The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Preparation
2.3. Membrane Characterization
2.3.1. Thickness
2.3.2. Porosity
2.3.3. Pore Size
2.3.4. Pure Water Permeability (PWP)
2.3.5. Contact Angle
2.3.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
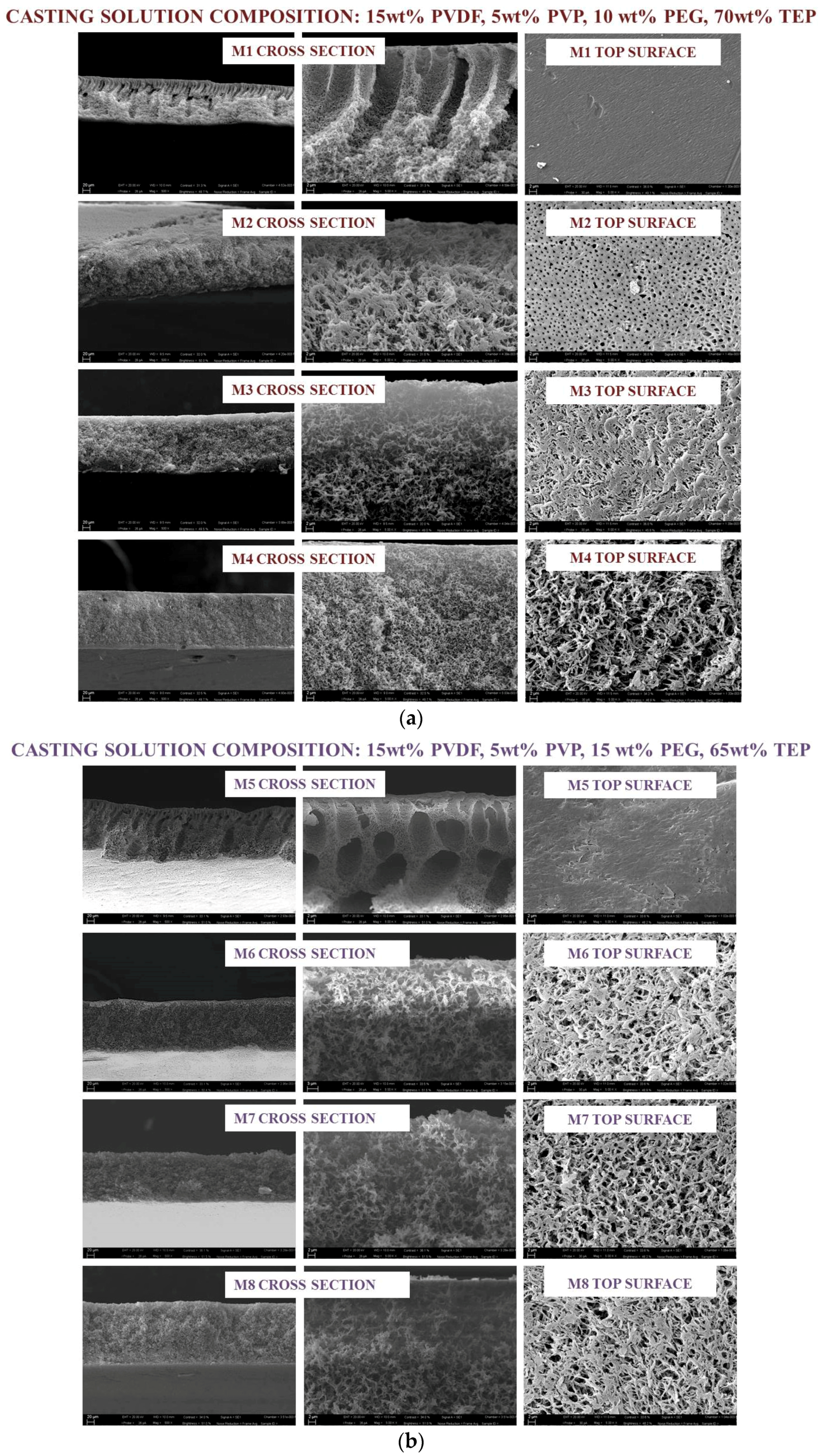
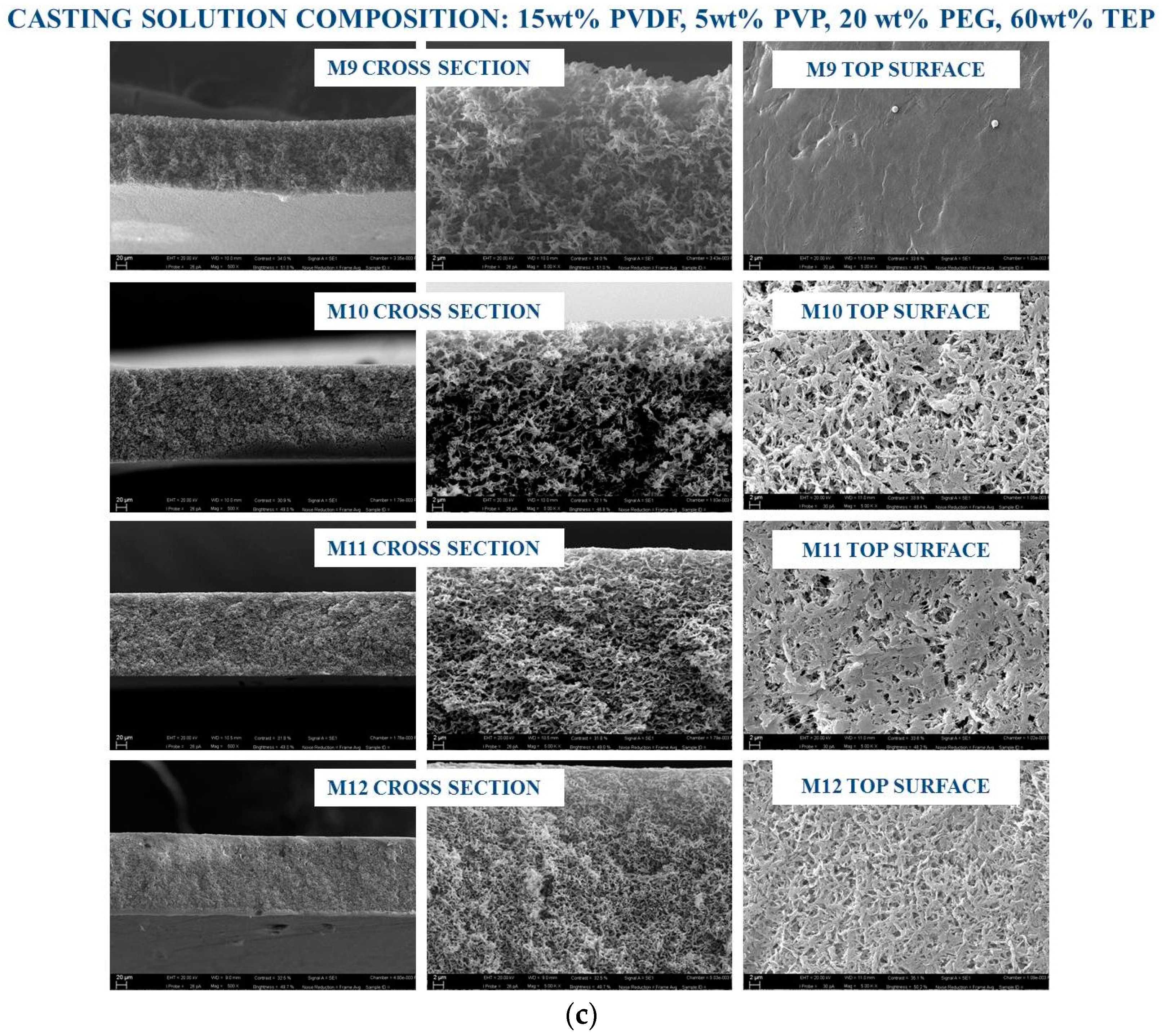
3.1. Membrane Morphology
3.2. Membrane Thickness, Porosity and Contact Angle
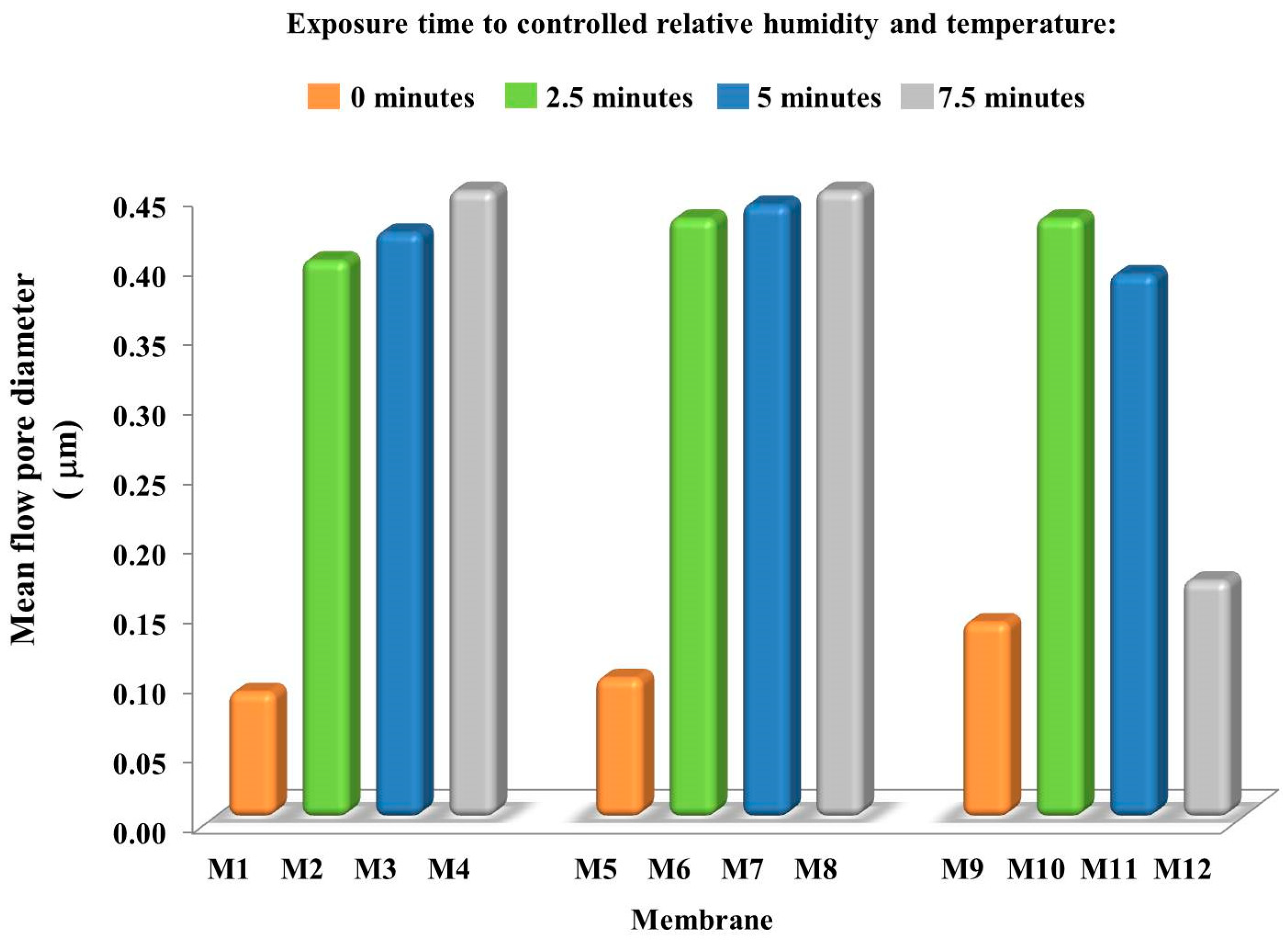
3.3. Membrane Pore Size
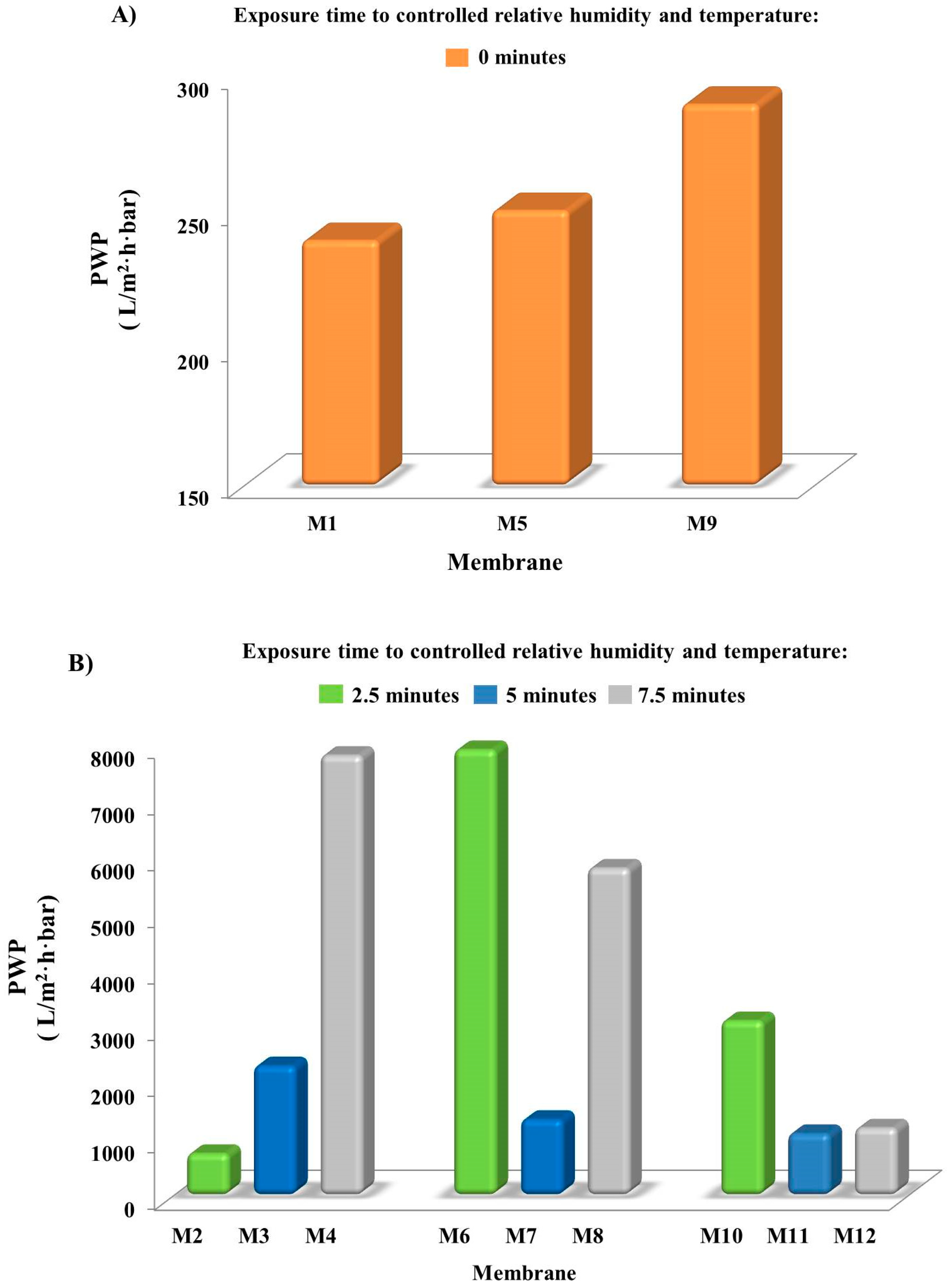
3.4. Membrane Pure Water Permeability (PWP)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Figoli, A.; Simone, S.; Drioli, E. Polymeric Membranes. In Membrane Fabrication; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1482210460. [Google Scholar]
- Sigma Aldrich Website. Available online: www.sigmaaldrich.com (accessed on 13 June 2018).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008. Available online: https://osha.europa.eu/it/legislation/directives/regulation-ec-no-1272-2008-classification-labelling-and-packaging-of-substances-and-mixtures (accessed on 28 August 2018).
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, E.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Safety Data Sheet of Triethyl Phosphate. Available online: http://www.cdhfinechemical.com/images/product/msds/37_1771179379_TRIETHYLPHOSPHATECASNO78-40-0MSDS.pdf (accessed on 27 August 2018).
- Organisation for Economic Co-Operation and Development Website. Available online: http://www.oecd.org (accessed on 13 June 2018).
- Fang, C.; Jeon, S.; Rajabzadeh, S.; Cheng, L.; Fang, L.; Matsuyama, H. Tailoring the surface pore size of hollow fiber membranes in the TIPS process. J. Mater. Chem. A 2018, 6, 535–547. [Google Scholar] [CrossRef]
- Tao, M.M.; Liu, F.; Ma, B.R.; Xue, L.X. Effect of solvent power on PVDF membrane polymorphism during phase inversion. Desalination 2013, 316, 137–145. [Google Scholar] [CrossRef]
- Chang, J.; Zuo, J.; Zhang, L.; O’Brien, G.S.; Chung, T.S. Using green solvent, triethyl phosphate (TEP), to fabricate highly porous PVDF hollow fiber membranes for membrane distillation. J. Membr. Sci. 2017, 539, 295–304. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.L.; Yu, L.Y. Effects of mixed solvents and PVDF types on performances of PVDF microporous membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.L.; Liu, M. Preparation and characterization of PVDF microporous membrane with highly hydrophobic surface. Polym. Adv. Technol. 2011, 22, 520–531. [Google Scholar] [CrossRef]
- Liu, F.; Tao, M.M.; Xue, L.X. PVDF membranes with inter-connected pores prepared via a Nat-ips process. Desalination 2012, 298, 99–105. [Google Scholar] [CrossRef]
- Fadhil, S.; Marino, T.; Makki, H.F.; Alsalhy, Q.F.; Blefari, S.; Macedonio, F.; Di Nicolò, E.; Giorno, L.; Drioli, E.; Figoli, A. Novel PVDF-HFP flat sheet membranes prepared by triethyl phosphate (TEP) solvent for direct contact membrane distillation. Chem. Eng. Process. Process Intensif. 2016, 102, 16–26. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological study of poly(vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Marino, T.; Blefari, S.; Di Nicolò, E.; Figoli, A. A more sustainable membrane preparation using triethyl phosphate as solvent. Green Process. Synth. 2017, 6, 295–300. [Google Scholar] [CrossRef]
- Nejati, S.; Boo, C.; Osuji, C.O.; Elimelech, M. Engineering flat sheet microporous PVDF films for membrane distillation. J. Membr. Sci. 2015, 492, 355–363. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9788578110796. [Google Scholar]
- Eastman Solvent Selector Chart. Available online: http://www.eastman.com/Literature_Center/S/SOL030.pdf (accessed on 13 June 2018).
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, C.; Liu, G.; Li, X.; Guan, Y.; Lv, J. Effect of TEP content in cooling bath on porous structure, crystalline and mechanical properties of PVDF hollow fiber membranes. Polym. Eng. Sci. 2014, 54, 2207–2214. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Chen, T.C.; Cheng, L.P. Microporous PVDF membrane formation by immersion precipitation from water/TEP/PVDF system. Desalination 2002, 145, 25–29. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with Rhodiasolv®Polarclean as water soluble green solvent. J. Membr. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Jena, A.; Gupta, K. Advances in pore structure evaluation by porometry. Chem. Eng. Technol. 2010, 33, 1241–1250. [Google Scholar] [CrossRef]
- Thomas, R.; Guillen-Burrieza, E.; Arafat, H.A. Pore structure control of PVDF membranes using a 2-stage coagulation bath phase inversion process for application in membrane distillation (MD). J. Membr. Sci. 2014, 452, 470–480. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Preparation and Characterization of Polyvinylidene Fluoride Membranes for Membrane Distillation. Ind. Eng. Chem. Res. 2001, 40, 5710–5718. [Google Scholar] [CrossRef]
- Mohammadi, T.; Safavi, M.A. Application of Taguchi method in optimization of desalination by vacuum membrane distillation. Desalination 2009, 249, 83–89. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; MacEdonio, F.; Al-Jlil, S.A.; Al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Ali, M.I.; Summers, E.K.; Arafat, H.A.; Lienhard V, J.H. Effects of membrane properties on water production cost in small scale membrane distillation systems. Desalination 2012, 306, 60–71. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology: In the Chemical Industry; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9783527313167. [Google Scholar]
- Peng, Y.; Fan, H.; Dong, Y.; Song, Y.; Han, H. Effects of exposure time on variations in the structure and hydrophobicity of polyvinylidene fluoride membranes prepared via vapor-induced phase separation. Appl. Surf. Sci. 2012, 258, 7872–7881. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Bouyer, D. A Review on Polymeric Membranes and Hydrogels Prepared by Vapor-Induced Phase Separation Process. Polym. Rev. 2013, 53, 568–626. [Google Scholar] [CrossRef]
- Abdulla AlMarzooqi, F.; Roil Bilad, M.; Ali Arafat, H. Improving Liquid Entry Pressure of Polyvinylidene Fluoride (PVDF) Membranes by Exploiting the Role of Fabrication Parameters in Vapor-Induced Phase Separation VIPS and Non-Solvent-Induced Phase Separation (NIPS) Processes. Appl. Sci. 2017, 7, 181. [Google Scholar] [CrossRef]
- Annamalai, P.K.; Pochat-Bohatier, C.; Bouyer, D.; Li, C.L.; Deratani, A.; Wang, D.M. Kinetics of mass transfer during vapour-induced phase separation (VIPS) process and its influence on poly-(vinylidene fluoride) (PVDF) membrane structure and surface morphology. Desalin. Water Treat. 2011, 34, 204–210. [Google Scholar] [CrossRef]
- Idris, A.; Yet, L.K. The effect of different molecular weight PEG additives on cellulose acetate asymmetric dialysis membrane performance. J. Membr. Sci. 2006, 280, 920–927. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, Y.; Fan, H.; Chen, P.; Li, Z.; Jiang, Q. Preparation of polysulfone membranes via vapor-induced phase separation and simulation of direct-contact membrane distillation by measuring hydrophobic layer thickness. Desalination 2013, 316, 53–66. [Google Scholar] [CrossRef]
- Wang, L.; Yong, W.F.; Yu, L.E.; Chung, T. Design of High Efficiency PVDF-PEG Hollow Fibers for Air Filtration of Ultrafine Particles. J. Membr. Sci. 2017, 535, 342–349. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Membr. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Venault, A.; Ballad, M.R.B.; Huang, Y.T.; Liu, Y.H.; Kao, C.H.; Chang, Y. Antifouling PVDF membrane prepared by VIPS for microalgae harvesting. Chem. Eng. Sci. 2016, 142, 97–111. [Google Scholar] [CrossRef]
- Tan, P.C.; Low, S.C. Role of hygroscopic triethylene glycol and relative humidity in controlling morphology of polyethersulfone ultrafiltration membrane. Desalin. Water Treat. 2016, 57, 19051–19061. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, J.; Feng, L.; Jiang, L.; Tang, W.; Luo, X.; Han, C.C. Facile Creation of a Super-Amphiphobic Coating Surface with Bionic Microstructure. Adv. Mater. 2004, 16, 302–305. [Google Scholar] [CrossRef]
- Peng, M.; Li, H.; Wu, L.; Zheng, Q.; Chen, Y.; Gu, W. Porous poly(vinylidene fluoride) membrane with highly hydrophobic surface. J. Appl. Polym. Sci. 2005, 98, 1358–1363. [Google Scholar] [CrossRef]
- Ditter, J.; Morris, R.; Zepf, R. Large Pore Synthetic Polymer Membranes. US Patent 5,846,422, 8 December 1998. [Google Scholar]
- Wang, L.F.; Ditter, J.F.; Zepf, R. Highly Porous Polyvinylidene Difluoride Membranes. US Patent No. 6,146,747, 14 November 2000. [Google Scholar]
- Gao, L.; Tang, B.; Wu, P. An experimental investigation of evaporation time and the relative humidity on a novel positively charged ultrafiltration membrane via dry-wet phase inversion. J. Membr. Sci. 2009, 326, 168–177. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y.; Li, Z.; Chen, P.; Jiang, Q.; Wang, S. Preparation and characterization of hydrophobic PVDF membranes by vapor-induced phase separation and application in vacuum membrane distillation. J. Polym. Res. 2013, 20, 134. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Wu, Z. Effects of solvent compositions on physicochemical properties and anti-fouling ability of PVDF microfiltration membranes for wastewater treatment. Desalination 2012, 297, 79–86. [Google Scholar] [CrossRef]
| Solvent | Hazard Statements (Classification according to Regulation (EC) No 1272/2008) | |
|---|---|---|
| DMF | H226 Flammable liquid and vapour. |  |
| H312 + H332 Harmful in contact with skin or if inhaled. | ||
| H319 Causes serious eye irritation. | ||
| H360D May damage the unborn child. | ||
| GERM CELL MUTAGENICITY:MOUSE, LYMPHOCYTE. MUTATION IN MAMMALIAN SOMATIC CELLS. | ||
| DMA | H312 + H332 Harmful in contact with skin or if inhaled. |  |
| H319 Causes serious eye irritation. | ||
| H360D May damage the unborn child. | ||
| MAY CAUSE CONGENITAL MALFORMATION IN THE FETUS. PRESUMED HUMAN REPRODUCTIVE TOXICANT OVEREXPOSURE MAY CAUSE REPRODUCTIVE DISORDER(S) BASED ON TESTS WITH LABORATORY ANIMALS. | ||
| NMP | H315 Causes skin irritation. |  |
| H319 Causes serious eye irritation. | ||
| H335 May cause respiratory irritation. | ||
| H360D May damage the unborn child. | ||
| DAMAGE TO FETUS POSSIBLE. | ||
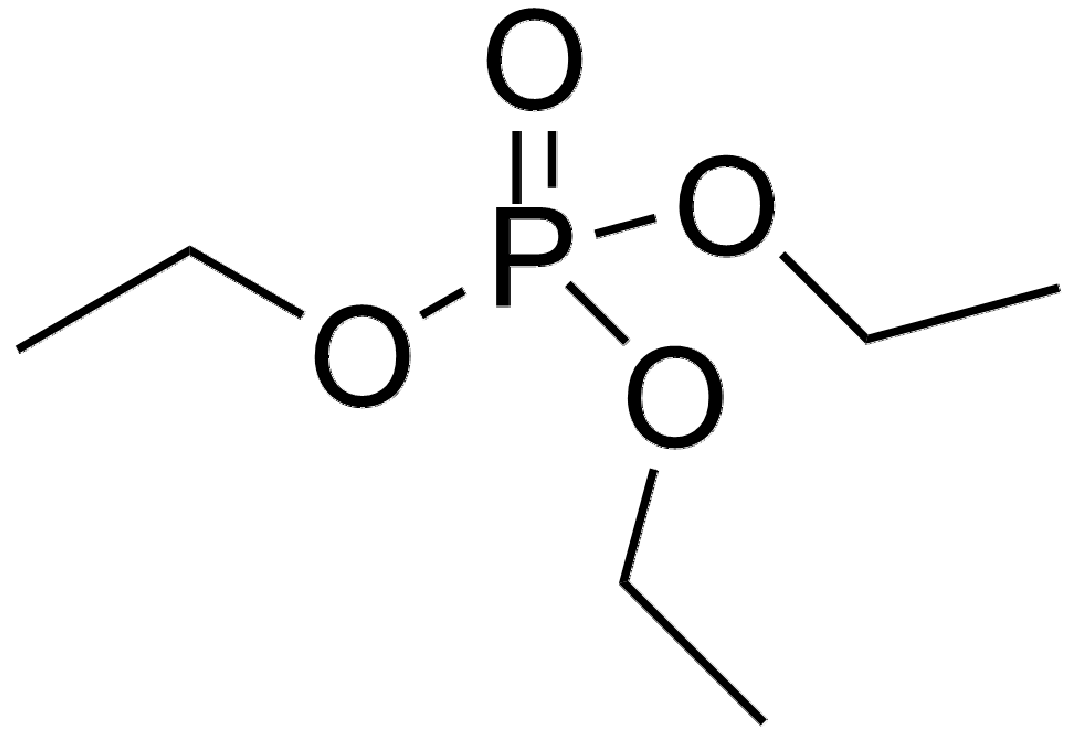
| TEP | H302 Harmful if swallowed. |  |
| H319 Causes serious eye irritation. | ||
| THIS SUBSTANCE/MIXTURE CONTAINS NO COMPONENTS CONSIDERED TO BE EITHER PERSISTENT, BIOACCUMULATIVE AND TOXIC, OR VERY PERSISTENT AND VERY BIOACCUMULATIVE AT LEVELS OF 0.1% OR HIGHER. | ||
| Compound | Hydrogen Bond Force | Dispersion Force | Polar Force | Solubility Parameter | Polymer–Solvent (S) Affinity | Solvent–Non-Solvent (NS) Affinity | Reference |
|---|---|---|---|---|---|---|---|
| δh | δd | δp | δsp | δPVDF−S | δS−NS | δh | |
| PVDF | 9.2 | 17.2 | 12.5 | - | - | - | [21] |
| TEP | 9.2 | 16.8 | 11.5 | 22.2 | 1.1 | 33.4 | [21] |
| DMF | 11.3 | 17.4 | 13.7 | 24.8 | 2.4 | 31.1 | [22] |
| DMA | 11.8 | 17.8 | 14.1 | 22.7 | 1.4 | 32.4 | [22] |
| NMP | 7.2 | 18.4 | 12.3 | 22.9 | 2.2 | 35.4 | [22] |
| WATER | 42.3 | 15.6 | 16.0 | 47.8 | - | - | [22] |
| Membrane Code | PVDF/wt % | PVP/wt % | PEG/wt % | TEP/wt % | Exposure Time to Rh/min |
|---|---|---|---|---|---|
| M1 | 15 | 5 | 10 | 70 | 0 |
| M2 | 15 | 5 | 10 | 70 | 2.5 |
| M3 | 15 | 5 | 10 | 70 | 5 |
| M4 | 15 | 5 | 10 | 70 | 7.5 |
| M5 | 15 | 5 | 15 | 65 | 0 |
| M6 | 15 | 5 | 15 | 65 | 2.5 |
| M7 | 15 | 5 | 15 | 65 | 5 |
| M8 | 15 | 5 | 15 | 65 | 7.5 |
| M9 | 15 | 5 | 20 | 60 | 0 |
| M10 | 15 | 5 | 20 | 60 | 2.5 |
| M11 | 15 | 5 | 20 | 60 | 5 |
| M12 | 15 | 5 | 20 | 60 | 7.5 |
| Membrane Code | Thickness (mm) | Porosity (%) | Contact Angle | |
|---|---|---|---|---|
| Air Side (°) | Glass Side (°) | |||
| M1 | 0.160 ± 0.002 | 83.3 ± 0.4 | 77 ± 2 | 98 ± 2 |
| M2 | 0.150 ± 0.001 | 80.6 ± 0.5 | 83 ± 1 | 99 ± 2 |
| M3 | 0.154 ± 0.001 | 81.8 ± 0.6 | 87 ± 2 | 99 ± 1 |
| M4 | 0.158 ± 0.001 | 82.4 ± 0.4 | 88 ± 2 | 100 ± 2 |
| M5 | 0.164 ± 0.004 | 85.5 ± 0.4 | 78 ± 2 | 99 ± 2 |
| M6 | 0.152 ± 0.001 | 82.8 ± 0.5 | 87 ± 2 | 100 ± 1 |
| M7 | 0.156 ± 0.003 | 84.5 ± 0.4 | 92 ± 2 | 101 ± 2 |
| M8 | 0.159 ± 0.001 | 84.9 ± 0.6 | 94 ± 3 | 104 ± 1 |
| M9 | 0.164 ± 0.002 | 86.5 ± 0.4 | 84 ± 2 | 99 ± 1 |
| M10 | 0.158 ± 0.001 | 85.6 ± 0.6 | 98 ± 2 | 101 ± 2 |
| M11 | 0.160 ± 0.002 | 86.4 ± 0.5 | 101 ± 1 | 103 ± 2 |
| M12 | 0.162 ± 0.002 | 86.6 ± 0.4 | 102 ± 2 | 106 ± 1 |
| Solvent Type | RH% | Exposure Time to Humid Air | Mean Pore Diameter | PWP | Potential Applications | Ref. |
|---|---|---|---|---|---|---|
| Min | mm | L/m2·h·BAR | ||||
| DMA | 100 | 0 | 0.11 | - | Vacuum membrane distillation (VMD) | [34] |
| 3 | 0.11 | |||||
| 5 | 0.11 | |||||
| 6 | 0.11 | |||||
| DMA | 60 | 2 | 0.06 | - | Direct contact membrane distillation (DCMD) | [36] |
| 5 | 0.06 | |||||
| 10 | 0.14 | |||||
| 80 | 2 | 0.07 | ||||
| 5 | 0.13 | |||||
| 10 | 0.14 | |||||
| DMA | 100 | 0 | 0.34 | - | VMD | [50] |
| 1 | 0.62 | |||||
| 2 | 0.8 | |||||
| 5 | 1.02 | |||||
| DMF | 30 ± 5 | 0.5 | - | 99.6 | MF | [51] |
| DMA | 87.7 | |||||
| TEP | 89.1 | |||||
| DMSO | 272.1 | |||||
| TEP | 55 | 0 | 0.14 | 290 | UF–MF | This work |
| 2.5 | 0.43 | 7900 | ||||
| 5 | 0.42 | 2300 | ||||
| 7.5 | 0.45 | 7800 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, T.; Russo, F.; Figoli, A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes 2018, 8, 71. https://doi.org/10.3390/membranes8030071
Marino T, Russo F, Figoli A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes. 2018; 8(3):71. https://doi.org/10.3390/membranes8030071
Chicago/Turabian StyleMarino, Tiziana, Francesca Russo, and Alberto Figoli. 2018. "The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation" Membranes 8, no. 3: 71. https://doi.org/10.3390/membranes8030071
APA StyleMarino, T., Russo, F., & Figoli, A. (2018). The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes, 8(3), 71. https://doi.org/10.3390/membranes8030071







