Treated Seawater as a Magnesium Source for Phosphorous Recovery from Wastewater—A Feasibility and Cost Analysis
Abstract
:1. Introduction
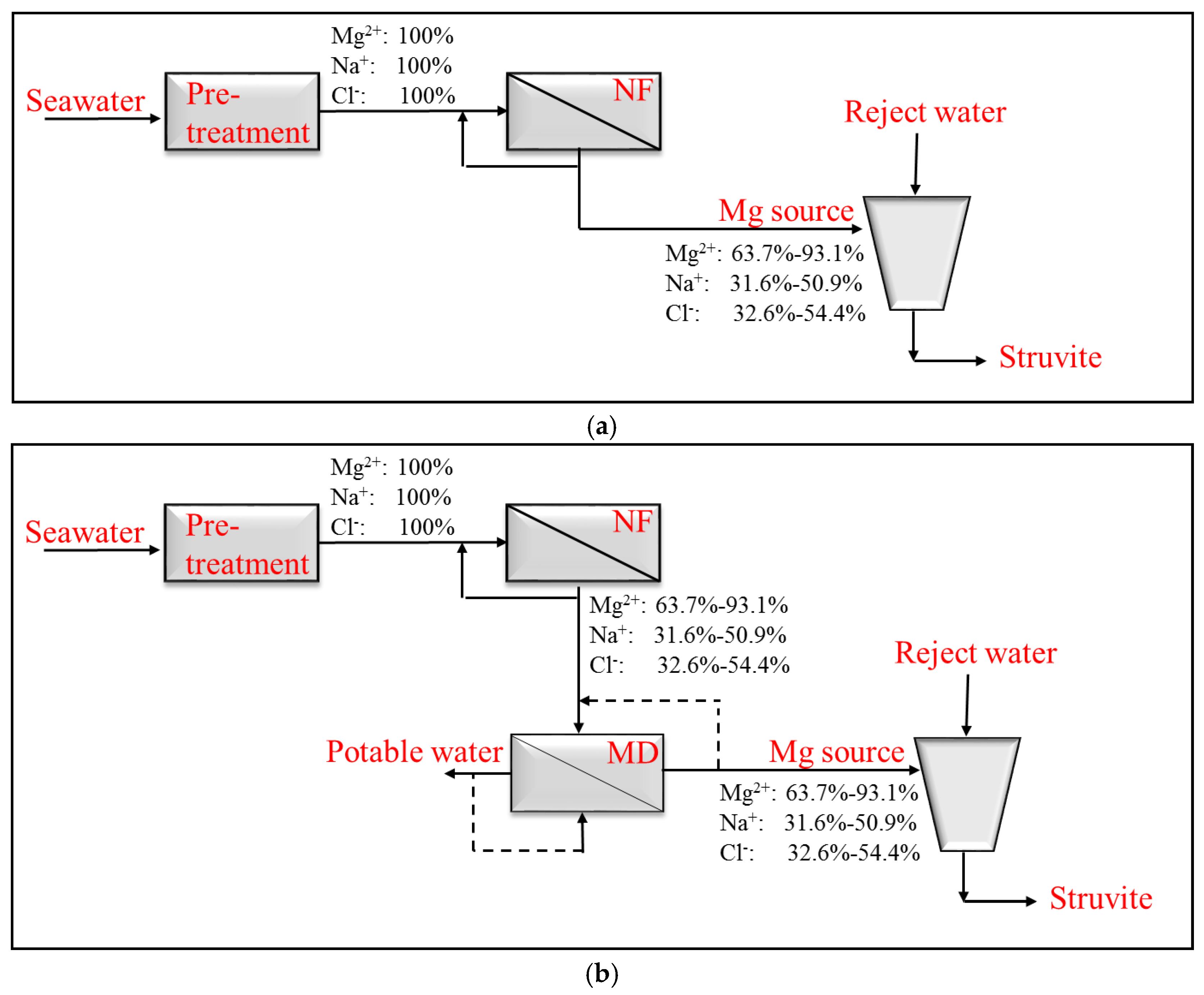
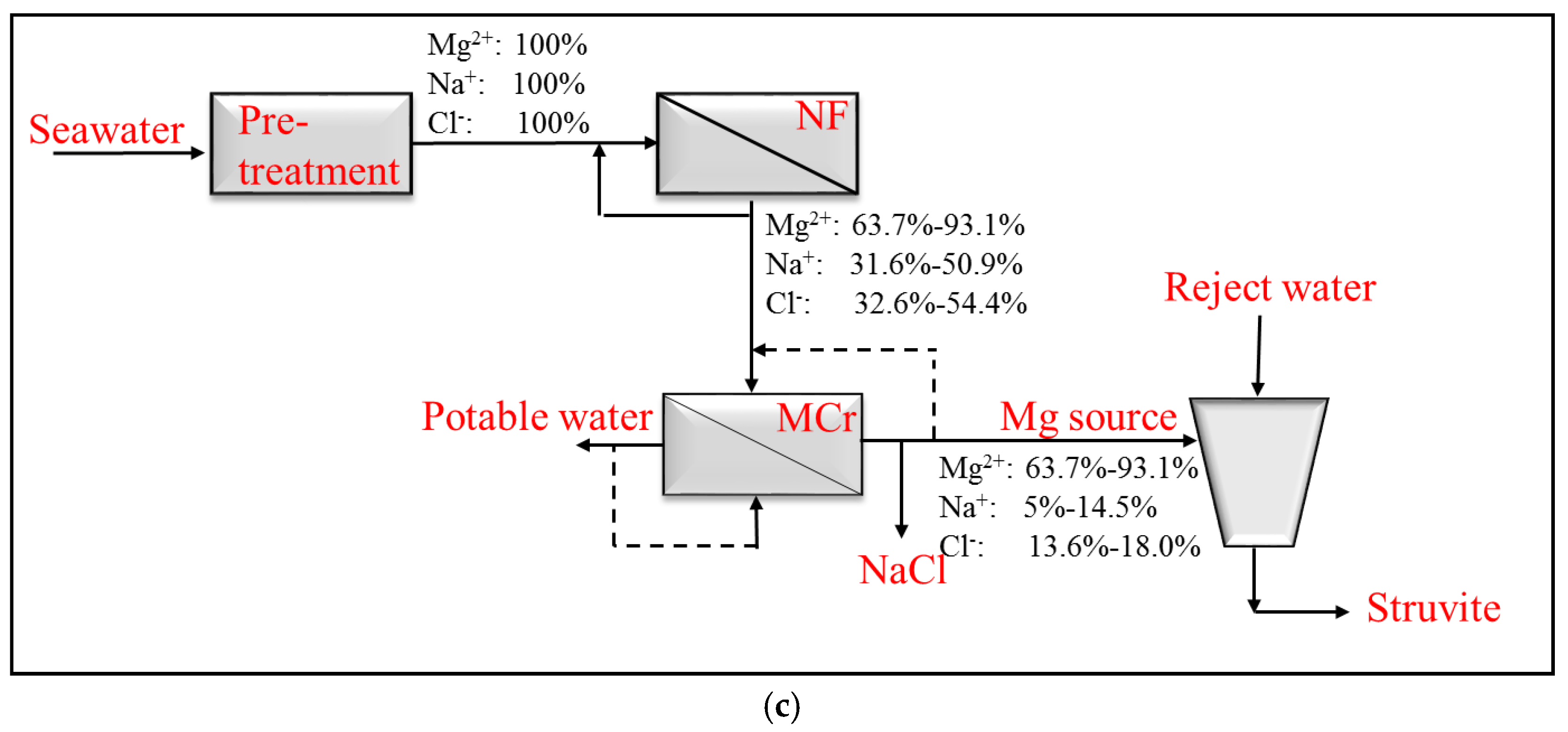
2. Materials and Methods
3. Results and Discussion
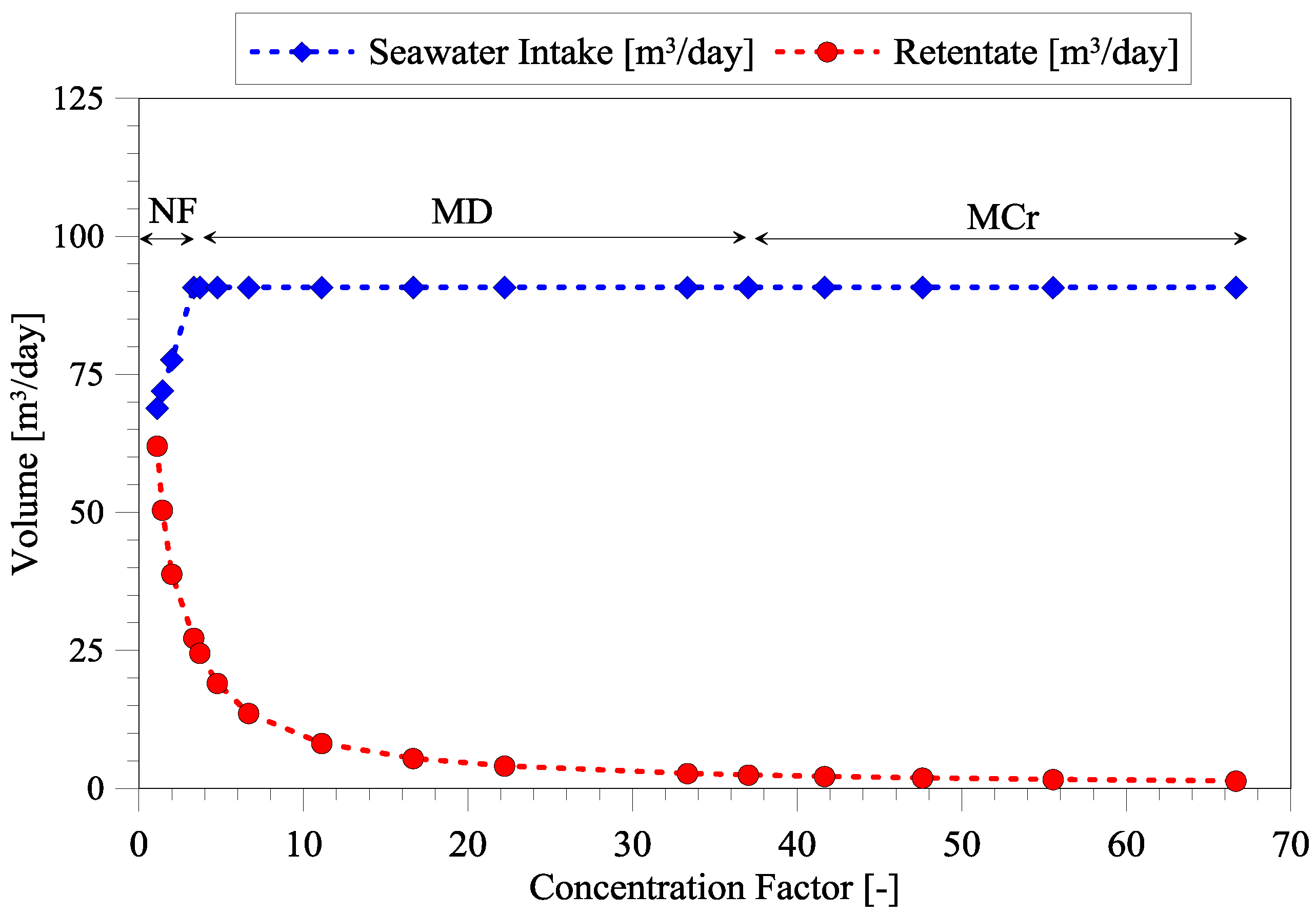
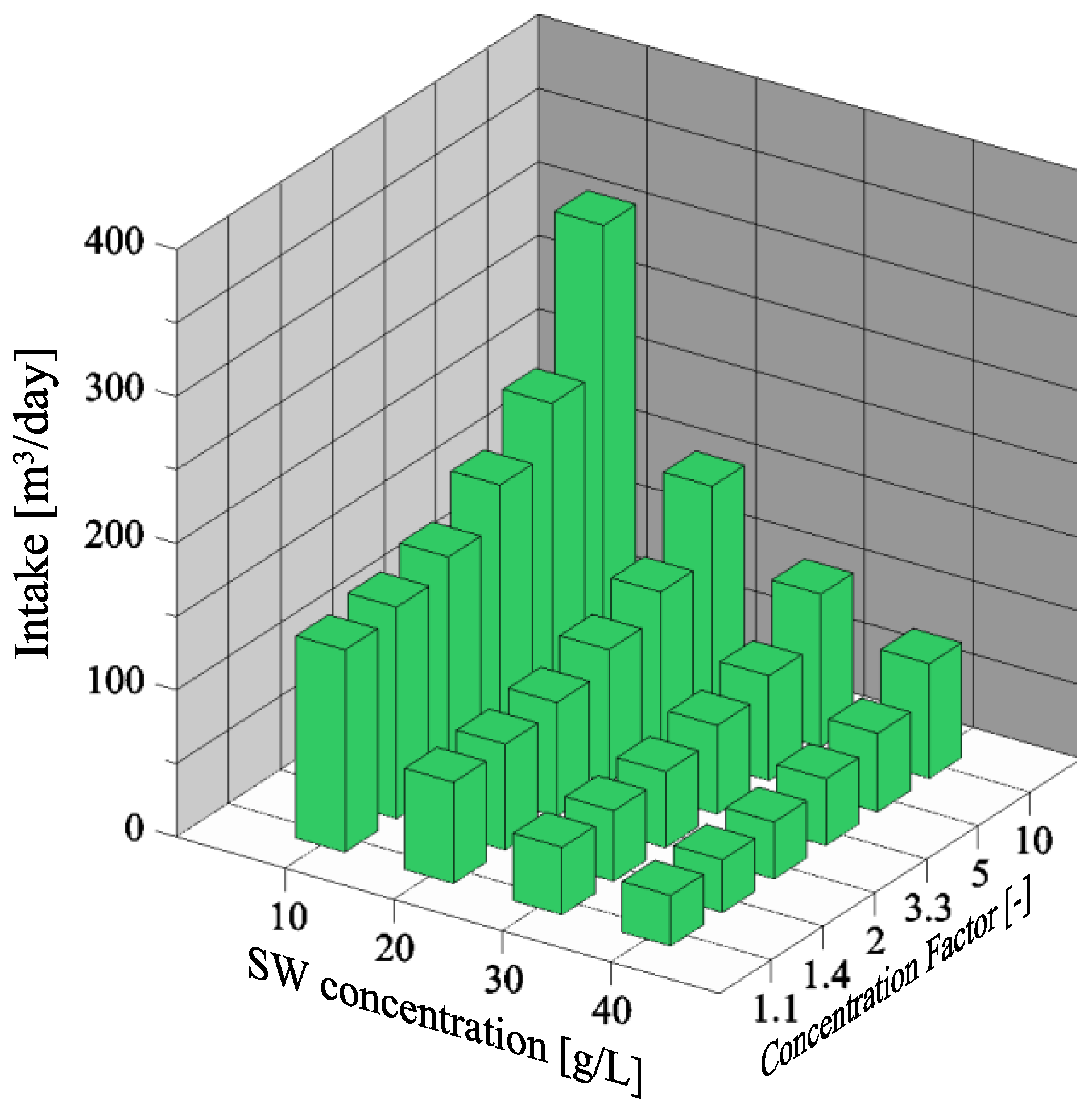
3.1. Dependency of Seawater Intake
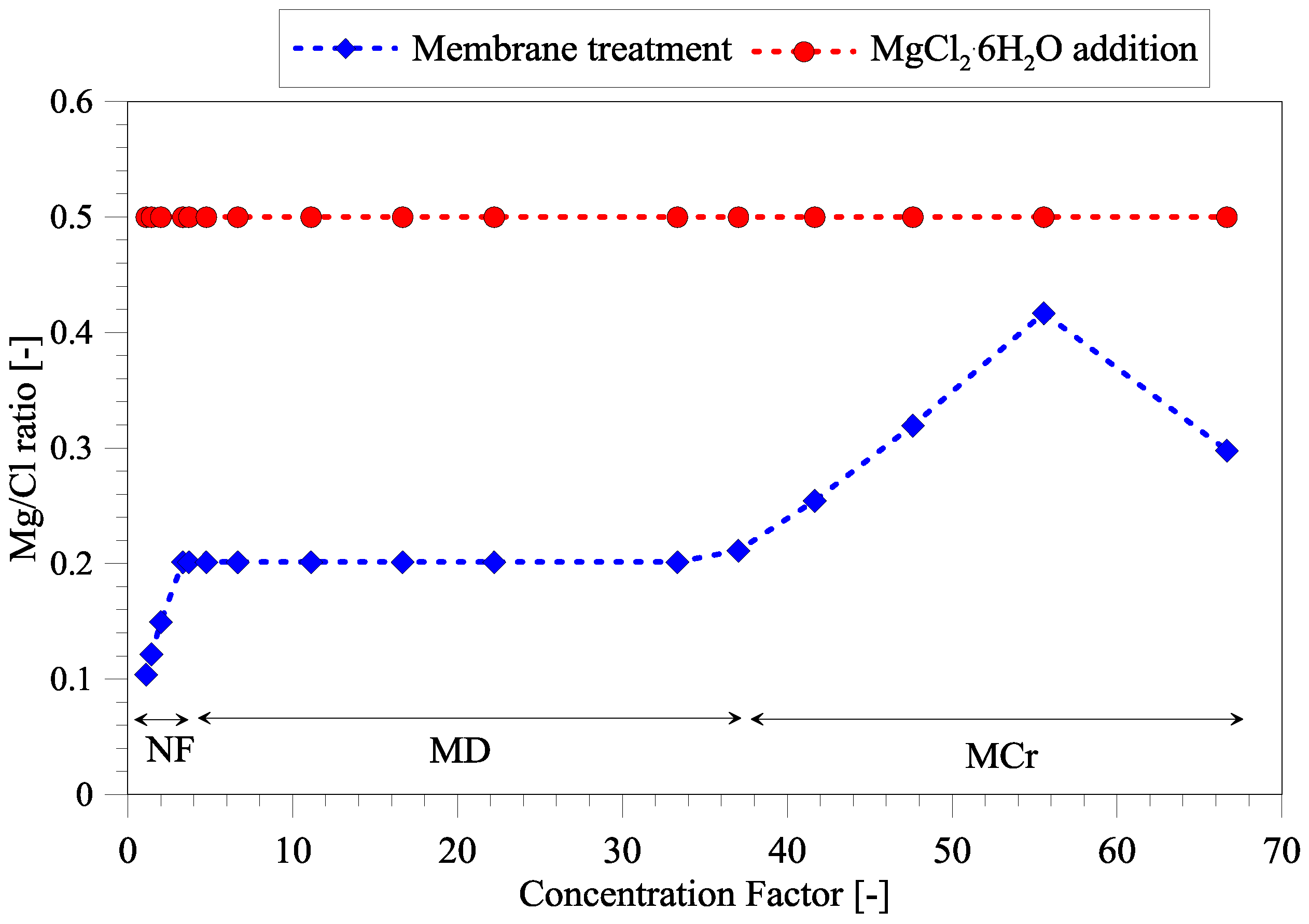
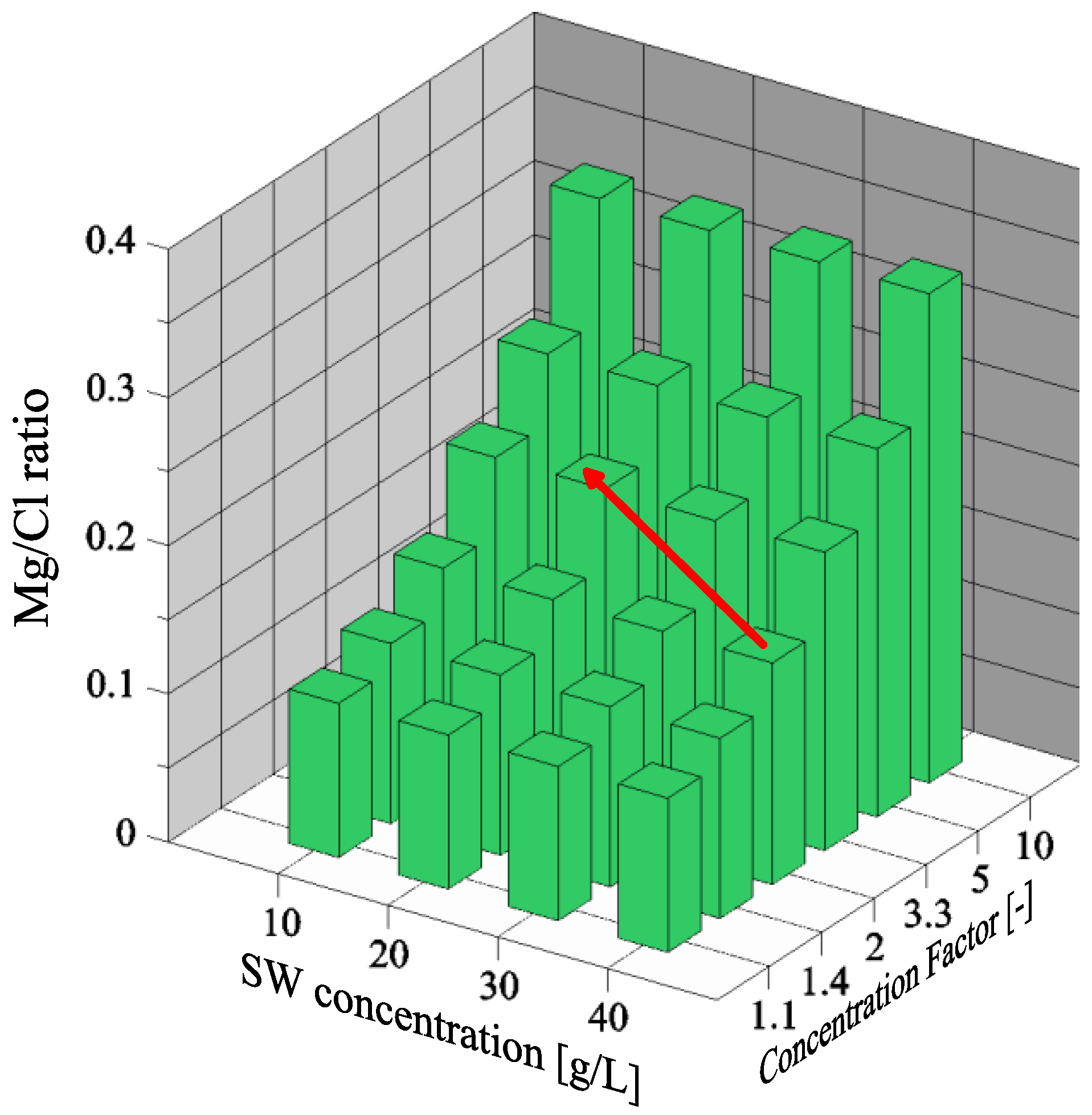
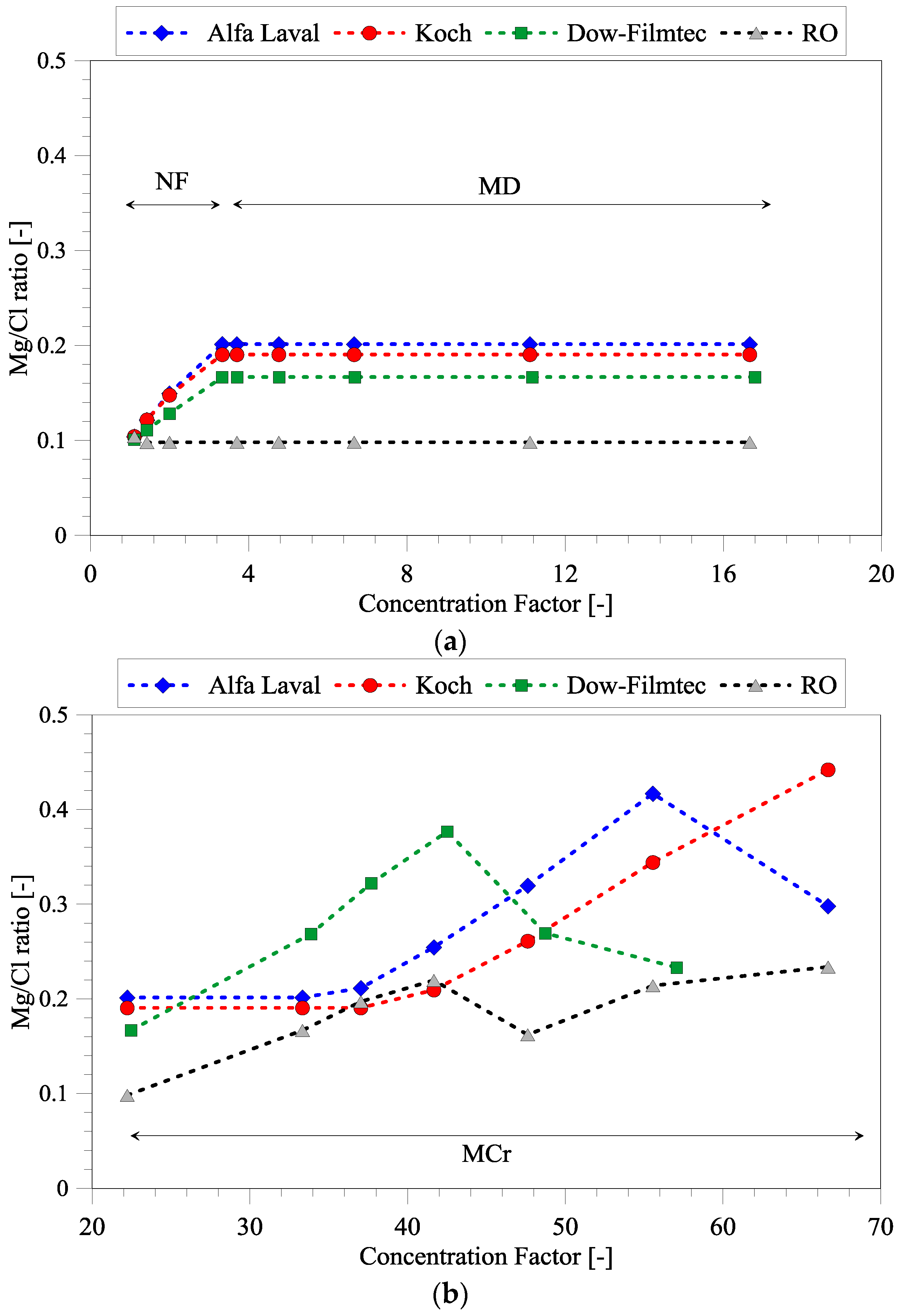
3.2. Magnesium and Chloride Separation
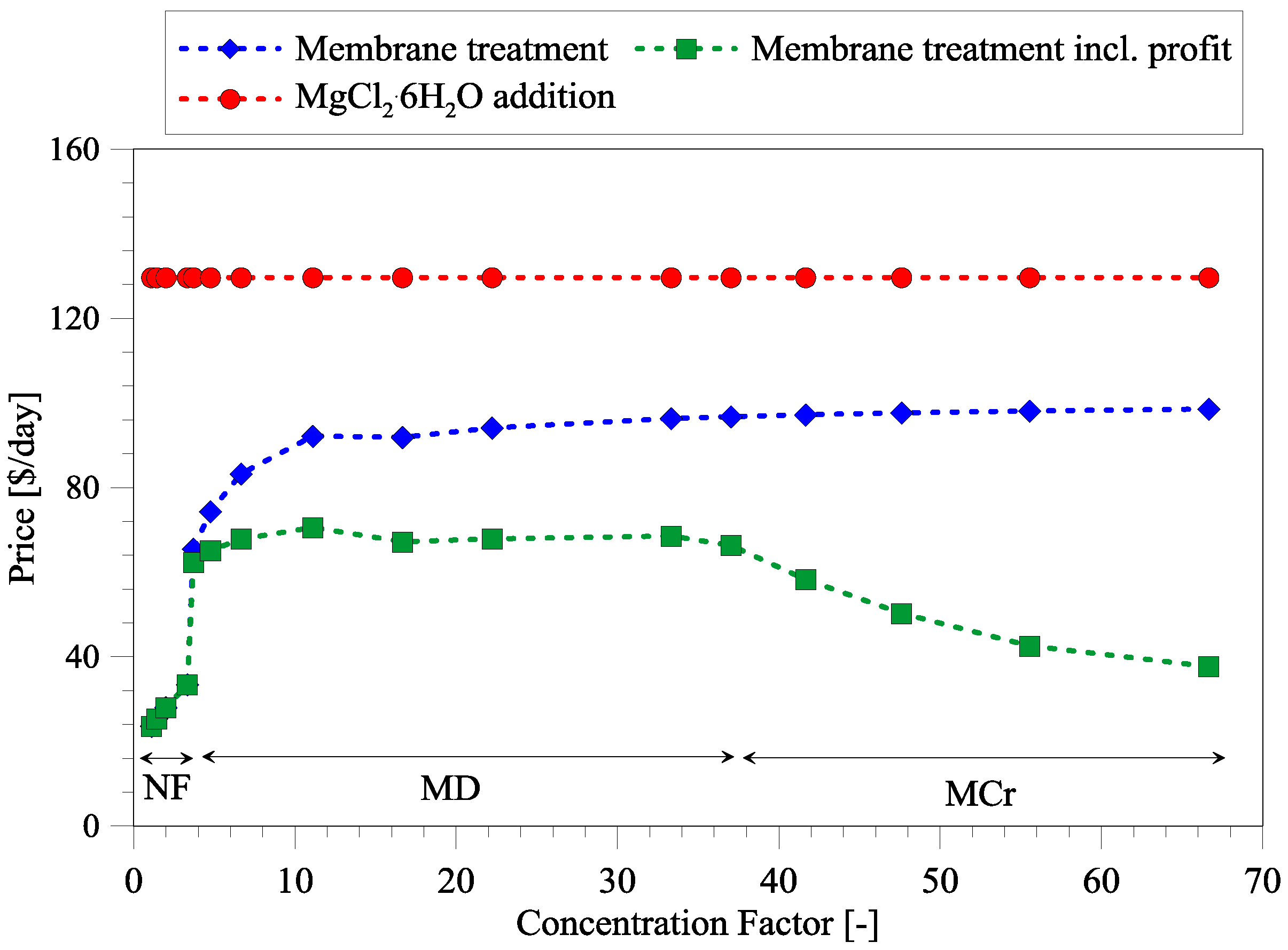
3.3. Cost Analysis of Integrated Membrane Systems
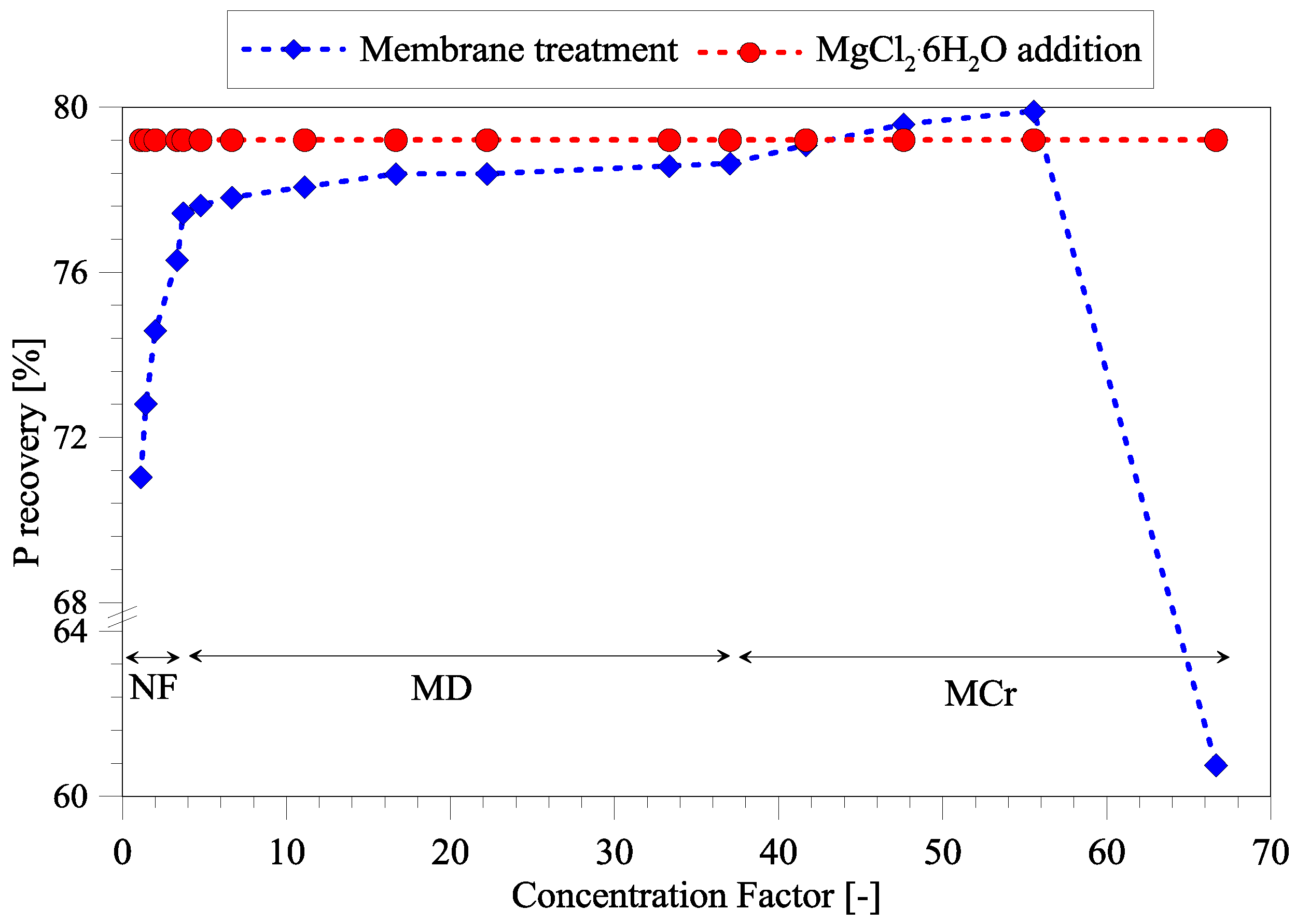
3.4. Efficiency of Phosphorous Removal Using Treated Seawater as Mg Source
3.5. Effect of Initial Seawater Composition
3.6. Comparison of Different NF Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| General Plant Description |
|---|
| Plant life time (n) = 15 years |
| Interest rate (i) = 0.15 |
| Amortization (ɑ) |
| Plant availability (f) = 0.7 |
| Pressure pretreatment (Ppre) = 5 × 105 Pa |
| NF Plant Description |
| Pressure NF (PNF) =1.5 × 106 Pa |
| Pressure drop NF (ΔPNF) = 0.5 |
| Pumps efficiencies (ηpump) = 0.7 |
| Recovery factor (RFNF) = 70% |
| Flux (JNF) = 30 L/(m2·h) |
| Area NF module (ANF) = 36 m2 (8 inch module) |
| Cost of NF module (CNF mod) = 1500 $ |
| NF membrane replacement = 20% |
| NF maximum concentration factor = 3.3 |
| MD/MCr Plant Description |
| Pressure MD (PMD) = 1.5 × 105 Pa |
| Flux (JNF) = 5 L/(m2·h) |
| Cost of MD membrane (CMD membrane) = 90 $/m2 |
| MD membrane replacement = 35% |
| Temperature NF retentate (TNF) = 15 °C |
| Temperature MD retentate (TMD) = 60 °C |
| Heat capacity water (Cp) = 4181.3 J/(kg·K) |
| Over all heat transfer coefficient (U) = 300 W/(m2·K) |
| Heat exchanger efficiency (ηhex) = 0.8 |
| Heat exchanger cost (c hex) = 2000 $/m2 |
| Latent heat of vaporization (λvap) = 2260 kJ/kg |
| Steam cost (Csteam) = 0.00705 $/kg |
| Cost Assumptions |
| Electricity (Danish industry) (celec): 0.1269 $/kWh |
| Labor (clabor): 0.05 $/m3 |
| Spare (cspare): 0.033 $/m3 |
| Chemicals (cchemicals): 0.025 $/m3 |
| MgCl2·6H2O: 310.6 $/ton |
| Struvite: 379 $/ton |
| Fresh water (From MD/MCr process): 1.6 $/m3 |
| NaCl (From MCr process): 121.3 $/ton |
Appendix A.1. Direct Capital Costs (DC)
Appendix A.1.1. Auxiliary Including Pipe Lines, Tanks etc. (Caux) [27]
Appendix A.1.2. Intake and Pretreatment
Appendix A.1.3. Pumps [28]
Appendix A.1.4. Membrane Costs
Appendix A.2. Indirect Capital Costs (IC)
Appendix A.3. Operation and Maintenance (O&M)
Appendix A.3.1. Electricity
Appendix A.3.2. Labor [26]
Appendix A.3.3. Membrane Replacement
Appendix A.3.4. Spare Costs [28]
Appendix A.3.5. Chemical Costs
Appendix A.3.6. Steam Costs
References
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Massey, M.S.; Davis, J.G.; Ippolito, J.A.; Sheffield, R.E. Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agron. J. 2009, 101, 323–329. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Phosphorus Use Efficiency of Bio-Based Fertilizers: Bioavailability and Fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Lee, S.; Weon, S.; Lee, C.; Koopman, B. Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhao, Q.L. MAP precipitation from landfill leachate and seawater bittern waste. Environ. Technol. 2002, 23, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.R.; Tilley, E.; Udert, K.M. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci. Total Environ. 2012, 419, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.-C.; Wang, J.-Y. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere 2013, 93, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, G.; Guan, D.; Li, J.; Wang, A.; Li, J.; Yu, Z.; Chen, Y.; Zhang, Z. Reverse osmosis brine for phosphorus recovery from source separated urine. Chemosphere 2016, 165, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jin, R.-C.; Yang, G.-F.; Yu, J.-J.; Xing, B.-S.; Zhang, Q.-Q. Impacts of transient salinity shock loads on Anammox process performance. Bioresour. Technol. 2012, 112, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Telzhensky, M.; Birnhack, L.; Lehmann, O.; Windler, E.; Lahav, O. Selective separation of seawater Mg 2 + ions for use in downstream water treatment processes. Chem. Eng. J. 2011, 175, 136–143. [Google Scholar] [CrossRef]
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite recovery from municipal-wastewater sludge centrifuge supernatant using seawater NF concentrate as a cheap Mg(II) source. Sep. Purif. Technol. 2013, 108, 103–110. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Henze, M.; Comeau, Y. Wastewater Characterization. In Biological Wastewater Treatment; IWA Publishing: London, UK, 2008; pp. 33–52. [Google Scholar]
- Jenicek, P.; Svehia, P.; Zabranska, J.; Dohanyos, M. Factors affecting nitrogen removal by nitritation/denitritation. Water Sci. Technol. 2004, 49, 73–79. [Google Scholar] [PubMed]
- Sode, S.; Bruhn, A.; Balsby, T.J.S.; Larsen, M.M.; Gotfredsen, A.; Rasmussen, M.B. Bioremediation of reject water from anaerobically digested waste water sludge with macroalgae (Ulva lactuca, Chlorophyta). Bioresour. Technol. 2013, 146, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhu, L.; Fu, Y.; Zhu, M.; Xue, L. Development of lower cost seawater desalination processes using nanofiltration technologies—A review. Desalination 2015, 376, 109–116. [Google Scholar] [CrossRef]
- Llenas, L.; Ribera, G.; Martínez-Lladó, X.; Rovira, M.; de Pablo, J. Selection of nanofiltration membranes as pretreatment for scaling prevention in SWRO using real seawater. Desalination Water Treat. 2013, 51, 930–935. [Google Scholar] [CrossRef]
- Drioli, E.; Curcio, E.; Criscuoli, A.; di Profio, G. Integrated system for recovery of CaCO3, NaCl and MgSO4·7H2O from nanofiltration retentate. J. Membr. Sci. 2004, 239, 27–38. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Hydrophobic membranes for salts recovery from desalination plants. Desalination Water Treat. 2010, 18, 224–234. [Google Scholar] [CrossRef]
- Drioli, E.; Criscuoli, A.; Macedonio, F. Membrane Based Desalination: An Integrated Approach (Medina) (European Water Research); IWA Publishing: London, UK, 2011. [Google Scholar]
- Alamdari, A.; Rahimpour, M.R.; Esfandiari, N.; Nourafkan, E. Kinetics of magnesium hydroxide precipitation from sea bittern. Chem. Eng. Process. Process Intensif. 2008, 47, 215–221. [Google Scholar] [CrossRef]
- Münch, E.V.; Barr, K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001, 35, 151–159. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Demirer, G.N.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geological Survey Techniques and Methods; Book 6; U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 2013. [Google Scholar]
- Macedonio, F.; Curcio, E.; Drioli, E. Integrated membrane systems for seawater desalination: Energetic and exergetic analysis, economic evaluation, experimental study. Desalination 2007, 203, 260–276. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Everaert, K.; Wilms, D.; Vandecasteele, C. Application of nanofiltration for removal of pesticides, nitrate and hardness from ground water: Rejection properties and economic evaluation. J. Membr. Sci. 2001, 193, 239–248. [Google Scholar] [CrossRef]
- Alobaidani, S.; Curcio, E.; Macedonio, F.; di profio, G.; Alhinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Membr. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
| Component | Range in Reject Water | Used in This Study |
|---|---|---|
| NH4+ (mg/L) | 120–2043 [14,15,16] | 900 |
| PO43− (mg/L) | 15–484 [14,16] | 300 |
| Ca2+ (mg/L) | <50 | – |
| Na+ (mg/L) | <200 | – |
| K+ (mg/L) | <200 | – |
| Mg2+ (mg/L) | <30 | – |
| Suspended matter (sludge, polymer) (mg/L) | 100–200 | – |
| Required Mg/P ratio | 1.3 | 1.3 |
| pH (controlled by NaOH) | 7.5 | 7.5 |
| Component | Composition (%) | Composition at 20 mg/L Dissolved Solids (mg/L) |
|---|---|---|
| Chlorine Cl− | 55.03 | 11,006 |
| Potassium K+ | 1.11 | 222 |
| Magnesium Mg2+ | 3.68 | 736 |
| Sodium Na+ | 30.59 | 6118 |
| Sulfate SO42− | 7.68 | 1536 |
| Calcium Ca2+ | 1.18 | 236 |
| Bicarbonate HCO3− | 0.41 | 82 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quist-Jensen, C.A.; Koustrup Jørgensen, M.; Christensen, M.L. Treated Seawater as a Magnesium Source for Phosphorous Recovery from Wastewater—A Feasibility and Cost Analysis. Membranes 2016, 6, 54. https://doi.org/10.3390/membranes6040054
Quist-Jensen CA, Koustrup Jørgensen M, Christensen ML. Treated Seawater as a Magnesium Source for Phosphorous Recovery from Wastewater—A Feasibility and Cost Analysis. Membranes. 2016; 6(4):54. https://doi.org/10.3390/membranes6040054
Chicago/Turabian StyleQuist-Jensen, Cejna Anna, Mads Koustrup Jørgensen, and Morten Lykkegaard Christensen. 2016. "Treated Seawater as a Magnesium Source for Phosphorous Recovery from Wastewater—A Feasibility and Cost Analysis" Membranes 6, no. 4: 54. https://doi.org/10.3390/membranes6040054
APA StyleQuist-Jensen, C. A., Koustrup Jørgensen, M., & Christensen, M. L. (2016). Treated Seawater as a Magnesium Source for Phosphorous Recovery from Wastewater—A Feasibility and Cost Analysis. Membranes, 6(4), 54. https://doi.org/10.3390/membranes6040054








