Optimization of Detergent-Mediated Reconstitution of Influenza A M2 Protein into Proteoliposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression, Purification and Spin-Labeling of M2 Protein
2.2. Liposome Preparation
2.3. Reconstitution of M2 Protein into Liposomes
2.4. Colorimetric Assay to Quantify Detergent Concentration
2.5. Dynamic Light Scattering
2.6. EPR Spectroscopy
3. Results: Optimization of OG-Mediated Reconstitution of Influenza M2 into Liposomes
3.1. Overview of Direct Insertion into Detergent-Saturated Liposomes Using OG
- preparing liposomes of a defined size, in our case by extrusion;
- adding detergent to the liposomes until they are saturated with detergent monomers;
- adding protein-detergent micelles to the detergent-saturated liposomes, and
- removing the detergent.
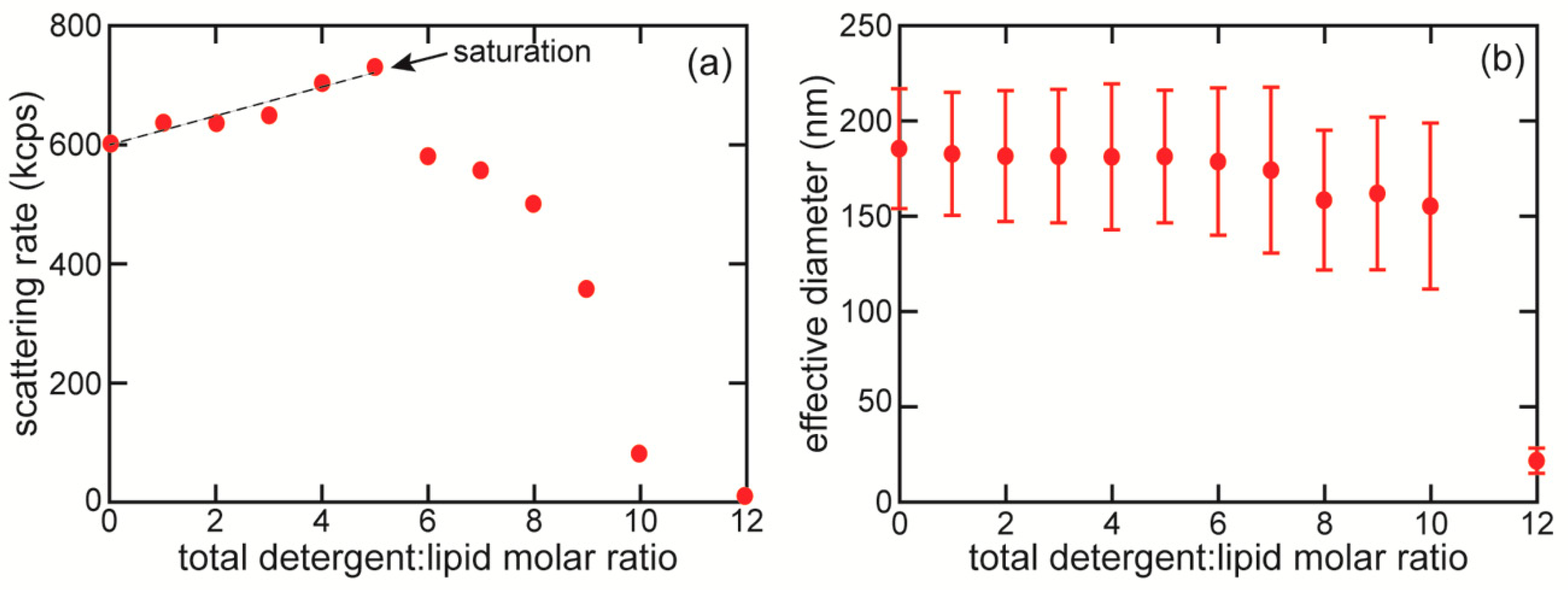
3.2. Detergent-Liposome Saturation Point Determination
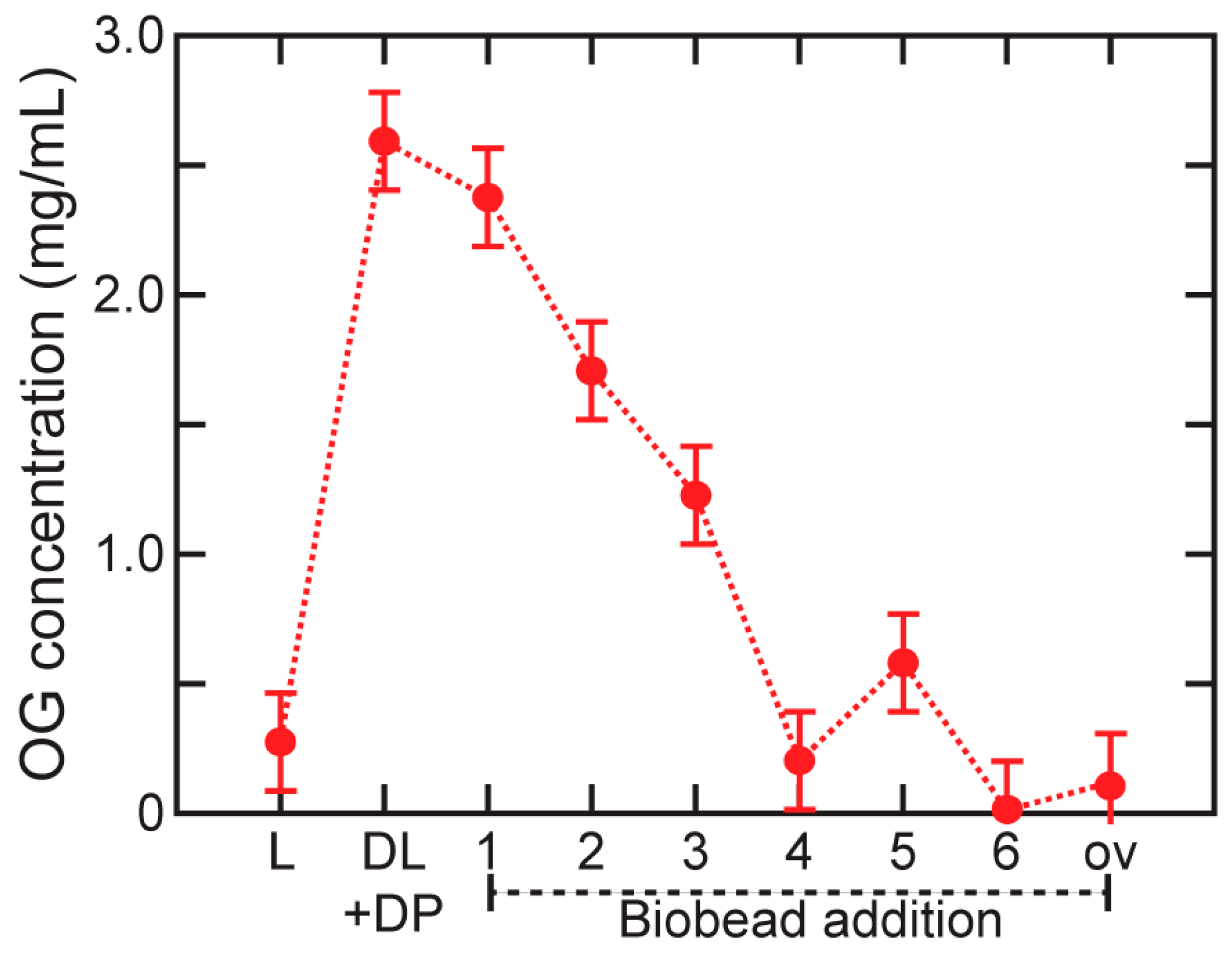
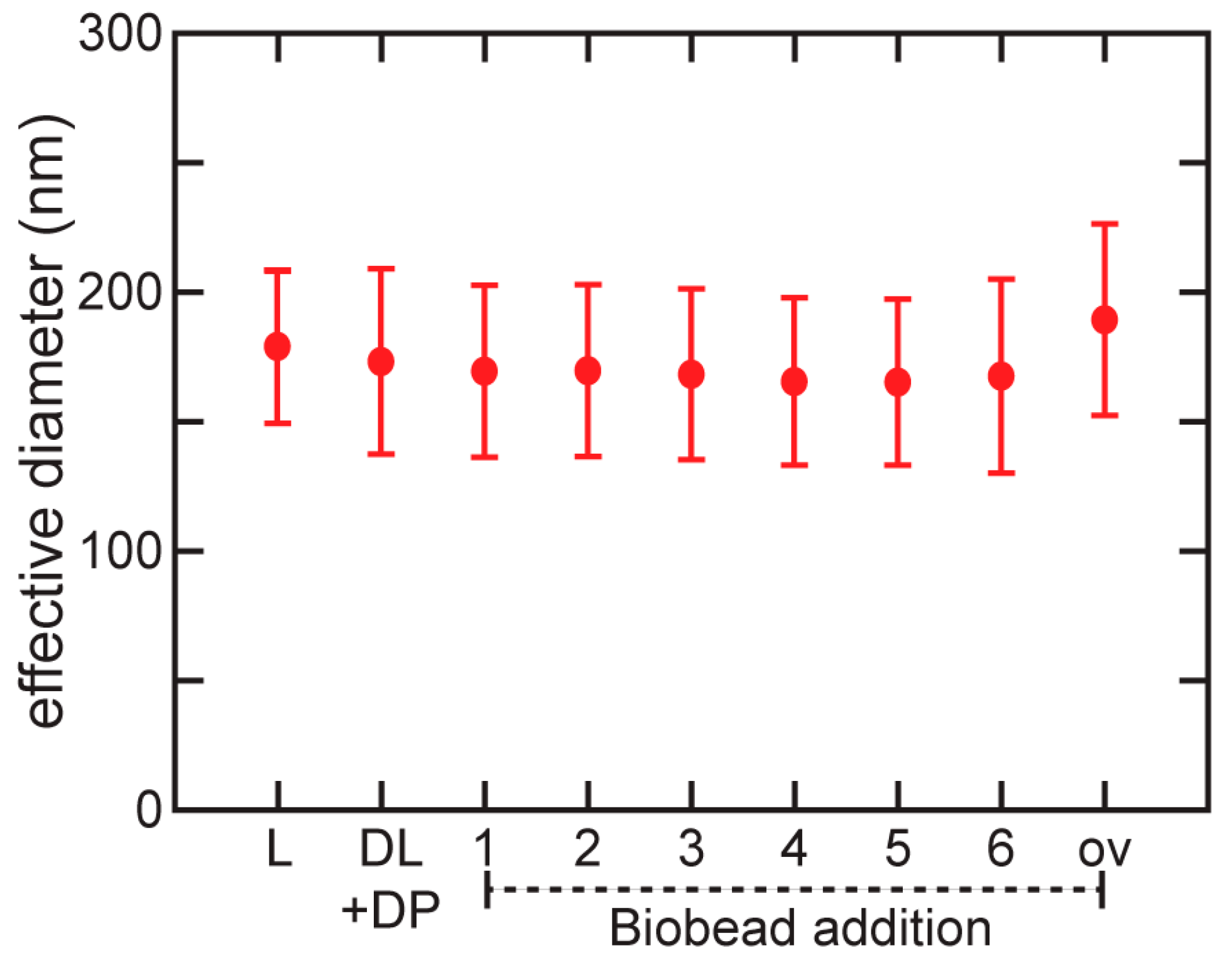
3.3. Characterization of Detergent Removal
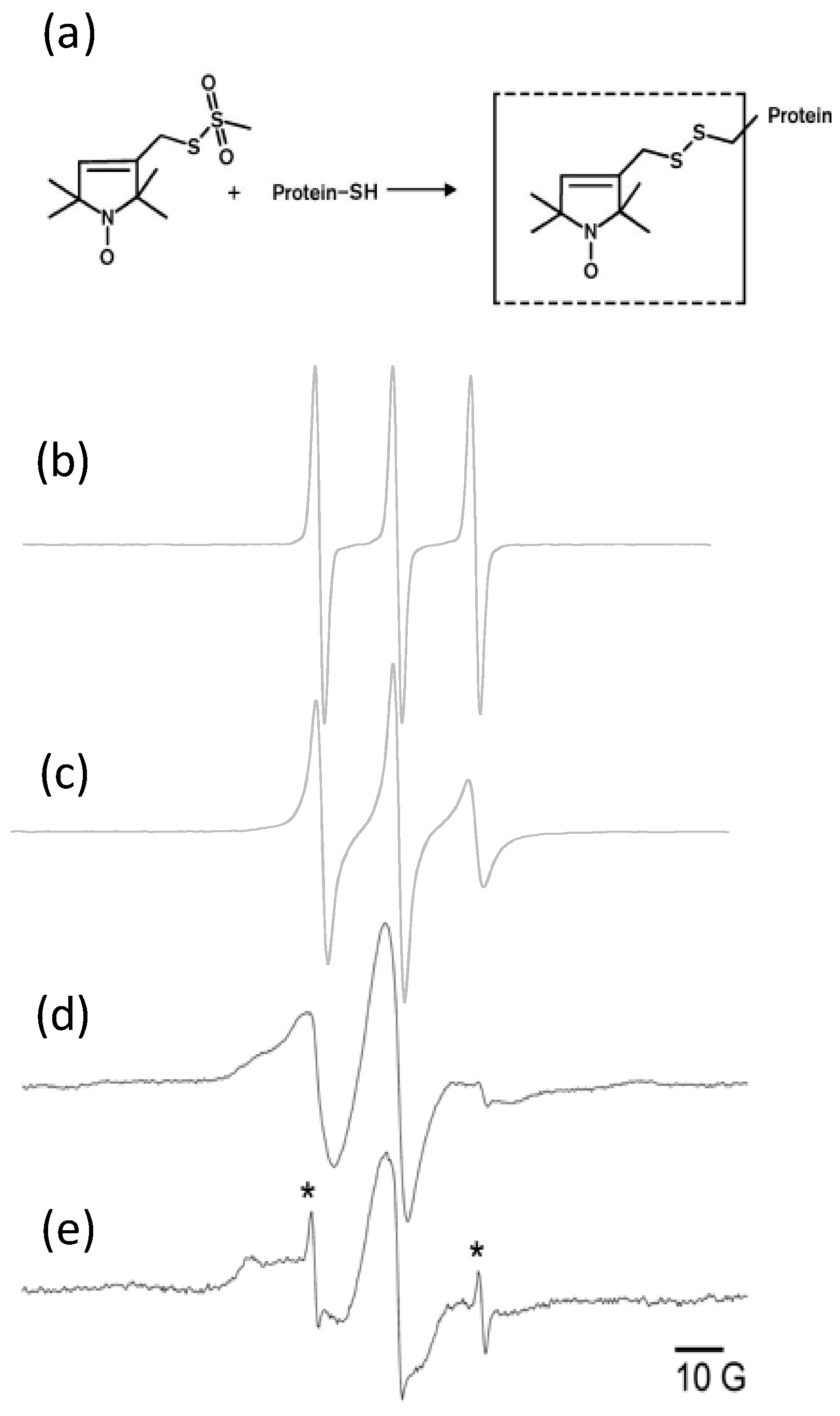
3.4. EPR Data from M2 Reconstituted into Liposomes Using Optimized Protocol
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fagerberg, L.; Jonasson, K.; von Heijne, G.; Uhlén, M.; Berglund, L. Prediction of the human membrane proteome. Proteomics 2010, 10, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, T.M.; Doig, A.J. Properties and identification of human protein drug targets. Bioinformatics 2009, 25, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.A.; Sharma, M.; Yi, M.; Zhou, H.X. Influence of solubilizing environments on membrane protein structures. Trends Biochem. Sci. 2011, 36, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, J.-L.; Pitard, B.; Lévy, D. Reconstitution of membrane proteins into liposomes: Application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1995, 1231, 223–246. [Google Scholar] [CrossRef]
- Rigaud, J.-L.; Lévy, D. Reconstitution of Membrane Proteins into Liposomes. Methods Enzym. 2003, 372, 65–86. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Tanford, C.; Reynolds, J.A. Phospholipid vesicle formation using nonionic detergents with low monomer solubility. Kinetic factors determine vesicle size and permeability. Biochemistry 1984, 23, 3070–3076. [Google Scholar] [CrossRef] [PubMed]
- Krämer, R.; Heberger, C. Functional reconstitution of carrier proteins by removal of detergent with a hydrophobic ion exchange column. Biochim. Biophys. Acta Biomembr. 1986, 863, 289–296. [Google Scholar] [CrossRef]
- Helenius, A.; Sarvas, M.; Simons, K. Asymmetric and Symmetric Membrane Reconstitution by Detergent Elimination. Eur. J. Biochem. 1981, 116, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Opatowski, E.; Kozlov, M.M. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim. Biophys. Acta Biomembr. 2000, 1508, 1–19. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Alonso, A.; Goñi, F.M. Detergent solubilization of lipid bilayers: A balance of driving forces. Trends Biochem. Sci. 2013, 38, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, J.X.; Soto, C.; DeGrado, W.F. Structural and dynamic mechanisms for the function and inhibition of the M2 proton channel from influenza A virus. Curr. Opin. Struct. Biol. 2011, 21, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Lamb, R.A. Influenza virus assembly and budding. Virology 2011, 411, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Green, B.; Thompson, M.; Chen, R.; Thomaston, J.; DeGrado, W.F.; Howard, K.P. C-terminal juxtamembrane region of full-length M2 protein forms a membrane surface associated amphipathic helix. Protein Sci. 2015, 24, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.; Soto, C.S.; Polishchuk, A.; Caputo, G.A.; Tatko, C.D.; Ma, C.L.; Ohigashi, Y.; Pinto, L.H.; DeGrado, W.F.; Howard, K.P. pH-induced conformational change of the influenza M2 protein C-terminal domain. Biochemistry 2008, 47, 9934–9936. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.H.; Lamb, R.A. The M2 proton channels of influenza A and B viruses. J. Biol. Chem. 2006, 281, 8997–9000. [Google Scholar] [CrossRef] [PubMed]
- Thomaston, J.L.; DeGrado, W.F. Crystal structure of the drug-resistant S31N influenza M2 proton channel. Protein Sci. 2016, 25, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Cady, S.D.; Schmidt-Rohr, K.; Wang, J.; Soto, C.S.; DeGrado, W.F.; Hong, M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 2010, 463, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Yi, M.; Dong, H.; Qin, H.; Peterson, E.; Busath, D.D.; Zhou, H.-X.; Cross, T.A. Insight into the Mechanism of the Influenza A Proton Channel from a Structure in a Lipid Bilayer. Science 2010, 330, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Herneisen, A.L.; Sahu, I.D.; McCarrick, R.M.; Feix, J.B.; Lorigan, G.A.; Howard, K.P. A Budding-Defective M2 Mutant Exhibits Reduced Membrane Interaction, Insensitivity to Cholesterol, and Perturbed Interdomain Coupling. Biochemistry 2017, 56, 5955–5963. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Upshur, M.A.; Saotome, K.; Sahu, I.D.; McCarrick, R.M.; Feix, J.B.; Lorigan, G.A.; Howard, K.P. Cholesterol-Dependent Conformational Exchange of the C-Terminal Domain of the Influenza A M2 Protein. Biochemistry 2015, 54, 7157–7167. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Duong-Ly, K.C.; Howard, K.P. Influenza A M2 protein conformation depends on choice of model membrane. Biopolymers 2015, 104, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Thomaston, J.L.; Nguyen, P.A.; Brown, E.C.; Upshur, M.A.; Wang, J.; Degrado, W.F.; Howard, K.P. Detection of drug-induced conformational change of a transmembrane protein in lipid bilayers using site-directed spin labeling. Protein Sci. 2013, 22, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cady, S.; Hong, M. Immobilization of the influenza A M2 transmembrane peptide in virus envelope-mimetic lipid membranes: A solid state NMR investigation. Biochemistry 2009, 48, 6361–6368. [Google Scholar] [CrossRef] [PubMed]
- Leiding, T.; Wang, J.; Martinsson, J.; DeGrado, W.F.; Arskold, S.P. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc. Natl. Acad. Sci. USA 2010, 107, 15409–15414. [Google Scholar] [CrossRef] [PubMed]
- le Maire, M.; Champeil, P.; Moller, J. V Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta Biomembr. 2000, 1508, 86–111. [Google Scholar] [CrossRef]
- Schubert, R. Liposome preparation by detergent removal. Methods Enzymol. 2003, 367, 46–70. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.Y.; Fritzsching, K.J.; Hong, M. Conformational analysis of the full-length M2 protein of the influenza A virus using solid-state NMR. Protein Sci. 2013, 22, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.Y.; Yang, Y.; Tietze, D.; Hong, M. The Influenza M2 Cytoplasmic Tail Changes the Proton-Exchange Equilibria and the Backbone Conformation of the Transmembrane Histidine Residue to Facilitate Proton Conduction. J. Am. Chem. Soc. 2015, 137, 6067–6077. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, B.M.; Columbus, L. Identification and removal of nitroxide spin label contaminant: Impact on PRE studies of a-helical membrane proteins in detergent. Protein Sci. 2012, 21, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Prince, C.C.; Jia, Z.C. Detergent quantification in membrane protein samples and its application to crystallization experiments. Amino Acids 2013, 45, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Prince, C.; Jia, Z.C. Measurement of detergent concentration using 2,6-dimethylphenol in membrane-protein crystallization. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.M.; Fu, R.Q.; Zhou, H.X.; Cross, T.A. Dynamic Short Hydrogen Bonds in Histidine Tetrad of Full-Length M2 Proton Channel Reveal Tetrameric Structural Heterogeneity and Functional Mechanism. Structure 2015, 23, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Proteoliposomes as tool for assaying membrane transporter functions and interactions with xenobiotics. Pharmaceutics 2013, 5, 472–497. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.T.; Griffin, J.; Cross, T.A. Detergent Optimized Membrane Protein Reconstitution in Liposomes for Solid State NMR. Biochemistry 2014, 53, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Ollivon, M.; Lesieur, S.; Grabielle-Madelmont, C.; Paternostre, M. Vesicle reconstitution from lipid-detergent mixed micelles. Biochim. Biophys. Acta Biomembr. 2000, 1508, 34–50. [Google Scholar] [CrossRef]
- Stuart, M.C.A.; Boekema, E.J. Two distinct mechanisms of vesicle-to-micelle and micelle-to-vesicle transition are mediated by the packing parameter of phospholipid-detergent systems. Biochim. Biophys. Acta 2007, 1768, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Gulik, A.; Seigneuret, M.; Rigaud, J.-L. Phospholipid Vesicle Solubilization and Reconstitution by detergents. Symmetrical Analysis of the Two Processes Using Octaethylene Glycol Mono-n-dodecyl Ether. Biochemistry 1990, 29, 9480–9488. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering With Applications to Chemistry, Biology, and Physics, 2nd ed.; Dover Publications: New York, NY, USA, 2000. [Google Scholar]
- Paternostre, M.T.; Roux, M.; Rigaud, J.L. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by Triton X-100, octyl glucoside, and sodium. Biochemistry 1988, 27, 2668–2677. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, J.L.; Levy, D.; Mosser, G.; Lambert, O. Detergent removal by non-polar polystyrene beads—Applications to membrane protein reconstitution and two-dimensional crystallization. Eur. Biophys. J. Biophys. Lett. 1998, 27, 305–319. [Google Scholar] [CrossRef]
- Rigaud, J.L.; Paternostre, M.T.; Bluzat, A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry 1988, 27, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, E. Site-Directed spin labeling of membrane proteins. Top. Curr. Chem. 2012, 121–157. [Google Scholar] [CrossRef]
- Miao, Y.M.; Qin, H.J.; Fu, R.Q.; Sharma, M.; Can, T.V.; Hung, I.; Luca, S.; Gor’kov, P.L.; Brey, W.W.; Cross, T.A. M2 Proton Channel Structural Validation from Full-Length Protein Samples in Synthetic Bilayers and E. coli Membranes. Angew. Chem.-Int. Ed. 2012, 51, 8383–8386. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crouch, C.H.; Bost, M.H.; Kim, T.H.; Green, B.M.; Arbuckle, D.S.; Grossman, C.H.; Howard, K.P. Optimization of Detergent-Mediated Reconstitution of Influenza A M2 Protein into Proteoliposomes. Membranes 2018, 8, 103. https://doi.org/10.3390/membranes8040103
Crouch CH, Bost MH, Kim TH, Green BM, Arbuckle DS, Grossman CH, Howard KP. Optimization of Detergent-Mediated Reconstitution of Influenza A M2 Protein into Proteoliposomes. Membranes. 2018; 8(4):103. https://doi.org/10.3390/membranes8040103
Chicago/Turabian StyleCrouch, Catherine H., Margaret H. Bost, Tae H. Kim, Bryan M. Green, D. Stuart Arbuckle, Carl H. Grossman, and Kathleen P. Howard. 2018. "Optimization of Detergent-Mediated Reconstitution of Influenza A M2 Protein into Proteoliposomes" Membranes 8, no. 4: 103. https://doi.org/10.3390/membranes8040103
APA StyleCrouch, C. H., Bost, M. H., Kim, T. H., Green, B. M., Arbuckle, D. S., Grossman, C. H., & Howard, K. P. (2018). Optimization of Detergent-Mediated Reconstitution of Influenza A M2 Protein into Proteoliposomes. Membranes, 8(4), 103. https://doi.org/10.3390/membranes8040103




