Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(ethylene-alt-tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(acrylamide)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Membrane Synthesis
2.2.1. Activation of the Films
2.2.2. Radiation-Induced Graft Polymerization
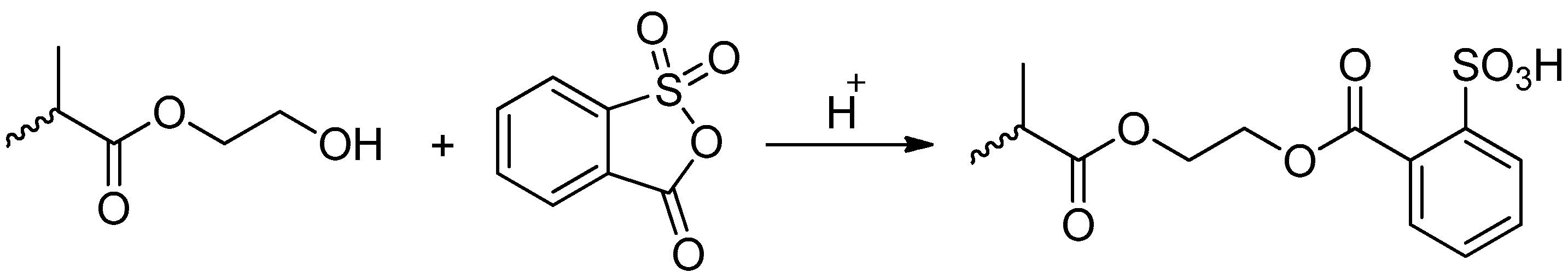
2.2.3. Sulfonation
2.3. Membrane Analysis
2.3.1. Fourier-Transformed Infrared Spectroscopy (FTIR)
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
- dM: thickness of the membrane (cm)
- A: area of the electrode (cm2)
- R: total resistance with the membrane (mΩ)
- R0: total resistance without the membrane (mΩ)
2.3.3. Ion Exchange Capacity (IEC) and Water Uptake (WU)
2.3.4. Fuel Cell Test
2.3.5. Mechanical Properties
2.3.6. VRFB
3. Results and Discussion
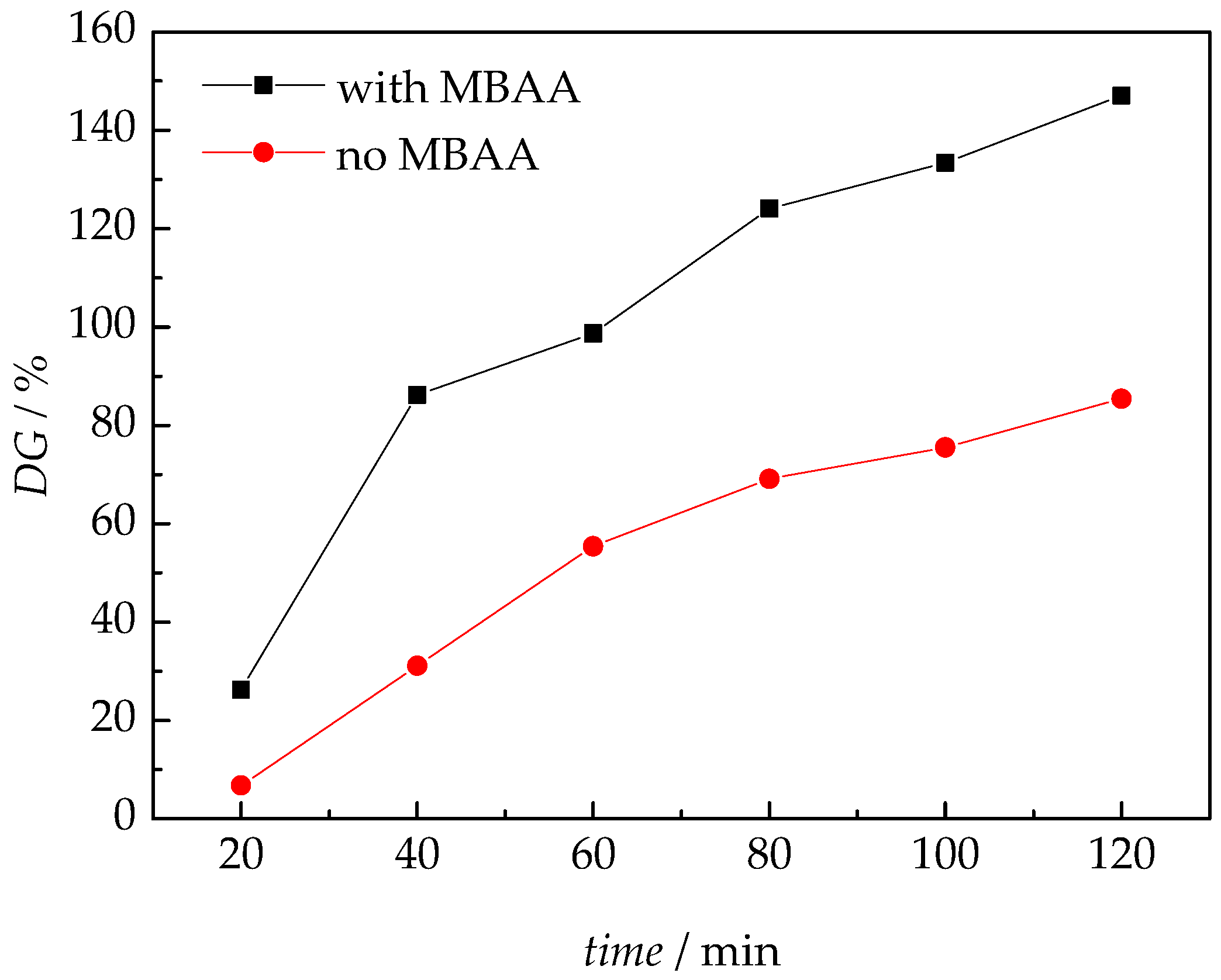
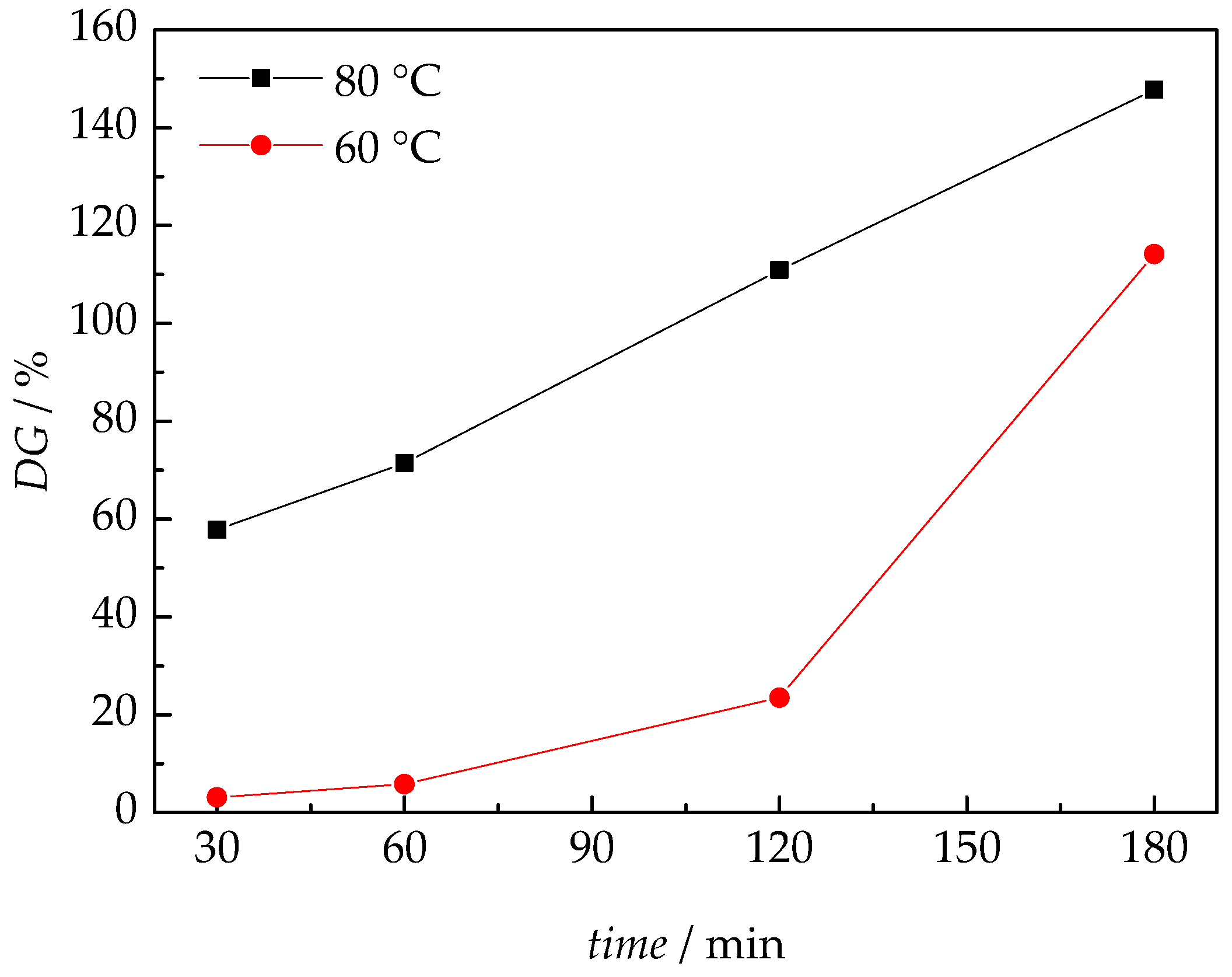
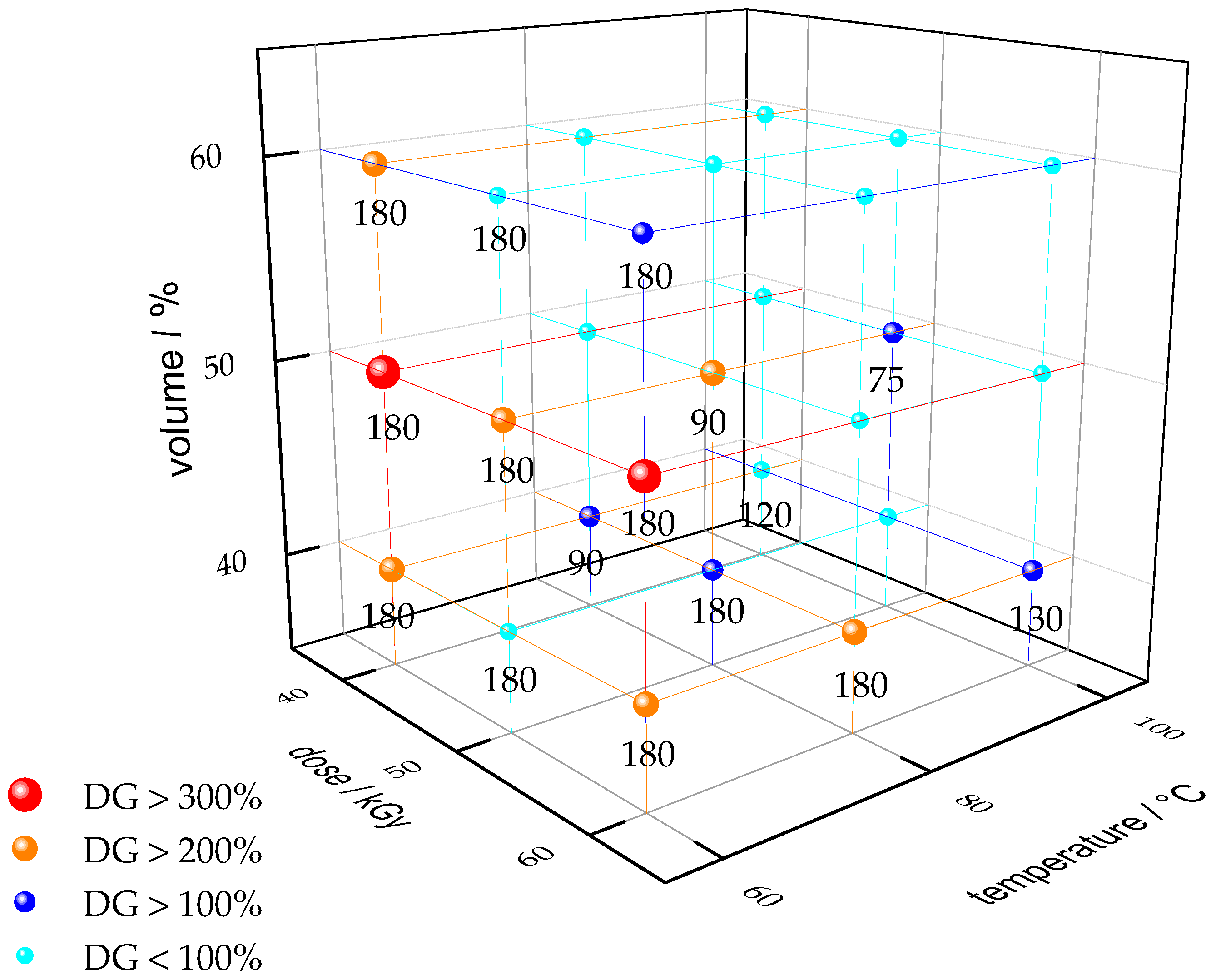
3.1. Membrane Synthesis
3.2. Analyses
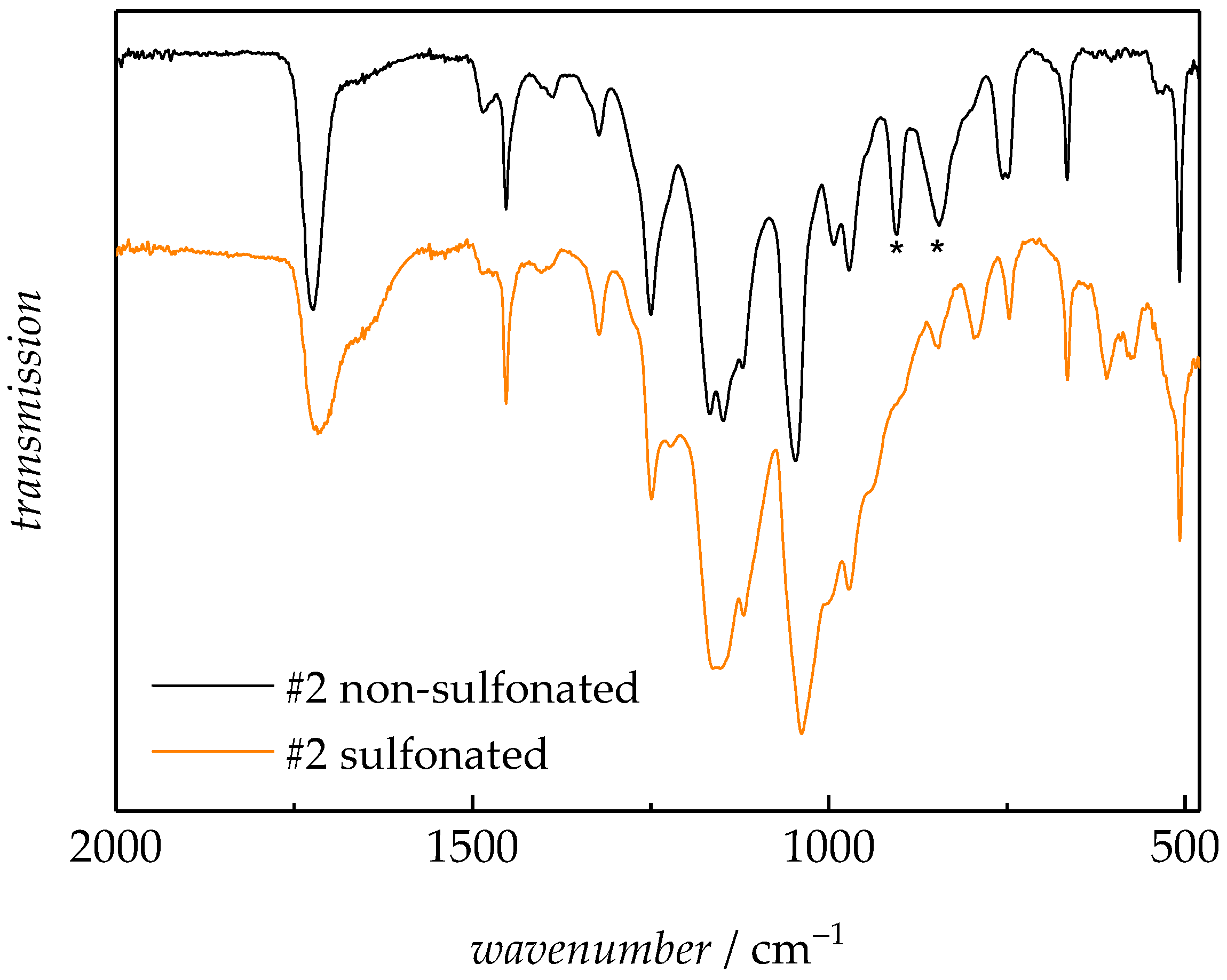
3.2.1. Fourier-Transformed Infrared Spectroscopy (FTIR)
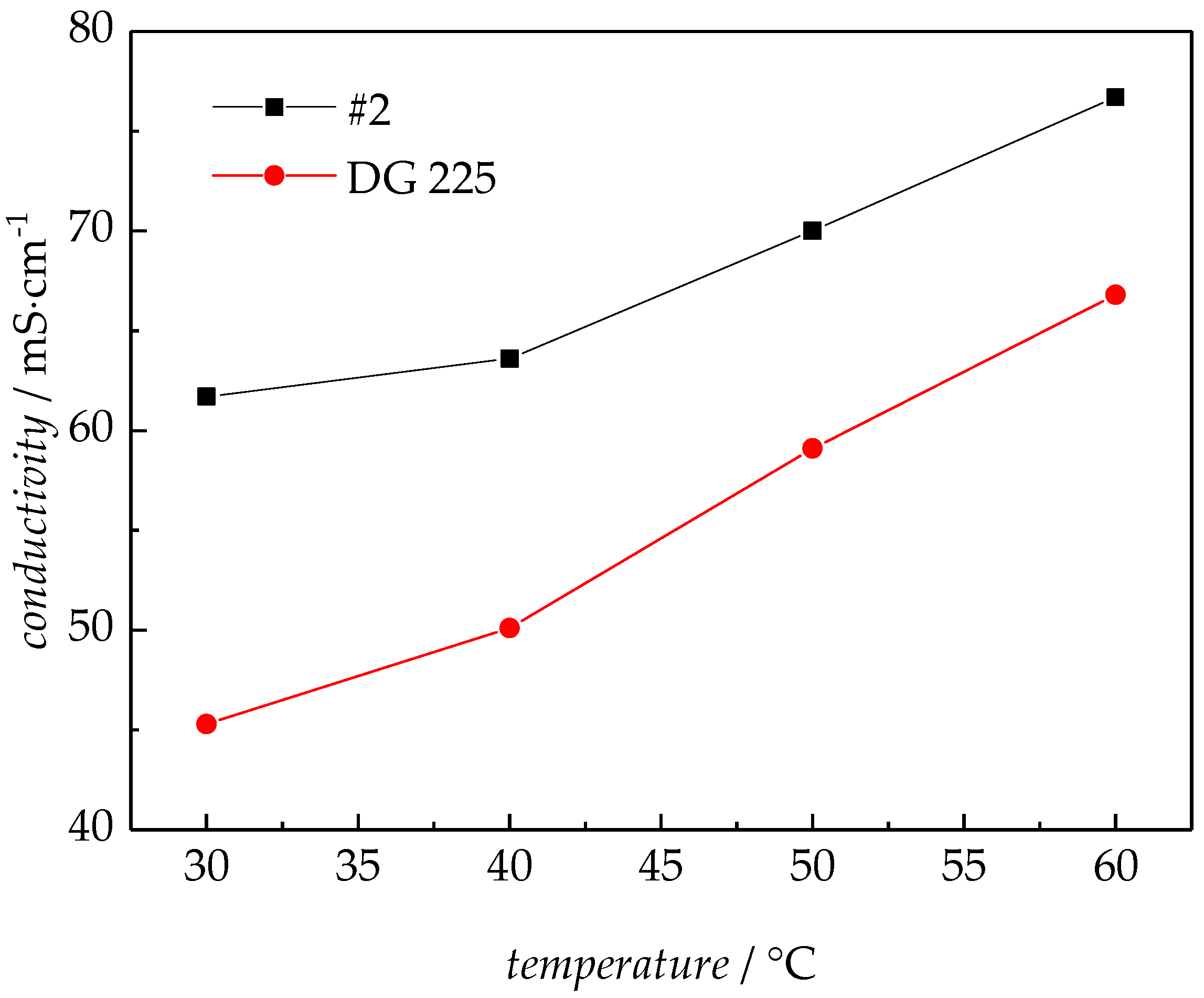
3.2.2. EIS
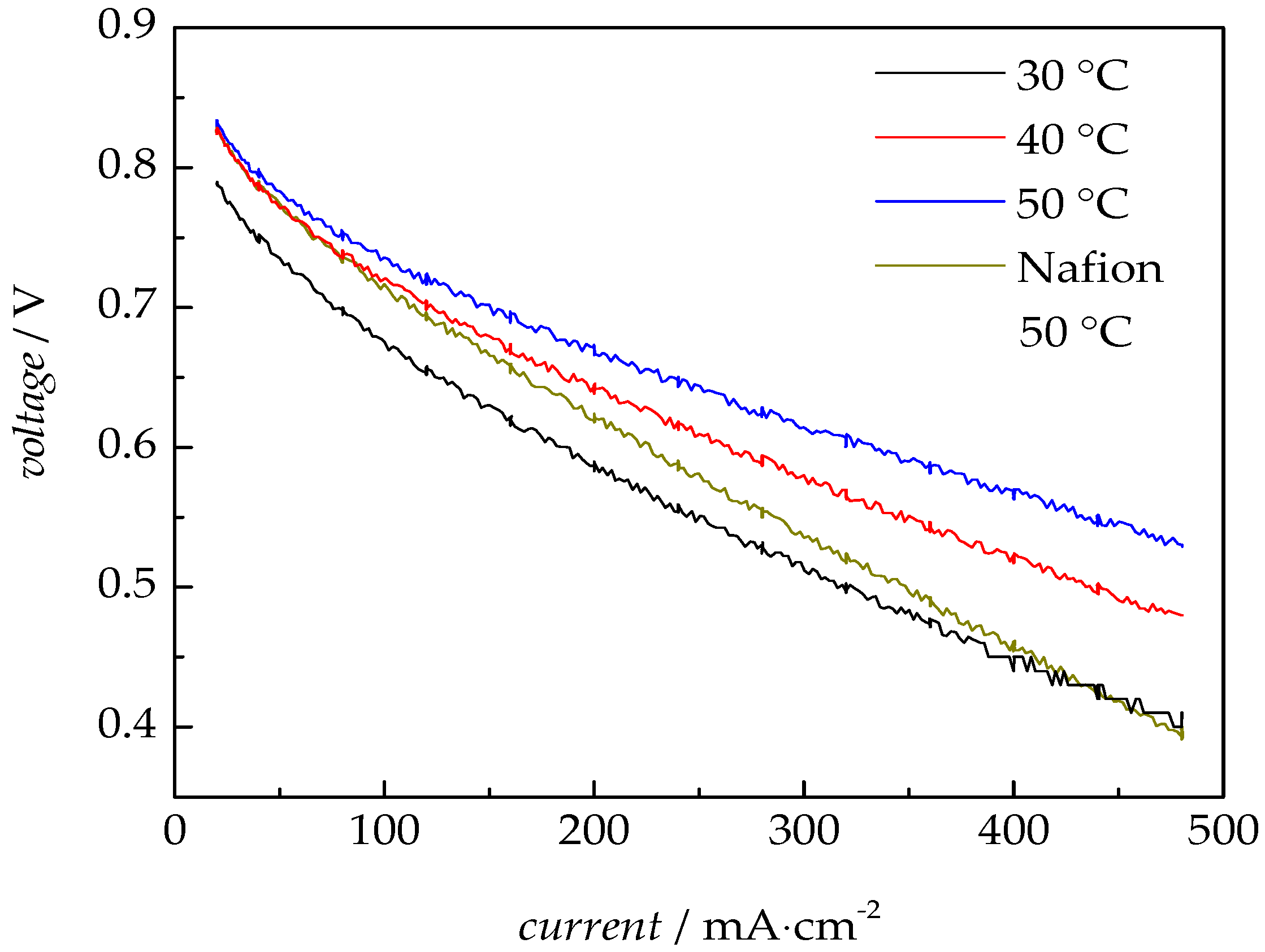
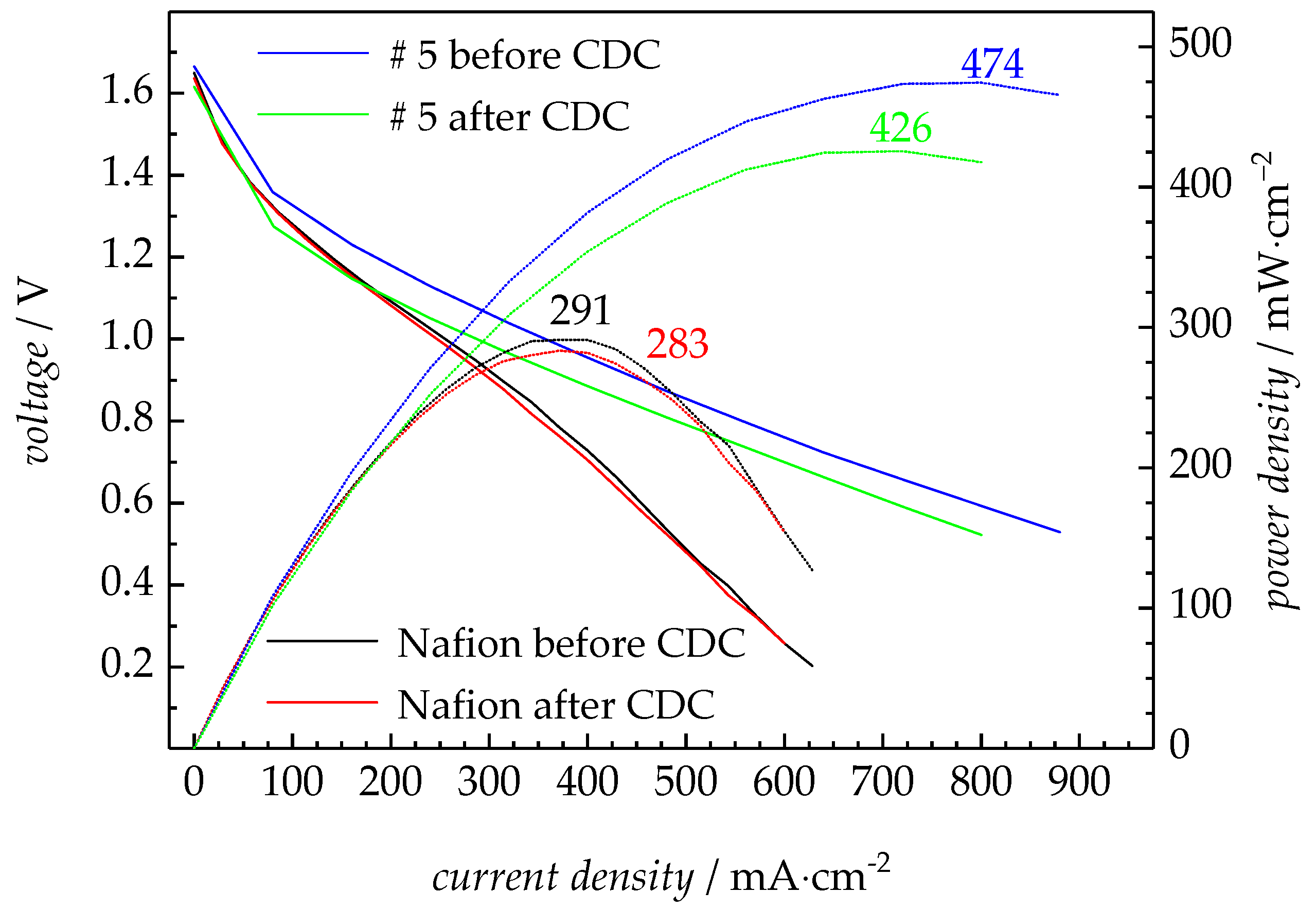
3.2.3. Fuel Cell Test
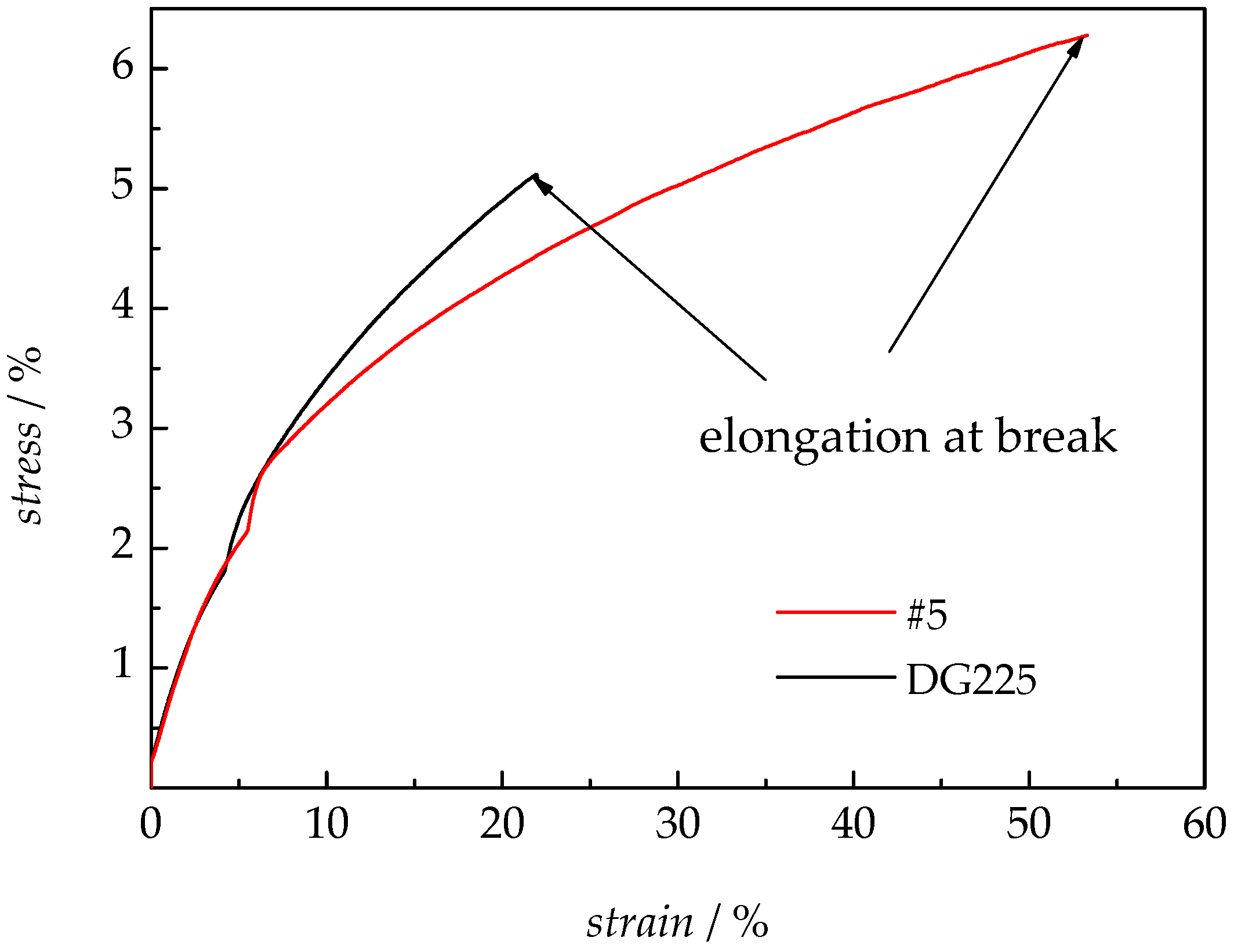
3.2.4. Mechanical Analysis
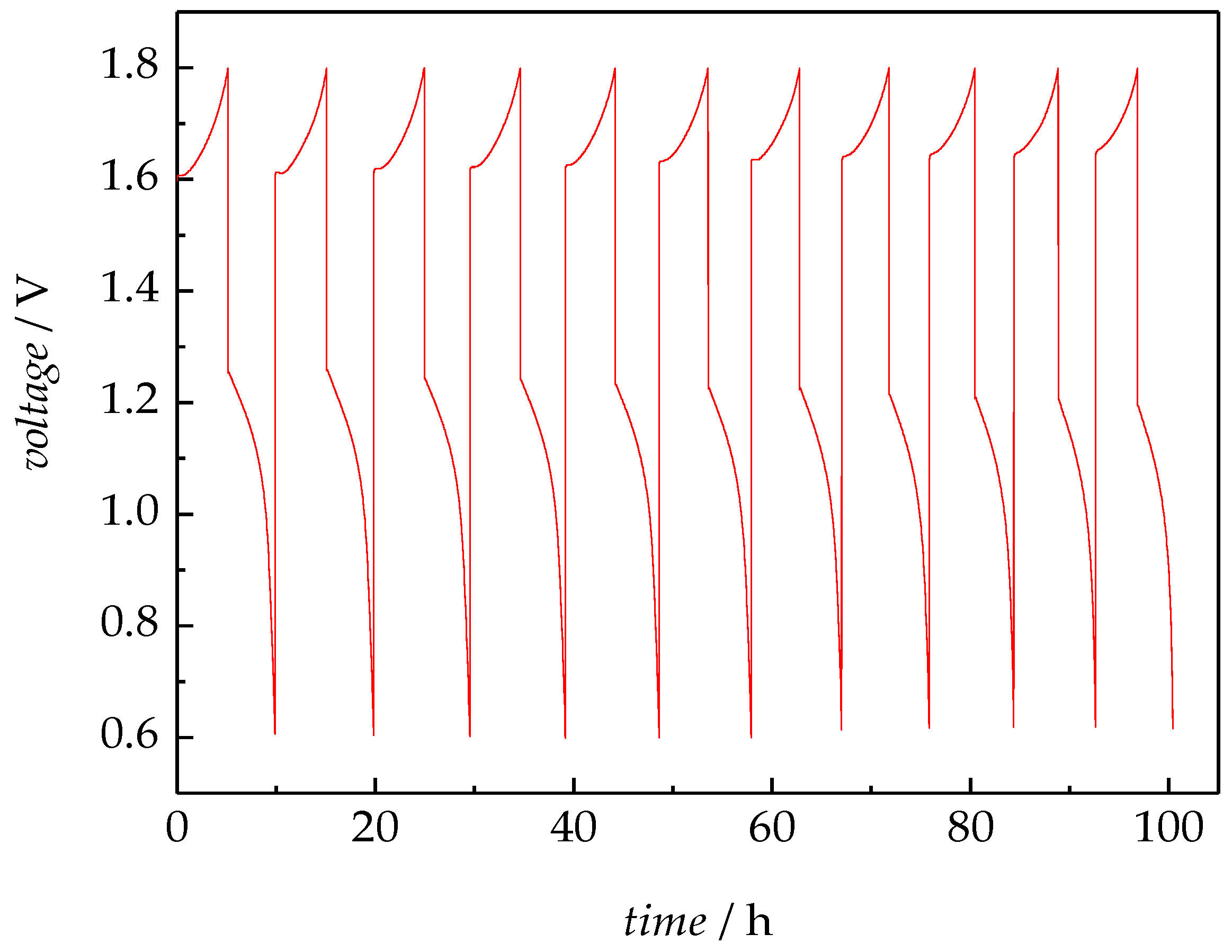
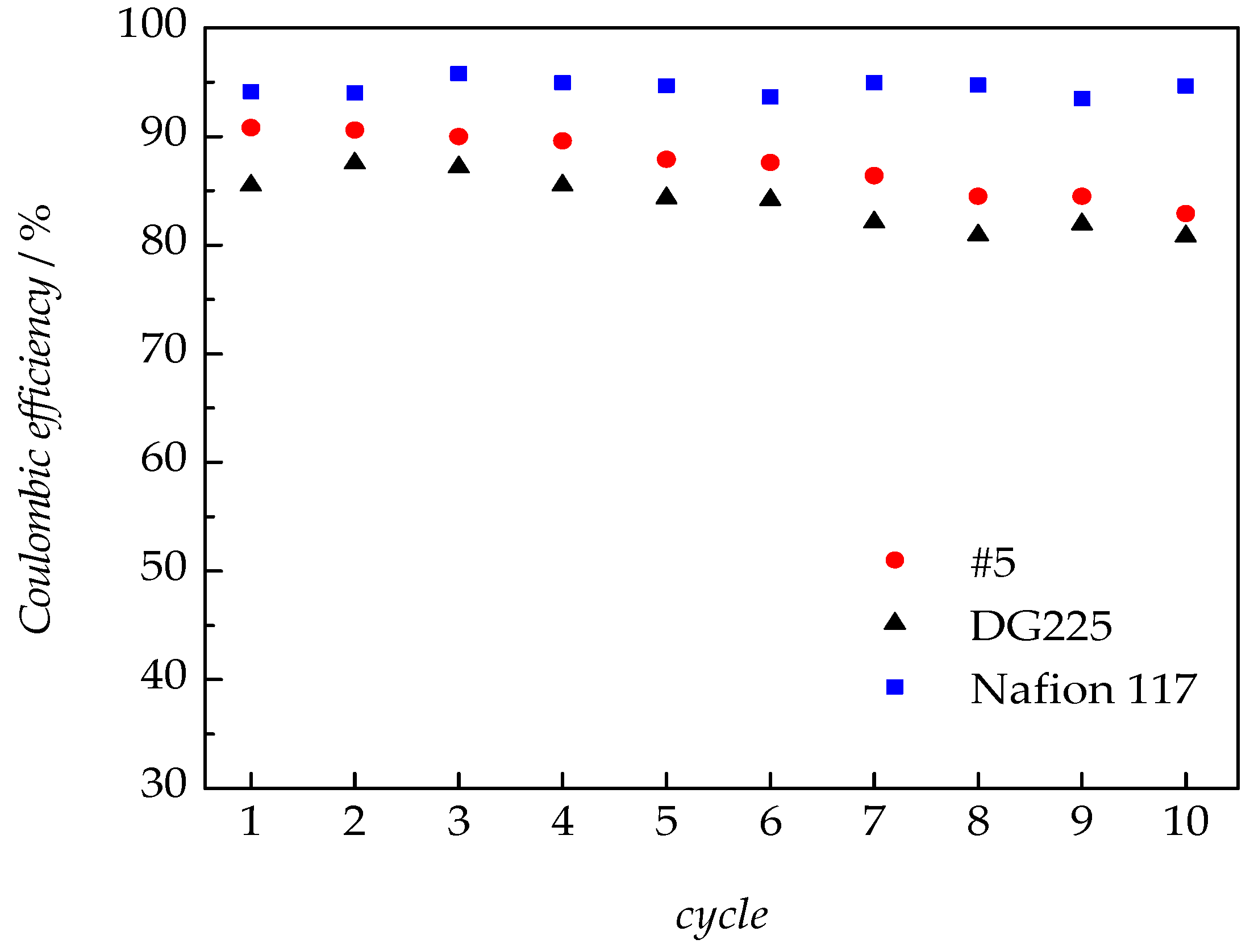
3.2.5. VRFB Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chalk, S.G.; Miller, J.F.; Wagner, F.W. Challenges for fuel cells in transport applications. J. Power Sources 2000, 86, 40–51. [Google Scholar] [CrossRef]
- Chu, D.; Jiang, R.; Gardner, K.; Jacobs, R.; Schmidt, J.; Quakenbush, T.; Stephens, J. Polymer electrolyte membrane fuel cells for communication applications. J. Power Sources 2001, 96, 174–178. [Google Scholar] [CrossRef]
- Lee, K.; Kang, S.; Ahn, K. Development of a highly efficient solid oxide fuel cell system. Appl. Energy 2017, 205, 822–833. [Google Scholar] [CrossRef]
- Tang, A.; Ting, S.; Bao, J.; Skyllas-Kazacos, M. Thermal modelling and simulation of the all-vanadium redox flow battery. J. Power Sources 2012, 203, 165–176. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Tang, A.; Qin, Y.; Liu, J.; Yan, C. Investigation of the use of electrolyte viscosity for online state–of–charge monitoring design in vanadium redox flow battery. Appl. Energy 2018, 211, 1050–1059. [Google Scholar] [CrossRef]
- Wang, Y.; Long, W.; Wang, L.; Yuan, R.; Ignaszak, A.; Fang, B.; Wilkinson, D.P. Unlocking the door to highly active ORR catalysts for PEMFC applications: Polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Saghali, Z.; Mahmoudimehr, J. Superiority of a novel conic tubular PEM fuel cell over the conventional cylindrical one. Int. J. Hydrog. Energy 2017, 42, 28865–28882. [Google Scholar] [CrossRef]
- Ding, Z.; He, L.; Dong, Z.; Gao, X. An integrated numerical model for a PEM fuel cell system. Adv. Mater. Res. 2013, 706–708, 1742–1745. [Google Scholar] [CrossRef]
- Chutichai, B.; Authayanun, S.; Assabumrungrat, S.; Arpornwichanop, A. Performance analysis of an integrated biomass gasification and PEMFC (proton exchange membrane fuel cell) system: Hydrogen and power generation. Energy 2013, 55, 98–106. [Google Scholar] [CrossRef]
- Rao, Z.; Zheng, C.; Geng, F. Proton conduction of fuel cell polymer membranes: Molecular dynamics simulation. Comput. Mater. Sci. 2018, 142, 122–128. [Google Scholar] [CrossRef]
- Junoh, H.; Jaafar, J.; Norddin, M.N.A.M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Ilbeygi, H. A review on the fabrication of electrospun polymer electrolyte membrane for direct methanol fuel cell. J. Nanomater. 2014, 2015. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skylla–Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Luo, T.; David, O.; Gendel, Y.; Wessling, M. Porous poly(benzimidazole) membrane for all vanadium redox flow battery. J. Power Sources 2016, 312, 45–54. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Liu, S.; He, Z.; Zhou, Z.; Li, A. A low-cost and high-performance sulfonated polyimide proton-conductive membrane for vanadium redox flow/static batteries. ACS Appl. Mater. Interfaces 2017, 9, 32643–32651. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Roy, A.; Banerjee, N.; Patra, S.; Saha, H. Precision dynamic equivalent circuit model of a vanadium redox flow battery and determination of circuit parameters for its optimal performance in renewable energy applications. J. Power Sources 2018, 396, 506–518. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Li, X.; Adams, F.; Luo, W.; Huang, Y.; Hu, L. Electrode materials of sodium–ion batteries toward practical application. ACS Energy Lett. 2018, 3, 1604–1612. [Google Scholar] [CrossRef]
- Wu, X.; Leonard, D.P.; Ji, X. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities. Chem. Mater. 2017, 29, 5031–5042. [Google Scholar] [CrossRef]
- Aalianvari, A. Optimum depth of grout curtain around pumped storage power cavern based on geological conditions. Bull. Eng. Geol. Environ. 2014, 73, 775–780. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G.L. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef] [PubMed]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox–flow batteries: From metals to organic redox-active materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, Q.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Weng, G.; Zou, Q.; Cong, G.; Lu, Y. A high-energy and low-cost polysulfide/iodide redox flow battery. Nano Energy 2016, 30, 283–292. [Google Scholar] [CrossRef]
- Gubler, L. Polymer design strategies for radiation-grafted fuel cell membranes. Adv. Energy. Mater. 2014, 4, 1300827. [Google Scholar] [CrossRef]
- Nasef, M.M. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem. Rev. 2014, 114, 12278–12329. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Li, X.; Drache, M.; Gohs, U.; Beuermann, S. Novel concept of polymer electrolyte membranes for high-temperature fuel cells based on ETFE grafted with neutral acrylic monomers. J. Membr. Sci. 2015, 495, 20–28. [Google Scholar] [CrossRef]
- Li, X.; Drache, M.; Ke, X.; Gohs, U.; Beuermann, S. Fuel cell application of high temperature polymer electrolyte membranes obtained by graft copolymerization of acrylic acid and 2-Hydroxyethylmethacrylate on ETFE backbone material. Macromol. Mater. Eng. 2016, 301, 56–64. [Google Scholar] [CrossRef]
- Luo, Z.; Chang, Z.; Zhang, Y.; Liu, Z.; Li, J. Electro–osmotic drag coefficient and proton conductivity in Nafion® membrane for PEMFC. Int. J. Hydrog. Energy 2010, 35, 3120–3124. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Mu, D.; Yu, L.; Liu, L.; Xi, J. Rice paper reinforced sulfonated poly(ether ether ketone) as low–cost membrane for vanadium flow batteries. ACS Sustain. Chem. Eng. 2017, 5, 2437–2444. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion membranes for direct alcohol fuel cells: An overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Alam, J.; Jafari, Y.; Sedighi, M.; Aljlil, S.A.; IIbeygi, H. Sulfonated poly ether ether ketone with different degree of sulphonation in microbial fuel cell: Application study and economical analysis. Int. J. Hydrog. Energy 2016, 41, 4862–4871. [Google Scholar] [CrossRef]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Sprenkle, V.; Wang, W. Polyvinyl chloride/silica nanoporous composite separator for all-vanadium redox flow battery applications. J. Electrochem. Soc. 2013, 160, A1215–A1218. [Google Scholar] [CrossRef]
- IAEA. Advances in Radiation Chemistry of Polymers; IAEA: Vienna, Austria, 2004; ISBN 92-0-112504-6. [Google Scholar]
- Li, X.; dos Santos, A.R.; Drache, M.; Ke, X.; Gohs, U.; Turek, T.; Becker, M.; Kunz, U.; Beuermann, S. Polymer electrolyte membranes prepared by pre-irradiation induced graft copolymerization on ETFE for vanadium redox flow battery applications. J. Membr. Sci. 2017, 524, 419–427. [Google Scholar] [CrossRef]
- Li, X. Synthese und Charakterisierung von Polymer–Elektrolyt–Membranen für die Anwendung in Brennstoffzellen und Vanadium–Redox–Flow–Batterien. Ph.D. Thesis, TU Clausthal, Clausthal-Zellerfeld, Germany, 7 July 2016. [Google Scholar]
- Zukowska, G.; Williams, J.; Stevens, J.R.; Jeffrey, K.R.; Lewera, A.; Kulesza, P.J. The application of acrylic monomers with acidic groups to the synthesis of proton-conducting polymer gels. Solid State Ion. 2004, 167, 123–130. [Google Scholar] [CrossRef]
- Kye, H.; Koh, Y.G.V.; Kim, Y.; Han, S.G.; Lee, H.; Lee, W. Tunable temperature response of a thermochromic photonic gel sensor containing N-Isopropylacrylamide and 4-Acryloyilmorpholine. Sensors 2017, 17, 1398. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.D.; Sanporean, C.G.; Christiansen, J.D.C. Mechanical response of HEMA gel under cyclic deformation: Viscoplasticity and swelling–induced recovery. Int. J. Solids Struct. 2015, 52, 220–234. [Google Scholar] [CrossRef]
- Santander-Borrego, M.; Green, D.W.; Chirila, T.V.; Whittaker, A.K.; Blakey, I. Click functionalization of methacrylate-based hydrogels and their cellular response. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1781–1789. [Google Scholar] [CrossRef]
- Schmidt, C.; Schmidt-Naake, G. Proton conducting membranes obtained by doping radiation–grafted basic membrane matrices with phosphoric acid. Macromol. Mater. Eng. 2007, 292, 1164–1175. [Google Scholar] [CrossRef]
- Schmidt-Naake, G. Polymer-Säure-Komposite auf Basis strahlungsinduzierter Pfropfpolymerisation für Hochtemperatur-PEM-Brennstoffzellen. Chem. Ing. Technol. 2009, 81, 599–609. [Google Scholar] [CrossRef]
- Schmidt, C.; Schmidt-Naake, G. Phosphorsäuredotierte protonenleiter auf basis von aminierten membranen aus ETFE-graft-poly-(glycidylmethacrylat)-derivaten. Chem. Ing. Technol. 2008, 80, 317–325. [Google Scholar] [CrossRef]
- Schmidt, C.; Glück, T.; Schmidt-Naake, G. Proton exchange membranes by radiation-induced graft polymerization of glycidyl methacrylate on poly(tetrafluoroethylene-alt-ethylene) (ETFE)-modification with butyl acrylate and acrylonitrile. Chem. Ing. Technol. 2007, 79, 137–145. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; EI-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M. Rate coefficients of free-radical polymerization deduced from pulsed laser experiments. Prog. Polym. Sci. 2002, 27, 191–254. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast fourier transform IR characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Nafion N115, N117, N1110, Ion Exchange Materials, Extrusion Cast Membranes. Available online: https://www.chemours.com/Nafion/en_US/assets/downloads/nafion-extrusion-cast-membranes-product-information.pdf. (accessed on 17 October 2018).
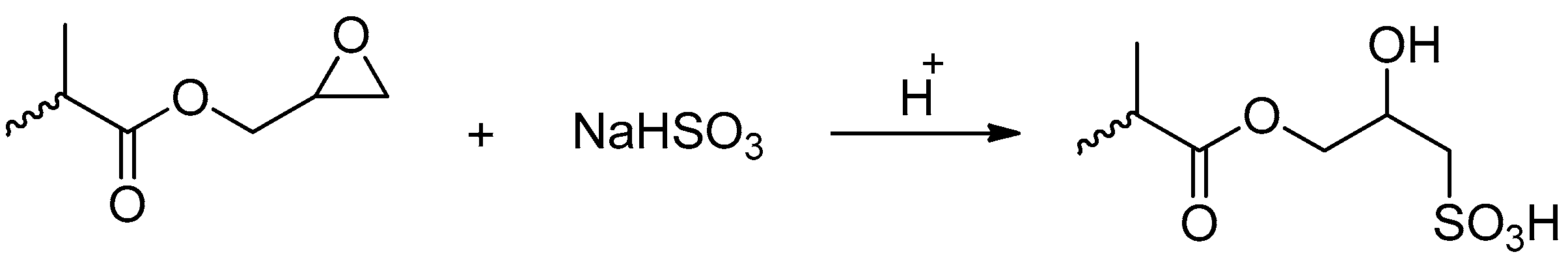
| Sample | Power Density after 5 h at 200 mA∙cm−2/mW∙cm−2 | Resistance/mΩ |
|---|---|---|
| #2 at 30 °C | 118 | 26 |
| #2 at 40 °C | 128 | 24 |
| #2 at 50 °C | 134 | 21 |
| DG225 at 50 °C [41] | 129 | 22 |
| Nafion 117 at 50 °C [41] | 124 | 34 |
| Sample | Rm/N∙mm−2 | E/N∙mm−2 | ε-Break/% |
|---|---|---|---|
| #5 | 6.28 | 43.3 | 53.3 |
| DG225 | 5.12 | 49.9 | 22.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Drache, M.; Gohs, U.; Kunz, U.; Beuermann, S. Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(ethylene-alt-tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(acrylamide). Membranes 2018, 8, 102. https://doi.org/10.3390/membranes8040102
Ke X, Drache M, Gohs U, Kunz U, Beuermann S. Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(ethylene-alt-tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(acrylamide). Membranes. 2018; 8(4):102. https://doi.org/10.3390/membranes8040102
Chicago/Turabian StyleKe, Xi, Marco Drache, Uwe Gohs, Ulrich Kunz, and Sabine Beuermann. 2018. "Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(ethylene-alt-tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(acrylamide)" Membranes 8, no. 4: 102. https://doi.org/10.3390/membranes8040102
APA StyleKe, X., Drache, M., Gohs, U., Kunz, U., & Beuermann, S. (2018). Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(ethylene-alt-tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(acrylamide). Membranes, 8(4), 102. https://doi.org/10.3390/membranes8040102





