permeation. Polymer 1996, 37, 485–492. [Google Scholar] [CrossRef]
- Shieh, J.J.; Chung, T.S.; Paul, D.R. Study on multi-layer composite hollow fiber membranes for gas separation. Chem. Eng. Sci. 1999, 54, 675–684. [Google Scholar] [CrossRef]
- Peng, F.B.; Liu, J.Q.; Li, J.T. Analysis of the gas transport performance through PDMS/PS composite membranes using the resistances-in-series model. J. Membr. Sci. 2003, 222, 225–234. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes 2018, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Hu, Y.; Masuda, T. Substituted Polyacetylenes. In Membrane Materials for Gas and Vapor Separation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–142. ISBN 9781119112747. [Google Scholar]
- Fritsch, D.; Bengtson, G.; Carta, M.; McKeown, N.B. Synthesis and Gas Permeation Properties of Spirobischromane-Based Polymers of Intrinsic Microporosity. Macromol. Chem. Phys. 2011, 212, 1137–1146. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.M.; Lillepaerg, J.; Abetz, V. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Membr. Sci. 2013, 436, 109–120. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lilleparg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Tena, A.; Rangou, S.; Shishatskiy, S.; Filiz, V.; Abetz, V. Claisen thermally rearranged (CTR) polymers. Sci. Adv. 2016, 2, e1501859. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Hao, P.J. Influence of the porous support on diffusion in composite membranes. J. Membr. Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Koester, S.; Lolsberg, J.; Lutz, L.; Marten, D.; Wessling, M. On individual resistances of selective skin, porous support and diffusion boundary layer in water vapor permeation. J. Membr. Sci. 2016, 507, 179–187. [Google Scholar] [CrossRef]
- Kattula, M.; Ponnuru, K.; Zhu, L.; Jia, W.; Lin, H.; Furlani, E.P. Designing ultrathin film composite membranes: The impact of a gutter layer. Sci. Rep. 2015, 5, 15016. [Google Scholar] [CrossRef] [PubMed]
- Pauls, J.R.; Fritsch, D.; Klassen, T.; Peinemann, K.-V. Gas permeation measurement under defined humidity via constant volume/variable pressure method. J. Membr. Sci. 2012, 389 (Suppl. C), 343–348. [Google Scholar] [CrossRef]
- Mason, E.A.; Malinauskas, A.P. Gas Transport in Porous Media: The Dusty-Gas Model; Elsevier: Amsterdam, The Netherlands, 1983; ISBN 9780444412959. [Google Scholar]
- Kipp, S. Charakterierung CO2-Selektiver Membranen zur Biogasaufbereitung; Diplomarbeit, TUHH HZG: Hamburg, Germany, 2010. [Google Scholar]
- Lilleparg, J.; Georgopanos, P.; Shishatskiy, S. Stability of blended polymeric materials for CO2 separation. J. Membr. Sci. 2014, 467, 269–278. [Google Scholar] [CrossRef]
- Scharnagl, N.; Buschatz, H. Polyacrylonitrile (PAN) membranes for ultra- and microfiltration. Desalination 2001, 139, 191–198. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.V.; Nunes, S.P.; Scharnagl, N.; et al. Developments in membrane research: From material via process design to industrial application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Peter, J.; Peinemann, K.V. Multilayer composite membranes for gas separation based on crosslinked PTMSP gutter layer and partially crosslinked Matrimid (R) 5218 selective layer. J. Membr. Sci. 2009, 340, 62–72. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Cabasso, I.; Lundy, K.A. Method of Making Membranes for Gas Separation and the Composite Membranes. U.S. Patent US4602922A, 29 July 1986. [Google Scholar]
- Lillepärg, J.; Georgopanos, P.; Emmler, T.; Shishatskiy, S. Effect of the reactive amino and glycidyl ether terminated polyethylene oxide additives on the gas transport properties of Pebax® bulk and thin film composite membranes. RSC Adv. 2016, 6, 11763–11772. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Nistor, C.; Shishatskiy, S.; Popa, M.; Nunes, S.P. CO2 Selective Membranes Based on Epoxy Silane. Rev. Roum. Chim. 2009, 54, 603–610. [Google Scholar]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J.A. Review article number 50—The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Epstein, N. On Tortuosity and the Tortuosity Factor in Flow and Diffusion through Porous-Media. Chem. Eng. Sci. 1989, 44, 777–779. [Google Scholar] [CrossRef]
- Stern, S.A.; Fang, S.M.; Frisch, H.L. Effect of pressure on gas permeability coefficients. A new application of “free volume” theory. J. Polym. Sci. Part A Polym. Phys. 1972, 10, 201–219. [Google Scholar] [CrossRef]
- Webb, S.W. Gas transport mechanisms. In Gas Transport in Porous Media; Springer: Amsterdam, The Netherlands, 2006; pp. 5–26. [Google Scholar]
- Brinkmann, T.; Naderipour, C.; Pohlmann, J.; Wind, J.; Wolff, T.; Esche, E.; Müller, D.; Wozny, G.; Hoting, B. Pilot scale investigations of the removal of carbon dioxide from hydrocarbon gas streams using poly (ethylene oxide)–poly (butylene terephthalate) PolyActive™) thin film composite membranes. J. Membr. Sci. 2015, 489, 237–247. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.; Nagai, K.; Freeman, B.D. Hydrocarbon and perfluorocarbon gas sorption in poly(dimethylsiloxane), poly(1-trimethylsilyl-1-propyne), and copolymers of tetrafluoroethylene and 2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole. Macromolecules 1999, 32, 370–374. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z.J. Pure- and mixed-gas permeation properties of polydimethylsiloxane for hydrocarbon/methane and hydrocarbon/hydrogen separation. J. Membr. Sci. 2004, 244, 227–233. [Google Scholar] [CrossRef]
- Flichy, N.M.B.; Kazarian, S.G.; Lawrence, C.J.; Briscoe, B.J. An ATR−IR Study of Poly (Dimethylsiloxane) under High-Pressure Carbon Dioxide: Simultaneous Measurement of Sorption and Swelling. J. Phys. Chem. B 2002, 106, 754–759. [Google Scholar] [CrossRef]
- Sirard, S.M.; Green, P.F.; Johnston, K.P. Spectroscopic ellipsometry investigation of the swelling of poly(dimethylsiloxane) thin films with high pressure carbon dioxide. J. Phys. Chem. B 2001, 105, 766–772. [Google Scholar] [CrossRef]
- Machado, D.R.; Hasson, D.; Semiat, R. Effect of solvent properties on permeate flow through nanofiltration membranes—Part II. Transport model. J. Membr. Sci. 2000, 166, 63–69. [Google Scholar] [CrossRef]
- Ohlrogge, K.; Wind, J.; Brinkmann, T. Membranes for Recovery of Volatile Organic Compounds. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010; pp. 213–242. ISBN 978-0-08-093250-7. [Google Scholar]
- Matyka, M.; Khalili, A.; Koza, Z. Tortuosity-porosity relation in porous media flow. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2008, 78 Pt 2, 026306. [Google Scholar] [CrossRef]
- Pisani, L. Simple Expression for the Tortuosity of Porous Media. Transp. Porous Media 2011, 88, 193–203. [Google Scholar] [CrossRef]
- Volkenandt, T.; Muller, E.; Gerthsen, D. Sample thickness determination by scanning transmission electron microscopy at low electron energies. Microsc. Microanal. 2014, 20, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; te Nijenhuis, K. (Eds.) Chapter 13—Mechanical Properties of Solid Polymers. In Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 383–503. ISBN 978-0-08-054819-7. [Google Scholar]
- Zhang, Y.X.; Chen, Y.; Yan, M.F.; Chen, F.L. New formulas for the tortuosity factor of electrochemically conducting channels. Electrochem. Commun. 2015, 60, 52–55. [Google Scholar] [CrossRef]
- Robeson, L.M.; Smith, Z.P.; Freeman, B.D.; Paul, D.R. Contributions of diffusion and solubility selectivity to the upper bound analysis for glassy gas separation membranes. J. Membr. Sci. 2014, 453, 71–83. [Google Scholar] [CrossRef]
- Coefficient of Linear Thermal Expansion (CLTE): Formula & Values. Available online: https://omnexus.specialchem.com/polymer-properties/properties/coefficient-of-linear-thermal-expansion#values (accessed on 20 October 2018).
- Pochan, D.J.; Lin, E.K.; Satija, S.K.; Wu, W.-L. Thermal Expansion of Supported Thin Polymer Films: A Direct Comparison of Free Surface vs. Total Confinement. Macromolecules 2001, 34, 3041–3045. [Google Scholar] [CrossRef]




| Designation | Description |
|---|---|
| Stamp 1 | PE / PAN porous support |
| Stamp 1-2 | PE / PAN / PDMS gutter layer |
| Stamp 1-3 | PE / PAN / PDMS / PolyActiveTM separation layer |
| Stamp 2 | PE / PAN porous support |
| Stamp 2-2 | PE / PAN / PDMS gutter layer |
| Stamp 2-3 | PE / PAN / PDMS / PolyActiveTM separation layer |
(10−15·mol·m·m−2·s−1·Pa−1) 1 | α (i/N2) | (10−15·mol·m·m−2·s−1·Pa−1) 1 | (kJ·mol−1) | |
|---|---|---|---|---|
| PDMS of gutter layer | ||||
| H2 | 127.0 | 2.8 | 33,424 | 14.0 |
| CH4 | 135.3 | 3.0 | 5567 | 9.4 |
| N2 | 45.1 | 1.0 | 8822 | 13.2 |
| O2 | 101.2 | 2.2 | 6067 | 10.3 |
| CO2 | 507.8 | 11.3 | 862 | 1.3 |
| PolyActiveTM | ||||
| H2 | 5.9 | 5.2 | 730,721 | 29.7 |
| CH4 | 3.5 | 3.1 | 854,055 | 31.1 |
| N2 | 1.1 | 1.0 | 607,510 | 33.2 |
| O2 | 2.9 | 2.6 | 538,272 | 30.7 |
| CO2 | 60.5 | 53.2 | 39,320 | 16.4 |
| Varied Parameters | Scenario | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Stamp No. | 1-2 | 1-2 | 1-2 | 2-2 |
| ε/τ | 0.056 | 0.056 | 0.056 | 0.099 |
| dpore (nm) | 133 | 133 | 133 | 119 |
| δPS (µm) | 30 | 23 | 40 | 30 |
| Gas | Thickness of gutter layer δG (nm) | |||
| H2 | 138 | 140 | 135 | 145 |
| CH4 | 130 | 166 | 161 | 147 |
| N2 | 136 | 136 | 135 | 154 |
| O2 | 149 | 149 | 145 | 155 |
| CO2 | 149 | 160 | n.a. | 160 |
| Average δG (nm) | 140 | 150 | 115 | 150 |
| SD | 7 | 13 | 65 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lillepärg, J.; Breitenkamp, S.; Shishatskiy, S.; Pohlmann, J.; Wind, J.; Scholles, C.; Brinkmann, T. Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation. Membranes 2019, 9, 22. https://doi.org/10.3390/membranes9020022
Lillepärg J, Breitenkamp S, Shishatskiy S, Pohlmann J, Wind J, Scholles C, Brinkmann T. Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation. Membranes. 2019; 9(2):22. https://doi.org/10.3390/membranes9020022
Chicago/Turabian StyleLillepärg, Jelena, Sabrina Breitenkamp, Sergey Shishatskiy, Jan Pohlmann, Jan Wind, Carsten Scholles, and Torsten Brinkmann. 2019. "Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation" Membranes 9, no. 2: 22. https://doi.org/10.3390/membranes9020022
APA StyleLillepärg, J., Breitenkamp, S., Shishatskiy, S., Pohlmann, J., Wind, J., Scholles, C., & Brinkmann, T. (2019). Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation. Membranes, 9(2), 22. https://doi.org/10.3390/membranes9020022







