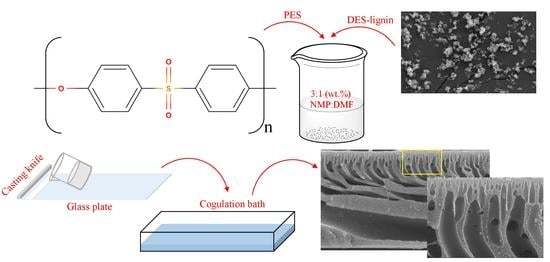
Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. General Procedure for DES Preparation
2.3. Lignin Extraction
2.4. Membrane Preparation
2.5. Membrane Characterization
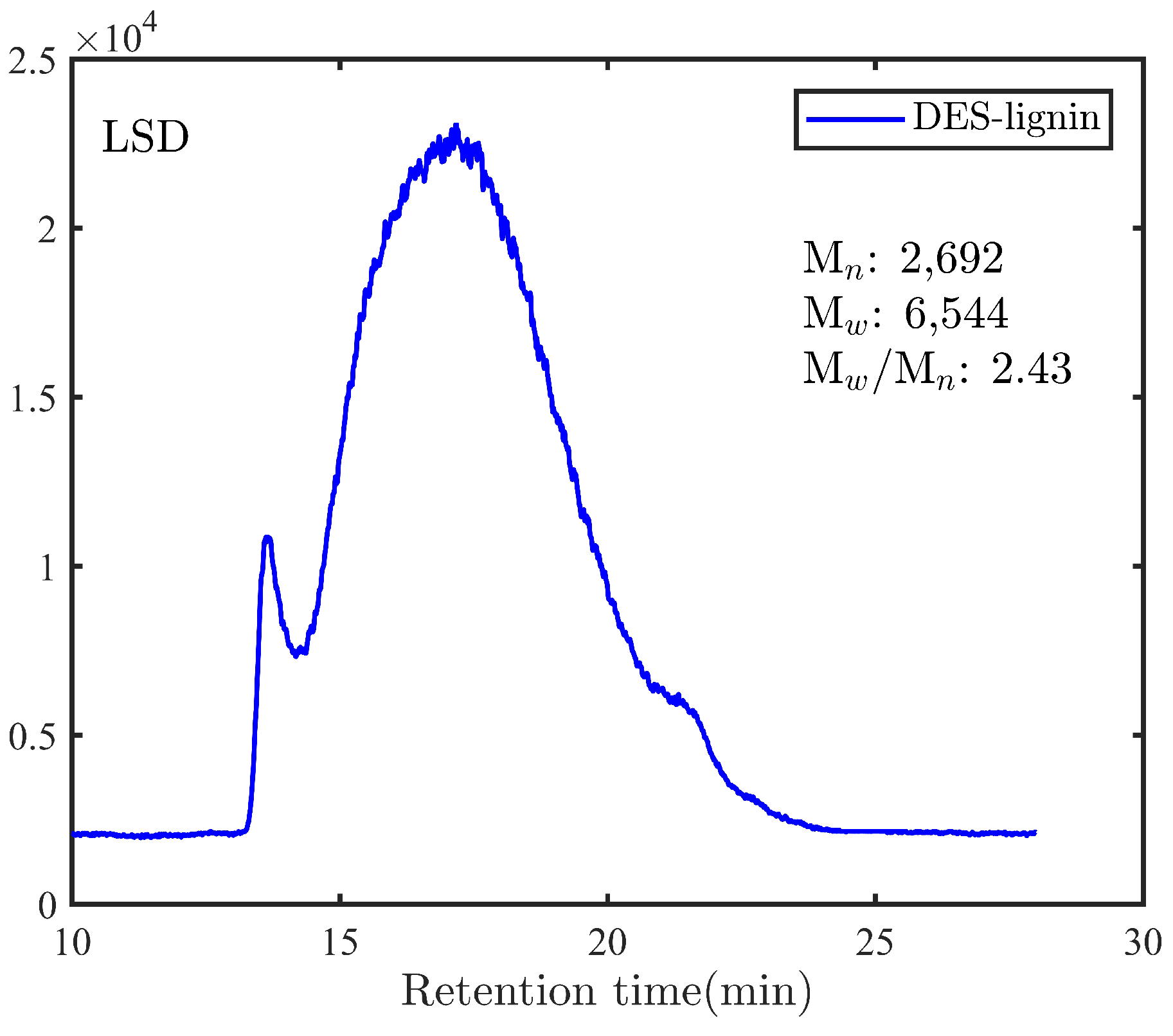
2.5.1. Molar Mass Distribution Analysis
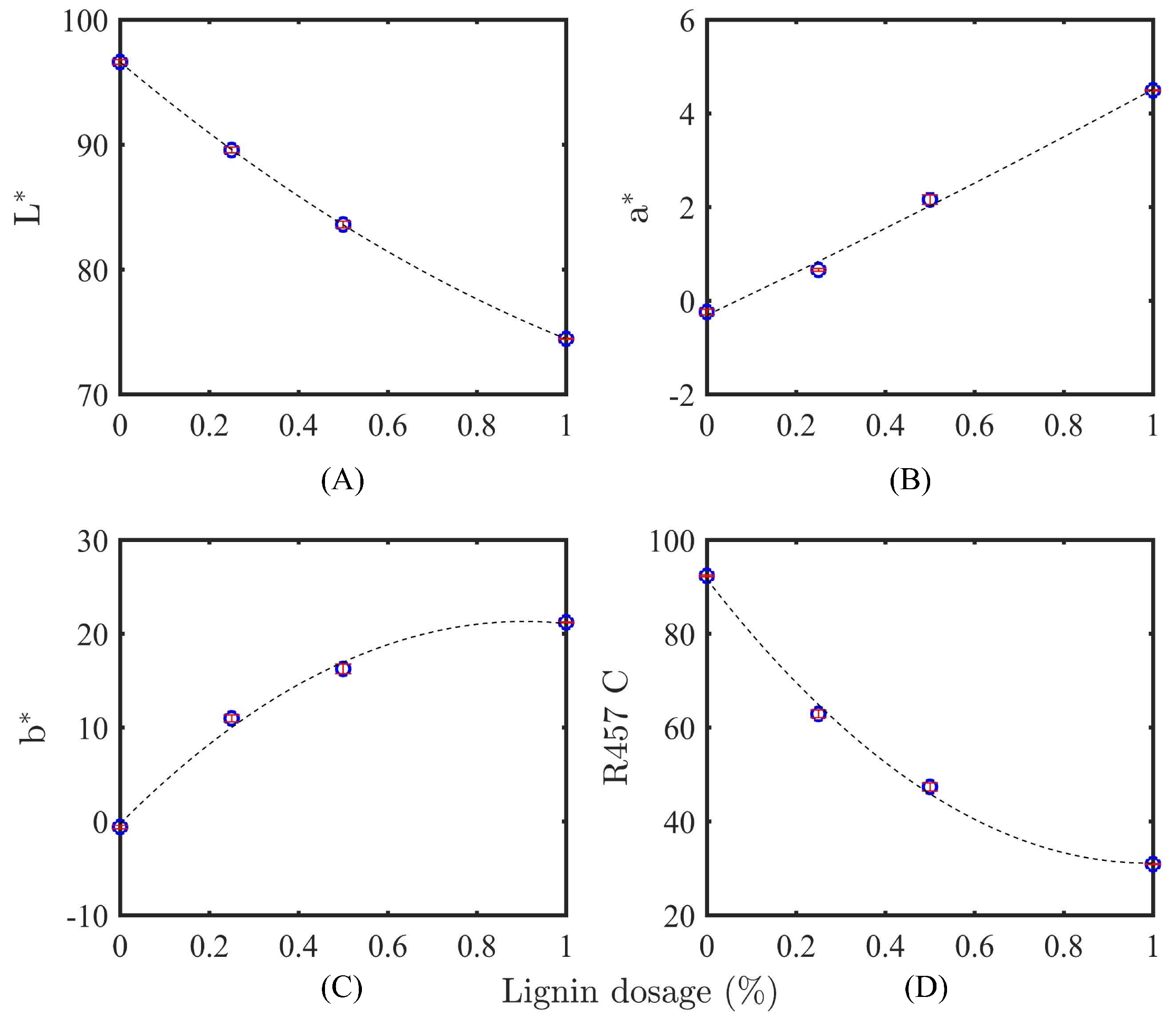
2.5.2. Color Parameter Measurements
2.5.3. Determination of Antioxidant Activity
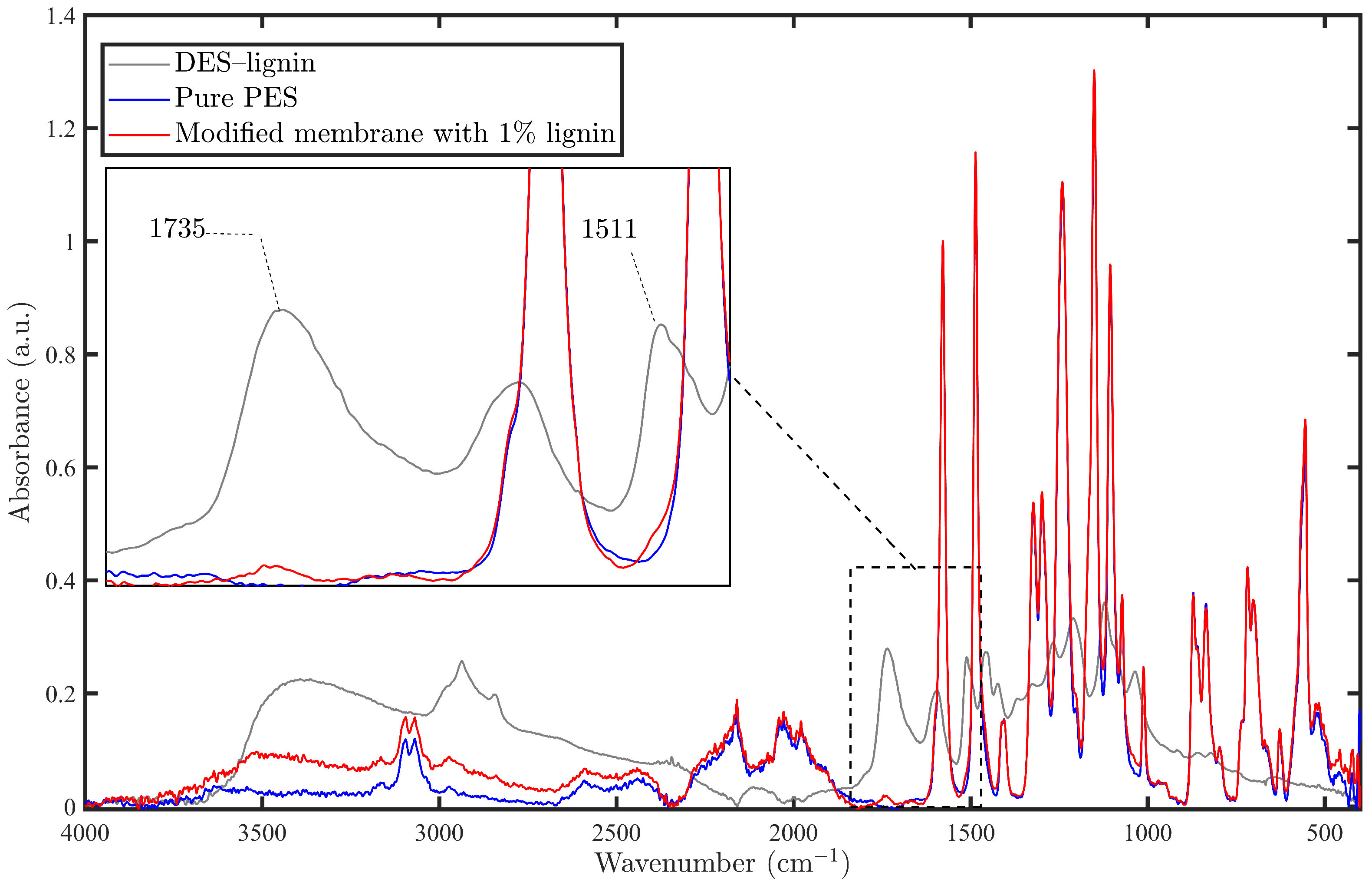
2.5.4. Fourier Transform Infrared Spectroscopy
2.5.5. Scanning Electron Microscopy
2.5.6. Casting Solution Viscosity Measurement
2.5.7. Contact Angle Measurements
2.5.8. Surface Charge Measurements
2.6. Experimental Design and Procedure
Pure Water Flux and Performance Analysis
3. Results and Discussion
3.1. Characterization of DES-Lignin
3.2. Characterization of the Membranes
3.2.1. FT-IR
3.2.2. Identification of Lignin by Color Measurement
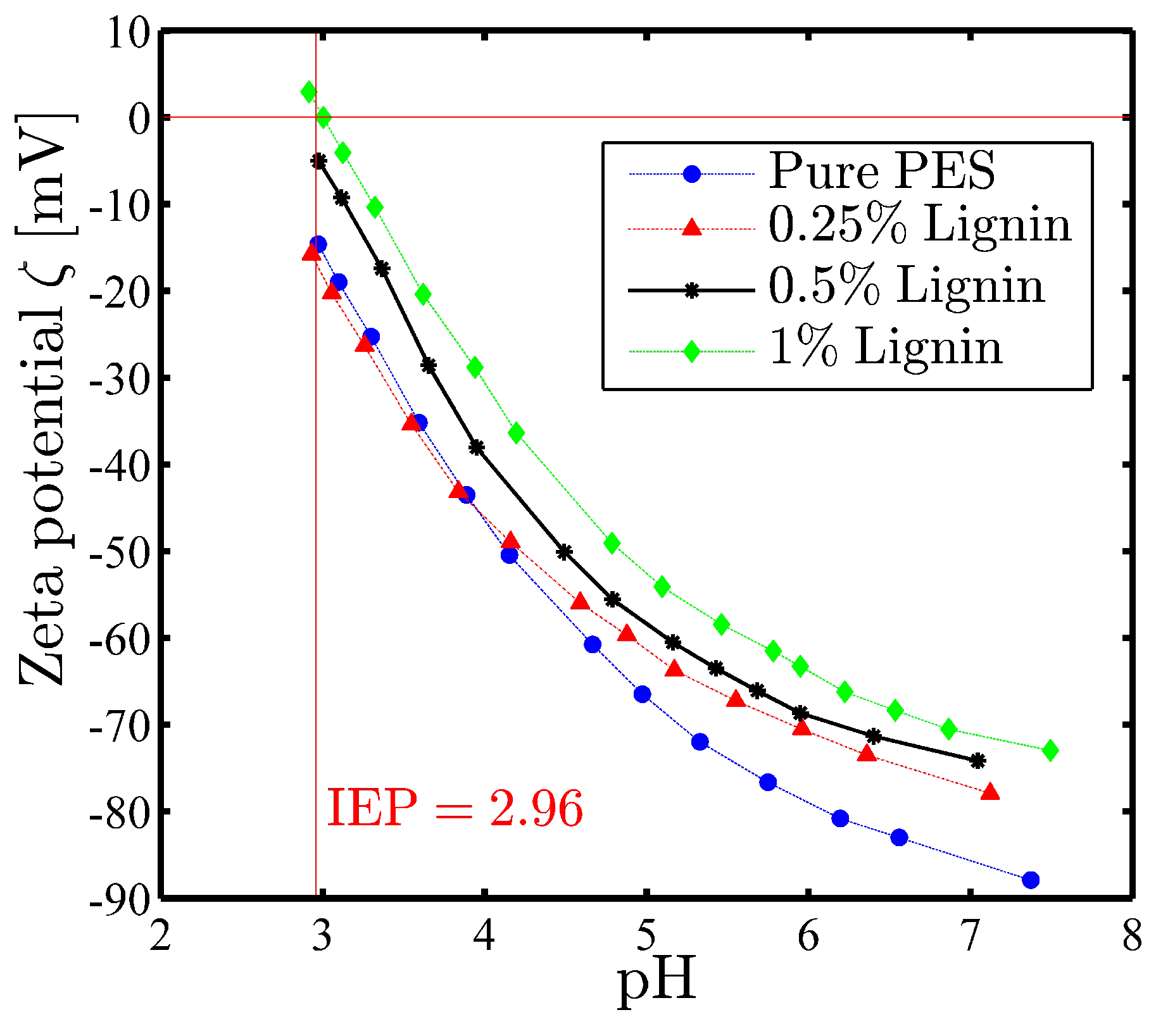
3.2.3. Surface Charge
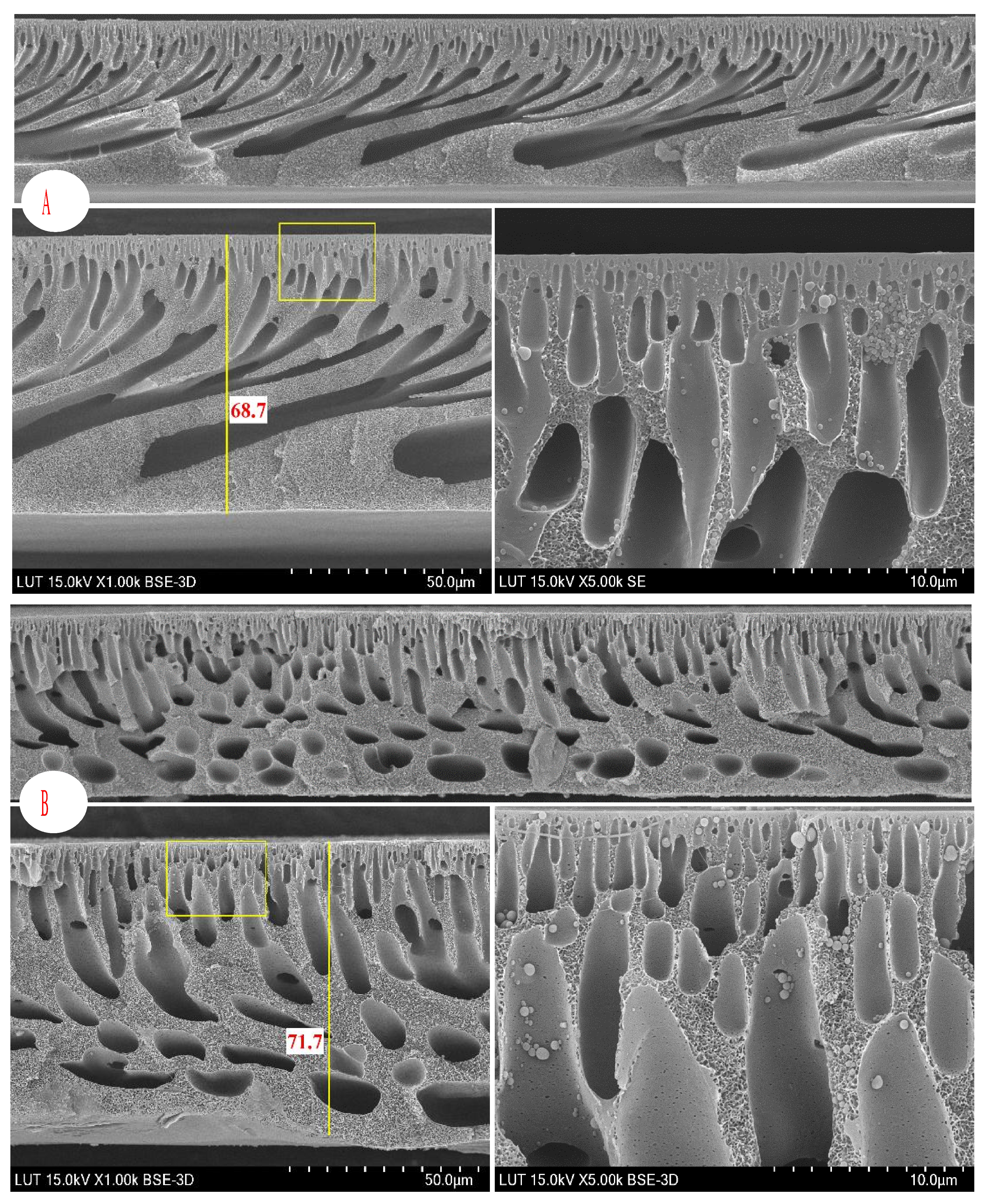
3.2.4. SEM
3.3. Effect of DES-Lignin on the Antioxidant Activity of Fabricated Membranes
3.4. Effect of DES-Lignin on Membrane Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.L.; Zhu, L.P.; Zhu, B.K.; Xu, Y.Y. High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent. Sep. Purif. Technol. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Egorov, V.M.; Smirnova, S.V.; Formanovsky, A.A.; Pletnev, I.V.; Zolotov, Y.A. Dissolution of cellulose in ionic liquids as a way to obtain test materials for metal-ion detection. Anal. Bioanal. Chem. 2007, 387, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2009, 27, 1391–1398. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjöholm, R.; Mikkola, J. Dissolution of lignocellulosic materials and its constituents using ionic liquids—A review. Ind. Crops Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Loow, Y.L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Maton, C.; Stevens, C.V. Biodegradation of imidazolium ionic liquids by activated sludge microorganisms. Biodegradation 2015, 26, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Santos, A.; Tojo, J.; Rodríguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.G.P.; Kollau, L.J.B.M.; van den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef] [PubMed]
- Majová, V.; Strišincová, P.; Jablonsky, M.; Andrea, S.; Vrška, M. Deep Eutectic Solvents: Fractionation of Wheat Straw. BioResources 2015, 10, 8039–8047. [Google Scholar]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018, 263, 193–199. [Google Scholar] [CrossRef]
- Majová, V.; Horanová, S.; Škulcová, A.; Šima, J.; Jablonský, M. Deep Eutectic Solvent Delignification: Impact of initial lignin. BioResources 2017, 12, 7301–7310. [Google Scholar]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Ding, Z.; Zhong, L.; Wang, X.; Zhang, L. Effect of lignin–cellulose nanofibrils on the hydrophilicity and mechanical properties of polyethersulfone ultrafiltration membranes. High Perform. Polym. 2016, 28, 1192–1200. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Hoek, E.M.; Mamba, B.B. Probing the mechanical and thermal properties of polysulfone membranes modified with synthetic and natural polymer additives. Polym. Test. 2014, 34, 202–210. [Google Scholar] [CrossRef]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Natarajan, G.; Cameron, D.C.; Nyström, M. Performance of RC and PES ultrafiltration membranes in filtration of pulp mill process waters. Desalination 2010, 264, 249–255. [Google Scholar] [CrossRef]
- Koivula, E.; Kallioinen, M.; Sainio, T.; Antón, E.; Luque, S.; Mänttäri, M. Enhanced membrane filtration of wood hydrolysates for hemicelluloses recovery by pretreatment with polymeric adsorbents. Bioresour. Technol. 2013, 143, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in lignin hydrogels and nanocomposites for water purification: Future perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Hoek, E.; Mamba, B.B. Investigating the Usability of Alkali Lignin as an Additive in Polysulfone Ultrafiltration Membranes. BioResources 2015, 10, 3079–3096. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Membr. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R.; Bodmeier, R. Polymer blends for controlled release coatings. J. Control. Release 2008, 125, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Causserand, C.; Rouaix, S.; Lafaille, J.P.; Aimar, P. Ageing of polysulfone membranes in contact with bleach solution: Role of radical oxidation and of some dissolved metal ions. Chem. Eng. Process. 2008, 47, 48–56. [Google Scholar] [CrossRef]
- Langsam, M.; Robeson, L.M. Substituted propyne polymers—Part II. Effects of aging on the gas permeability properties of poly[1-(trimethylsilyl)propyne] for gas separation membranes. Polym. Eng. Sci. 1989, 29, 44–54. [Google Scholar] [CrossRef]
- Raccach, M. The Antimicrobial Activity of Phenolic Antioxidants in Foods: A review. J. Food Saf. 1984, 6, 141–170. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Lacasse, M.; Wang, J.; Luchian, C. Lignin and Its Polyblends. J. Macromol. Sci. Part A 1995, 32, 1613–1619. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Mishra, A.K.; Mishra, S.B.; Mamba, B.B.; Thwala, J.M. Influence of TiO2-Modification on the Mechanical and Thermal Properties of Sugarcane Bagasse–EVA Composites. J. Inorg. Organomet. Polym. Mater. 2010, 20, 802–808. [Google Scholar] [CrossRef]
- Knyazkova, T.V.; Zhurayev, O.Z. Dynamic Polymer Layers on Membranes as Antifoulants in Membrane Filtration. In Role of Interfaces in Environmental Protection; Barany, S., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 181–189. [Google Scholar]
- Zhang, X.; Benavente, J.; Garcia-Valls, R. Lignin-based membranes for electrolyte transference. J. Power Sources 2005, 145, 292–297. [Google Scholar] [CrossRef]
- Nevárez, L.M.; Casarrubias, L.B.; Canto, O.S.; Celzard, A.; Fierro, V.; Gómez, R.I.; Sánchez, G.G. Biopolymers-based nanocomposites: Membranes from propionated lignin and cellulose for water purification. Carbohydr. Polym. 2011, 86, 732–741. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Hoek, E.M.V.; Mamba, B.B. Investigating the structure and water permeation of membranes modified with natural and synthetic additives using tensile, porosity, and glass transition temperature studies. J. Appl. Polym. Sci. 12014, 131, 1–8. [Google Scholar] [CrossRef]
- Vilakati, G.D.; Wong, M.C.; Hoek, E.M.; Mamba, B.B. Relating thin film composite membrane performance to support membrane morphology fabricated using lignin additive. J. Membr. Sci. 2014, 469, 216–224. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Pan, Y.H.; Jin, W.Z.; Xu, J.M.; Lao, K.K.; Gu, L. Preparation of sodium lignin sulfonate modified polysulfone membranes and their use as supports for forward osmosis membranes. Acta Polym. Sin. 2017, 851–857. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Li, W.; Xing, W.; Wang, Y. Depositing lignin on membrane surfaces for simultaneously upgraded reverse osmosis performances: An upscalable route. AIChE J. 2017, 63, 2221–2231. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Idris, A.; Lee, K.Y.; Hing, H.K. Preparation of cellulose acetate dialysis membrane for separation of bovine serum albumin. J. Teknol. 2005, 42, 35–46. [Google Scholar]
- Sun, S.N.; Cao, X.F.; Xu, F.; Sun, R.C.; Jones, G.L. Structural Features and Antioxidant Activities of Lignins from Steam-Exploded Bamboo (Phyllostachys pubescens). J. Agric. Food Chem. 2014, 62, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Ferlita, R.R.; Phipps, D.; Safarik, J.; Yeh, D.H. Cryo-snap: A simple modified freeze-fracture method for SEM imaging of membrane cross-sections. Environ. Prog. Sustain. Energy 2008, 27, 204–209. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM–41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative stabilisation of kraft lignin for carbon fibre production. Holzforschung 2012, 66, 141–147. [Google Scholar] [CrossRef]
- Esmaeili, M.; Virtanen, T.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Use of Vanillin as a hydrophilicity promoter in polyethersulphone membranes to improve their performance in wood extract treatment. 2018; submitted. [Google Scholar]
- Virtanen, T.; Parkkila, P.; Koivuniemi, A.; Lahti, J.; Viitala, T.; Kallioinen, M.; Mänttäri, M.; Bunker, A. Characterization of membrane–foulant interactions with novel combination of Raman spectroscopy, surface plasmon resonance and molecular dynamics simulation. Sep. Purif. Technol. 2018, 205, 263–272. [Google Scholar] [CrossRef]
- Shahzad, A.; Isaac, D.H. Weathering of Lignocellulosic Polymer Composites. In Lignocellulosic Polymer Composites; Thakur, V.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 325–367. [Google Scholar]
- Cheng, S.Y.; Wang, B.J.; Weng, Y.M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT–Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.; Avena-Bustillos, R.; Friedman, M.; McHugh, T. Physical and Antibacterial Properties of Edible Films Formulated with Apple Skin Polyphenols. J. Food Sci. 2011, 76, M149–M155. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Su, Y.; Zhu, S.; Li, C.; Zhao, Y.; Jiang, Z. A facile method for synthesis of pegylated polyethersulfone and its application in fabrication of antifouling ultrafiltration membrane. J. Membr. Sci. 2007, 303, 204–212. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Influence of ultrafiltration membrane characteristics on adsorptive fouling with dextrans. J. Membr. Sci. 2005, 266, 132–142. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Hubner, A.; Wiltowski, T.S. Chromium Adsorption by Lignin. Energy Sources 2000, 22, 45–56. [Google Scholar] [CrossRef]
- Lou, H.; Zhu, J.Y.; Lan, T.Q.; Lai, H.; Qiu, X. pH-Induced Lignin Surface Modification to Reduce Nonspecific Cellulase Binding and Enhance Enzymatic Saccharification of Lignocelluloses. ChemSusChem 2013, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, T.; Chen, C.; Li, J. A study on membrane morphology by digital image processing. J. Membr. Sci. 2007, 305, 93–102. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Isothermal phase diagrams and phase-inversion behavior of poly(vinylidene fluoride)/solvents/additives/water systems. J. Appl. Polym. Sci. 2003, 90, 2150–2155. [Google Scholar] [CrossRef]
- Alshawi, H.; Aubaid, A.; Al-Dujaili, N.H. A novel antibiotic-like substance isolation from a dermatophyte, Trichophyton rubrum. Rev. Med. Microbiol. 2018, 29, 89–100. [Google Scholar] [CrossRef]

| Membrane | PES (%) | Lignin Content (%) | Bulk Porosity (%) | Antioxidant DPPH (%) | Capacities ABTS (%) |
|---|---|---|---|---|---|
| #1 | 20 | - | 69.3 | 19.3 ± 0.03 | 33.5 ± 0.1 |
| #2 | 20 | 0.25 | 71.5 | 32.4 ± 0.06 | 42.5 ± 0.03 |
| #3 | 20 | 0.5 | 72.5 | 43.1 ± 0.1 | 47.9 ± 0.06 |
| #4 | 20 | 1 | 79.9 | 70.6 ± 0.2 | 71.7 ± 0.5 |
| Membrane | PES (%) | Lignin Content (%) | Contact Angle () | Pure water Flux (L/mh) | Permeability (L/mh bar) | Leakage (mg/L) | Rejection (%) (PEG 35 kDa) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 bar | 2 bar | 3 bar | |||||||
| #1 | 20 | - | 51.9 ± 0.4 | 67.7 ± 1.8 | 137.9 ± 0.7 | 210.0 ± 0.9 | 74.9 | 0.9 | 85.7 |
| 75.9 ± 1.7 | 151.1 ± 0.5 | 233.3 ± 0.8 | 0.6 | 82.8 | |||||
| #2 | 20 | 0.25 | 48.6 ± 0.3 | 85.9 ± 2.0 | 171.2 ± 1.7 | 254.8 ± 1.4 | 81.5 | 0.5 | 88.7 |
| 78.8 ± 1.9 | 154.5 ± 1.6 | 235.8 ± 0.9 | 0.4 | 84.9 | |||||
| #3 | 20 | 0.5 | 46.9 ± 0.4 | 94.2 ± 1.7 | 189.9 ± 0.7 | 286.0 ± 0.7 | 91.5 | 0.4 | 75.7 |
| 86.4 ± 0.9 | 181.2 ± 0.8 | 260.8 ± 0.7 | 0.4 | 83.6 | |||||
| #4 | 20 | 1 | 45.6 ± 1.2 | 105.6 ± 1.3 | 206.0 ± 1.0 | 309.4 ± 0.9 | 96.9 | 0.6 | 86.6 |
| 104.6 ± 1.4 | 183.7 ± 0.4 | 288.5 ± 0.5 | 0.8 | 81.4 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili, M.; Anugwom, I.; Mänttäri, M.; Kallioinen, M. Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes. Membranes 2018, 8, 80. https://doi.org/10.3390/membranes8030080
Esmaeili M, Anugwom I, Mänttäri M, Kallioinen M. Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes. Membranes. 2018; 8(3):80. https://doi.org/10.3390/membranes8030080
Chicago/Turabian StyleEsmaeili, Mohammadamin, Ikenna Anugwom, Mika Mänttäri, and Mari Kallioinen. 2018. "Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes" Membranes 8, no. 3: 80. https://doi.org/10.3390/membranes8030080
APA StyleEsmaeili, M., Anugwom, I., Mänttäri, M., & Kallioinen, M. (2018). Utilization of DES-Lignin as a Bio-Based Hydrophilicity Promoter in the Fabrication of Antioxidant Polyethersulfone Membranes. Membranes, 8(3), 80. https://doi.org/10.3390/membranes8030080