Application of Semipermeable Membranes in Glucose Biosensing
Abstract
:1. Introduction
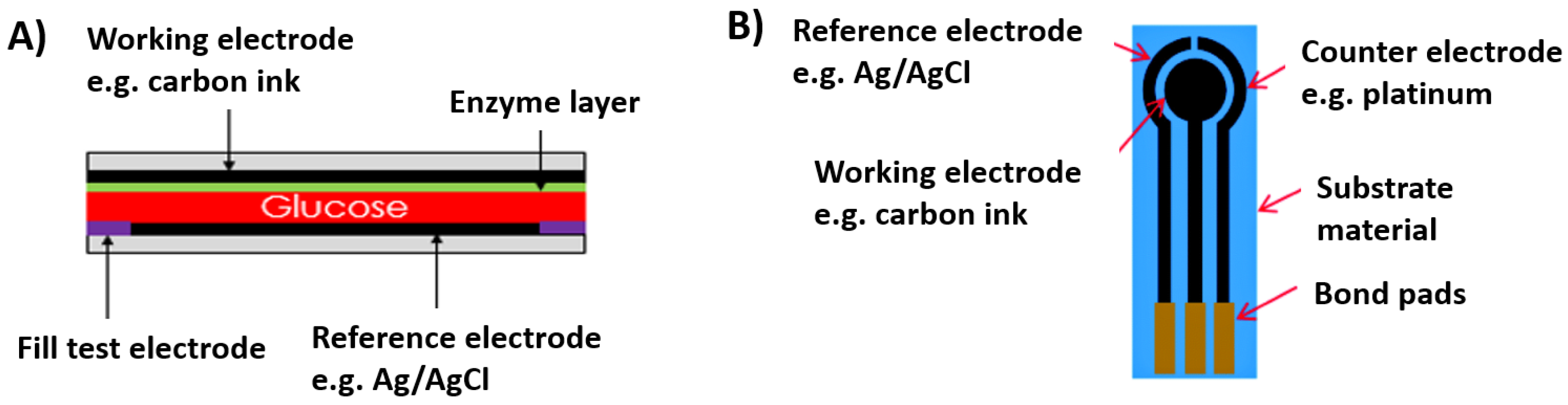
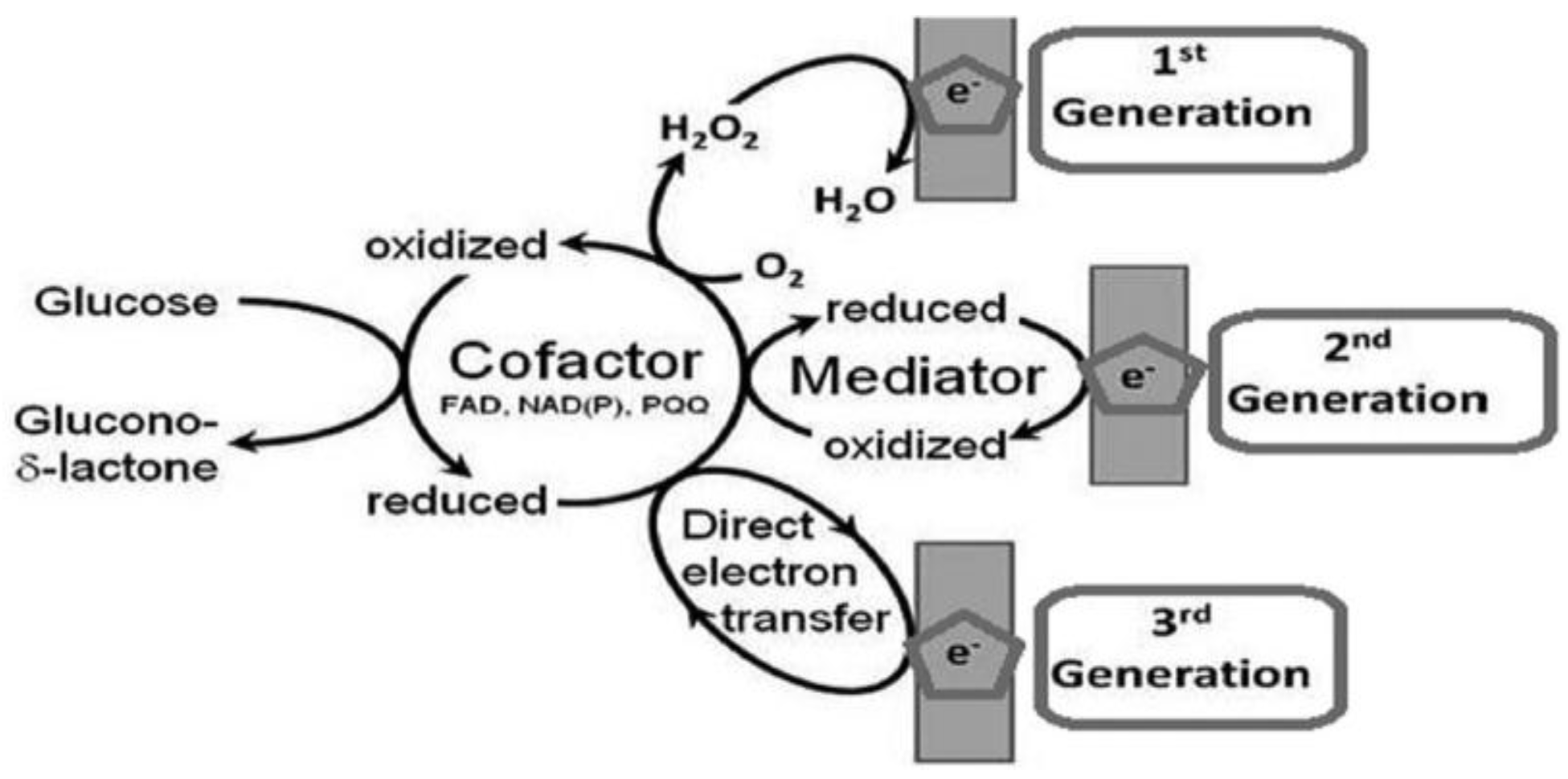
2. Generations of Glucose Biosensors
- Accuracy: The ability of the glucose biosensor to produce the output reading close to the true value. The sensor needs to be accurate since any discrepancies can result in drifted glucose readings, which can prove to be fatal.
- Sensitivity: Sensitivity is determined from the slope of the calibration curve. It is a measure of the change in the biosensor’s output current over the change in glucose concentration. An ideal biosensor will exhibit high and constant sensitivity.
- Selectivity: Blood is a complex matrix and consists of components other than glucose. Interference species such as ascorbic acid and uric acid and competing species such as fructose, xylose, sucrose, and galactose are present in the body. An ideal glucose biosensor will be highly selective towards glucose compared to the other interfering and competing species.
- Dynamic range: An ideal glucose biosensor must have a wide dynamic range. Dynamic range is defined as the range of glucose concentration over which the sensor produces linear response. It is essential for a glucose sensor to detect hypoglycemic glucose (<70 mg/dL) levels as well as hyperglycemic glucose (>100 mg/dL) levels along with the normal glucose levels (70–100 mg/dL).
- Testing volume: From the point of view of patient convenience, an ideal biosensor should be able to operate with a minimal amount of blood sample. Initial glucose biosensor designs required approximately 30 µL of blood. But with advancement in microtechnology and improvement in the design of glucose biosensors, total volume of blood required for testing is presently as low as 0.3 µL.
- Response time: An ideal biosensor should have fast response time. The response time varies for various glucose biosensors. It ranges between 3 and 60 s. Since the glucose concentration is proportional to the steady state current, it is essential for a sensor to reach steady state response as quickly as possible.
- Calibration: This is a very important characteristic of a glucose biosensor. It is a measure of the stability of the glucose biosensor. An ideal glucose biosensor should not require frequent recalibration. It should be able to detect glucose for days, sometimes up to months without recalibration. However, current glucose biosensors need calibration whenever new batch of test strips are used.
- Specificity: This refers to the ability of the glucose biosensor to correctly determine the glucose concentration in the blood sample. The choice of enzyme plays an important role in determining the specificity of the glucose biosensor. At times, the enzyme will be specific to certain functional group instead of an individual analyte. An ideal glucose sensor will have high specificity.
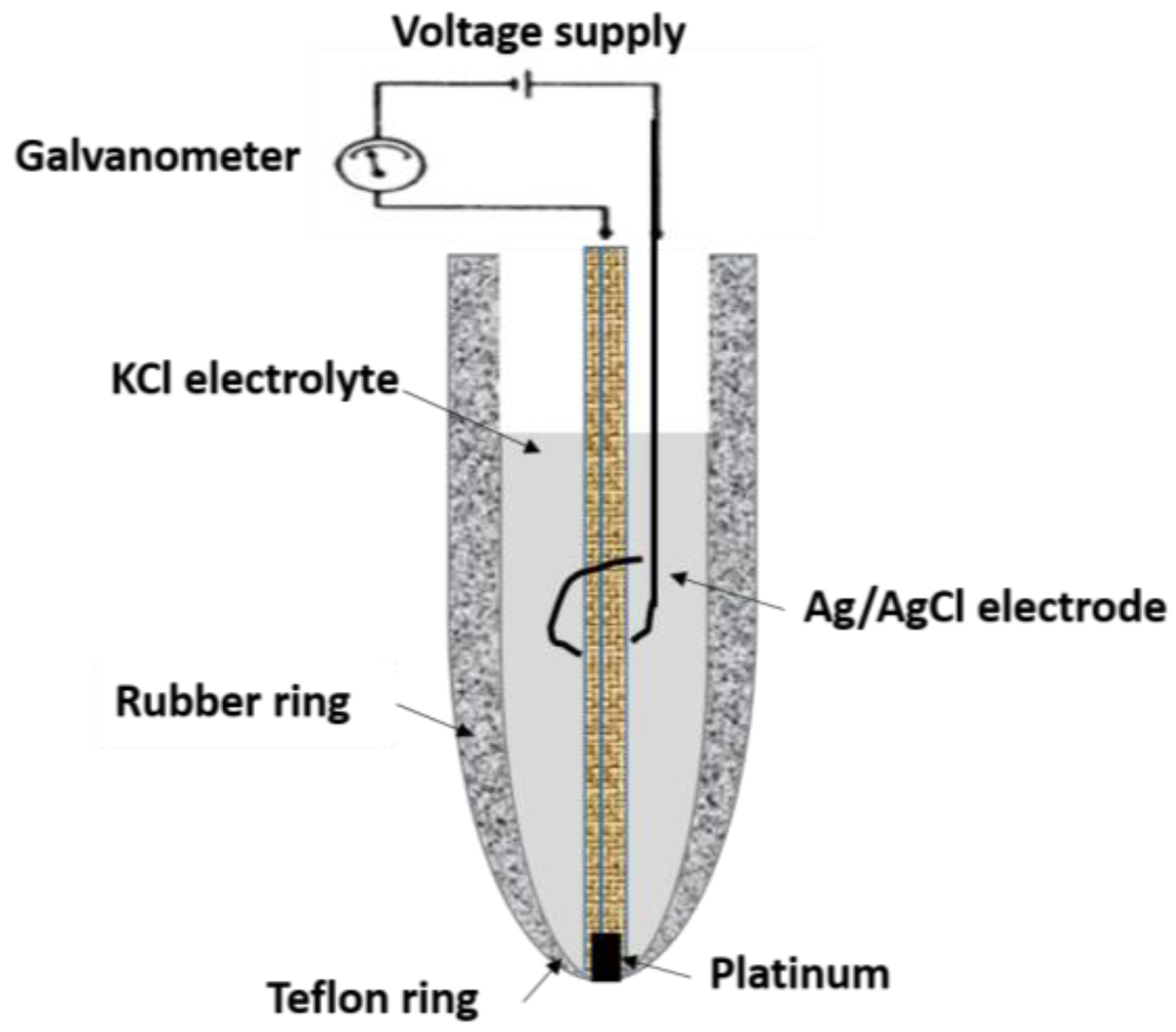
3. Semipermeable Membranes
4. Non-Enzymatic Glucose Biosensor Membranes
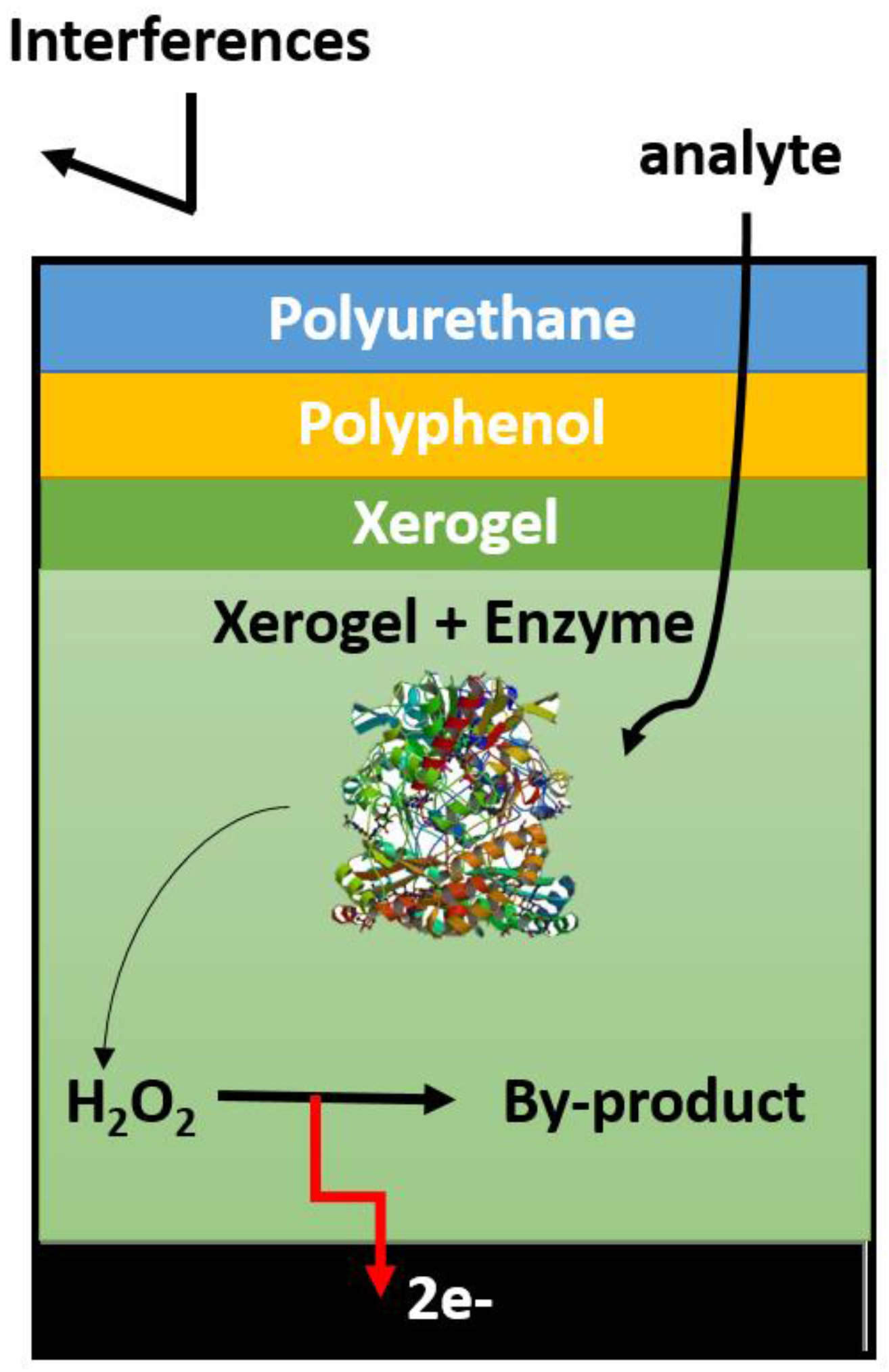
5. Enzymatic Glucose Biosensor Membranes
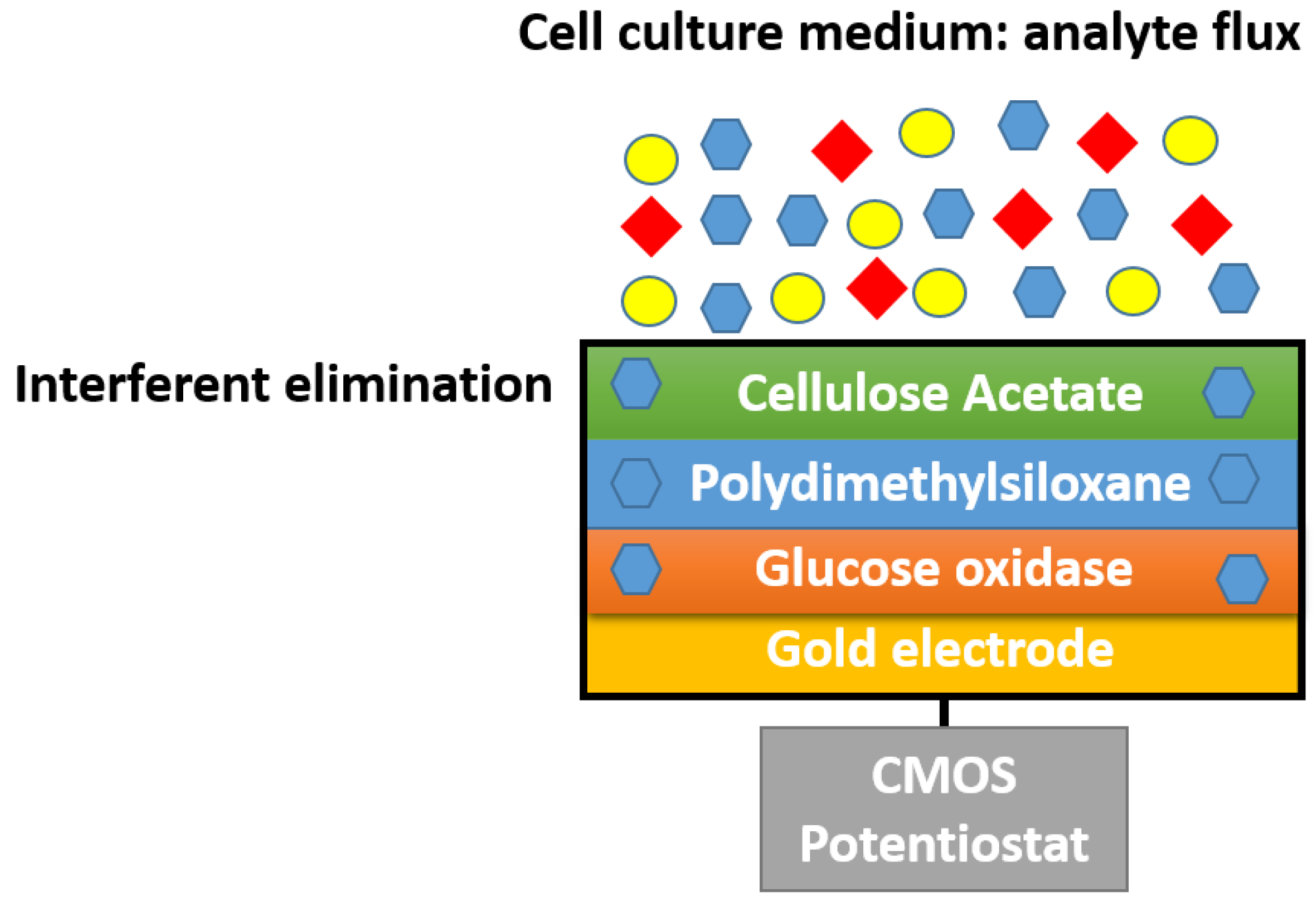
5.1. Cellulose Acetate-Based Membranes
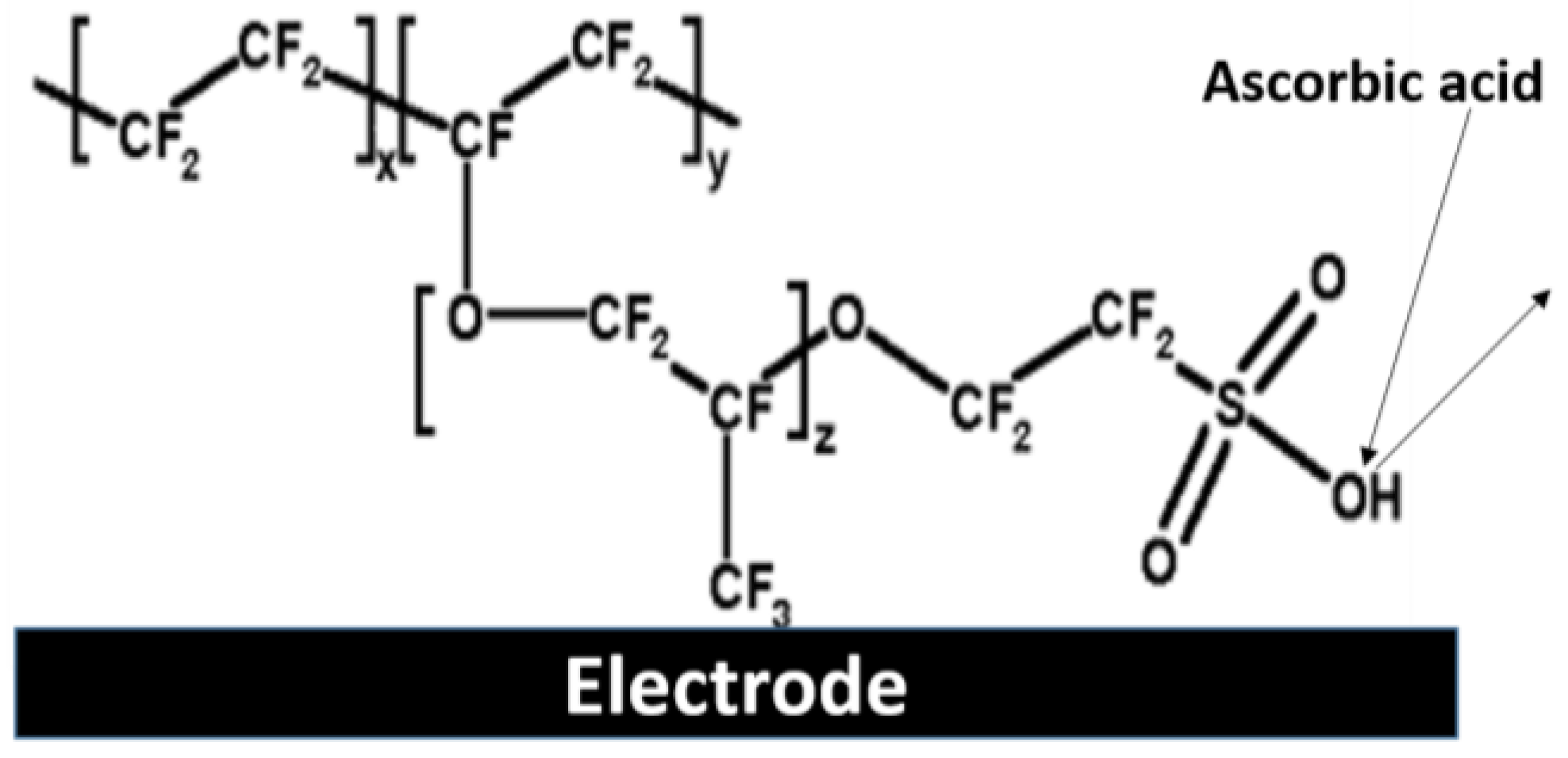
5.2. Nafion-Based Membranes
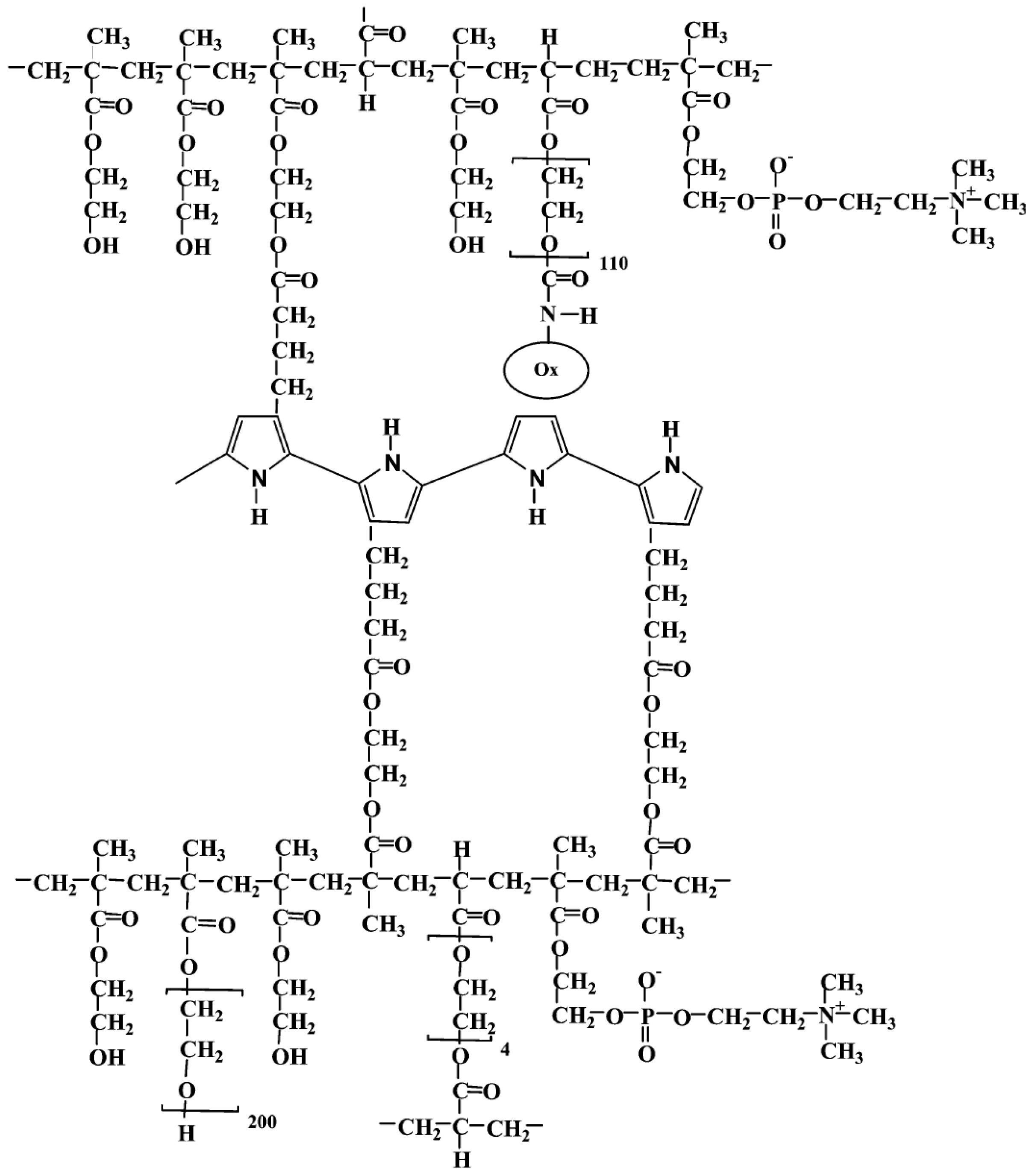
5.3. Other Polymer-Based Membranes
5.4. Chitosan-Based Membrane
5.5. Poly(2-hydroxyethyl Methacrylate) (pHEMA)-Based Membranes
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fioretto, P.; Steffes, M.W.; Sutherland, D.E.; Goetz, F.C.; Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 1998, 339, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, F.; Marzorati, S.; Secchi, A. Islet cell transplantation. Curr. Mol. Med. 2006, 6, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Colton, C.K.; Avgoustiniatos, E.S. Bioengineering in development of the hybrid artificial pancreas. J. Biomech. Eng. 1991, 113, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Karnieli, O.; Izhar-Prato, Y.; Bulvik, S.; Efrat, S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 2007, 25, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G.; Kulkarni, T. A self-powered glucose biosensing system. Biosens. Bioelectron. 2016, 78, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.Z.; Wang, K.; Xia, X.H. Elimination of electrochemical interferences in glucose biosensors. TrAC Trends Anal. Chem. 2010, 29, 306–318. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Updike, S.J.; Hicks, G.P. The enzyme electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Updike, S.J.; Hicks, G.P. Reagentless substrate analysis with immobilized enzymes. Science 1967, 158, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Lowry, J.P.; McAteer, K.; El Atrash, S.S.; Duff, A.; O’Neill, R.D. Characterization of glucose oxidase-modified poly(phenylenediamine)-coated electrodes in vitro and in vivo: Homogeneous interference by ascorbic acid in hydrogen peroxide detection. Anal. Chem. 1994, 66, 1754–1761. [Google Scholar] [CrossRef]
- Cass, A.E.; Davis, G.; Francis, G.D.; Hill, H.A.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.E.; Hill, H.A. Electrochemical biosensors. Anal. Chem. 1987, 59, A933–A944. [Google Scholar] [CrossRef]
- Shichiri, M.; Yamasaki, Y.; Kawamori, R.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 1982, 320, 1129–1131. [Google Scholar] [CrossRef]
- Chaubey, A.; Malhotra, B.D. Mediated biosensors. Biosens. Bioelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef]
- Heller, A. Electrical wiring of redox enzymes. Acc. Chem. Res. 1990, 23, 128–134. [Google Scholar] [CrossRef]
- Wang, J.; Naser, N.; Ozsoz, M. Plant tissue-based amperometric electrode for eliminating ascorbic acid interferences. Anal. Chim. Acta 1990, 234, 315–320. [Google Scholar] [CrossRef]
- Wollenberger, U.; Wang, J.; Ozsoz, M.; Gonzalez-Romero, E.; Scheller, F. Bulk modified enzyme electrodes for reagentless detection of peroxides. J. Electroanal. Chem. Interfacial Electrochem. 1991, 321, 287–296. [Google Scholar] [CrossRef]
- Wang, J.; Naser, N.; Wollenberger, U. Use of tyrosinase for enzymatic elimination of acetaminophen interference in amperometric sensing. Anal. Chim. Acta 1993, 281, 19–24. [Google Scholar] [CrossRef]
- Halámková, L.; Halámek, J.; Bocharova, V.; Szczupak, A.; Alfonta, L.; Katz, E. Implanted biofuel cell operating in a living snail. J. Am. Chem. Soc. 2012, 134, 5040–5043. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chou, A.; Rahmat, W.; Paddon-Row, M.N.; Gooding, J.J. Achieving direct electrical connection to glucose oxidase using aligned single walled carbon nanotube arrays. Electroanalysis 2005, 17, 38–46. [Google Scholar] [CrossRef]
- Murugaiyan, S.B.; Ramasamy, R.; Gopal, N.; Kuzhandaivelu, V. Biosensors in clinical chemistry: An overview. Adv. Biomed. Res. 2014, 3. [Google Scholar] [CrossRef]
- Katz, E.; Bückmann, A.F.; Willner, I. Self-powered enzyme-based biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef] [PubMed]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Wilson, G.S.; Hu, Y. Enzyme-based biosensors for in vivo measurements. Chem. Rev. 2000, 100, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Butterfield, A.D. (Eds.) New Insights into Membrane Science and Technology: Polymeric and Biofunctional Membranes; Elsevier: Amsterdam, The Netherlands, 2003.
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Chung, C.H. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sens. Actuators B Chem. 2009, 142, 216–223. [Google Scholar] [CrossRef]
- Chen, X.M.; Lin, Z.J.; Chen, D.J.; Jia, T.T.; Cai, Z.M.; Wang, X.R.; Chen, X.; Chen, G.N.; Oyama, M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2010, 25, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chung, T.D.; Kim, H.C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003, 75, 3046–3049. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wenkin, M.; Ruiz, P.; Delmon, B.; Devillers, M. The role of bismuth as promoter in Pd–Bi catalysts for the selective oxidation of glucose to gluconate. J. Mol. Catal. A Chem. 2002, 180, 141–159. [Google Scholar] [CrossRef]
- Cao, X.; Wang, N.; Jia, S.; Shao, Y. Detection of glucose based on bimetallic PtCu nanochains modified electrodes. Anal. Chem. 2013, 85, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, R.; Chai, Y.; Che, X.; Li, W.; Zhong, X. Nonenzymatic glucose sensor based on a glassy carbon electrode modified with chains of platinum hollow nanoparticles and porous gold nanoparticles in a chitosan membrane. Microchim. Acta 2011, 172, 163–169. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, T. A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochim. Acta 2013, 102, 104–107. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Lu, X.; Li, Z.; Sun, L.; Song, Y. Dendritic copper-cobalt nanostructures/reduced graphene oxide-chitosan modified glassy carbon electrode for glucose sensing. Sens. Actuators B Chem. 2014, 195, 1–7. [Google Scholar] [CrossRef]
- Shen, C.; Su, J.; Li, X.; Luo, J.; Yang, M. Electrochemical sensing platform based on Pd–Au bimetallic cluster for non-enzymatic detection of glucose. Sens. Actuators B Chem. 2015, 209, 695–700. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, W.; Li, P.; Wang, X.; Zheng, Q.; Wu, H.; Ma, Y.; Guang, W.; Yu, Y.; Ding, K. Highly sensitive nonenzymatic glucose sensor based on Nickel nanoparticle–attapulgite-reduced graphene oxide-modified glassy carbon electrode. Talanta 2016, 159, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Becerik, I.; Süzer, Ş.; Kadırgan, F. Platinum–palladium loaded polypyrrole film electrodes for the electrooxidation of d-glucose in neutral media. J. Electroanal. Chem. 1999, 476, 171–176. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Li, J.; Jia, L.; Wang, C. Improvement of sensitive Ni(OH)2 nonenzymatic glucose sensor based on carbon nanotube/polyimide membrane. Carbon 2013, 63, 367–375. [Google Scholar] [CrossRef]
- Ahammad, A.S.; Al Mamun, A.; Akter, T.; Mamun, M.A.; Faraezi, S.; Monira, F.Z. Enzyme-free impedimetric glucose sensor based on gold nanoparticles/polyaniline composite film. J. Solid State Electrochem. 2016, 20, 1933–1939. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Zhang, X.; Liu, N.; Tan, W.; Zhang, X.; Zhang, L. Facile synthesis of NiCo2O4@Polyaniline core–shell nanocomposite for sensitive determination of glucose. Biosens. Bioelectron. 2016, 75, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Shaolin, M.; Huaiguo, X.; Bidong, Q. Bioelectrochemical responses of the polyaniline glucose oxidase electrode. J. Electroanal. Chem. Interfacial Electrochem. 1991, 304, 7–16. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Sternberg, R.; Coulet, P.R.; Laurent, J.; Gautheron, D.C. Enzyme collagen membrane for electrochemical determination of glucose. Anal. Chem. 1979, 51, 96–100. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Coulet, P.R.; Sternberg, R.; Gautheron, D.C. A highly sensitive glucose electrode using glucose oxidase collagen film. Bioelectrochem. Bioenerg. 1978, 5, 548–553. [Google Scholar] [CrossRef]
- Sternberg, R.; Bindra, D.S.; Wilson, G.S.; Thevenot, D.R. Covalent enzyme coupling on cellulose acetate membranes for glucose sensor development. Anal. Chem. 1988, 60, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Mross, S.; Fürst, P.; Pierrat, S.; Zimmermann, T.; Vogt, H.; Kraft, M. Enzyme Sensor with Polydimethylsiloxane Membrane and CMOS Potentiostat for Wide-Range Glucose Measurements. IEEE Sens. J. 2015, 15, 7096–7104. [Google Scholar] [CrossRef]
- Yuan, C.J.; Hsu, C.L.; Wang, S.C.; Chang, K.S. Eliminating the interference of ascorbic acid and uric acid to the amperometric glucose biosensor by cation exchangers membrane and size exclusion membrane. Electroanalysis 2005, 17, 2239–2245. [Google Scholar] [CrossRef]
- Harrison, D.J.; Turner, R.F.; Baltes, H.P. Characterization of perfluorosulfonic acid polymer coated enzyme electrodes and a miniaturized integrated potentiostat for glucose analysis in whole blood. Anal. Chem. 1988, 60, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Poyard, S.; Jaffrezic-Renault, N.; Martelet, C.; Cosnier, S.; Labbe, P. Optimization of an inorganic/bio-organic matrix for the development of new glucose biosensor membranes. Anal. Chim. Acta 1998, 364, 165–172. [Google Scholar] [CrossRef]
- Lim, S.H.; Wei, J.; Lin, J.; Li, Q.; KuaYou, J. A glucose biosensor based on electrodeposition of palladium nanoparticles and glucose oxidase onto Nafion-solubilized carbon nanotube electrode. Biosens. Bioelectron. 2005, 20, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; Mburu, N.; Slaughter, G. Characterization of a Self-powered Glucose Monitor. Sens. Transducers 2016, 203, 1–7. [Google Scholar]
- Ahmad, F.; Yusof, A.P.; Bainbridge, M.; Ab Ghani, S. The application of glucose biosensor in studying the effects of insulin and anti-hypertensive drugs towards glucose level in brain striatum. Biosens. Bioelectron. 2008, 23, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Wilson, G.S.; Moatti-Sirat, D.; Poitout, V.; Reach, G. Elimination of the acetaminophen interference in an implantable glucose sensor. Anal. Chem. 1994, 66, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Ramanavičius, A.; Kaušaitė, A.; Ramanavičienė, A. Polypyrrole-coated glucose oxidase nanoparticles for biosensor design. Sens. Actuators B Chem. 2005, 111, 532–539. [Google Scholar] [CrossRef]
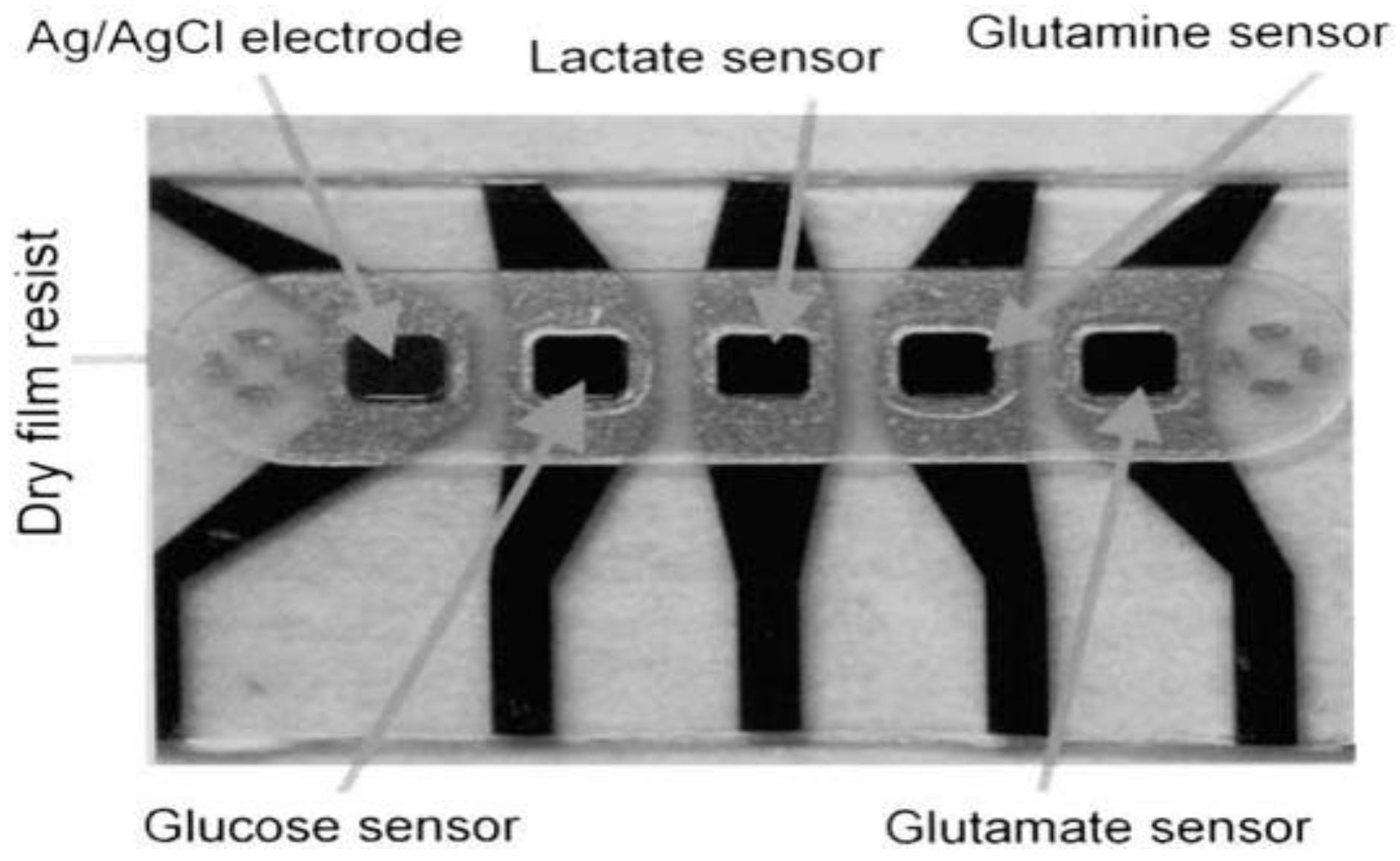
- Schuvailo, O.M.; Soldatkin, O.O.; Lefebvre, A.; Cespuglio, R.; Soldatkin, A.P. Highly selective microbiosensors for in vivo measurement of glucose, lactate and glutamate. Anal. Chim. Acta 2006, 573, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Moser, I.; Jobst, G.; Urban, G.A. Biosensor arrays for simultaneous measurement of glucose, lactate, glutamate, and glutamine. Biosens. Bioelectron. 2002, 17, 297–302. [Google Scholar] [CrossRef]
- Poyard, S.; Martelet, C.; Jaffrezic-Renault, N.; Cosnier, S.; Labbe, P. Association of a poly(4-vinylpyridine-co-styrene) membrane with an inorganic/organic mixed matrix for the optimization of glucose biosensors. Sens. Actuators B Chem. 1999, 58, 380–383. [Google Scholar] [CrossRef]
- Poulos, N.G.; Hall, J.R.; Leopold, M.C. Functional Layer-By-Layer design of xerogel-based first-generation amperometric glucose biosensors. Langmuir 2015, 31, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens. Bioelectron. 2005, 21, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.S.; Liu, X.F.; Bai, H.T.; Jiang, J.H. Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011, 26, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Development of an amperometric-based glucose biosensor to measure the glucose content of fruit. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chung, J.H.; Park, H.K.; Lee, G.J. A Simple and Facile Glucose Biosensor Based on Prussian Blue Modified Graphite String. J. Sens. 2016, 2016. [Google Scholar] [CrossRef]
- Dušek, K.; Patterson, D. Transition in swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. A-2 Polym. Phys. 1968, 6, 1209–1216. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Quinn, C.A.; Connor, R.E.; Heller, A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials 1997, 18, 1665–1670. [Google Scholar] [CrossRef]
- Arica, Y.; Hasirci, V.N. Immobilization of glucose oxidase in poly(2-hydroxyethyl methacrylate) membranes. Biomaterials 1987, 8, 489–495. [Google Scholar] [CrossRef]
- Quinn, C.P.; Pathak, C.P.; Heller, A.; Hubbell, J.A. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials 1995, 16, 389–396. [Google Scholar] [CrossRef]
- Slaughter, G.; Sunday, J. Fabrication of enzymatic glucose hydrogel biosensor based on hydrothermally grown ZnO nanoclusters. IEEE Sens. J. 2014, 14, 1573–1576. [Google Scholar] [CrossRef]
- Slaughter, G. Fabrication of Nano-Indented-Electrodes for Glucose Detection. J. Diabetes Sci. Technol. 2010, 4, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A.; Brahim, S.; Slaughter, G.; Ward, K. Design of a Subcutaneous Implantable Biochip for Monitoring of Glucose and Lactate. IEEE Sens. J. 2005, 5, 345–355. [Google Scholar] [CrossRef]
- Brahim, S.; Slaughter, G.; Guiseppi-Elie, A. Electrical and electrochemical characterization of electroconductive PPy-p(HEMA) composite hydrogels. In Proceedings of the Smart Structures and Materials. International Society for Optics and Photonics, San Diego, CA, USA, 12 August 2003; Volume 5053, pp. 1–12.
- Shaw, G.W.; Claremont, D.J.; Pickup, J.C. In vitro testing of a simply constructed, highly stable glucose sensor suitable for implantation in diabetic patients. Biosens. Bioelectron. 1991, 6, 401–406. [Google Scholar] [CrossRef]
- Morales, A.; Céspedes, F.; Alegret, S. Graphite–methacrylate biocomposite material with renewable sensing surface for reagentless amperometric biosensors based on glucose dehydrogenase. Mater. Sci. Eng. C 1999, 7, 99–104. [Google Scholar] [CrossRef]
- Minteer, S.D.; Akers, N.L.; Moore, C.M.; Saint Louis University. Enzyme Immobilization for Use in Biofuel Cells and Sensors. U.S. Patent US7,638,228, 29 December 2009. [Google Scholar]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Basura, V.; Beattie, P.; Holdcroft, S. Solid-state electrochemical oxygen reduction at Pt|Nafion® 117 and Pt|BAM3G™ 407 interfaces. J. Electroanal. Chem. 1998, 458, 1–5. [Google Scholar] [CrossRef]
- Jung, U.H.; Park, K.T.; Park, E.H.; Kim, S.H. Improvement of low-humidity performance of PEMFC by addition of hydrophilic SiO2 particles to catalyst layer. J. Power Sources 2006, 159, 529–532. [Google Scholar] [CrossRef]
- Machida, S.; Miyata, S.; Techagumpuch, A. Chemical synthesis of highly electrically conductive polypyrrole. Synth. Met. 1989, 31, 311–318. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dwevedi, A. Implication of Enzyme Immobilization in Therapeutics as Well as Diagnostics of Various Diseases. In Enzyme Immobilization; Springer: Heidelberg, Germany, 2016; pp. 65–86. [Google Scholar]
- Desseaux, S.; Hinestrosa, J.P.; Schüwer, N.; Lokitz, B.S.; Ankner, J.F.; Kilbey, S.M.; Voitchovsky, K.; Klok, H.A. Swelling Behavior and Nanomechanical Properties of (Peptide-Modified) Poly(2-hydroxyethyl methacrylate) and Poly(poly(ethylene glycol) methacrylate) Brushes. Macromolecules 2016, 49, 4609–4618. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Wafi, N.I.; Daud, W.R.; Majlan, E.H.; Somalu, M.R.; Ahmad, A. Application of poly(2-hydroxyethyl methacrylate) gel electrolyte in electrochemical device: An Overview. Int. J. Appl. Eng. Res. 2016, 11, 10043–10047. [Google Scholar]
- Tipnis, R.; Vaddiraju, S.; Jain, F.; Burgess, D.J.; Papadimitrakopoulos, F. Layer-by-layer assembled semipermeable membrane for amperometric glucose sensors. J. Diabetes Sci. Technol. 2007, 1, 193–200. [Google Scholar] [CrossRef] [PubMed]

| Cellulose Acetate |
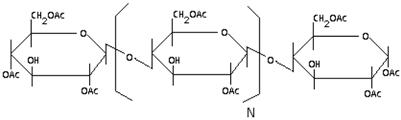 |
| Nafion |
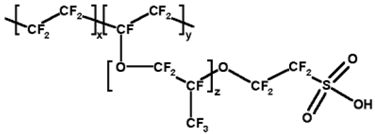 |
| Polypyrrole |
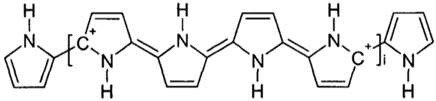 |
| Polyurethane |
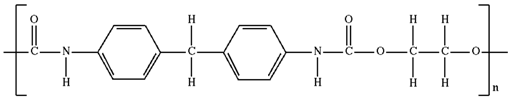 |
| Chitosan |
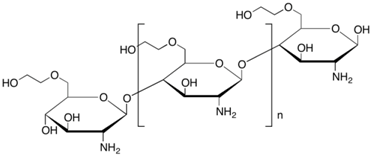 |
| Poly(2-hydroxyethyl methacrylate) |
 |
| Substrate | Enzyme | Analyte | Membrane | Sensitivity (µA/mM·cm2) | Linear Dynamic Range (mM) | Author | Reference Number |
|---|---|---|---|---|---|---|---|
| Biological- and water-based inks | Glucose oxidase | Glucose | Cellulose acetate | 6.43 (µA/M·cm2) | Upto 60 mM | Setti, L., et al. (2005) | [49] |
| Ceramic | Glucose oxidase | Glucose | Polydimethylsiloxane, Cellulose acetate | 0.1922 (µA/mM·cm2) | Upto 200 mM | Mross, Stefan, et al. (2015) | [50] |
| Platinum | Glucose oxidase | Glucose | Nafion | 176.18 (µA/mM·cm2) | Upto 28 mM | Harrison, D., et al. (1988) | [52] |
| Platinum | Glucose oxidase | Glucose | Nafion | 132 (mA/mM·cm2) | 0.01–20 mM | Poyard, S., et al. (1998) | [53] |
| Carbon nanotube | Glucose oxidase | Glucose | Nafion | – | Upto 12 mM | Lim, San Hua, et al. (2005) | [54] |
| Carbon fiber + Ruthenium | Glucose oxidase + Lactate oxidase + Glutamate oxidase | Glucose, Glutamate and Lactate | m-phenylene diamine | – | Glucose (upto 4 mM), Glutamate (upto 0.25 mM), Lactate (upto 1.75 mM) | Schuvailo, O.M., et al. (2006) | [59] |
| Platinum | Glucose oxidase + Lactate oxidase + Glutamate oxidase | Glucose, Glutamine, Glutamate and Lactate | 1,3-Diaminobenzene | Glucose (5–20 (nA/mM·mm2)), Lactate (10–40 (nA/mM·mm2)), Glutamine (30 (nA/mM·mm2)), Glutamate (20–400 (nA/mM·mm2)) | Glucose (0.1–35 mM), Lactate (0.05–15 mM), Glutamine (0.05–10 mM), Glutamate (0.001–5 mM) | Moser, I., et al. (2002) | [60] |
| Platinum | Glucose oxidase | Glucose | Poly(4-vinylpyridine-co-styrene) | 30 (mA/mM·cm2) | 0.01–1.5 mM | Poyard, S., et al. (1999) | [61] |
| Platinum | Glucose oxidase | Glucose | Polyphenol + Polyurethane | 354.23 (µA/mM·cm2) | ≥24–28 mM | Poulos, N.G., et al. (2015) | [62] |
| Carbon nanotube | Glucose oxidase | Glucose | Chitosan | 184.4 (µA/mM·cm2) | 0–7.8 mM | Liu, Ying, et al. (2005) | [63] |
| Palladium nanoparticles + graphene | Glucose oxidase | Glucose | Chitosan | 31.2 (µA/mM·cm2) | 0.001–1 mM | Zeng, Qiong, et al. (2011) | [64] |
| Platinum | Glucose oxidase | Glucose | Chitosan | 10.18 (mA/mM·cm2) | 0.01–15 mM | Ang, L.F., et al. (2015) | [59] |
| Prussian blue graphite strings | Glucose oxidase | Glucose | Chitosan | 641.3 (µA/mM·cm2) | 0.03–1 mM | Lee, Seung Ho, et al. (2016) | [60] |
| Gold wire | Glucose oxidase | Glucose | Poly(ethylene glycol) (PEG) | 616.11 (µA/mM·cm2) | 0–30 mM | Quinn, C.A., et al. (1997) | [63] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, T.; Slaughter, G. Application of Semipermeable Membranes in Glucose Biosensing. Membranes 2016, 6, 55. https://doi.org/10.3390/membranes6040055
Kulkarni T, Slaughter G. Application of Semipermeable Membranes in Glucose Biosensing. Membranes. 2016; 6(4):55. https://doi.org/10.3390/membranes6040055
Chicago/Turabian StyleKulkarni, Tanmay, and Gymama Slaughter. 2016. "Application of Semipermeable Membranes in Glucose Biosensing" Membranes 6, no. 4: 55. https://doi.org/10.3390/membranes6040055
APA StyleKulkarni, T., & Slaughter, G. (2016). Application of Semipermeable Membranes in Glucose Biosensing. Membranes, 6(4), 55. https://doi.org/10.3390/membranes6040055







