Abstract
When Saccharomyces cerevisiae is starved of glucose, the gluconeogenic enzymes fructose-1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase, isocitrate lyase, and malate dehydrogenase, as well as the non-gluconeogenic enzymes glyceraldehyde-3-phosphate dehydrogenase and cyclophilin A, are secreted into the periplasm. In the extracellular fraction, these secreted proteins are associated with small vesicles that account for more than 90% of the total number of extracellular structures observed. When glucose is added to glucose-starved cells, FBPase is internalized and associated with clusters of small vesicles in the cytoplasm. Specifically, the internalization of FBPase results in the decline of FBPase and vesicles in the extracellular fraction and their appearance in the cytoplasm. The clearance of extracellular vesicles and vesicle-associated proteins from the extracellular fraction is dependent on the endocytosis gene END3. This internalization is regulated when cells are transferred from low to high glucose. It is rapidly occurring and is a high capacity process, as clusters of vesicles occupy 10%–20% of the total volume in the cytoplasm in glucose re-fed cells. FBPase internalization also requires the VPS34 gene encoding PI3K. Following internalization, FBPase is delivered to the vacuole for degradation, whereas proteins that are not degraded may be recycled.
1. Introduction
Saccharomyces cerevisiae is an excellent model system to study cellular responses to environmental changes such as temperature, oxidative stress, and the availability of carbon sources [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Yeast cells can obtain energy through the utilization of sucrose, galactose, glucose, fructose, melibiose, and maltose [1,2,3,4,5,6]. Yeast cells also use non-fermentable carbon sources such as glycerol, pyruvate, acetate, and lactate to produce energy [1,2,3,4,5,6,16]. The addition of glucose to cells grown in non-fermentable carbon sources results in a rapid change in transcription [6,17]. An estimated 40% of genes in yeast alter their expression by more than two-fold within minutes after addition of glucose [17]. Glucose increases the expression of genes involved in ribosomal functions, glycolysis, and cell division [1,2,3,4,5,6,16,17,18,19,20,21,22,23,24,25,26,27]. Glucose also represses genes required for mitochondrial functions and genes encoding for gluconeogenic enzymes that include FBP1 (fructose-1,6-bisphosphatase), ICL1 (isocitrate lyase), PCK1 (phosphoenolpyruvate carboxykinase), and MLS1 (malate synthase) [4,6,17,26,28,29,30]. Likewise, glucose represses genes involved in the metabolism of sugars such as maltose and galactose. The repression of genes by glucose is known as “catabolite repression” [4,16,17,28,31,32,33].
Additionally, glucose causes changes in the turnover rates of mRNA and proteins. It decreases the turnover rates of mRNAs for the 40S and 60S ribosomal subunits [17,34], and increases the turnover rates of mRNAs for PCK1 and FBP1 [4,5,16,35,36,37]. At the protein level, glucose inactivates gluconeogenic enzymes through an increase in the rate of degradation. This is referred to as “catabolite inactivation” [38,39,40,41]. Catabolite inactivation has been observed in gluconeogenic enzymes that include fructose-1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase (Pck1p), isocitrate lyase (Icl1p), and malate dehydrogenase (MDH2) [37,42,43,44,45,46,47,48,49]. Glucose also inactivates mitochondrial enzymes such as cytochrome c oxidase, aconitase, mitochondrial ATPase, and NADH dehydrogenase [35,50,51,52].
Gluconeogenic enzymes are induced with half-lives greater than 100 h when yeast cells are grown in media containing low glucose. However, when glucose is added to glucose-starved cells, these proteins are inactivated and degraded with half-lives of 20–40 min [45,49,53,54]. This inactivation and degradation of gluconeogenic enzymes during glucose re-feeding prevents energy futile cycles that may be detrimental to cells. The key gluconeogenic enzyme fructose-1,6-bisphosphatase (FBPase) has been studied extensively for catabolite inactivation. FBPase is either ubiquitinated and degraded in the proteasome [55,56], or phosphorylated and degraded in the vacuole [42,57,58,59,60]. The site of FBPase degradation is dependent on the duration of starvation [49]. When glucose is added to cells that are starved of glucose for 1 day, this protein is degraded in the proteasome [49]. In contrast, when glucose is added to cells that are starved for 3 days, FBPase is degraded in the vacuole [49]. For the vacuole-dependent pathway, the RAS2/PKA signaling pathway is activated leading to FBPase phosphorylation and subsequent degradation in the vacuole [58,59,60,61,62,63,64]. Other gluconeogenic enzymes that are degraded in the vacuole following glucose replenishment include malate dehydrogenase (MDH2), malate synthase (Mls1p), phosphoenolpyruvate carboxykinase (Pck1p), and isocitrate lyase (Icl1p) [45,49].
2. Small Vesicles Carry Gluconeogenic Enzymes to the Vacuole for Degradation via the Vacuole Import and Degradation Pathway
The vacuole import and degradation (Vid) pathway utilizes small vesicles to carry the gluconeogenic enzymes FBPase, MDH2, Pck1p, and Icl1p to the vacuole for degradation following glucose replenishment to glucose-starved cells. Mutants defective in the glucose-induced degradation of FBPase in the vacuole were isolated [54]. These mutants were classified into two groups based on FBPase distribution patterns determined by immunofluorescence microscopy [54]. Some mutants exhibited diffuse FBPase staining, while others displayed FBPase staining in punctate structures [54]. Distribution of FBPase in punctate structures suggests that FBPase is associated with membranous structures. Using S-1000 size chromatography, four FBPase-containing peaks were identified. The first peak contained FBPase and the plasma membrane protein Pma1p [54]. The second and third peaks were purified and contained clusters of Vid vesicles of different sizes [44]. The fourth peak was purified and consisted of Vid vesicles, as shown by negative staining and TEM to have diameters of 30–50 nm [65]. The biogenesis of Vid vesicles requires the ubiquitin conjugation enzyme 1 (Ubc1p) and the formation of ubiquitin chains [66]. The sequestration of FBPase into Vid vesicles is dependent on the cytosolic heat shock protein Ssa1p/Ssa2p and the peptidylprolyl isomerase cyclophilin A [43,46]. In addition, COPI coatomer proteins are localized to Vid vesicles as peripheral proteins and are trafficked to endosomes during anterograde trafficking [48]. They are distributed on retrograde vesicles that form on the vacuole membrane in response to glucose re-feeding [48]. Vid24p, Vid28p, and Vid30p are also localized to Vid vesicles and are required for FBPase degradation in the vacuole [67,68,69].
3. Gluconeogenic Enzymes Are Secreted into the Periplasm during Glucose Starvation
Recent evidence indicates that gluconeogenic enzymes are secreted into the periplasm when yeast cells are starved of glucose for 3 days [70,71,72]. To observe the distribution of gluconeogenic proteins at the ultra-structural level, immuno-TEM was used [71,73]. Wild-type cells were grown in low glucose for 3 days and processed for immuno-TEM. Thin sections of cells were incubated with affinity purified antibodies and then with goat anti-rabbit secondary antibodies conjugated with 10 nm gold particles. In wild-type cells grown in low glucose for 3 days, about 80% of the FBPase was in the periplasm and 20% was in the cytoplasm (Figure 1A). Likewise, significant amounts of MDH2, Icl1p, and Pck1p were in the periplasm in glucose-starved cells [71]. In addition, non-gluconeogenic enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and cyclophilin A (Cpr1p) were distributed in the periplasm in cells grown in low glucose [71]. Quantification of immuno-TEM gold particles indicated that 76.7% of FBPase, 36.9% of MDH2, 46.9% of Icl1p, 57.6% of Pck1p, 33.7% of GAPDH, and 42.7% of Cpr1p were in the periplasm in glucose-starved wild-type cells [71].
The secretion of gluconeogenic enzymes has been reported in multiple secretomic studies [74,75,76,77,78]. For instance, fructose-1,6-bisphosphatase, phosphoenolpyruvate carboxykinase, and malate dehydrogenase are found in the secretome of Clonorchis sinensis [78], while fructose-1,6-bisphosphatase, malate dehydrogenase, and isocitrate lyase are detected in the secretome of Bacillus anthracis [74]. Malate dehydrogenase is also present in the secretome of Schistosoma mansoni and phosphoenolpyruvate carboxykinase is detectable in the secretome of Schistosoma mansoni and Echinostoma caproni [76]. Additionally, gluconeogenic enzymes are found in extracellular vesicles released from Histoplasma capsultatum [79] and are present in exosomes from a mouse insulinoma cell line [80]. Thus, the secretion of gluconeogenic enzymes is widely observed from bacterial to animal cells. Similarly, the secretion of glyceraldehyde-3-phosphate dehydrogenase and cyclophilin A is also observed from yeast grown in high glucose [81,82] and from other organism ranging from bacteria to humans [75,76,77,78,83,84,85,86].

Figure 1.
FBPase is re-distributed from the periplasm to the cytoplasm following glucose addition. Wild-type cells were grown in low glucose media for 3 days and harvested (t = 0 min) (A); or transferred to high glucose media for 30 min and harvested (t = 30 min) (B). Cells were processed and FBPase was visualized by immuno-TEM. Bars: 200 nm, PM: plasma membrane, CW: cell wall. This figure is in a manuscript published in Proteome Science [73].
4. The Use of an Extraction Protocol to Detect Gluconeogenic Enzymes in the Extracellular Fraction in Glucose-Starved Cells
As shown by immuno-TEM, FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p are secreted into the periplasm in glucose-starved cells. In order to obtain extracellular proteins from this location for further analysis, an extraction protocol was used [68,71,72,87]. The method of extraction has previously been utilized to study the secretion of mammalian galectin-1 expressed in Saccharomyces cerevisiae [88]. A comparable method has been used to identify proteins associated with the cell wall in Candida albicans [89,90]. Proteins that are secreted directly into the media can be collected from the culture media without the use of this method.
To extract gluconeogenic enzymes from the periplasm, cells were incubated in buffer containing the reducing agent β-mercaptoethanol (βME) and Tris with pH 9.4 at 37 °C [91,92]. In control experiments, extracted cells were able to exclude trypan blue and also internalized from the media and actively transported the vital dye FM 4-64 to the vacuole membrane [73]. This indicates that cells are viable and the plasma membrane is intact after the extraction procedure. Furthermore, this protocol detects the presence of known periplasmic proteins such as Scw4p and Scw11p in the extracellular fraction [73]. In contrast, signaling molecules involved in the inactivation and degradation of gluconeogenic enzymes are distributed mostly in the intracellular fraction [72]. Vps34p is the major phosphatidylinositol 3-kinase (PI3K) in yeast and Tor1p is a subunit of the TORC1 complex. Both were detected in the intracellular fraction [73]. Tpk1p, a subunit of the protein kinase A that phosphorylates FBPase [58,59,60] in response to glucose replenishment, was also distributed in the intracellular fraction [73].
Most importantly, the extraction procedure detects the presence of FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in the extracellular fraction in glucose-starved cells [93]. Wild-type cells that expressed tagged proteins were grown in low glucose media for 3 days and then extracted. Proteins that were released into the supernatant following the extraction procedure were precipitated with TCA, washed and then solubilized in SDS sample buffer to form the extracellular (E) fraction. Proteins from the cell-associated fraction were solubilized in SDS sample buffer to form the intracellular (I) fraction. Western blotting was used to determine the distribution of tagged proteins in the intracellular and extracellular fractions. In glucose-starved cells, FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p were present in the extracellular fraction [93]. In contrast, Sec28p and signaling molecules involved in the Vid pathway were mainly distributed in the intracellular fraction [72].
As mentioned, the site of FBPase degradation is dependent on the duration of starvation. Having established that the extraction protocol was useful in detecting the presence of FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in the extracellular fraction, this protocol was used to examine whether or not secretion of these proteins into the extracellular fraction was dependent on the duration of starvation. Wild-type cells expressing tagged proteins were starved of glucose for 1 d, 2 d, and 3 d. Cells were then extracted and the distribution of these proteins in the extracellular fraction was determined [93]. Levels of FBPase, MDH2, and GAPDH in the extracellular fraction were low in 1 d-starved wild-type cells and increased in 3 d-starved cells. Conversely, amounts of extracellular Cpr1p were high in 1 d-starved cells and decreased in 3 d-starved cells. Levels of Icl1p and Pck1p in the extracellular fraction were similar in 1 d- vs. 3 d-starved cells. Thus, the amounts of FBPase, MDH2, GAPDH, and Cpr1p in the extracellular fraction vary depending on the duration of starvation [93].
5. Proteomic Approach to Identify Extracellular Proteins in Glucose-Starved Cells
Now established as an effective method for detecting extracellular proteins present in the periplasm, this extraction protocol was utilized to obtain extracellular proteins for a large-scale proteomic analysis. Wild-type cells were starved of glucose for 3 days and extracted. Extracellular proteins were digested by trypsin and the resulting trypsin fragments were subjected to proteomic analysis [93]. Ninety-two extracellular proteins were identified. As expected, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p were identified in this study. The extracellular proteins were further classified into multiple functional groups that included enzymes in the metabolisms of carbohydrates, amino acids, nucleotides, and lipids. Heat shock proteins, anti-oxidative proteins and proteins involved in other functions were also identified [93]. Of the 92 extracellular proteins, more than 95% do not contain the classical ER sequences and, as a result, are secreted by the non-classical pathway. Hence, the non-classical pathway is the major pathway to secrete proteins in glucose-starved cells. This conclusion is also observed in a previous proteomic study using yeast cells grown in high glucose [94]. In that study, 99 extracellular proteins were identified and only 17 proteins contained the ER sequence [94]. Many of these extracellular proteins found in yeast have also been identified in multiple large-scale secretomic/proteomic studies from organisms that ranged from bacteria to humans [75,76,77,78,83,84,85,86], suggesting that the secretion of these proteins via the non-classical pathways is conserved across species.
6. Vid Vesicles are Secreted as Extracellular Vesicles in Glucose-Starved Cells
During glucose starvation, Vid vesicles that carry gluconeogenic enzymes are distributed in multiple locations. Free Vid vesicles are in the cytoplasm and are enriched in the 200,000× g pellet fraction [65]. These Vid vesicles can aggregate to form clusters and associate with actin patches in the cytoplasm [48,69]. Interestingly, Vid vesicles are also secreted as extracellular vesicles and enriched in the 200,000× g pellet fraction in total extracts [93]. To observe extracellular Vid vesicles, total extracts were obtained from wild-type cells that were grown in low glucose for 3 days. The extract was centrifuged first at 3000× g and at 200,000× g. The 200,000× g pellets were then stained with uranyl acetate and visualized by TEM (Figure 2A). At least two types of structures were observed in total extracts from glucose-starved wild-type cells. Approximately 93%–96% of the extracellular structures consisted of Vid vesicles that were 30–50 nm in diameter, while the remaining 4%–7% were larger structures with diameters ranging from 100 to 300 nm (Figure 2A). As such, extracellular Vid vesicles account for the vast majority of the structures present in total extracts in glucose-starved cells.

Figure 2.
Glucose induces a rapid decline of small vesicles in the extracellular fraction. Wild-type and ∆end3 cells were glucose starved for 3 days and re-fed with glucose for 30 min. Total extracts were obtained and centrifuged at 3000× g and then at 200,000× g. The 200,000× g pellet fraction was fixed, stained with uranyl acetate, and visualized by TEM (A). Bars: 200 nm. The number of 30–50 nm small vesicles per µm2 at t = 0 and t = 30 min in wild-type (B) and ∆end3 (C) cells was quantified. The number of 100–300 nm large structures per µm2 at t = 0 and t = 30 min in wild-type (D) and ∆end3 (E) cells was quantified. This figure is in a manuscript published in the Journal of Extracellular Vesicles [93].
7. Extracellular FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p are Associated with Membranes in the Vesicle-Enriched Fraction in Total Extracts
In the extracellular fraction of glucose-starved wild-type cells, secreted gluconeogenic enzymes are associated with extracellular Vid vesicles that were enriched in the 200,000× g pellet fraction [93]. Wild-type cells expressing tagged proteins were grown in low-glucose media for 3 days and extracted. Total extracts were first centrifuged at 3000× g and then at 200,000× g. The pellet fraction was resuspended in PBS buffer, aliquoted and incubated in the absence and presence of detergent (2% SDS). Following incubation, samples were re-centrifuged for 2 h at 200,000× g and the distribution of proteins in the supernatant (S) and pellet (P) fractions was determined (Figure 3). FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p were in the 200,000× g pellet fraction in the absence of detergent (Figure 3, left panels). In contrast, these proteins were in the 200,000× g supernatant fraction when membranes were solubilized by 2% SDS (Figure 3, right panels). Therefore, membrane integrity is required for the distribution of extracellular gluconeogenic enzymes (FBPase, MDH2, Icl1p and Pck1p) and non-gluconeogenic enzymes (GAPDH and Cpr1p) in the vesicle-enriched fraction.

Figure 3.
FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p are associated with vesicles in the extracellular fraction. Total extracts were obtained from wild-type cells starved of glucose for 3 days and subjected to ultracentrifugation. The final 200,000× g pellet fraction was aliquoted, and incubated with or without 2% SDS for 30 min. Samples were re-centrifuged at 200,000× g for 2 h following incubation. Western blotting was used to examine the distribution of FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in the 200,000× g supernatant (S) and pellet (P) fractions. This figure is in a manuscript published in the Journal of Extracellular Vesicles [93].
8. Glucose Induces a Rapid Clearance of Vid Vesicles and Vesicle-Associated Proteins from the Extracellular Fraction
In order to observe the effects of glucose in the secretome/extracellular fraction, wild-type cells were starved of glucose for 3 days and harvested (t = 0) or transferred to glucose for 30 min and then harvested (t = 30). Cells were subsequently extracted and the total extracts were centrifuged at 3000× g and then at 200,000× g. For visualization using TEM, the 200,000× g pellet fraction was suspended in PBS buffer, fixed, and then stained with uranyl acetate (Figure 2). When total extracts were prepared from wild-type cells grown in low glucose (t = 0 min), more than 90% of total extracts contained small vesicles and less than 10% were 100–300 nm large structures (Figure 2A, left panels). Following addition of glucose for 30 min (t = 30), most of the small vesicles were no longer present, while the 100–300 nm structures were still observed. The number of small vesicles was 234.3 ± 4.7 per µm2 in t = 0 cells and was 10.7 ± 2.5 per µm2 in t = 30 cells (Figure 2B). The number of 100–300 nm large structures was 13.5 ± 1.1 per µm2 before glucose addition and 10.3 ± 1.2 per µm2 after glucose addition (Figure 2D). Hence, the addition of glucose caused a 95.5% reduction in small vesicles while 76.3% of the large structures remained [93].
Given that FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p are associated with vesicles and that these vesicles disappear from the extracellular fraction following glucose re-feeding, these vesicle-associated proteins should be cleared from the extracellular fraction in response to glucose replenishment. Wild-type cells were starved of glucose for 3 days and transferred to media containing glucose for 0, 15, and 30 min. Cells were extracted and levels of proteins in the extracellular fraction were examined by Western blotting (Figure 4). High levels of FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p were observed in the extracellular fraction at t = 0 min (Figure 4A). These proteins decreased levels at the t = 30 min time point (Figure 4A). Hence, glucose causes a rapid reduction of vesicle-associated proteins in the extracellular fraction.

Figure 4.
The decline of extracellular FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in response to glucose re-feeding is dependent on END3. Wild-type cells (A) and the ∆end3 cells (B) were starved of glucose for 3 days and re-fed with glucose for 0, 15, and 30 min. Western blotting was used to examine levels of FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in the intracellular (I) and extracellular (E) fractions. This figure is in a manuscript published in the Journal of Extracellular Vesicles [93].
9. FBPase is Re-Distributed from the Periplasm to the Cytoplasm in Response to Glucose
The rapid decline of vesicle-associated proteins in response to glucose addition may result from their internalization into the cytoplasm or release into the culture media. Because FBPase is degraded in the vacuole following glucose addition, secreted FBPase that associates with extracellular vesicles should be internalized after glucose addition prior to being delivered to the vacuole. The distribution of FBPase in response to glucose addition was examined using immuno-TEM [72,73]. Wild-type cells were grown in low glucose media for 3 days and harvested or transferred to glucose-enriched media for 30 min and then harvested. Cells were processed for immuno-TEM. The majority of the FBPase was observed in the periplasm when cells were grown in low glucose for 3 days (Figure 1A, arrow). At the t = 30 min time point, most of the FBPase was found in intracellular structures that contained clusters of vesicles (Figure 1B, arrow). Taken together, these results suggest that FBPase and vesicles are internalized in response to glucose addition resulting in the appearance of FBPase and vesicles in the cytoplasm in glucose re-fed cells. To rule out the possibility that small vesicles were released into culture media following glucose addition, culture media was collected from cells that were starved and re-fed with glucose, subjected to ultracentrifugation and followed by observation using TEM. Very few vesicles were observed in the culture media, indicating that glucose does not cause the release of vesicles into the culture media.
10. VPS34 is Required for the Decline of Extracellular FBPase in Response to Glucose
Internalization of FBPase following glucose addition requires the VPS34 gene, which encodes the major PI3K in yeast [72]. The role that VPS34 plays in FBPase distribution was examined using immuno-TEM. The ∆vps34 mutant was grown in low glucose for 3 days and transferred to glucose. Thin sections of the ∆vps34 mutant were incubated with affinity purified anti-FBPase and then followed by incubation in goat anti-rabbit secondary antibodies conjugated with 10 nm gold particles. When the ∆vps34 mutant was starved of glucose, most of the FBPase was in the periplasm while small amounts were in the cytoplasm (Figure 5A, arrow). Following glucose addition to the ∆vps34 mutant, the majority of FBPase remained in the periplasm (Figure 5B, arrow). To confirm these immuno-TEM results, levels of extracellular FBPase before and after glucose addition were examined with Western blotting [72]. In wild-type cells, levels of extracellular FBPase were high at t = 0 min and decreased following glucose addition for 30 min. In the ∆vps34 mutant, high levels of FBPase were observed in glucose-starved cells and levels did not decrease after glucose addition [72]. Therefore, the VPS34 gene is required for the decline and internalization of extracellular FBPase in response to glucose addition (Table 1).
The N736 residue and the C-terminal 11 amino acids of Vps34p are essential for PI3K activity. When either the N736 residue was mutated or when the C-terminal amino acids were deleted, levels of FBPase did not decrease rapidly after glucose addition to glucose-starved mutant cells [72]. Thus, the N736 residue and the C-terminal 11 amino acids of Vps34p are critical for the decline of extracellular FBPase in response to glucose addition.
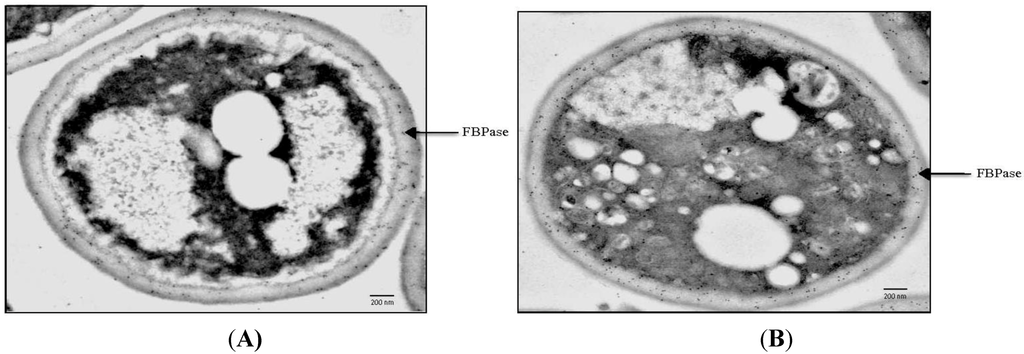
Figure 5.
FBPase fails to be internalized in response to glucose in cells lacking the VPS34 gene. The ∆vps34 mutant was starved of glucose for 3 days (A) and then re-fed with glucose for 2 h (B). Cells were processed and examined for the distribution of FBPase using immuno-TEM. This figure is in a manuscript published in the Journal of Biological Chemistry [72].

Table 1.
FBPase distribution in wild-type, ∆vps34, and ∆end3 mutant strains during glucose starvation and following glucose re-feeding.
| Strain | Glucose Starvation | Glucose Re-Feeding |
|---|---|---|
| WT | Secretion | Internalization |
| ∆vps34 | Normal secretion | Defective internalization |
| ∆end3 | Normal secretion | Defective internalization |
11. END3 is Essential for the Glucose-Induced Decrease of Extracellular Vesicles and Vesicle-Associated Proteins
The endocytosis gene END3 plays an important role in the decline of extracellular Vid vesicles and vesicle-associated proteins in response to glucose [93]. The ∆end3 strain was grown in low glucose media and harvested (t = 0) or transferred to glucose-rich media for 30 min and then harvested (t = 30). Total extracts were centrifuged at 3000× g and the resulting supernatant was further centrifuged at 200,000× g. The final pellet fraction was re-suspended in buffer, fixed, stained with uranyl acetate, and visualized by TEM (Figure 2A, right panels). Total extracts isolated from ∆end3 cells contained 239.2 ± 9.7 small vesicles (30–50 nm diameter) per µm2 before glucose addition and 231.1 ± 6.6 per µm2 after glucose addition (Figure 2C). The number of large structures in total extracts isolated from ∆end3 cells was 19.7 ± 2.5 per µm2 in glucose-starved cells and was 18.6 ± 1.6 per µm2 in glucose re-fed cells (Figure 2E). Given that small vesicles were not cleared from the extracellular fraction when glucose was added to glucose-starved ∆end3 cells, the END3 gene is critical for the decline of extracellular vesicles in response to glucose replenishment.
Because the END3 gene is required for the glucose-induced decline of extracellular Vid vesicles, proteins that associate with these extracellular vesicles should also show an END3-dependent decrease in levels following glucose addition. Wild-type and the ∆end3 strain were starved of glucose for 3 days and then transferred to glucose-rich media for 15 and 30 min. Levels of extracellular FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p were examined. In glucose-starved wild-type cells, these proteins were present in the extracellular fraction (Figure 4A). Their levels decreased after glucose addition to wild-type cells (Figure 4A). In the ∆end3 strain, these proteins were also observed in the extracellular fraction at t = 0 (Figure 4B). However, levels of these proteins did not decrease following a transfer to glucose for 30 min (Figure 4B). Thus, the END3 gene is required for the decline and internalization of extracellular vesicles and vesicle-associated proteins in response to glucose addition (Table 1).
Although the molecular mechanisms for the internalization of extracellular vesicles from the periplasm are largely unknown, several features of this endocytosis process suggest that this is a new type of endocytosis. First, it is utilized to transport vesicles from the periplasm to the cytoplasm. This vesicle-mediated transport is distinct from the receptor-mediated process in which ligands bind to their receptors on the plasma membrane before they are internalized. Secondly, this is regulated by changes in glucose concentrations in the environment. It occurs when cells are transferred from low to high glucose. Therefore, this is different from the fluid phase endocytosis, which is constitutive. Thirdly, this is a very high capacity process. At the t = 30 min time point, clusters of small vesicles occupy about 10%–20% of the total volume of cytoplasm (Figure 1B). Finally, the endocytosis process is rapidly occurring, as the decline of proteins/vesicles in the extracellular fraction is almost complete within the first 30 min after glucose addition. The rapid clearance of extracellular vesicles and vesicle-associated proteins may enable cells to adjust quickly to the changing environment. Based on these features, this vesicle-mediated endocytosis is fundamentally different from the conventional receptor-mediated endocytosis and the fluid phase endocytosis.
12. Conclusions
The Vid pathway that was originally described for the trafficking of gluconeogenic enzymes to the vacuole for degradation can be divided into three major parts: (a) secretion of gluconeogenic and non-gluconeogenic enzymes in vesicles into the periplasm during glucose starvation (Figure 6A); (b) internalization of these proteins following glucose addition; and (c) trafficking of vesicles to the vacuole (Figure 6B). In glucose-starved cells, these proteins are secreted via the non-classical pathway and are associated with Vid vesicles in the extracellular fraction. Vid vesicles exist as free form and aggregated form in the cytoplasm. The biogenesis of Vid vesicles is dependent on Ubc1p (ubiquitin conjugating enzyme 1) [66], whereas the association of FBPase with Vid vesicles requires the cytosolic 70 kD heat shock protein Ssa1p/Ssa2p [46], Vid22p [47], and Cpr1p [43]. These Vid vesicles also aggregate to form large clusters and associate with actin patches. This step requires Vid28p and Vid30p [68,95]. It remains to be determined whether or not extracellular vesicles are released from the intracellular free vesicles or from aggregated vesicles in the cytoplasm. In cells grown in low glucose, 100–300 nm structures are also distributed in the extracellular fraction (Figure 6A). Using a large-scale proteomic approach, 92 proteins that were present in the extracellular fraction in glucose-starved cells were identified. More than 95% of these extracellular proteins do not contain the typical signal sequence for the ER-Golgi pathway, suggesting that they are secreted via the non-classical pathway. Therefore, the non-classical pathway is the major pathway to secrete proteins into the extracellular fraction. This conclusion is observed in cells grown in low glucose as well as in cells grown in high glucose.
Interestingly, secreted gluconeogenic enzymes and non-gluconeogenic enzymes are associated with extracellular Vid vesicles in glucose-starved cells (Figure 6A). These extracellular vesicles account for more than 90% of the total structures present in total extracts. Vid vesicles are 30–50 nm in diameter, have densities of 1.2 g/mL [65], and are associated with secreted gluconeogenic enzymes in the total extracts. Exosomes with a diameter of 40–100 nm and a density of 1.1–1.2 g/mL are secreted from a variety of mammalian cells [95,96,97]. Gluconeogenic enzymes FBPase and MDH2 have also been identified in exosomes purified from the mouse insulinoma NIT-1 cells [80]. Thus, extracellular Vid vesicles that carry gluconeogenic enzymes in yeast cells share features similar to those observed in exosomes secreted from mammalian cells. Secreted gluconeogenic enzymes that associate with extracellular vesicles during glucose starvation are internalized into the cytoplasm after glucose addition. This internalization is dependent on the VPS34 gene and the END3 gene (Figure 6B). Internalization results in a decline in extracellular gluconeogenic enzymes and the disappearance of small vesicles from the extracellular fraction at the t = 30 time point. Moreover, internalization also leads to the appearance of clusters of FBPase and small vesicles in the cytoplasm [44,72]. Vid/endosomes have been purified to near homogeneity from the cytoplasm of wild-type cells re-fed with glucose [44]. Inside these Vid/endosomes, FBPase was associated with small vesicles [44], indicating that FBPase is internalized in these vesicles. Internalization is also consistent with the findings that the endocytosis gene END3 is required for the decline of extracellular vesicles and vesicle-associated proteins including FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p in response to glucose addition [93]. Following internalization, FBPase is delivered to the vacuole for degradation. This post-internalization step requires Vid24p, Sec28p (COPI coatomer subunit), Reg1p/Glc7p phosphatase, the vacuole-ATPase, HOPS (homtypic fusion vacuole protein sorting complex Vps39p and Vps41p), v-SNARE (Ykt6p, Nyv1p, Vti1p), and t-SNARE (Vam3p) [44,48,98,99,100].
As mentioned above, Vid vesicles share many similar physical properties with extracellular vesicles (EVs) or exosomes released from the mammalian cells. However, the biogenesis, secretion and internalization of Vid vesicles may not be the same as those described for EVs. The formation, exocytosis, and endocytosis of EVs have been reviewed extensively by several investigators [101,102,103,104,105,106,107]. Readers are encouraged to view these articles for a complete understanding of these processes. Briefly, EVs are produced by inward budding of MVBs (multivesicular bodies) using the ESCRT complex. Rabs proteins are required for MVBs trafficking to the plasma membrane. Following the v-SNARE and t-SNARE pairing, MVBs then fuse with the plasma membrane to release EVs into the extracellular space. Because EVs are produced by inward budding of the MVB membranes, these vesicles should not be present in the cytoplasm. For the Vid pathway, free Vid vesicles are present in the cytoplasm constitutively. Likewise, clusters of Vid vesicles are also present in the cytoplasm before and after glucose addition. Furthermore, MVBs are enclosed by a single layer of outer membrane, whereas an outer membrane was not observed in clusters of Vid vesicles. However, both Vid vesicle clusters and MVBs contain numerous small vesicles within these organelles.
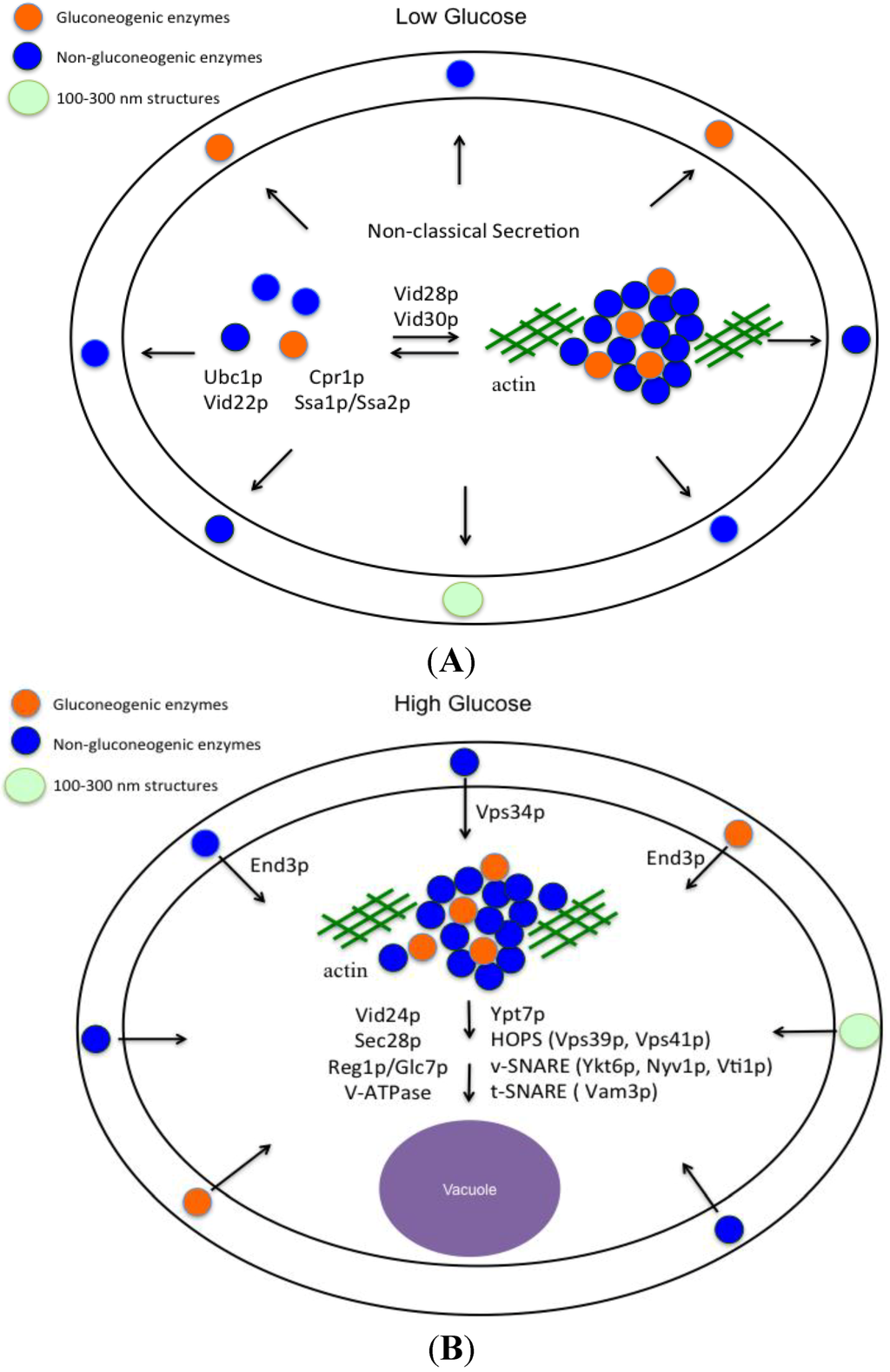
Figure 6.
Vid vesicles are secreted into the periplasm during glucose starvation and are internalized following glucose addition. When yeast cells are grown in low glucose, Vid vesicles that carry gluconeogenic enzymes and non-gluconeogenic enzymes are secreted into the periplasm via the non-classical secretory pathway (A). In the periplasm, 100–300 nm structures are also present (A). In the cytoplasm, FBPase is associated with Vid vesicles. The association is dependent on Ubc1p, Ssa1p/Ssa2p, Vid22p, and Cpr1p. Vid vesicles also aggregate to form large clusters and associate with actin patches. This step requires the VID28 and VID30 genes. Following glucose addition to glucose-starved cells for 30 min, FBPase is internalized (B). The internalization requires the endocytosis gene END3 and the VPS34 gene encoding PI3K (B). The 100–300 nm structures decrease in number but are still observed at the t = 30 min time point. Following internalization, FBPase is delivered to the vacuole to be degraded, while proteins that are not degraded may be recycled. Vid24p, Sec28p, Reg1p/Glc7p, V-ATPase, Ypt7p, HOPS, v-SNARE and t-SNARE are critical for the post-internalization step in the Vid pathway [44,48,98,99,100].
For endocytosis of EVs, different mechanisms such as fusion, clathrin-mediated endocytosis, and macropinocytosis have been implicated [102,103,108]. During heterotypic membrane fusion, the donor membrane and acceptor membrane fuse which results in the integration of these two different membranes into one membrane. If EVs fuse with the plasma membrane, EVs’ membranes become part of the plasma membrane, whereas the internal contents from EVs will be released directly into the cytoplasm. However, EVs labeled with fluorescent lipid dye were observed using fluorescence microscopy to be in the cytoplasm in acceptor cells as punctate structures [108]. This suggests that the exosome membranes are not integrated into the plasma membrane. Therefore, fusion is unlikely to explain the uptake of EVs into acceptor cells. Likewise, fusion is unlikely to explain the internalization of FBPase following glucose addition. If extracellular Vid vesicles fuse with the plasma membrane, then FBPase should be distributed near the plasma membrane or as free protein in the cytoplasm. Because FBPase was distributed in clusters of vesicles in the cytoplasm following internalization, fusion of extracellular Vid vesicles with the plasma membrane should not happen. It remains to be determined whether or not clathrin-mediated endocytosis is used for FBPase uptake into the cells.
At the present time, the molecular mechanisms required for Vid vesicle trafficking in and out of the cell are not well understood. In the future, it will be important to purify the 100–300 nm structures and determine whether or not these structures are involved in the secretion or internalization of vesicles. It will also be imperative to identify genes required for the biogenesis and trafficking of Vid vesicles across the plasma membrane.
Acknowledgments
The work was supported by Penn State Bridge Fund to Hui-Ling Chiang.
Author Contributions
Both authors contributed equally to the drafting and editing of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carlson, M. Regulation of glucose utilization in yeast. Curr. Opin. Genet. Dev. 1998, 8, 560–564. [Google Scholar]
- Ludin, K.; Jiang, R.; Carlson, M. Glucose-regulated interaction of a regulatory subunit of protein phosphatase 1 with the Snf1 protein kinase in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1998, 95, 6245–6250. [Google Scholar]
- Fraenkel, D.G. The top genes: On the distance from transcript to function in yeast glycolysis. Curr. Opin. Microbiol. 2003, 6, 198–201. [Google Scholar]
- Gancedo, J.M. The early steps of glucose signalling in yeast. FEMS Microbiol. Rev. 2008, 32, 673–704. [Google Scholar]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar]
- Zaman, S.; Lippman, S.I.; Zhao, X.; Broach, J.R. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 2008, 42, 27–81. [Google Scholar]
- Van den Brink, J.; Akeroyd, M.; van der Hoeven, R.; Pronk, J.T.; de Winde, J.H.; Daran-Lapujade, P. Energetic limits to metabolic flexibility: Responses of Saccharomyces cerevisiae to glucose-galactose transitions. Microbiology 2009, 155, 1340–1350. [Google Scholar]
- Haurie, V.; Sagliocco, F.; Boucherie, H. Dissecting regulatory networks by means of two-dimensional gel electrophoresis: Application to the study of the diauxic shift in the yeast Saccharomyces cerevisiae. Proteomics 2004, 4, 364–373. [Google Scholar]
- Kolkman, A.; Daran-Lapujade, P.; Fullaondo, A.; Olsthoorn, M.M.; Pronk, J.T.; Slijper, M.; Heck, A.J. Proteome analysis of yeast response to various nutrient limitations. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef]
- Francesca, G.; Francesca, M.; Tania, G.; Marina, B.; Maurizio, S.; Alessandra, M. Effect of different glucose concentrations on proteome of Saccharomyces cerevisiae. Biochim. Biophys. Acta 2010, 1804, 1516–1525. [Google Scholar]
- De Groot, M.J.; Daran-Lapujade, P.; van Breukelen, B.; Knijnenburg, T.A.; de Hulster, E.A.; Reinders, M.J.; Pronk, J.T.; Heck, A.J.; Slijper, M. Quantitative proteomics and transcriptomics of anaerobic and aerobic yeast cultures reveals post-transcriptional regulation of key cellular processes. Microbiology 2007, 153, 3864–3878. [Google Scholar]
- Usaite, R.; Wohlschlegel, J.; Venable, J.D.; Park, S.K.; Nielsen, J.; Olsson, L.; Yates Iii, J.R. Characterization of global yeast quantitative proteome data generated from the wild-type and glucose repression saccharomyces cerevisiae strains: The comparison of two quantitative methods. J. Proteome Res. 2008, 7, 266–275. [Google Scholar]
- Costenoble, R.; Picotti, P.; Reiter, L.; Stallmach, R.; Heinemann, M.; Sauer, U.; Aebersold, R. Comprehensive quantitative analysis of central carbon and amino-acid metabolism in Saccharomyces cerevisiae under multiple conditions by targeted proteomics. Mol. Syst. Biol. 2011, 7, 464. [Google Scholar]
- Kolkman, A.; Olsthoorn, M.M.; Heeremans, C.E.; Heck, A.J.; Slijper, M. Comparative proteome analysis of Saccharomyces cerevisiae grown in chemostat cultures limited for glucose or ethanol. Mol. Cell Proteomics 2005, 4, 1–11. [Google Scholar]
- Pham, T.K.; Chong, P.K.; Gan, C.S.; Wright, P.C. Proteomic analysis of Saccharomyces cerevisiae under high gravity fermentation conditions. J. Proteome Res. 2006, 5, 3411–3419. [Google Scholar]
- Klein, C.J.; Olsson, L.; Nielsen, J. Glucose control in Saccharomyces cerevisiae: The role of Mig1 in metabolic functions. Microbiology 1998, 144, 13–24. [Google Scholar]
- Yin, Z.; Wilson, S.; Hauser, N.C.; Tournu, H.; Hoheisel, J.D.; Brown, A.J. Glucose triggers different global responses in yeast, depending on the strength of the signal, and transiently stabilizes ribosomal protein mRNAs. Mol. Microbiol. 2003, 48, 713–724. [Google Scholar]
- Warner, J.R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 1999, 24, 437–440. [Google Scholar]
- Venema, J.; Tollervey, D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999, 33, 261–311. [Google Scholar]
- Planta, R.J.; Goncalves, P.M.; Mager, W.H. Global regulators of ribosome biosynthesis in yeast. Biochem. Cell Biol. 1995, 73, 825–834. [Google Scholar]
- Goncalves, P.M.; Griffioen, G.; Minnee, R.; Bosma, M.; Kraakman, L.S.; Mager, W.H.; Planta, R.J. Transcription activation of yeast ribosomal protein genes requires additional elements apart from binding sites for Abf1p or Rap1p. Nucleic Acids Res. 1995, 23, 1475–1480. [Google Scholar]
- Newcomb, L.L.; Diderich, J.A.; Slattery, M.G.; Heideman, W. Glucose regulation of Saccharomyces cerevisiae cell cycle genes. Eukaryot. Cell 2003, 2, 143–149. [Google Scholar]
- Goncalves, P.M.; Griffioen, G.; Bebelman, J.P.; Planta, R.J. Signalling pathways leading to transcriptional regulation of genes involved in the activation of glycolysis in yeast. Mol. Microbiol. 1997, 25, 483–493. [Google Scholar]
- De Groot, E.; Bebelman, J.P.; Mager, W.H.; Planta, R.J. Very low amounts of glucose cause repression of the stress-responsive gene HSP12 in Saccharomyces cerevisiae. Microbiology 2000, 146, 367–375. [Google Scholar]
- Griffioen, G.; Mager, W.H.; Planta, R.J. Nutritional upshift response of ribosomal protein gene transcription in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1994, 123, 137–144. [Google Scholar]
- Sierkstra, L.N.; Sillje, H.H.; Verbakel, J.M.; Verrips, C.T. The glucose-6-phosphate-isomerase reaction is essential for normal glucose repression in Saccharomyces cerevisiae. Eur. J. Biochem. 1993, 214, 121–127. [Google Scholar]
- Moore, P.A.; Sagliocco, F.A.; Wood, R.M.; Brown, A.J. Yeast glycolytic mRNAs are differentially regulated. Mol. Cell Biol. 1991, 11, 5330–5337. [Google Scholar]
- Sierkstra, L.N.; Nouwen, N.P.; Verbakel, J.M.; Verrips, C.T. Regulation of glycolytic enzymes and the Crabtree effect in galactose-limited continuous cultures of Saccharomyces cerevisiae. Yeast 1993, 9, 787–795. [Google Scholar]
- Beullens, M.; Mbonyi, K.; Geerts, L.; Gladines, D.; Detremerie, K.; Jans, A.W.; Thevelein, J.M. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1988, 172, 227–231. [Google Scholar]
- Lee, F.J.; Lin, L.W.; Smith, J.A. A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J. Biol. Chem. 1990, 265, 7413–7418. [Google Scholar]
- Elbing, K.; Stahlberg, A.; Hohmann, S.; Gustafsson, L. Transcriptional responses to glucose at different glycolytic rates in Saccharomyces cerevisiae. Eur. J. Biochem. 2004, 271, 4855–4864. [Google Scholar]
- Cohen, R.; Holland, J.P.; Yokoi, T.; Holland, M.J. Identification of a regulatory region that mediates glucose-dependent induction of the Saccharomyces cerevisiae enolase gene ENO2. Mol. Cell Biol. 1986, 6, 2287–2297. [Google Scholar]
- Lascaris, R.; Piwowarski, J.; van der Spek, H.; Teixeira de Mattos, J.; Grivell, L.; Blom, J. Overexpression of HAP4 in glucose-derepressed yeast cells reveals respiratory control of glucose-regulated genes. Microbiology 2004, 150, 929–934. [Google Scholar]
- Scheffler, I.E.; de la Cruz, B.J.; Prieto, S. Control of mRNA turnover as a mechanism of glucose repression in Saccharomyces cerevisiae. Int. J. Biochem. Cell Biol. 1998, 30, 1175–1193. [Google Scholar]
- Gancedo, J.M. Carbon catabolite repression in yeast. Eur. J. Biochem. 1992, 206, 297–313. [Google Scholar]
- Yin, Z.; Hatton, L.; Brown, A.J. Differential post-transcriptional regulation of yeast mRNAs in response to high and low glucose concentrations. Mol. Microbiol. 2000, 35, 553–565. [Google Scholar]
- Regelmann, J.; Schule, T.; Josupeit, F.S.; Horak, J.; Rose, M.; Entian, K.D.; Thumm, M.; Wolf, D.H. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: A genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol. Biol. Cell 2003, 14, 1652–1663. [Google Scholar]
- Gancedo, J.M.; Gancedo, C. Inactivation of gluconeogenic enzymes in glycolytic mutants of Saccharomyces cerevisiae. Eur. J. Biochem. 1979, 101, 455–460. [Google Scholar]
- Entian, K.D.; Droll, L.; Mecke, D. Studies on rapid reversible and non-reversible inactivation of fructose-1,6-bisphosphatase and malate dehydrogenase in wild-type and glycolytic block mutants of Saccharomyces cerevisiae. Arch. Microbiol. 1983, 134, 187–192. [Google Scholar]
- Entian, K.D.; Frohlich, K.U.; Mecke, D. Regulation of enzymes and isoenzymes of carbohydrate metabolism in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1984, 799, 181–186. [Google Scholar]
- Holzer, H. Proteolytic catabolite inactivation in Saccharomyces cerevisiae. Revis. Biol. Cel. 1989, 21, 305–319. [Google Scholar]
- Brown, C.R.; Chiang, H.L. A selective autophagy pathway that degrades gluconeogenic enzymes during catabolite inactivation. Commun. Integr. Biol. 2009, 2, 177–183. [Google Scholar]
- Brown, C.R.; Cui, D.Y.; Hung, G.G.; Chiang, H.L. Cyclophilin A mediates Vid22p function in the import of fructose-1,6-bisphosphatase into Vid vesicles. J. Biol. Chem. 2001, 276, 48017–48026. [Google Scholar]
- Brown, C.R.; Dunton, D.; Chiang, H.L. The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation. J. Biol. Chem. 2010, 285, 1516–1528. [Google Scholar]
- Brown, C.R.; Hung, G.C.; Dunton, D.; Chiang, H.L. The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway. J. Biol. Chem. 2010, 285, 23359–23370. [Google Scholar]
- Brown, C.R.; McCann, J.A.; Chiang, H.L. The heat shock protein Ssa2p is required for import of fructose-1, 6-bisphosphatase into Vid vesicles. J. Cell Biol. 2000, 150, 65–76. [Google Scholar]
- Brown, C.R.; McCann, J.A.; Hung, G.G.; Elco, C.P.; Chiang, H.L. Vid22p, a novel plasma membrane protein, is required for the fructose-1,6-bisphosphatase degradation pathway. J. Cell Sci. 2002, 115, 655–666. [Google Scholar]
- Brown, C.R.; Wolfe, A.B.; Cui, D.; Chiang, H.L. The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation. J. Biol. Chem. 2008, 283, 26116–26127. [Google Scholar]
- Hung, G.C.; Brown, C.R.; Wolfe, A.B.; Liu, J.; Chiang, H.L. Degradation of the gluconeogenic enzymes fructose-1,6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J. Biol. Chem. 2004, 279, 49138–49150. [Google Scholar]
- Polakis, E.S.; Bartley, W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem. J. 1965, 97, 284–297. [Google Scholar]
- Polakis, E.S.; Bartley, W.; Meek, G.A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem. J. 1965, 97, 298–302. [Google Scholar]
- Satrustegui, J.; Machado, A. The synthesis of yeast matrix mitochondrial enzymes is regulated by different levels of mitochondrial function. Arch. Biochem. Biophys. 1977, 184, 355–363. [Google Scholar]
- Chiang, H.L.; Schekman, R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature 1991, 350, 313–318. [Google Scholar]
- Hoffman, M.; Chiang, H.L. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics 1996, 143, 1555–1566. [Google Scholar]
- Schork, S.M.; Thumm, M.; Wolf, D.H. Catabolite inactivation of fructose-1,6-bisphosphatase of Saccharomyces cerevisiae. Degradation occurs via the ubiquitin pathway. J. Biol. Chem. 1995, 270, 26446–26450. [Google Scholar]
- Schule, T.; Rose, M.; Entian, K.D.; Thumm, M.; Wolf, D.H. Ubc8p functions in catabolite degradation of fructose-1, 6-bisphosphatase in yeast. EMBO J. 2000, 19, 2161–2167. [Google Scholar]
- Gancedo, C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J. Bacteriol. 1971, 107, 401–405. [Google Scholar]
- Pohlig, G.; Holzer, H. Phosphorylation and inactivation of yeast fructose-1,6-bisphosphatase by cyclic AMP-dependent protein kinase from yeast. J. Biol. Chem. 1985, 260, 13818–13823. [Google Scholar]
- Rittenhouse, J.; Moberly, L.; Marcus, F. Phosphorylation in vivo of yeast (Saccharomyces cerevisiae) fructose-1,6-bisphosphatase at the cyclic AMP-dependent site. J. Biol. Chem. 1987, 262, 10114–10119. [Google Scholar]
- Jiang, Y.; Davis, C.; Broach, J.R. Efficient transition to growth on fermentable carbon sources in Saccharomyces cerevisiae requires signaling through the Ras pathway. EMBO J. 1998, 17, 6942–6951. [Google Scholar]
- Gancedo, J.M.; Mazon, M.J.; Gancedo, C. Inactivation and phosphorylation of yeast fructose 1,6-bisphosphatase. Biochem. Soc. Trans. 1982, 10, 326–327. [Google Scholar]
- Lamponi, S.; Galassi, C.; Tortora, P.; Guerritore, A. Glucose-induced degradation of yeast fructose-1,6-bisphosphatase requires additional triggering events besides protein phosphorylation. FEBS Lett. 1987, 216, 265–269. [Google Scholar]
- Mazon, M.J.; Gancedo, J.M.; Gancedo, C. Inactivation of yeast fructose-1,6-bisphosphatase. In vivo phosphorylation of the enzyme. J. Biol. Chem. 1982, 257, 1128–1130. [Google Scholar]
- Toyoda, Y.; Fujii, H.; Miwa, I.; Okuda, J.; Sy, J. Anomeric specificity of glucose effect on cAMP, fructose 1,6-bisphosphatase, and trehalase in yeast. Biochem. Biophys. Res. Commun. 1987, 143, 212–217. [Google Scholar]
- Huang, P.H.; Chiang, H.L. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J. Cell Biol. 1997, 136, 803–810. [Google Scholar]
- Shieh, H.L.; Chen, Y.; Brown, C.R.; Chiang, H.L. Biochemical analysis of fructose-1,6-bisphosphatase import into vacuole import and degradation vesicles reveals a role for UBC1 in vesicle biogenesis. J. Biol. Chem. 2001, 276, 10398–10406. [Google Scholar]
- Chiang, M.C.; Chiang, H.L. Vid24p, a novel protein localized to the fructose-1,6-bisphosphatase-containing vesicles, regulates targeting of fructose-1,6-bisphosphatase from the vesicles to the vacuole for degradation. J. Cell Biol. 1998, 140, 1347–1356. [Google Scholar]
- Giardina, B.J.; Dunton, D.; Chiang, H.L. Vid28 protein is required for the association of vacuole import and degradation (Vid) vesicles with actin patches and the retention of Vid vesicle proteins in the intracellular fraction. J. Biol. Chem. 2013, 288, 11636–11648. [Google Scholar]
- Alibhoy, A.A.; Giardina, B.J.; Dunton, D.D.; Chiang, H.L. Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway. Autophagy 2012, 8, 29–46. [Google Scholar]
- Giardina, B.J.; Chiang, H.L. The key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted during prolonged glucose starvation and is internalized following glucose re-feeding via the non-classical secretory and internalizing pathways in Saccharomyces cerevisiae. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef]
- Giardina, B.J.; Chiang, H.L. Fructose-1,6-bisphosphatase, Malate Dehydrogenase, Isocitrate Lyase, Phosphoenolpyruvate Carboxykinase, Glyceraldehyde-3-phosphate Dehydrogenase, and Cyclophilin A are secreted in Saccharomyces cerevisiae grown in low glucose. Commun. Integr. Biol. 2013, 6, e27216. [Google Scholar]
- Alibhoy, A.A.; Giardina, B.J.; Dunton, D.D.; Chiang, H.L. Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway. J. Biol. Chem. 2012, 287, 33080–33093. [Google Scholar]
- Giardina, B.J.; Stanley, B.A.; Chiang, H.L. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci. 2014, 12. [Google Scholar] [CrossRef]
- Lamonica, J.M.; Wagner, M.; Eschenbrenner, M.; Williams, L.E.; Miller, T.L.; Patra, G.; DelVecchio, V.G. Comparative secretome analyses of three Bacillus anthracis strains with variant plasmid contents. Infect. Immun. 2005, 73, 3646–3658. [Google Scholar]
- Schaumburg, J.; Diekmann, O.; Hagendorff, P.; Bergmann, S.; Rohde, M.; Hammerschmidt, S.; Jansch, L.; Wehland, J.; Karst, U. The cell wall subproteome of Listeria monocytogenes. Proteomics 2004, 4, 2991–3006. [Google Scholar]
- Cass, C.L.; Johnson, J.R.; Califf, L.L.; Xu, T.; Hernandez, H.J.; Stadecker, M.J.; Yates, J.R., 3rd; Williams, D.L. Proteomic analysis of Schistosoma mansoni egg secretions. Mol. Biochem. Parasitol. 2007, 155, 84–93. [Google Scholar]
- Sotillo, J.; Valero, M.L.; Sanchez Del Pino, M.M.; Fried, B.; Esteban, J.G.; Marcilla, A.; Toledo, R. Excretory/secretory proteome of the adult stage of Echinostoma caproni. Parasitol. Res. 2010, 107, 691–697. [Google Scholar]
- Zheng, M.; Hu, K.; Liu, W.; Hu, X.; Hu, F.; Huang, L.; Wang, P.; Hu, Y.; Huang, Y.; Li, W.; et al. Proteomic analysis of excretory secretory products from Clonorchis sinensis adult worms: Molecular characterization and serological reactivity of a excretory-secretory antigen-fructose-1,6-bisphosphatase. Parasitol. Res. 2011, 109, 737–744. [Google Scholar]
- Albuquerque, P.C.; Nakayasu, E.S.; Rodrigues, M.L.; Frases, S.; Casadevall, A.; Zancope-Oliveira, R.M.; Almeida, I.C.; Nosanchuk, J.D. Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008, 10, 1695–1710. [Google Scholar]
- Lee, H.S.; Jeong, J.; Lee, K.J. Characterization of vesicles secreted from insulinoma NIT-1 cells. J. Proteome Res. 2009, 8, 2851–2862. [Google Scholar]
- Rowe, J.D.; Harbertson, J.F.; Osborne, J.P.; Freitag, M.; Lim, J.; Bakalinsky, A.T. Systematic identification of yeast proteins extracted into model wine during aging on the yeast lees. J. Agric. Food Chem. 2010, 58, 2337–2346. [Google Scholar]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimaraes, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; Nimrichter, L.; Rodrigues, M.L. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 2010, 5, e11113. [Google Scholar] [CrossRef]
- Shin, Y.K.; Yoo, B.C.; Hong, Y.S.; Chang, H.J.; Jung, K.H.; Jeong, S.Y.; Park, J.G. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis 2009, 30, 2182–2192. [Google Scholar]
- Yamashita, R.; Fujiwara, Y.; Ikari, K.; Hamada, K.; Otomo, A.; Yasuda, K.; Noda, M.; Kaburagi, Y. Extracellular proteome of human hepatoma cell, HepG2 analyzed using two-dimensional liquid chromatography coupled with tandem mass spectrometry. Mol. Cell Biochem. 2007, 298, 83–92. [Google Scholar]
- Pavlou, M.P.; Diamandis, E.P. The cancer cell secretome: A good source for discovering biomarkers? J. Proteomics 2010, 73, 1896–1906. [Google Scholar]
- Martinez-Gomariz, M.; Perumal, P.; Mekala, S.; Nombela, C.; Chaffin, W.L.; Gil, C. Proteomic analysis of cytoplasmic and surface proteins from yeast cells, hyphae, and biofilms of Candida albicans. Proteomics 2009, 9, 2230–2252. [Google Scholar]
- Giardina, B.J.; Stanley, B.A.; Chiang, H.L. Comparative Proteomic Analysis of Transition of Saccharomyces cerevisiae from Glucose-Deficient Medium to Glucose-Rich Medium. Proteome Sci. 2012, 10, 40:1–40:21. [Google Scholar]
- Cleves, A.E.; Cooper, D.N.; Barondes, S.H.; Kelly, R.B. A new pathway for protein export in Saccharomyces cerevisiae. J. Cell Biol. 1996, 133, 1017–1026. [Google Scholar]
- Chaffin, W.L.; Lopez-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martinez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar]
- Nombela, C.; Gil, C.; Chaffin, W.L. Non-conventional protein secretion in yeast. Trends Microbiol. 2006, 14, 15–21. [Google Scholar]
- Mrsa, V.; Seidl, T.; Gentzsch, M.; Tanner, W. Specific labelling of cell wall proteins by biotinylation. Identification of four covalently linked O-mannosylated proteins of Saccharomyces cerevisiae. Yeast 1997, 13, 1145–1154. [Google Scholar] [CrossRef]
- Klis, F.M.; de Jong, M.; Brul, S.; de Groot, P.W. Extraction of cell surface-associated proteins from living yeast cells. Yeast 2007, 24, 253–258. [Google Scholar]
- Giardina, B.J.; Stein, K.; Chiang, H.L. The endocytosis gene END3 is essential for the glucose-induced rapid decline of small vesicles in the extracellular fraction in Saccharomyces cerevisiae. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Insenser, M.R.; Hernaez, M.L.; Nombela, C.; Molina, M.; Molero, G.; Gil, C. Gel and gel-free proteomics to identify Saccharomyces cerevisiae cell surface proteins. J. Proteomics 2010, 73, 1183–1195. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteomics 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Cui, D.Y.; Brown, C.R.; Chiang, H.L. The type 1 phosphatase Reg1p-Glc7p is required for the glucose-induced degradation of fructose-1,6-bisphosphatase in the vacuole. J. Biol. Chem. 2004, 279, 9713–9724. [Google Scholar]
- Liu, J.; Brown, C.R.; Chiang, H.L. Degradation of the gluconeogenic enzyme fructose-1,6-bisphosphatase is dependent on the vacuolar ATPase. Autophagy 2005, 1, 146–156. [Google Scholar] [CrossRef]
- Brown, C.R.; Liu, J.; Hung, G.C.; Carter, D.; Cui, D.; Chiang, H.L. The Vid vesicle to vacuole trafficking event requires components of the SNARE membrane fusion machinery. J. Biol. Chem. 2003, 278, 25688–25699. [Google Scholar]
- Atay, S.; Godwin, A.K. Tumor-derived exosomes: A message delivery system for tumor progression. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; Belting, M. Exosome and microvesicle mediated phene transfer in mammalian cells. Semin. Cancer Biol. 2014. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162:1–162:12. [Google Scholar]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29C, 116–125. [Google Scholar]
- Sun, Y.; Liu, J. Potential of Cancer Cell-Derived Exosomes in Clinical Application: A Review of Recent Research Advances. Clin. Ther. 2014, 36, 863–872. [Google Scholar]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef]
- Ye, S.B.; Li, Z.L.; Luo, D.H.; Huang, B.J.; Chen, Y.S.; Zhang, X.S.; Cui, J.; Zeng, Y.X.; Li, J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014, 5, 5439–5452. [Google Scholar]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).