Biomimetic Membranes as a Technology Platform: Challenges and Opportunities
Abstract
1. Introduction
2. Biomimetic Membrane Technology Development Status
2.1. Membranes for Water Treatment Technology
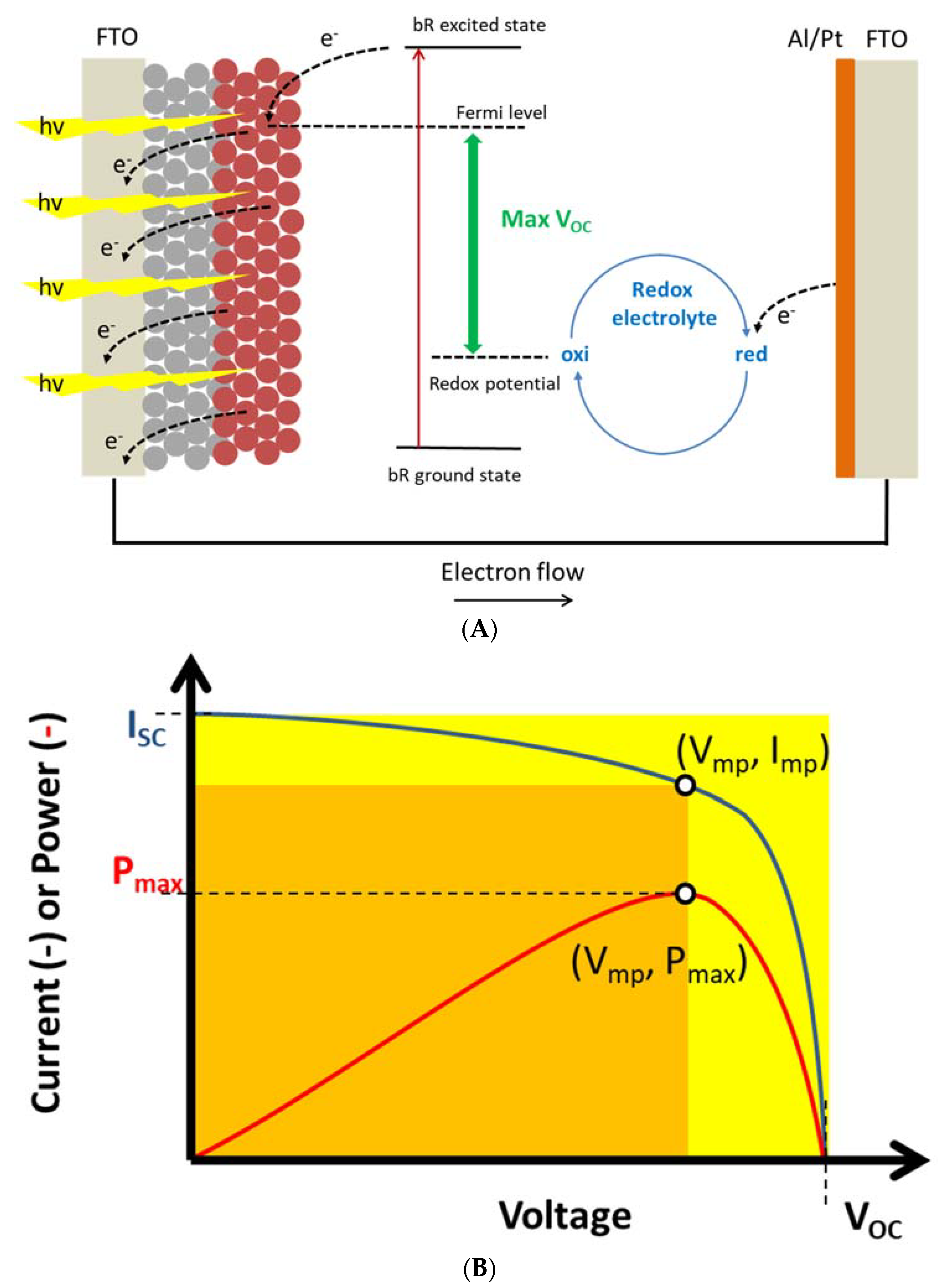
2.2. Membranes for Energy Conversion
2.3. Biomimetic Membranes and Membrane Processes for Biomedical Applications
3. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Hockfield, S. A New Century’s New Technologies; Project Syndicate—The World’s Opinion Page: Prague, Czech Republic, 2015. [Google Scholar]
- Nielsen, C.H. Biomimetic membranes for sensor and separation applications. Anal. Bioanal. Chem. 2009, 395, 697–718. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.T.; Abdelmohsen, L.; Rutjes, F.; van Hest, J.C.M. Nanoreactors for green catalysis. Beilstein J. Org. Chem. 2018, 14, 716–733. [Google Scholar] [CrossRef] [PubMed]
- LaVan, D.A.; Cha, J.N. Approaches for biological and biomimetic energy conversion. Proc. Natl. Acad. Sci. USA 2006, 103, 5251–5255. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.R.; Granja, J.R.; Buehler, L.K. Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature 1994, 369, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.E. Biomimetic Approaches for Biomaterials Development; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier: New York, NY, USA, 2015; p. 1168. [Google Scholar]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of h-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Robinson, C.V.; Edelman, I.S. Self-diffusion and structure of liquid water. III. Measurements of the self-diffusion of liquid water with H2, H3 and O18 as tracers. J. Am. Chem. Soc. 1953, 75, 466–470. [Google Scholar] [CrossRef]
- Zhong, P.S.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A. Aquaporin-embedded biomimetic membranes for nanofiltration. J. Membr. Sci. 2012, 407–408, 27–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Hélix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin based biomimetic membrane by interfacial polymerization—Membrane preparation and ro performance characterization. J. Membr. Sci. 2012, 423–424, 422–428. [Google Scholar] [CrossRef]
- Perry, M.; Madsen, S.U.; Jorgensen, T.; Braekevelt, S.; Lauritzen, K.; Helix-Nielsen, C. Challenges in commercializing biomimetic membranes. Membranes 2015, 5, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Z.; Petrinic, I.; Fane, A.G.; Helix-Nielsen, C. Biomimetic aquaporin membranes coming of age. Desalination 2015, 368, 89–105. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Helix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; legrand, Y.M.; Roualdes, S.; Barboiu, M. Highly selective reverse osmosis membranes incorporating biomimetic artificial water channels. In Proceedings of the Artificial Water Channels Faraday Discussion, Glasgow, UK, 25–27 June 2018; p. 14. [Google Scholar]
- Warne, T.; Serrano-Vega, M.J.; Tate, C.G.; Schertler, G.F.X. Development and crystallization of a minimal thermostabilised g protein-coupled receptor. Protein Expr. Purif. 2009, 65, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Bomholt, J.; Hélix-Nielsen, C.; Scharff-Poulsen, P.; Amstrup Pedersen, P. Production of human aquaporin-1 in saccharomyces cerevisiae to a high membrane density. PLoS ONE 2013, 8, e56431. [Google Scholar] [CrossRef] [PubMed]
- Bjørkskov, F.B.; Krabbe, S.L.; Nurup, C.N.; Missel, J.W.; Spulber, M.; Bomholt, J.; Molbaek, K.; Helix-Nielsen, C.; Gotfryd, K.; Gourdon, P.; et al. Purification and functional comparison of nine human aquaporins produced in saccharomyces cerevisiae for the purpose of biophysical characterization. Sci. Rep. 2017, 7, 16899. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organix nanotubes based on a cyclic peptide architechture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Arseniev, A.S.; Lomize, A.L.; Barsukov, I.L.; Bystrov, V.F. Gramicidin a transmembrane ion-channel. Three-dimensional structure reconstruction based on nmr spectroscopy and energy refinement. Biol. Membr. 1986, 3, 1077–1104. [Google Scholar]
- Percec, V.; Dulcey, A.E.; Balagurusamy, V.S.K.; Miura, Y.; Smidrkal, J.; Peterca, M.; Nummelin, S.; Edlund, U.; Hudson, S.D.; Heiney, P.A.; et al. Self-assembly of amphiphilic dendritic dipeptides into helical pores. Nature 2004, 430, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Kaucher, M.S.; Peterca, M.; Dulcey, A.E.; Kim, A.J.; Vinogradov, S.A.; Hammer, D.A.; Heiney, P.A.; Percec, V. Selective transport of water mediated by porous dendritic dipeptides. J. Am. Chem. Soc. 2007, 129, 11698–11699. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, Y.; Michau, M.; Gilles, A.; Gence, V.; Legrand, Y.M.; van der Lee, A.; Tingry, S.; Barboiu, M. Imidazole-quartet water and proton dipolar channels. Angew. Chem. Int. Ed. Engl. 2011, 50, 11366–11372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sheng, S.; Hong, Y.; Zeng, H. Proton gradient-induced water transport mediated by water wires inside narrow aquapores of aquafoldamer molecules. J. Am. Chem. Soc. 2014, 136, 14270–14276. [Google Scholar] [CrossRef] [PubMed]
- Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Vögele, M.; Köfinger, J.; Hummer, G. Molecular dynamics simulations of carbon nanotube porins in lipid bilayers. Faraday Discuss. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, F.; Park, H.G.; Holt, J.K.; Stadermann, M.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl. Acad. Sci. USA 2008, 105, 17250–17255. [Google Scholar] [CrossRef] [PubMed]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.-C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.; Pohl, P. Comment on “enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins”. Science 2018, 359, eaap9173. [Google Scholar] [CrossRef] [PubMed]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.-C.; Pham, T.A.; Wanunu, M.; Noy, A. Response to comment on “enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins”. Science 2018, 359, eaaq1214. [Google Scholar] [CrossRef] [PubMed]
- Barboiu, M. Artificial water channels—Incipient innovative developments. Chem. Commun. (Camb.) 2016, 52, 5657–5665. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Jorgensen, T. Global Product Executive; Aquaporin A/S: Kongens Lyngby, Denmark, 2018. [Google Scholar]
- Bradley, E.L.; Castle, L.; Jickells, S.M.; Mountfort, K.A.; Read, W.A. Use of overall migration methodology to test for food-contact substances with specific migration limits. Food Addit. Contam. Part A 2009, 26, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2008, 36, 189–217. [Google Scholar] [CrossRef]
- Li, Z.; Valladares Linares, R.; Bucs, S.; Fortunato, L.; Hélix-Nielsen, C.; Vrouwenvelder, J.S.; Ghaffour, N.; Leiknes, T.; Amy, G. Aquaporin based biomimetic membrane in forward osmosis: Chemical cleaning resistance and practical operation. Desalination 2017, 420, 208–215. [Google Scholar] [CrossRef]
- Hey, T.; Zarebska, A.; Bajraktari, N.; Vogel, J.; Hélix-Nielsen, C.; La Cour Jansen, J.; Jönsson, K. Influences of mechanical pre-treatment on the non-biological treatment of municipal wastewater by forward osmosis. Environ. Technol. 2017, 18, 1–10. [Google Scholar]
- Luo, W.; Xie, M.; Song, X.; Guo, W.; Ngo, H.H.; Zhou, J.L.; Nghiem, L.D. Biomimetic aquaporin membranes for osmotic membrane bioreactors: Membrane performance and contaminant removal. Bioresour. Technol. 2018, 249, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.A.; Bjørkskov, F.; Alvisse, S.; Helix-Nielsen, C. From channel proteins to industrial biomimetic membrane technology. Faraday Discuss. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuis, R.P.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1449. [Google Scholar] [CrossRef]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Guo, Z. Recent advances in biomimetic thin membranes applied in emulsified oil/water separation. J. Mater. Chem. A 2016, 4, 15749–15770. [Google Scholar] [CrossRef]
- Peinemann, K.-V.; Nunes, S.P. Membranes for energy conversion. In Membranes for Energy Conversion; Peinemann, K.-V., Nunes, S.P., Eds.; Wiley: Weinheim, Germany, 2008; pp. xi–xiii. [Google Scholar]
- Shishatskiy, S.; Nistor, C.; Popa, M.; Nunes, S.P.; Peinemann, K.V. Polyimide asymmetric membranes for hydrogen separation: Influence of formation conditions on gas transport properties. Adv. Eng. Mater. 2006, 8, 390–397. [Google Scholar] [CrossRef]
- Xin, Q.; Zhang, Y.; Huo, T.; Ye, H.; Ding, X.; Lin, L.; Zhang, Y.; Wu, H.; Jiang, Z. Mixed matrix membranes fabricated by a facile in situ biomimetic mineralization approach for efficient CO2 separation. J. Membr. Sci. 2016, 508, 84–93. [Google Scholar] [CrossRef]
- Yao, K.; Wang, Z.; Wang, J.; Wang, S. Biomimetic material-poly(N-vinylimidazole)-zinc complex for CO2 separation. Chem. Commun. (Camb.) 2012, 48, 1766–1768. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lim, J.; Hamdan, S.H.; Madsen, H.T.; Søgaard, E.G.; Hélix-Nielsen, C.; Luis, P.; van der Bruggen, B. Enhanced performance of a biomimetic membrane for Na2CO3 crystallization in the scenario of CO2 capture. J. Membr. Sci. 2015, 498, 75–85. [Google Scholar] [CrossRef]
- Skilhagen, S.E. Osmotic power—A new, renewable energy source. Desalination Water Treat. 2010, 15, 271–278. [Google Scholar] [CrossRef]
- Bajraktari, N.; Hélix-Nielsen, C.; Madsen, H.T. Pressure retarded osmosis from hypersaline sources—A review. Desalination 2017, 413, 65–85. [Google Scholar] [CrossRef]
- Choi, W.; Bae, H.; Lee, H.-H.; Lee, J.; Kin, J.H.; Park, C.H. Performance analysis of pressure-retarded osmosis power using biomimetic aquaporin membrane. Polymer 2015, 39, 317–322. [Google Scholar] [CrossRef]
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959–6989. [Google Scholar] [CrossRef] [PubMed]
- Tributsch, H. Application of electrochemical kinetics to photosynthesis and oxidative phosphorylation: The redox element hypothesis and the principle of parametric energy coupling. J. Bioenerg. 1971, 2, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Stoeckenius, W. Functions of a new photoreceptor membrane. Proc. Natl. Acad. Sci. USA 1973, 70, 2853–2857. [Google Scholar] [CrossRef] [PubMed]
- Kayushin, L.P.; Skulachev, V.P. Bacteriorhodopsin as an electrogenic proton pump: Reconstitution of bacteriorhodopsin proteoliposomes generating delta psi and delta ph. FEBS Lett. 1974, 39, 39–42. [Google Scholar] [CrossRef]
- Thavasi, V.; Lazarova, T.; Filipek, S.; Kolinski, M.; Quero, E.; Kumar, A.; Ramakrishna, S.; Padrós, E.; Renugopalakrishnan, V. Study on the feasibility of bacteriorhodopsin as bio-photosensitizer in excitonic solar cell: A first report. J. NanoSci. Nanotech. 2008, 8, 1679–1687. [Google Scholar] [CrossRef]
- Saga, Y.; Watanabe, T.; Koyama, K.; Miyasaka, T. Mechanism of photocurrent generation from bacteriorhodopsin on gold electrodes. J. Phys. Chem. B 1999, 103, 234–238. [Google Scholar] [CrossRef]
- Heyes, C.D.; El-Sayed, M.A. Thermal properties of bacteriorhodopsin. J. Phys. Chem. B 2003, 107, 12045–12053. [Google Scholar] [CrossRef]
- Brouillette, C.G.; McMichens, R.B.; Stern, L.J.; Khorana, H.G. Structure and thermal stability of monomeric bacteriorhodopsin in mixed phospholipid/detergent micelles. Proteins 1989, 5, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, T.; Koyama, K. Image sensing and processing by a bacteriorhodopsin-based artificial photoreceptor. Appl. Opt. 1993, 32, 6371–6379. [Google Scholar] [CrossRef] [PubMed]
- Simmeth, R.; Rayfield, G.W. Evidence that the photoelectric response of bacteriorhodopsin occurs in less than 5 picoseconds. Biophys. J. 1990, 57, 1099–1101. [Google Scholar] [CrossRef]
- Goushcha, A.O.; Tabbert, B. On response time of semiconductor photodiodes. Opt. Eng. 2017, 56, 097101. [Google Scholar] [CrossRef]
- Rodrigo-Banos, M.; Garbayo, I.; Vilchez, C.; Bonete, M.J.; Martinez-Espinosa, R.M. Carotenoids from haloarchaea and their potential in biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [PubMed]
- Griep, M.H.; Winder, E.M.; Lueking, D.R.; Garrett, G.A.; Karna, S.P.; Friedrich, C.R. Forster resonance energy transfer between core/shell quantum dots and bacteriorhodopsin. Mol. Biol. Int. 2012, 2012, 910707. [Google Scholar] [CrossRef] [PubMed]
- Renugopalakrishnan, V.; Barbiellini, B.; King, C.; Molinari, M.; Mochalov, K.; Sukhanova, A.; Nabiev, I.; Fojan, P.; Tuller, H.L.; Chin, M.; et al. Engineering a robust photovoltaic device with quantum dots and bacteriorhodopsin. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 16710–16717. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.J.; Griep, M.H.; Giri, A.; Hirsch, S.G.; Rodriguez-Santiago, V.; Bujanda, A.A.; McCauley, J.E.; Karna, S.P. Tunable TiO2 Nanotube Arrays for Flexible Bio-Sensitized Solar Cells; Army Research Laboratory (ARL) TR-6075; Army Research Laboratory: Aberdeen, MD, USA, 2012; pp. 1–16. [Google Scholar]
- Mohammadpour, R.; Janfaza, S. Efficient nanostructured biophotovoltaic cell based on bacteriorhodopsin as biophotosensitizer. ACS Sustain. Chem. Eng. 2015, 3, 809–813. [Google Scholar] [CrossRef]
- Janfaza, S.; Molaeirad, A.; Mohamadpour, R.; Khayati, M.; Mehrvand, J. Efficient bio-nano hybrid solar cells via purple membrane as sensitizer. BioNanoScience 2013, 4, 71–77. [Google Scholar] [CrossRef]
- Molaeirad, A.; Janfaza, S.; Karimi-Fard, A.; Mahyad, B. Photocurrent generation by adsorption of two main pigments of halobacterium salinarum on tio2 nanostructured electrode. Biotechnol. Appl. Biochem. 2015, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Graetzel, M. Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 1993, 97, 6272–6277. [Google Scholar] [CrossRef]
- Fahrenbruch, A.L.; Bube, R.H. Survey of basic concepts. In Fundamentals of Solar Cells; Fahrenbruch, A.L., Bube, R.H., Eds.; Academic Press: New York, NY, USA, 1983; pp. 1–25. [Google Scholar]
- Espina Palanco, M.; Skovgaard, N.; Hansen, J.S.; Berg-Sorensen, K.; Helix-Nielsen, C. Tuning biomimetic membrane barrier properties by hydrocarbon, cholesterol and polymeric additives. Bioinspir. Biomim. 2017, 13, 016005. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H.A. New concept for macromolecular therapeutics in cancer-chemotherapy—Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Liu, D.; Auguste, D.T. Cancer targeted therapeutics: From molecules to drug delivery vehicles. J. Control. Release 2015, 219, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Nagy, J.A.; Hipp, J.; Dvorak, H.F.; Dvorak, A.M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J. Exp. Med. 1996, 183, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical translation of nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Schoonen, L.; van Hest, J.C. Compartmentalization approaches in soft matter science: From nanoreactor development to organelle mimics. Adv. Mater. 2016, 28, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Voit, B. Progress on multi-compartment polymeric capsules. Polym. Chem. 2013, 4, 435–443. [Google Scholar] [CrossRef]
- Groschel, A.H.; Muller, A.H. Self-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876. [Google Scholar] [CrossRef] [PubMed]
- Einfalt, T.; Witzigmann, D.; Edlinger, C.; Sieber, S.; Goers, R.; Najer, A.; Spulber, M.; Onaca-Fischer, O.; Huwyler, J.; Palivan, C.G. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat. Commun. 2018, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Wodzki, R.; Szczepanski, P. Coupled membrane processes and their biomimetic fundamentals. Chem. Pap. 2000, 54, 430–436. [Google Scholar]
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Zhang, P.; Zhang, Z.; Yu, H.; Li, Y. Bioinspired nanoparticles with nir-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv. Funct. Mater. 2016, 26, 7495–7506. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]


| BSSC Substrate | Cell Area | Short Circuit Current | Open Circuit Voltage | Illumination Intensity j | Efficiency | Fill Factor | Ref |
|---|---|---|---|---|---|---|---|
| A [cm2] | ISC [A/m2] | VOC [V] | P [W/m2] | H [%] | FF - | ||
| bR-TiO2 g | 0.5 | 0.9 | 0.35 | 400 | 0.002 a | 0.24 b | [59] |
| bR-TiO2 h | <2 | 2.3 | 0.22 | 600 | 0.03 c | 0.67 d | [69] |
| bR-TiO2 e | 0.25 | 10 | 0.53 | 1000 | 0.35 | 0.66 | [70] |
| bR-TiO2 e | 0.25 | 2.8 | 0.52 | 1000 | 0.09 | 0.62 | [71] |
| bR-TiO2 f | 0.25 | 2.1 | 0.53 | 1000 | n.a. | n.a. | [71] |
| bR-TiO2 e | 0.25 | 4 | 0.5 | 1000 | 0.11 | n.a. | [72] |
| bRu-TiO2 e | 0.25 | 2.1 | 0.53 | 1000 | 0.11 | n.a. | [72] |
| (bRu + bR)-TiO2 e | 0.25 | 4.5 | 0.57 | 1000 | 0.16 | 0.62 | [72] |
| Cu-2-α-oxymesoisochlorin e4-TiO2 i | 1.0 | 90 | 0.52 | 1000 | 2.6 | 0.7 b | [73] |
| N719 (Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II))-TiO2 e | 0.25 | 90.5 | 0.77 | 1000 | 5.9 | - | [71] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hélix-Nielsen, C. Biomimetic Membranes as a Technology Platform: Challenges and Opportunities. Membranes 2018, 8, 44. https://doi.org/10.3390/membranes8030044
Hélix-Nielsen C. Biomimetic Membranes as a Technology Platform: Challenges and Opportunities. Membranes. 2018; 8(3):44. https://doi.org/10.3390/membranes8030044
Chicago/Turabian StyleHélix-Nielsen, Claus. 2018. "Biomimetic Membranes as a Technology Platform: Challenges and Opportunities" Membranes 8, no. 3: 44. https://doi.org/10.3390/membranes8030044
APA StyleHélix-Nielsen, C. (2018). Biomimetic Membranes as a Technology Platform: Challenges and Opportunities. Membranes, 8(3), 44. https://doi.org/10.3390/membranes8030044




