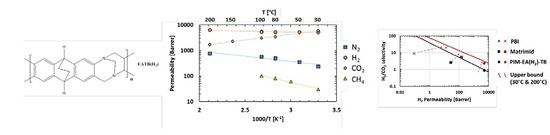
Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer
Abstract
1. Introduction
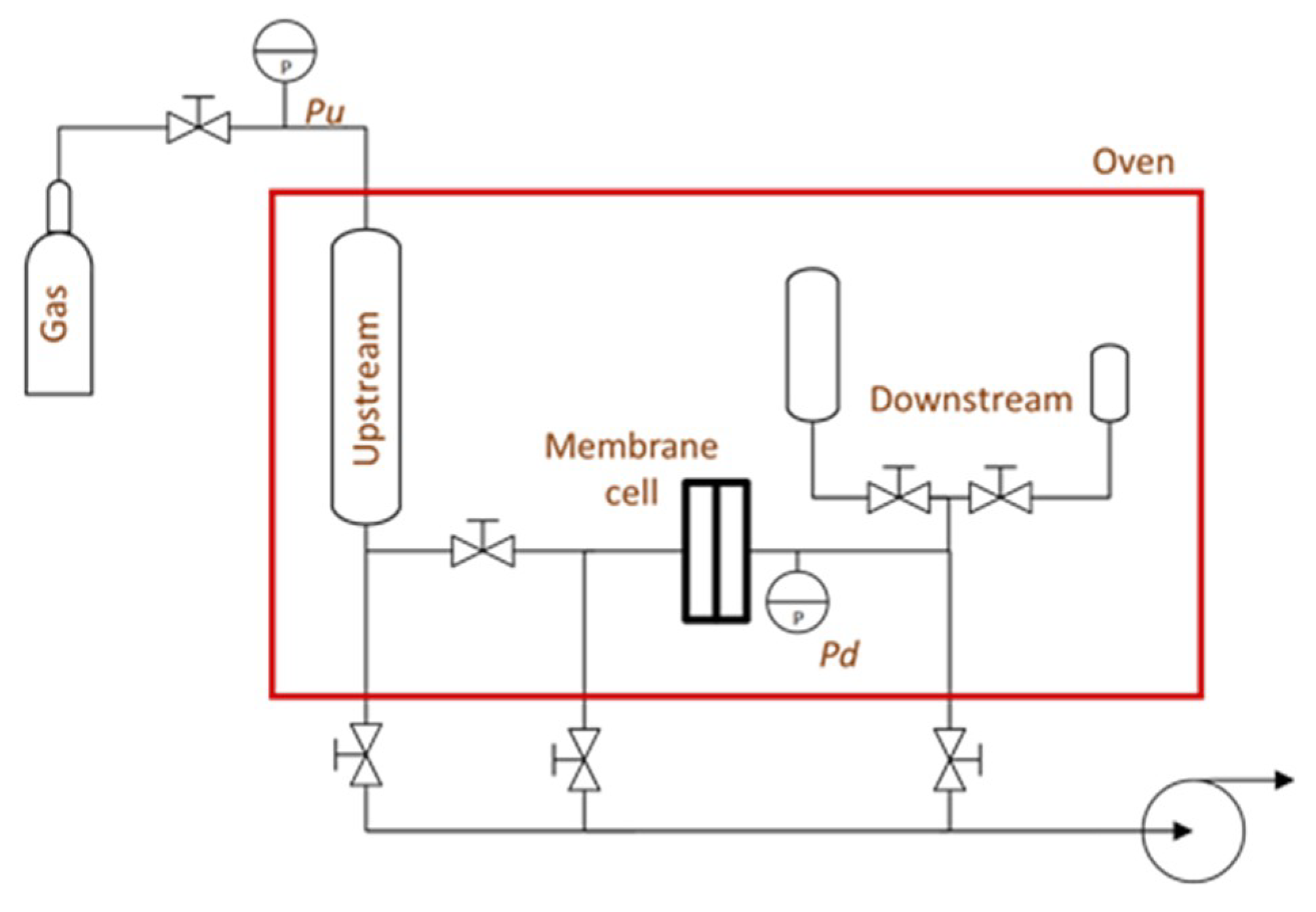
2. Experimental Section
3. Results
3.1. Permeability
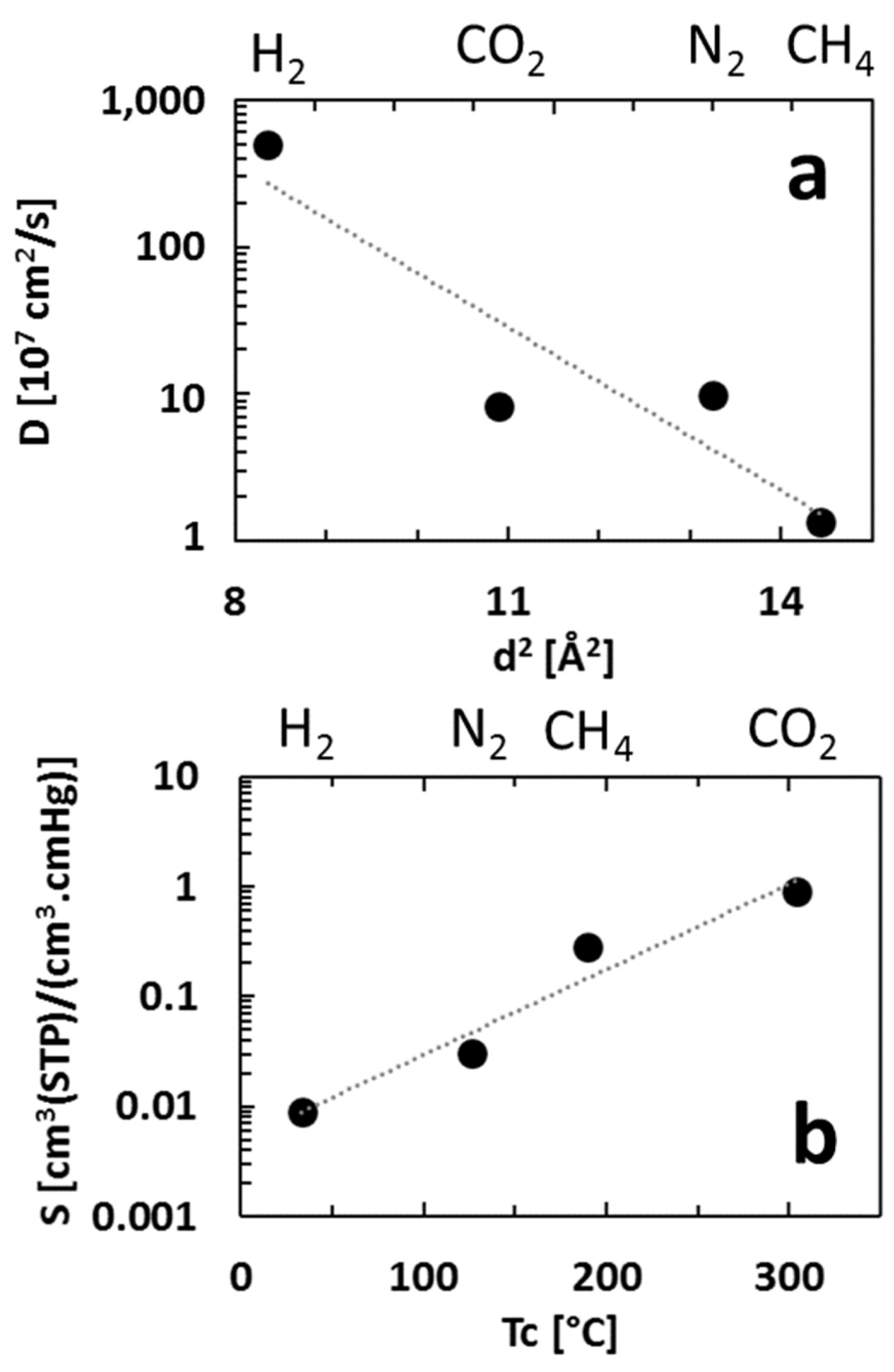
3.2. Diffusivity and Solubility Coefficients
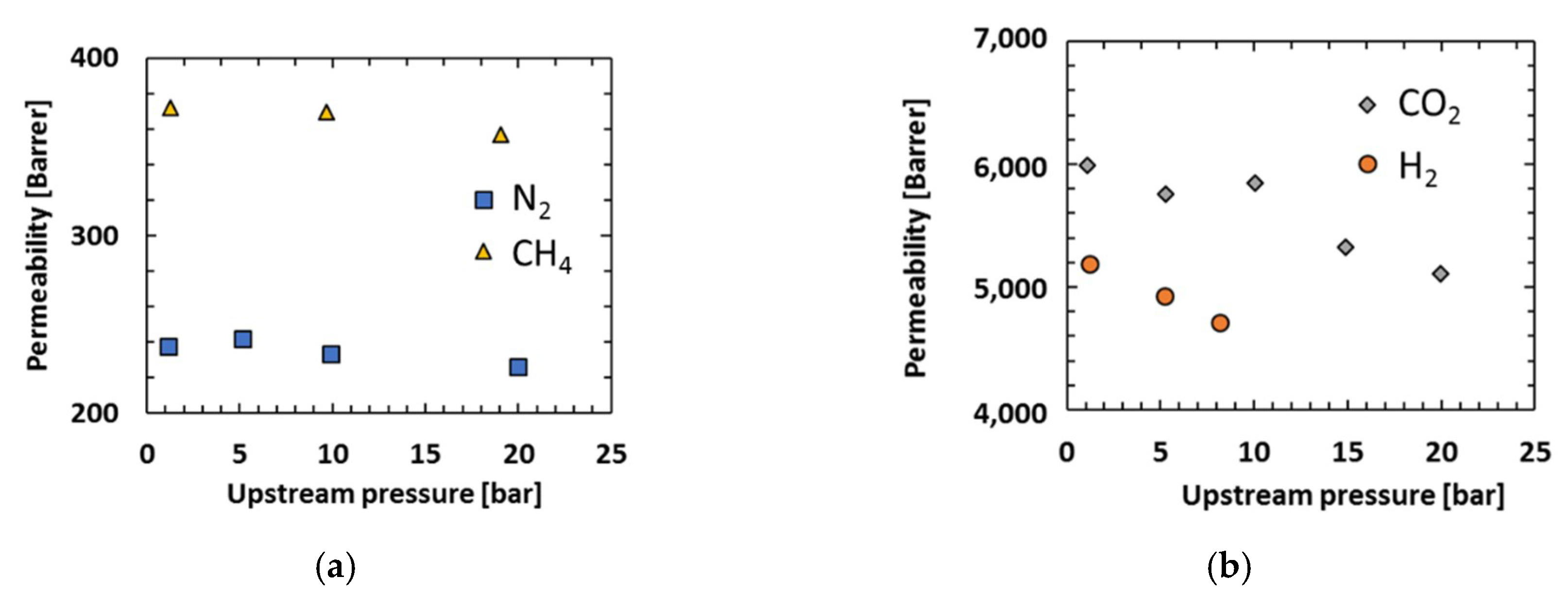
3.3. Effect of Pressure
3.4. Effect of Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Merkel, T.; Zhou, M.; Baker, R. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Franz, J.; Scherer, V. An evaluation of CO2 and H2 selective polymeric membranes for CO2 separation in IGCC processes. J. Membr. Sci. 2010, 359, 173–183. [Google Scholar] [CrossRef]
- Singh, R.; Berchtold, K. H2 Selective Membranes for Precombustion Carbon Capture. In Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 177–206. [Google Scholar]
- Abanades, J.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.; Li, H.; Hoc, M.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control 2015, 40, 126–166. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.; Paul, D. Temperature dependence of gas sorption and permeation in PIM-1. J. Membr. Sci. 2014, 450, 380–388. [Google Scholar] [CrossRef]
- Merkel, T.; Gupta, R.; Turk, B.; Freeman, B. Mixed gas permeation of syngas components in poly(dimethylsiloxane) and poly(1-trimethysilyl-1-propyne) at elevated temperatures. J. Membr. Sci. 2001, 191, 85–94. [Google Scholar] [CrossRef]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Urtiaga, A.; Ortiz, I. Mixed gas separation study for the hydrogen recovery from H2/CO/N2/CO2 post combustion mixtures using a Matrimid membrane. J. Membr. Sci. 2011, 378, 359–368. [Google Scholar] [CrossRef]
- Pesiri, D.R.; Jorgensen, B.; Dye, R.C. Thermal optimization of polybenzimidazole meniscus membranes for the separation of hydrogen, methane, and carbon dioxide. J. Membr. Sci. 2003, 218, 11–18. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Fuoco, A.; Comesaña-Gándara, B.; Longo, M.; Esposito, E.; Monteleone, M.; Rose, I.; Bezzu, C.; Carta, M.; McKeown, N.; Jansen, J. Temperature Dependence of Gas Permeation and Diffusion in Triptycene-Based Ultrapermeable Polymers of Intrinsic Microporosity. ACS Appl. Mater. Interfaces 2018, 10, 36475–36482. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.; Iniesta, J.; Leguey, V.M.; Armstrong, R.; Taylor, S.; Madrid, E.; Rong, Y.; Castaing, R.; Malpass-Evans, R.; Carta, M.; et al. Carbonization of polymers of intrinsic porosity to microporous heterocarbon: Capacitive pH measurements. Appl. Mater. Today 2017, 9, 136–144. [Google Scholar] [CrossRef]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Membr. Sci. 2019, 569, 17–31. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanč, M.; McKeown, N. Triptycene Induced Enhancement of Membrane Gas Selectivity for Microporous Tröger’s Base Polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Tocci, E.; Lorenzo, L.D.; Bernardo, P.; Clarizia, G.; Bazzarelli, F.; Mckeown, N.B.; Carta, M.; Malpass-Evans, R.; Friess, K.; Pilnáček, K.; et al. Molecular Modeling and Gas Permeation Properties of a Polymer of Intrinsic Microporosity Composed of Ethanoanthracene and Tröger’s Base Units. Macromolecules 2014, 47, 7900–7916. [Google Scholar] [CrossRef]
- Lau, C.; Konstas, K.; Doherty, C.; Carta, M.; Lasseuguette, E.; Ferrari, M.; McKeown, N.; Hill, M. Tailoring compatibility to deplasticize ageless, ultrapermeable blends for molecular sieving. In Proceedings of the ICOM 2017, San Francisco, CA, USA, 29 July–4 August 2017. [Google Scholar]
- Benito, J.; Sánchez-Laínez, J.; Zornoza, B.; Martín, S.; Carta, M.; Malpass-Evans, R.; Téllez, C.; McKeown, N.B.; Coronas, J.; Gascón, I. Ultrathin Composite Polymeric Membranes for CO2/N2 separation with Minimum Thickness and High CO2 permeance. ChemSusChem 2017, 10, 4014–4017. [Google Scholar] [CrossRef]
- Baker, R. Membrane and Technology and Applications; John Wiley & Sons: London, UK, 2004. [Google Scholar]
- Robeson, L. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Mason, C.R.; Maynard-Atem, L.; Heard, K.W.J.; Satilmis, B.; Budd, P.M.; Friess, K.; Lanč, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Polymer of Intrinsic Microporosity Incorporating Thioamide Functionality: Preparation and Gas Transport Properties. Macromolecules 2014, 47, 1021–1029. [Google Scholar] [CrossRef]
- Ghosal, K.; Chern, R.T.; Freeman, B.D.; Daly, W.H.; Negulescu, I.I. Effect of Basic Substituents on Gas Sorption and Permeation in Polysulfone. Macromolecules 1996, 29, 4360–4369. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.; Paul, D. Gas sorption and permeation in PIM-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical Aging, Plasticization and Their Effects on Gas Permeation in “Rigid” Polymers of Intrinsic Microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef]
- Thomas, S.; Pinnau, I.; Du, N.; Guiver, M.D. Pure- and mixed-gas permeation properties of a microporous spirobisindane-based ladder polymer (PIM-1). J. Membr. Sci. 2009, 333, 125–131. [Google Scholar] [CrossRef]
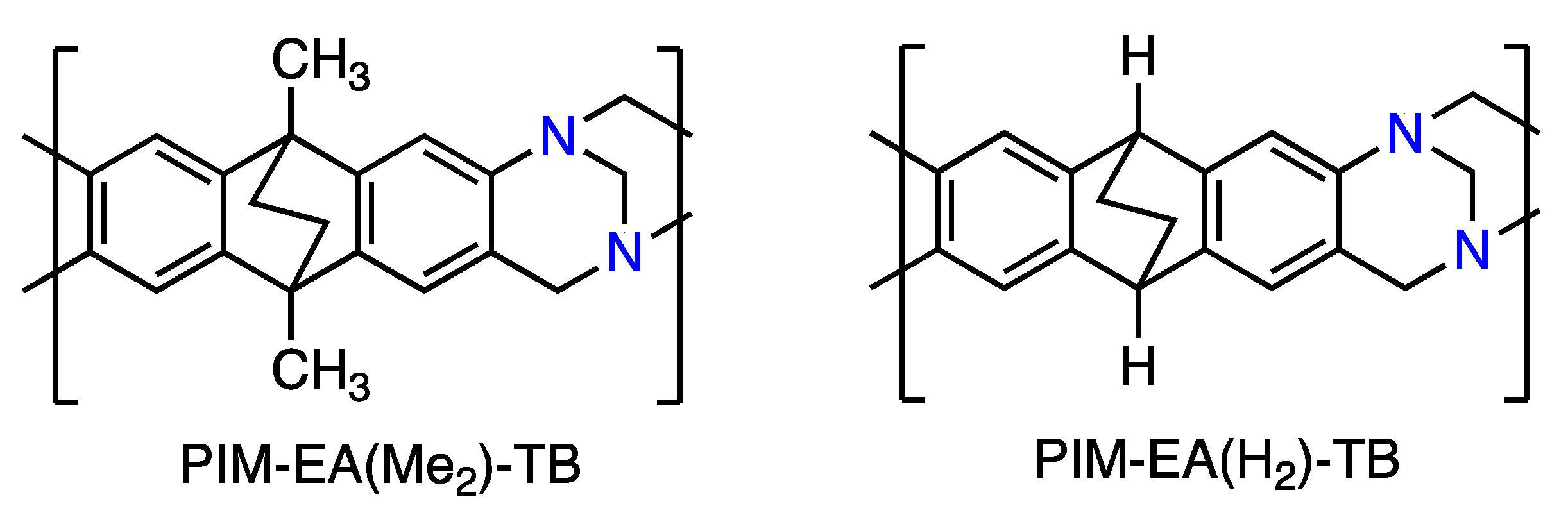
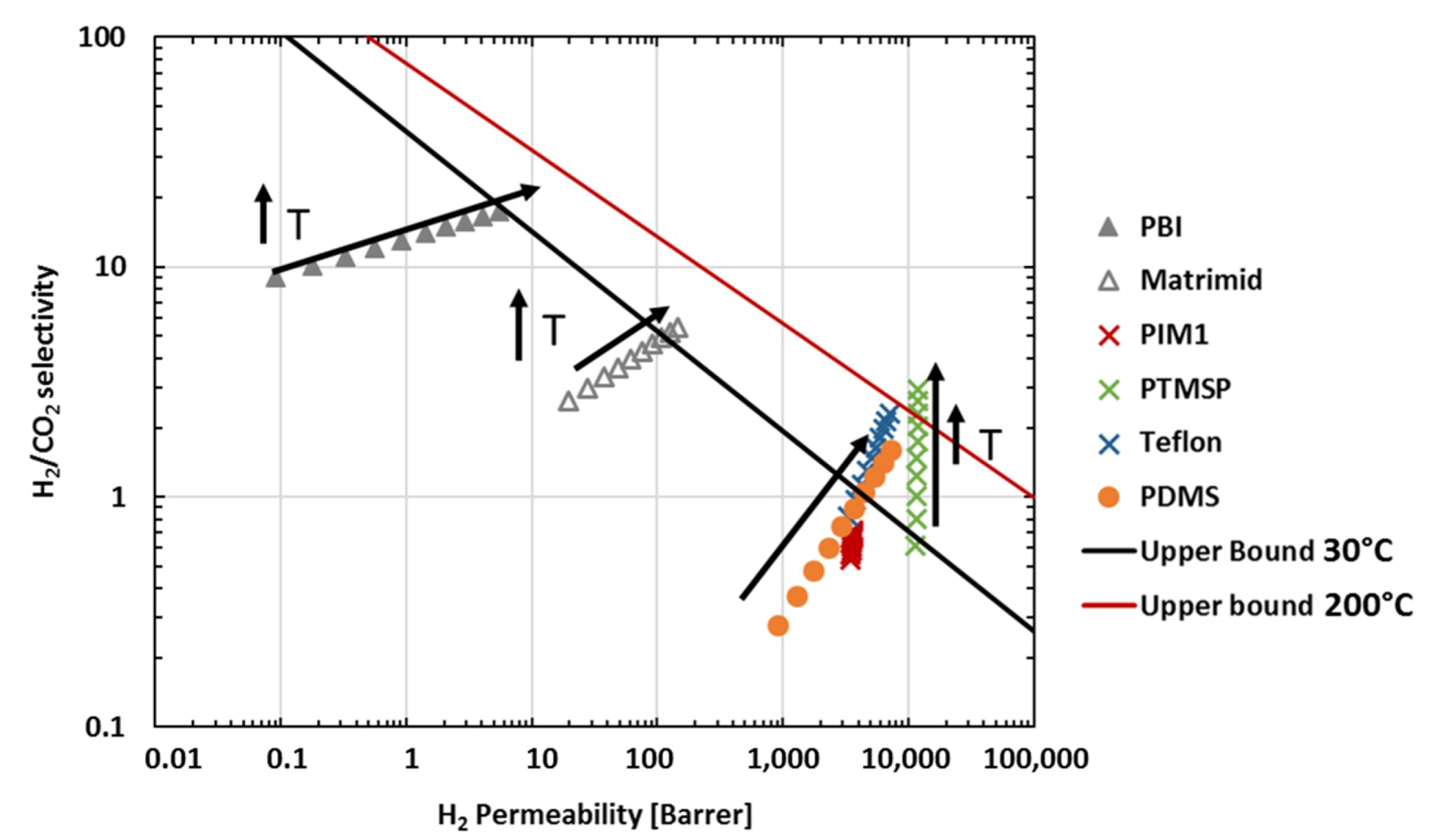
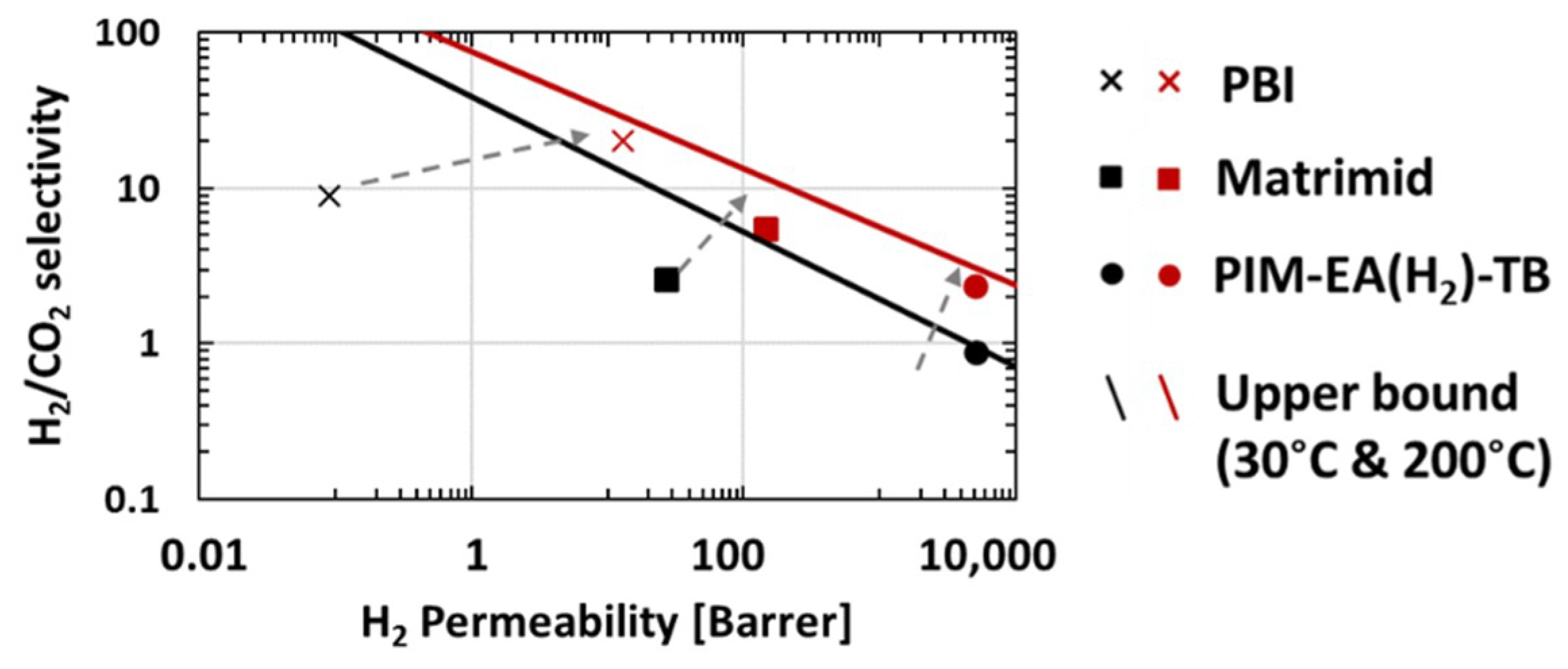
 ), PIM-EA(Me2)-TB [16] (
), PIM-EA(Me2)-TB [16] ( ) and PIM-EA(H2)-TB [our study] (
) and PIM-EA(H2)-TB [our study] ( ) at 30 °C and 1 bar. The lines represents the 2008 upper bound for each gas pair [21].
) at 30 °C and 1 bar. The lines represents the 2008 upper bound for each gas pair [21].


| Gas | Kinetic Diameter (d) (Å) | Critical Temperature (Tc) (K) |
|---|---|---|
| H2 | 2.89 | 33.2 |
| N2 | 3.64 | 126.2 |
| CH4 | 3.8 | 190.6 |
| CO2 | 3.3 | 304.2 |
| 30 °C, 1 bar | N2 | H2 | CO2 | CH4 | |
|---|---|---|---|---|---|
| PIM-1 [8] | Permeability (Barrer) | 252 | 2936 | 5303 | 440 |
| Selectivity CO2/Gas | 21 | 1.8 | - | 12 | |
| Selectivity H2/Gas | 12 | - | 0.5 | 6.7 | |
| PIM-EA(Me2)-TB [16] | Permeability (Barrer) | 525 | 7760 | 7140 | 699 |
| Selectivity CO2/Gas | 13.6 | 0.9 | - | 10 | |
| Selectivity H2/Gas | 14.8 | - | 1.1 | 11 | |
| PIM-EA(H2)-TB (This study) | Permeability (Barrer) (± Error) | 238 (± 3%) | 5188 (± 1%) | 5990 (± 1%) | 372 (± 3%) |
| Selectivity CO2/Gas | 25 | 1 | - | 16 | |
| Selectivity H2/Gas | 22 | - | 1 | 14 | |
| 30 °C, 1 bar | N2 | H2 | CO2 | CH4 |
|---|---|---|---|---|
| D (10−7 cm2/s) (± Error) | 9.7 (± 12%) | 500.0 (± 9%) | 8.2 (± 3%) | 1.3 (± 11%) |
| S (cm3(STP)/(cm3·cmHg)) (± Error) | 3 × 10−2 (± 15%) | 9 × 10−3 (± 10%) | 9 × 10−1 (± 4%) | 3 × 10−1 (± 14%) |
| Selectivity, 30 °C | H2/CO2 | H2/N2 | H2/CH4 | CO2/N2 | CO2/CH4 | CH4/N2 |
|---|---|---|---|---|---|---|
| 1 bar | 1 | 22 | 14 | 25 | 16 | 2 |
| 5 bar | 1 | 20 | - | 24 | - | - |
| 10 bar | 1 | 20 | 14 | 25 | 16 | 2 |
| 20 bar | - | - | - | 23 | 14 | 2 |
| Gas | EP (kJ/mol) | |||
|---|---|---|---|---|
| PIM-EA(H2)-TB (This Study) | PIM-1 [26] | PIM-TMN-Trip [12] | PTMSP [26] | |
| H2 | 0.5 | −0.4 | −2.8 | −2.1 |
| N2 | 8.6 | 14.3 | 4.4 | −3.5 |
| CH4 | 13.1 | 19.4 | 9.5 | −5.3 |
| CO2 | −8.6 | −1 | −7.7 | −11.7 |
| 1 bar | EP (kJ/mol) | ED (kJ/mol) | ΔHs (kJ/mol) |
|---|---|---|---|
| CO2 | −8.6 | 8.1 | −16.7 |
| N2 | 8.6 | 18.5 | −9.9 |
| H2 | 0.5 | 5.2 | −4.6 |
| CH4 | 13.1 | 17.9 | −4.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasseuguette, E.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Ferrari, M.-C. Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer. Membranes 2018, 8, 132. https://doi.org/10.3390/membranes8040132
Lasseuguette E, Malpass-Evans R, Carta M, McKeown NB, Ferrari M-C. Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer. Membranes. 2018; 8(4):132. https://doi.org/10.3390/membranes8040132
Chicago/Turabian StyleLasseuguette, Elsa, Richard Malpass-Evans, Mariolino Carta, Neil B. McKeown, and Maria-Chiara Ferrari. 2018. "Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer" Membranes 8, no. 4: 132. https://doi.org/10.3390/membranes8040132
APA StyleLasseuguette, E., Malpass-Evans, R., Carta, M., McKeown, N. B., & Ferrari, M.-C. (2018). Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger’s Base Polymer. Membranes, 8(4), 132. https://doi.org/10.3390/membranes8040132