Calreticulin: Roles in Cell-Surface Protein Expression
Abstract
:1. Introduction
2. Calreticulin in the ER
3. Calreticulin Outside the ER
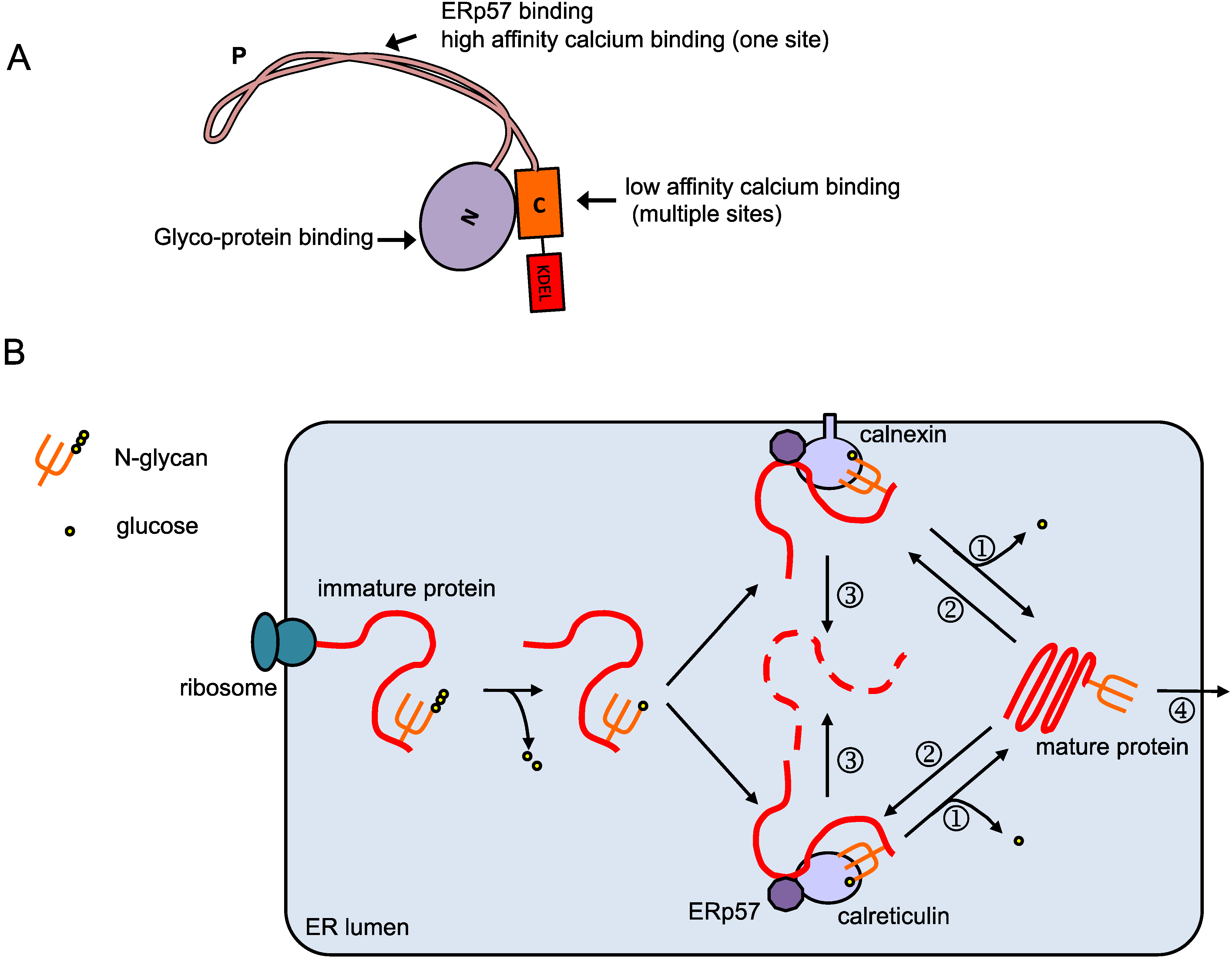
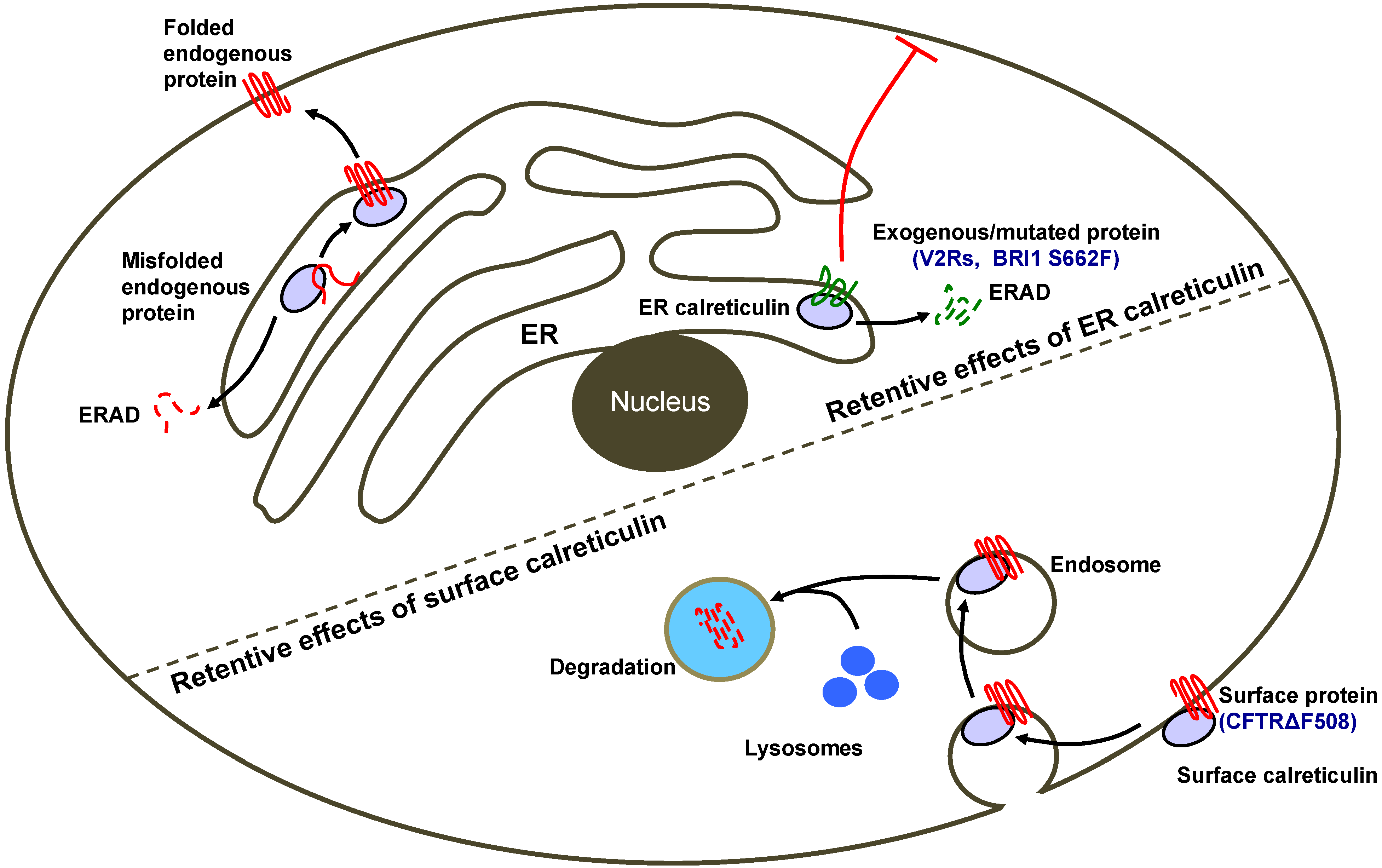
4. Calreticulin as an ER-Retention Factor
5. Calreticulin at the Cell Surface Can Destabilize Cell-Surface Protein
6. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ostwald, T.J.; MacLennan, D.H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 1974, 249, 974–979. [Google Scholar] [PubMed]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of lrp on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.I.; Eggleton, P.; Sweetwyne, M.T.; Van Duyn, L.B.; Greives, M.R.; Naylor, S.M.; Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 665–683. [Google Scholar]
- Martins, I.; Kepp, O.; Galluzzi, L.; Senovilla, L.; Schlemmer, F.; Adjemian, S.; Menger, L.; Michaud, M.; Zitvogel, L.; Kroemer, G. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann. N.Y. Acad. Sci. 2010, 1209, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Okiyoneda, T.; Hashimoto, Y.; Ueno, K.; Nakamura, K.; Yamahira, K.; Sugahara, T.; Shuto, T.; Wada, I.; Suico, M.A.; et al. Calreticulin negatively regulates the cell surface expression of cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2006, 281, 12841–12848. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.A. Protein processing: A role in the pathophysiology of genetic disease. FEBS Lett. 1997, 409, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Koch, G.L. Multiple zones in the sequence of calreticulin (crp55, calregulin, hacbp), a major calcium binding er/sr protein. EMBO J. 1989, 8, 3581–3586. [Google Scholar] [PubMed]
- Fliegel, L.; Burns, K.; MacLennan, D.H.; Reithmeier, R.A.; Michalak, M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1989, 264, 21522–21528. [Google Scholar] [PubMed]
- Kozlov, G.; Pocanschi, C.L.; Rosenauer, A.; Bastos-Aristizabal, S.; Gorelik, A.; Williams, D.B.; Gehring, K. Structural basis of carbohydrate recognition by calreticulin. J. Biol. Chem. 2010, 285, 38612–38620. [Google Scholar] [CrossRef] [PubMed]
- Mesaeli, N.; Nakamura, K.; Zvaritch, E.; Dickie, P.; Dziak, E.; Krause, K.H.; Opas, M.; MacLennan, D.H.; Michalak, M. Calreticulin is essential for cardiac development. J. Cell Biol. 1999, 144, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.H.; Michalak, M. Calreticulin. Cell 1997, 88, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Bergeron, J.J.; Wada, I.; Degen, E.; Williams, D.B. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J. Biol. Chem. 1992, 267, 10914–10918. [Google Scholar] [PubMed]
- Degen, E.; Williams, D.B. Participation of a novel 88-kd protein in the biogenesis of murine class i histocompatibility molecules. J. Cell Biol. 1991, 112, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Wada, I.; Rindress, D.; Cameron, P.H.; Ou, W.J.; Doherty, J.J., 2nd; Louvard, D.; Bell, A.W.; Dignard, D.; Thomas, D.Y.; Bergeron, J.J. Ssr alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991, 266, 19599–19610. [Google Scholar]
- Pamer, E.; Cresswell, P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Rutkevich, L.A.; Williams, D.B. Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr. Opin. Cell Biol. 2011, 23, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kleizen, B.; Braakman, I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, J.J.; Parodi, A.J. Getting in and out from calnexin/calreticulin cycles. J. Biol. Chem. 2008, 283, 10221–10225. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; van der Wal, F.J.; Bulleid, N.J.; High, S. Interaction of the thiol-dependent reductase erp57 with nascent glycoproteins. Science 1997, 275, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Jessop, C.E.; Tavender, T.J.; Watkins, R.H.; Chambers, J.E.; Bulleid, N.J. Substrate specificity of the oxidoreductase erp57 is determined primarily by its interaction with calnexin and calreticulin. J. Biol. Chem. 2009, 284, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Van Duyn Graham, L.; Sweetwyne, M.T.; Pallero, M.A.; Murphy-Ullrich, J.E. Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix. J. Biol. Chem. 2010, 285, 7067–7078. [Google Scholar]
- Sugahara, T.; Koga, T.; Ueno-Shuto, K.; Shuto, T.; Watanabe, E.; Maekawa, A.; Kitamura, K.; Tomita, K.; Mizuno, A.; Sato, T.; et al. Calreticulin positively regulates the expression and function of epithelial sodium channel. Exp. Cell Res. 2009, 315, 3294–3300. [Google Scholar]
- Ramos, R.R.; Swanson, A.J.; Bass, J. Calreticulin and hsp90 stabilize the human insulin receptor and promote its mobility in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2007, 104, 10470–10475. [Google Scholar] [CrossRef] [PubMed]
- Okiyoneda, T.; Kono, T.; Niibori, A.; Harada, K.; Kusuhara, H.; Takada, T.; Shuto, T.; Suico, M.A.; Sugiyama, Y.; Kai, H. Calreticulin facilitates the cell surface expression of abcg5/g8. Biochem. Biophys. Res. Commun. 2006, 347, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Arosa, F.A.; de Jesus, O.; Porto, G.; Carmo, A.M.; de Sousa, M. Calreticulin is expressed on the cell surface of activated human peripheral blood t lymphocytes in association with major histocompatibility complex class i molecules. J. Biol. Chem. 1999, 274, 16917–16922. [Google Scholar] [CrossRef] [PubMed]
- Sadasivan, B.; Lehner, P.J.; Ortmann, B.; Spies, T.; Cresswell, P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of mhc class i molecules with tap. Immunity 1996, 5, 103–114. [Google Scholar] [CrossRef] [PubMed]
- White, T.K.; Zhu, Q.; Tanzer, M.L. Cell surface calreticulin is a putative mannoside lectin which triggers mouse melanoma cell spreading. J. Biol. Chem. 1995, 270, 15926–15929. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Pedraza, C.E.; Pallero, M.A.; Elzie, C.A.; Goicoechea, S.; Strickland, D.K.; Murphy-Ullrich, J.E. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003, 161, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Elzie, C.A.; Kucik, D.F.; Murphy-Ullrich, J.E. Thrombospondin signaling through the calreticulin/ldl receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci. 2003, 116, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.R.; Lynch, N.J.; Day, A.J.; Schwaeble, W.J.; Sim, R.B. The c1q and collectin binding site within c1q receptor (cell surface calreticulin). Immunopharmacology 1997, 38, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.A.; deCathelineau, A.; Hoffmann, P.R.; Bratton, D.; Ghebrehiwet, B.; Fadok, V.A.; Henson, P.M. C1q and mannose binding lectin engagement of cell surface calreticulin and cd91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001, 194, 781–795. [Google Scholar] [PubMed]
- Vandivier, R.W.; Ogden, C.A.; Fadok, V.A.; Hoffmann, P.R.; Brown, K.K.; Botto, M.; Walport, M.J.; Fisher, J.H.; Henson, P.M.; Greene, K.E. Role of surfactant proteins a, d, and c1q in the clearance of apoptotic cells in vivo and in vitro: Calreticulin and cd91 as a common collectin receptor complex. J. Immunol. 2002, 169, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Willis, A.C.; Jensenius, J.C.; Jackson, J.; Sim, R.B. Structure and homology of human c1q receptor (collectin receptor). Immunology 1993, 78, 341–348. [Google Scholar] [PubMed]
- Eggleton, P.; Lieu, T.S.; Zappi, E.G.; Sastry, K.; Coburn, J.; Zaner, K.S.; Sontheimer, R.D.; Capra, J.D.; Ghebrehiwet, B.; Tauber, A.I. Calreticulin is released from activated neutrophils and binds to c1q and mannan-binding protein. Clin. Immunol. Immunopathol. 1994, 72, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar]
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; van Endert, P.; et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009, 28, 578–590. [Google Scholar]
- Tufi, R.; Panaretakis, T.; Bianchi, K.; Criollo, A.; Fazi, B.; Di Sano, F.; Tesniere, A.; Kepp, O.; Paterlini-Brechot, P.; Zitvogel, L.; et al. Reduction of endoplasmic reticulum Ca2+ levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ. 2008, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M.; Eriksson, K.K.; Calanca, V.; Galli, C.; Cresswell, P.; Michalak, M.; Helenius, A. Contrasting functions of calreticulin and calnexin in glycoprotein folding and er quality control. Mol. Cell 2004, 13, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.I.; Paduraru, C.; Dwek, R.A.; Petrescu, S.M. Soluble tyrosinase is an endoplasmic reticulum (er)-associated degradation substrate retained in the er by calreticulin and bip/grp78 and not calnexin. J. Biol. Chem. 2005, 280, 13833–13840. [Google Scholar] [CrossRef] [PubMed]
- Herrada, G.; Dulac, C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 1997, 90, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Ryba, N.J.; Tirindelli, R. A new multigene family of putative pheromone receptors. Neuron 1997, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Buck, L.B. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 1997, 90, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Matsunami, H. Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 16651–16656. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Groenendyk, J.; Steiner, S.S.; Guo, L.; Dabrowska, M.; Parker, J.M.; Muller-Esterl, W.; Opas, M.; Michalak, M. Identification by mutational analysis of amino acid residues essential in the chaperone function of calreticulin. J. Biol. Chem. 2006, 281, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: Clarifying the pathway from ligand perception to gene expression. Mol. Cell 2002, 10, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, Z.; Nam, K.H.; Li, J. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of uggt in er quality control. Mol. Cell 2007, 26, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Hong, Z.; Su, W.; Li, J. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2009, 106, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Jin, H.; Fitchette, A.C.; Xia, Y.; Monk, A.M.; Faye, L.; Li, J. Mutations of an alpha1,6 mannosyltransferase inhibit endoplasmic reticulum-associated degradation of defective brassinosteroid receptors in arabidopsis. Plant Cell 2009, 21, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Kajiura, H.; Su, W.; Jin, H.; Kimura, A.; Fujiyama, K.; Li, J. Evolutionarily conserved glycan signal to degrade aberrant brassinosteroid receptors in arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11437–11442. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Liu, Y.; Xia, Y.; Hong, Z.; Li, J. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Gregory, R.J.; Marshall, J.; Paul, S.; Souza, D.W.; White, G.A.; O'Riordan, C.R.; Smith, A.E. Defective intracellular transport and processing of cftr is the molecular basis of most cystic fibrosis. Cell 1990, 63, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Okiyoneda, T.; Hashimoto, Y.; Oyokawa, K.; Nakamura, K.; Suico, M.A.; Shuto, T.; Kai, H. Curcumin enhances cystic fibrosis transmembrane regulator expression by down-regulating calreticulin. Biochem. Biophys. Res. Commun. 2007, 353, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Karnabi, E.; Qu, Y.; Yue, Y.; Boutjdir, M. Calreticulin negatively regulates the surface expression of cav1.3 l-type calcium channel. Biochem. Biophys. Res. Commun. 2013, 437, 497–501. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, Y.; Dey, S.; Matsunami, H. Calreticulin: Roles in Cell-Surface Protein Expression. Membranes 2014, 4, 630-641. https://doi.org/10.3390/membranes4030630
Jiang Y, Dey S, Matsunami H. Calreticulin: Roles in Cell-Surface Protein Expression. Membranes. 2014; 4(3):630-641. https://doi.org/10.3390/membranes4030630
Chicago/Turabian StyleJiang, Yue, Sandeepa Dey, and Hiroaki Matsunami. 2014. "Calreticulin: Roles in Cell-Surface Protein Expression" Membranes 4, no. 3: 630-641. https://doi.org/10.3390/membranes4030630
APA StyleJiang, Y., Dey, S., & Matsunami, H. (2014). Calreticulin: Roles in Cell-Surface Protein Expression. Membranes, 4(3), 630-641. https://doi.org/10.3390/membranes4030630



