Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis
Abstract
:1. Introduction
2. Theory
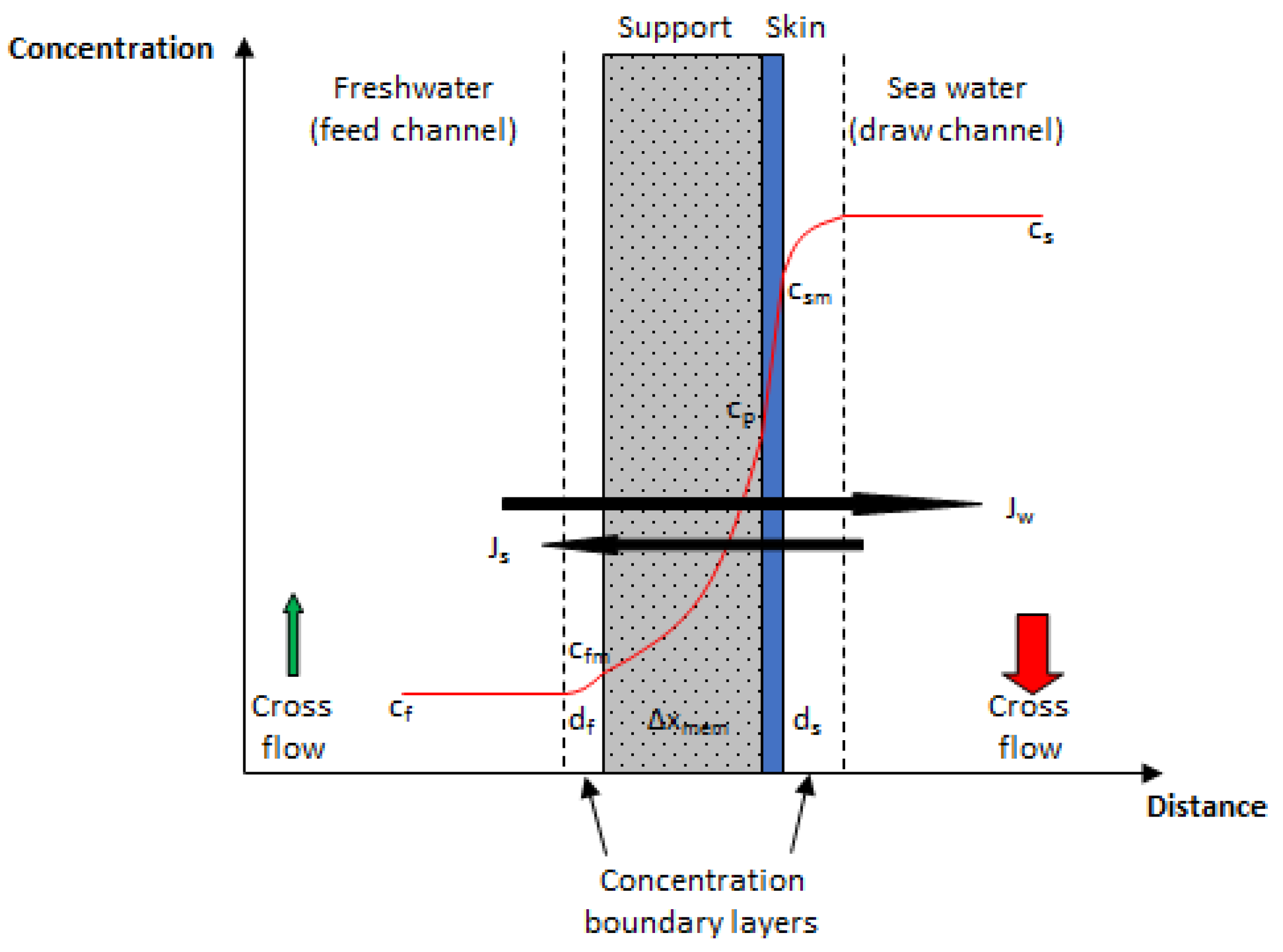
2.1. Transport Model
2.2. Impact of Temperature and Concentration on PRO Performance
3. Materials and Methods
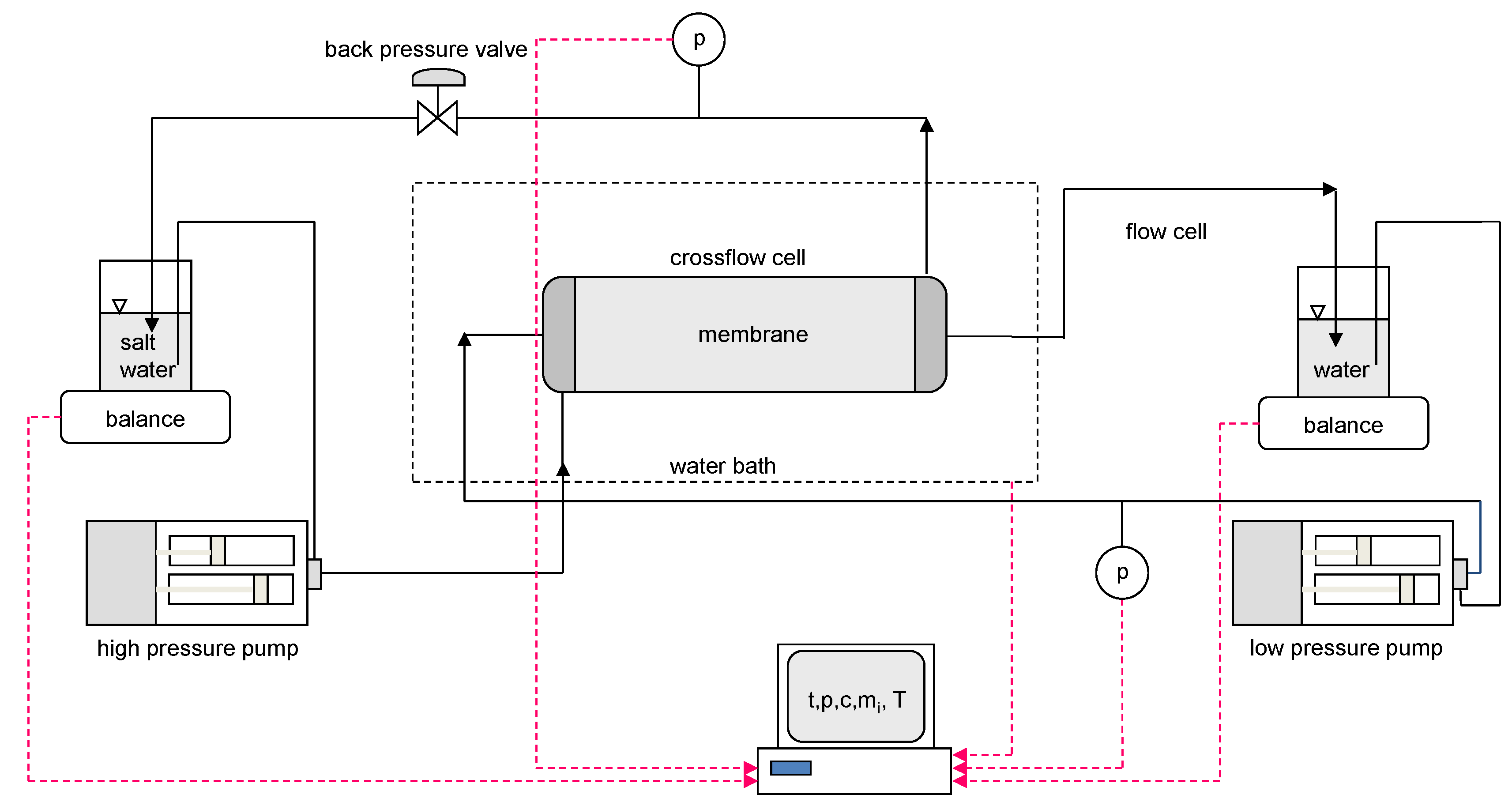
3.1. Apparatus
3.2. Standard Test Protocol
3.3. Membranes
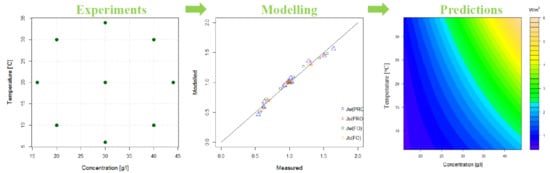
3.4. Experimental Design
4. Results and Discussion
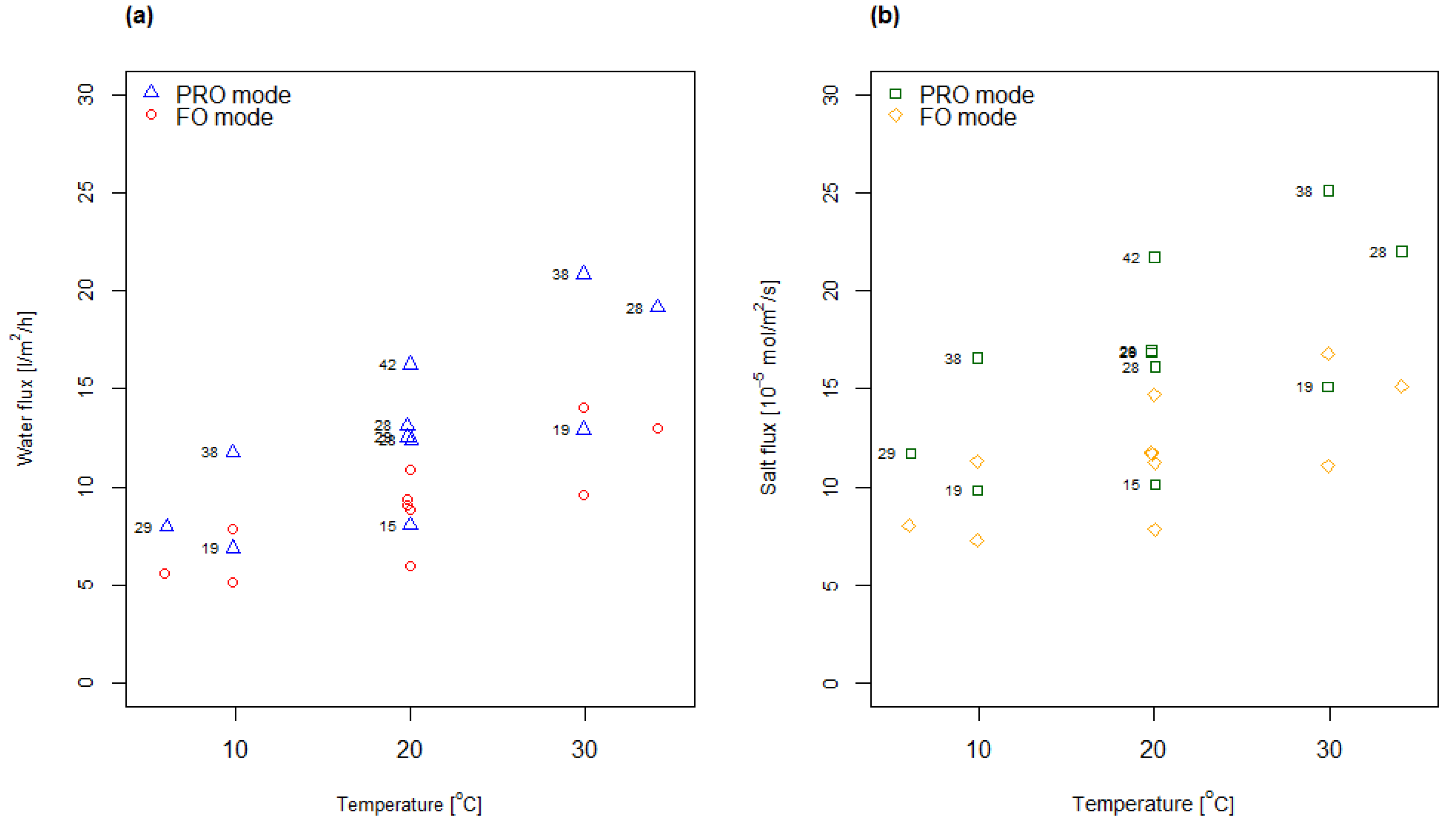
4.1. Measured Water and Salt Fluxes
4.2. Analysis of Variance of Flux Data
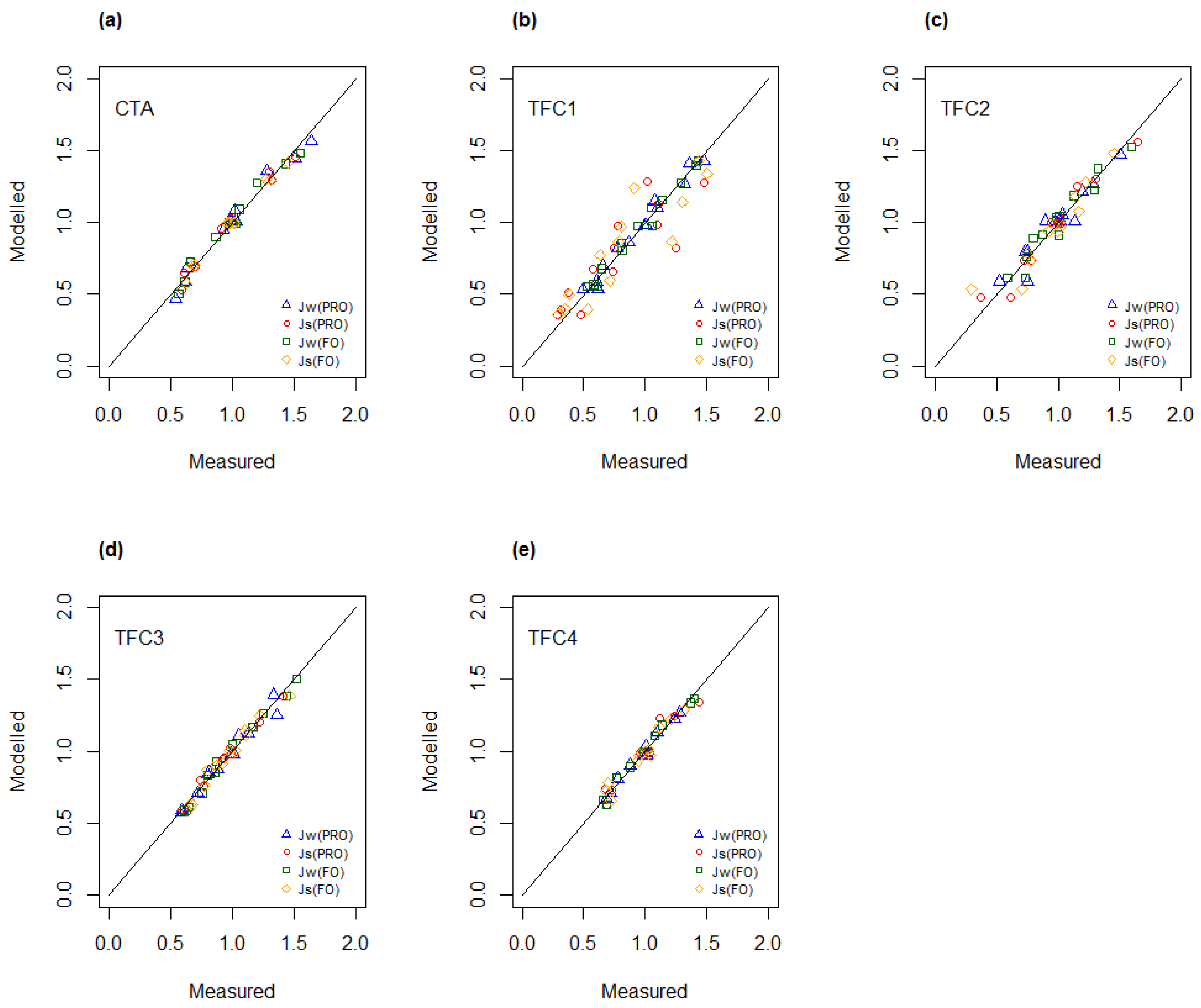
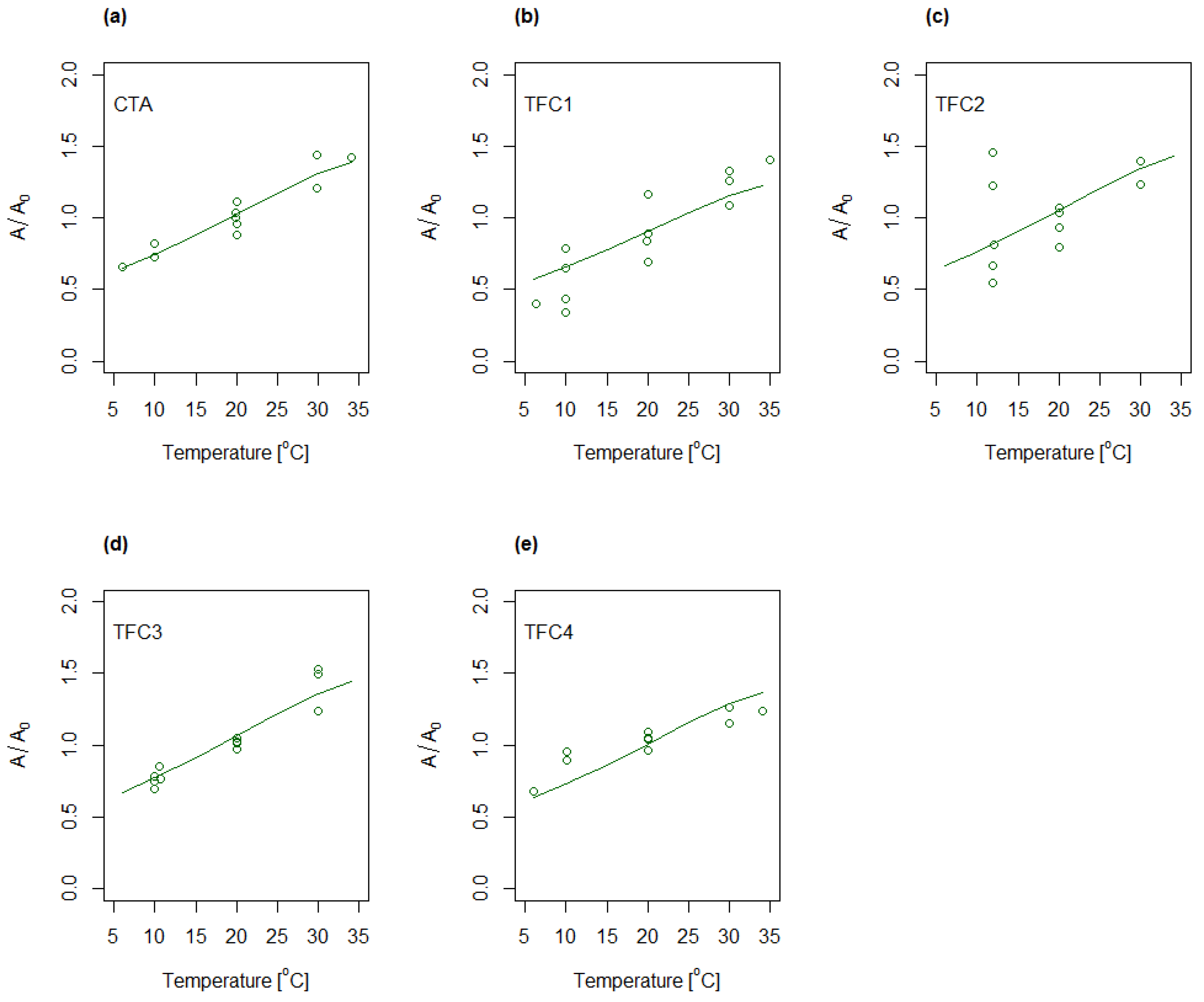
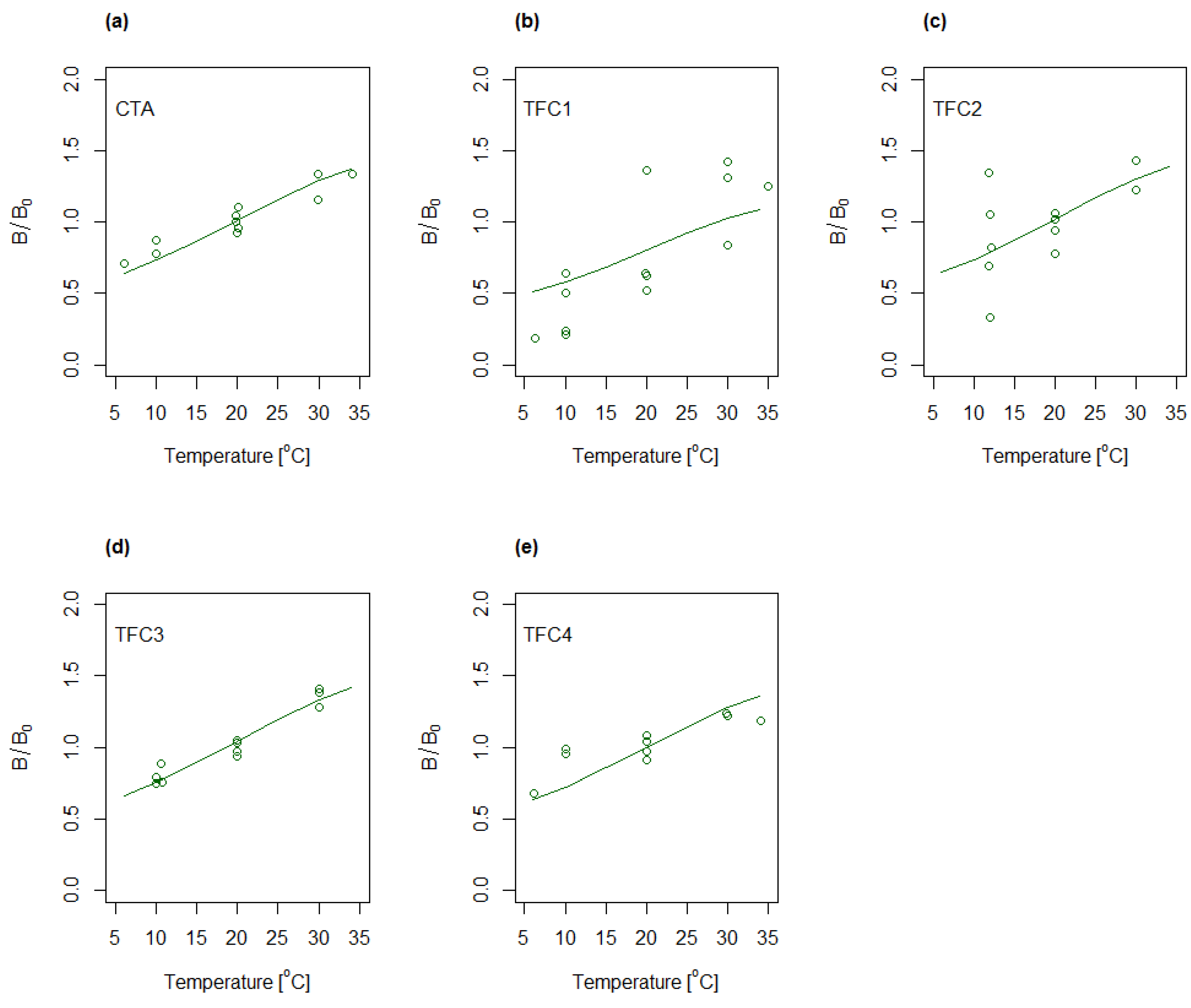
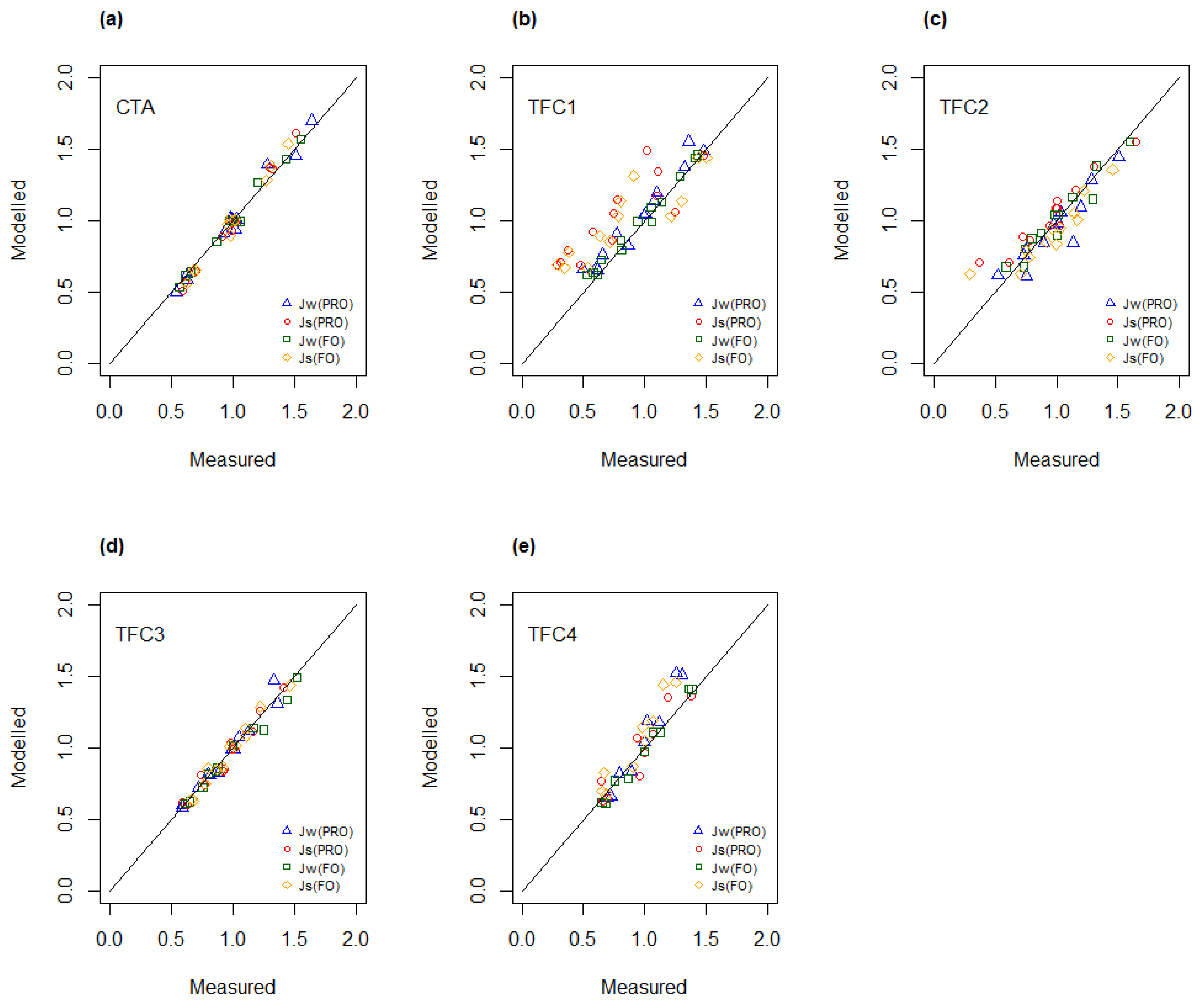
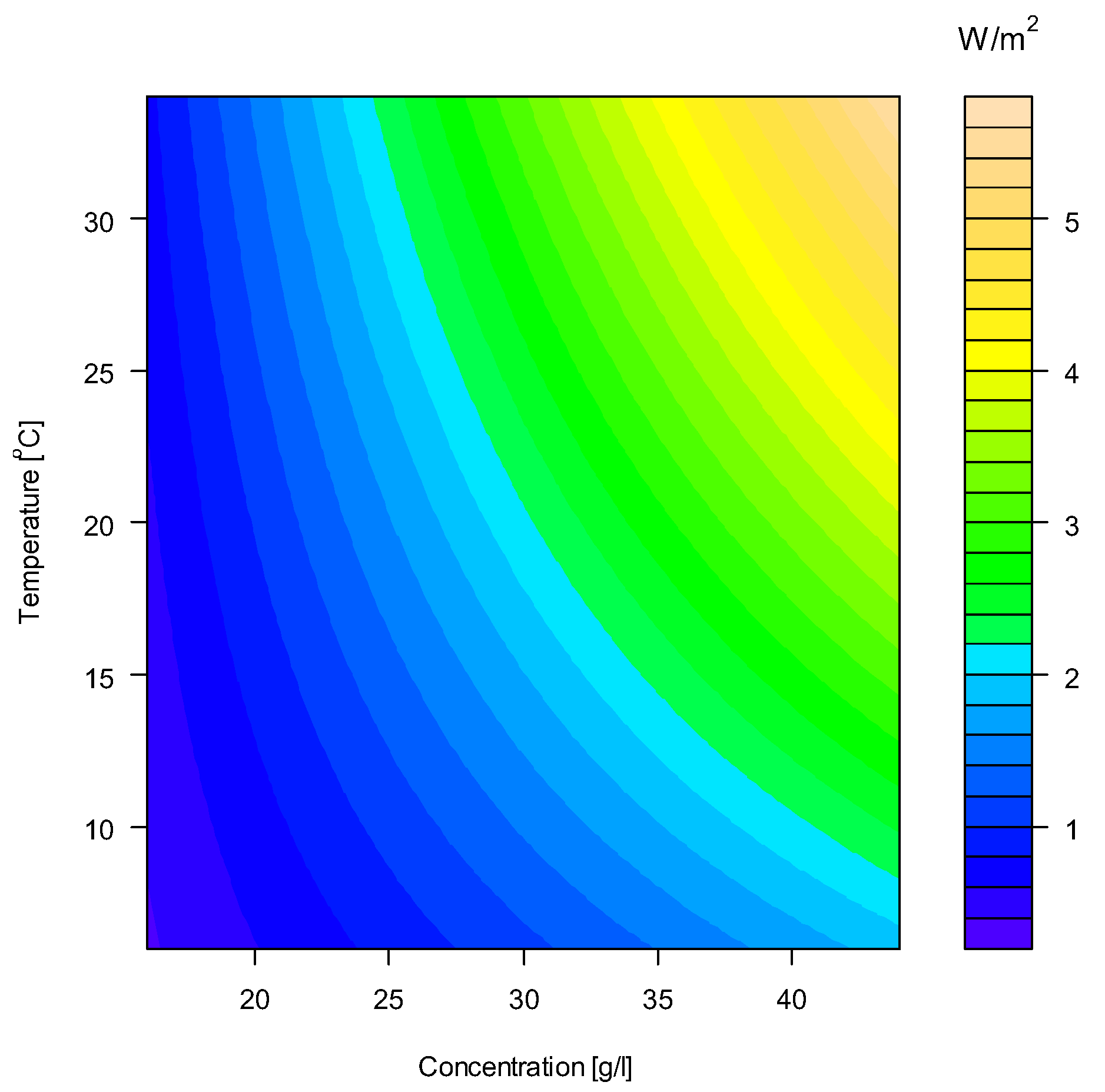
4.3. Determination of A, B and S as Function of Concentration and Temperature
4.4. Modelling of A and B
5. Implications in River Water/Seawater PRO
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Achilli, A.; Childress, A.E. Pressure retarded osmosis: From the vision of sidney loeb to the first prototype installation-review. Desalination 2010, 261, 205–211. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Skilhagen, S.E.; Dugstad, J.E.; Aaberg, R.J. Osmotic power-power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 2008, 220, 476–482. [Google Scholar] [CrossRef]
- Thorsen, T.; Holt, T. The potential for power production from salinity gradients by pressure retarded osmosis. J. Membr. Sci. 2009, 335, 103–110. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 2012, 46, 5230–5239. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.F.; Chung, T.S. Techno-economic evaluation of various RO plus PRO and RO plus FO integrated processes. Appl. Energy 2018, 212, 1038–1050. [Google Scholar] [CrossRef]
- Li, M.H. Reducing specific energy consumption of seawater desalination: Staged Ro or RO-PRO? Desalination 2017, 422, 124–133. [Google Scholar] [CrossRef]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Ng, B.C. The water-energy nexus: Solutions towards energy-efficient desalination. Energy Technol. 2017, 5, 1136–1155. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Elfil, H. Osmotic energy recovery from reverse osmosis using two-stage pressure retarded osmosis. Energy 2017, 132, 213–224. [Google Scholar] [CrossRef]
- Altaee, A.; Zaragoza, G.; Drioli, E.; Zhou, J. Evaluation the potential and energy efficiency of dual stage pressure retarded osmosis process. Appl. Energy 2017, 199, 359–369. [Google Scholar] [CrossRef]
- Attarde, D.; Jain, M.; Singh, P.K.; Gupta, S.K. Energy-efficient seawater desalination and wastewater treatment using osmotically driven membrane processes. Desalination 2017, 413, 86–100. [Google Scholar] [CrossRef]
- Chung, K.; Yeo, I.H.; Lee, W.I.; Oh, Y.K.; Park, T.S.; Park, Y.G. Pilot study of the key design and operation parameters of a pressure retarded osmosis (PRO) system for SWRO-PRO hybrid desalination. Desalin. Water Treat. 2017, 73, 215–224. [Google Scholar] [CrossRef]
- Chung, K.; Yeo, I.H.; Lee, W.; Oh, Y.K.; Kim, D.; Park, Y.G. Investigation into design parameters in seawater reverse osmosis (SWRO) and pressure retarded osmosis (PRO) hybrid desalination process: A semi-pilot scale study. Desalin. Water Treat. 2016, 57, 24636–24644. [Google Scholar] [CrossRef]
- Kim, J.E.; Phuntsho, S.; Chekli, L.; Choi, J.Y.; Shon, H.K. Environmental and economic assessment of hybrid FO-RO/NF system with selected inorganic draw solutes for the treatment of mine impaired water. Desalination 2018, 429, 96–104. [Google Scholar] [CrossRef]
- Kim, D.I.; Choi, J.; Hong, S. Evaluation on suitability of osmotic dewatering through forward osmosis (FO) for xylose concentration. Sep. Purif. Technol. 2018, 191, 225–232. [Google Scholar] [CrossRef]
- Phuntsho, S.; Kim, J.E.; Hong, S.; Ghaffour, N.; Leiknes, T.; Choi, J.Y.; Shon, H.K. A closed-loop forward osmosis-nanofiltration hybrid system: Understanding process implications through full-scale simulation. Desalination 2017, 421, 169–178. [Google Scholar] [CrossRef]
- Ortega-Bravo, J.C.; Ruiz-Filippi, G.; Donoso-Bravo, A.; Reyes-Caniupan, I.E.; Jeison, D. Forward osmosis: Evaluation thin-film-composite membrane for municipal sewage concentration. Chem. Eng. J. 2016, 306, 531–537. [Google Scholar] [CrossRef]
- Han, G.; Wang, P.; Chung, T.S. Highly robust thin-film composite pressure retarded osmosis (PRO) hollow fiber membranes with high power densities for renewable salinity-gradient energy generation. Environ. Sci. Technol. 2013, 47, 8070–8077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.F.; Zou, L.D. Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 2011, 278, 157–164. [Google Scholar] [CrossRef]
- She, Q.H.; Jin, X.; Tang, C.Y.Y. Osmotic power production from salinity gradient resource by pressure retarded osmosis: Effects of operating conditions and reverse solute diffusion. J. Membr. Sci. 2012, 401–402, 262–273. [Google Scholar] [CrossRef]
- Kim, Y.C.; Elimelech, M. Potential of osmotic power generation by pressure retarded osmosis using seawater as feed solution: Analysis and experiments. J. Membr. Sci. 2013, 429, 330–337. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Hanel, C.; Schiestel, T. Effect of the operating temperature on hydrodynamics and membrane parameters in pressure retarded osmosis. Desalin. Water Treat. 2016, 57, 10477–10489. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Schiestel, T. Impact of tempearture on power recovery in osmotic power production by pressure retarded osmosis. Energy Proceda 2014, 50, 960–969. [Google Scholar] [CrossRef]
- Sivertsen, E.; Holt, T.; Thelin, W.; Brekke, G. Modelling mass transport in hollow fibre membranes used for pressure retarded osmosis. J. Membr. Sci. 2012, 417–418, 69–79. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Modeling water flux in forward osmosis: Implications for improved membrane design. AIChE J. 2007, 53, 1736–1744. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Forward (direct) osmosis desailination using polymeric membranes. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2004; Volume 228, p. U633. [Google Scholar]
- Tang, C.Y.Y.; She, Q.H.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Tang, W.L.; Ng, H.Y. Concentration of brine by forward osmosis: Performance and influence of membrane structure. Desalination 2008, 224, 143–153. [Google Scholar] [CrossRef]
- Dytnerskij, J.I. Membranprozesse-Theorie und Berechnung; Verlag Chimija: Moskau, Russia, 1986. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: An experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Gray, G.T.; McCutcheon, J.R.; Elimelech, M. Internal concentration polarization in forward osmosis: Role of membrane orientation. Desalination 2006, 197, 1–8. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivertsen, E.; Holt, T.; Thelin, W.R. Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis. Membranes 2018, 8, 39. https://doi.org/10.3390/membranes8030039
Sivertsen E, Holt T, Thelin WR. Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis. Membranes. 2018; 8(3):39. https://doi.org/10.3390/membranes8030039
Chicago/Turabian StyleSivertsen, Edvard, Torleif Holt, and Willy R. Thelin. 2018. "Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis" Membranes 8, no. 3: 39. https://doi.org/10.3390/membranes8030039
APA StyleSivertsen, E., Holt, T., & Thelin, W. R. (2018). Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis. Membranes, 8(3), 39. https://doi.org/10.3390/membranes8030039