Abstract
The ubiquitous and highly conserved flotillin proteins, flotillin-1 and flotillin-2, have been shown to be involved in various cellular processes such as cell adhesion, signal transduction through receptor tyrosine kinases as well as in cellular trafficking pathways. Due to the fact that flotillins are acylated and form hetero-oligomers, they constitutively associate with cholesterol-enriched lipid microdomains. In recent years, such microdomains have been appreciated as platforms that participate in endocytosis and other cellular trafficking steps. This review summarizes the current findings on the role of flotillins in membrane-bound cargo endocytosis and endosomal trafficking events. We will discuss the proposed function of flotillins in endocytosis in the light of recent findings that point towards a role for flotillins in a step that precedes the actual endocytic uptake of cargo molecules. Recent findings have also revealed that flotillins may be important for endosomal sorting and recycling of specific cargo molecules. In addition to these aspects, the cellular trafficking pathway of flotillins themselves as potential cargo in the context of growth factor signaling will be discussed.
1. Lipid Microdomains and Endocytosis
Initially, lipid microdomains were described in the early 1990s as membrane structures that are insoluble in cold non-ionic detergents such as Triton X-100 and thus float in low density fractions [1]. Ever since Simons and Ikonen proposed the principle of lipid rafts in 1997 [2], it has been refined over the years. Nowadays, such lipid microdomains are considered as specific nanoscale assemblies enriched in cholesterol and sphingolipids that constitute a liquid-ordered phase in cellular membranes. These microdomains are dynamic and can coalesce to serve as signaling platforms or to function in membrane trafficking [3].
Certain modifications and properties of proteins increase their propensity to associate with lipid microdomains. For example, the association of glycosylphosphatidyl-inositol (GPI) anchored proteins with rafts is mediated by their glycolipid anchor [4,5]. Multiple acylation has been shown to enhance the affinity of proteins for the liquid-ordered membrane phase. For example, tyrosine kinases of the Src family are doubly acylated and thus associate with rafts [5,6,7,8]. Palmitoylation, which is a reversible process that takes place in Cys residues, was suggested to serve as regulatory means to recruit or exclude proteins from lipid microdomains [9]. However, single palmitoylation alone is not sufficient to recruit proteins into rafts, as evidenced by the transferrin receptor which can be palmitoylated but is constitutively localized outside of rafts [9]. Apart from GPI anchors and acylation, oligomerization of proteins enhances their affinity for rafts and can also serve to stabilize the respective microdomain in a scaffolding manner [9,10,11,12].
Endocytosis can be roughly classified in two categories: clathrin mediated endocytosis (CME) and clathrin independent endocytosis (CIE). For recent reviews, the reader is referred to [13,14,15,16]. In contrast to the detailed mechanistic insights that are available for CME and its structural component clathrin, CIE is far less understood. So far, it appears that a major hallmark of CIE is that even uncoated membrane pits can be invaginated and internalized into the cell. While CME depends on dynamin for vesicle scission, both dynamin dependent and independent CIE pathways have been described. The fission of caveolae, invaginated structures in the plasma membrane that are decorated with caveolins and cavins [17], depends on dynamin [18,19]. On the other hand, flotillin mediated endocytosis of some cargo molecules was suggested to be dynamin independent [20,21], whereas the growth factor induced internalization of flotillins clearly depends on dynamin [22]. In this review, we will only shortly summarize the suggested role of flotillins in CIE. For a more comprehensive review on flotillins in the endocytosis of specific cargo molecules, please refer to a recent review by Otto and Nichols [23]. The purpose of the present review is to critically discuss recent findings that suggest that in the case of some cargo molecules, flotillins might not actively participate in endocytosis but rather in a step preceding the endocytic uptake that may even take place by means of CME. In addition, we will provide insights into the emerging role of flotillins in cargo sorting within endosomes.
2. The Flotillin Protein Family
Flotillin-1/reggie-2 and flotillin-2/reggie-1 constitutively associate with specific membrane microdomains by acylation (a single palmitate in flotillin-1, a myristate and three palmitates in flotillin-2) [11,24,25], oligomerization [11,12,21,26] and cholesterol binding ([27]; our unpublished data). Upon the discovery by Bickel et al., flotillins were implicated to exert a functional role in membrane trafficking processes [28]. Originally, it was proposed that flotillins associate with caveolae [28,29], but later findings clearly have shown that flotillins participate in the formation of specific non-caveolar microdomains [20,30]. Furthermore, our unpublished results from flotillin-2 knockout mice do not reveal any significant changes in caveolin protein expression. Nowadays, flotillins are commonly used as marker proteins for non-caveolar rafts. Their ability to float in low density fractions of Triton X-100 insoluble membrane preparations coined their name as flotillins and indicated their association with rafts [28].
Structurally, flotillins are composed of two domains, the function of which has not been clarified in detail. The N-terminal SPFH (stomatin/prohibitin/flotillin/HflK/C) domain contains the sites for acylation [11,24,25,27,31], whereas the so-called flotillin domain in the C-terminus mediates the oligomerization and contains Ala-Glu repeats and phosphorylatable tyrosines that are important for flotillin function [11,12,26,32,33,34]. Both flotillins are ubiquitously expressed, conserved among species and homologous to each other [35,36], although they appear to be functionally distinct. However, the expression of one flotillin depends on that of the other one, and depletion or deletion of one flotillin also reduces the stability of the other. However, flotillin-1 appears to be more dependent on flotillin-2 than vice versa [26,37,38]. Functionally, flotillins have been implicated in several cellular processes, such as cellular migration and adhesion, signaling by receptor tyrosine kinases and mitogen activated protein kinases (MAPK) as well as membrane trafficking. For detailed information on the role of flotillins in signal transduction and putative roles in cancer, we would like to refer the reader to our recent review articles [34,39,40].
Flotillins display a dynamic cellular localization that considerably varies between different cell types [21,31]. Under growth conditions, flotillins predominantly localize to the plasma membrane and endosomal structures, i.e., late endosomes, recycling endosomes and exosomes [12,27,31,41,42,43,44,45,46]. However, under growth factor deprivation, flotillins relocate to the plasma membrane by means of recycling from intracellular compartments. Upon stimulation with epidermal growth factor (EGF), Src family kinases phosphorylate flotillins at several tyrosine residues, and flotillin oligomers increase in size and translocate to late endosomes [12,32,33]. Furthermore, flotillins actively participate in signaling pathways, e.g., receptor tyrosine kinase signaling and MAPK signaling [37,38,39,40,47].
3. Discovery of the Putative Flotillin Dependent Endocytosis Pathway
To date, several cargo molecules, such as the GPI-anchored protein CD59, cholera toxin B subunit (CTxB), cationic molecules and polyplexes, proteoglycans and proteoglycan bound ligands, as well as the Niemann-Pick C1-like 1 protein (NPC1L1) [20,21,48,49,50] have been suggested to utilize an internalization pathway that depends on flotillin-1 (Table 1). The initial idea that flotillins would establish their own CIE pathway, was suggested by Glebov et al., who found increasing amounts of flotillin-1 in early endocytic vesicles after fluid-phase uptake of magnetic nanoparticles (ferrofluid) [20]. However, they did not observe a colocalization of flotillin-1 with transferrin (Tfn), a classical cargo for CME, or with clathrin in these early endocytic vesicles. Due to these findings, together with the observation that flotillin-1 colocalizes in HeLa and COS-7 cells with the GPI-anchored protein CD59 and the ganglioside GM1, Glebov et al. reasoned that flotillins participate in an internalization pathway that is different from CME. This was further supported by the findings demonstrating that upon expression of a dominant negative version of AP180, a molecule required for the formation of clathrin coated pits (CCPs) [51], ectopically expressed flotillin-1-GFP still colocalized with CTxB in endocytic vesicles, and depletion of flotillin-1 partially inhibited the uptake of an antibody directed towards CD59 [20,52]. However, CTxB, which binds to its receptor GM1, is somewhat controversial as a raft marker, since CTxB/GM1 have been found to be internalized not only by CIE, but also via CCPs and thus CME [53,54]. Upon immunolabeling of ultra-thin cryosections, vesicles positive for flotillin-1-GFP and CTxB were detected. However, according to the authors, only 15% of the total flotillin-1-GFP was found in these vesicles, and neither CTxB nor CD59 were significantly enriched in flotillin-1-GFP positive vesicles and invaginations at the plasma membrane. Live imaging with total internal reflection of fluorescence (TIRF) showed a very dynamic behavior of flotillin-1-GFP at the plasma membrane, with vesicles that disappeared towards the cellular interior. It was observed that flotillin-1-GFP positive vesicles and microdomains at the plasma membrane are very dynamic and move with a high mean velocity as compared to CCVs [20,21]. The dynamic movement of flotillins at the plasma membrane is in line with the fluctuating and varying lifetime of lipid microdomains [55,56]. However, flotillin-1-GFP containing vesicles bud into the cell at a frequency that is less than one third of that of CCPs [20]. Pursuing the idea that flotillins would define a CIE pathway, Frick and colleagues proposed that flotillins might serve as structural components for this pathway [21]. They observed that ectopic expression of flotillin-1-GFP and flotillin-2-GFP induces their coassembly to specific flotillin microdomains which induce membrane curvature and thus generate membrane buds that in turn bud towards the cellular interior. They suggested that the highly dynamic flotillin microdomains become static just prior to their internalization [21], which might be caused by coalescence of flotillin oligomers into larger oligomeric structures, as we have shown to occur upon EGF stimulation of the cells [12]. Since the study of Frick et al. was based on overexpression of GFP-tagged flotillins, which do not necessarily fully resemble the endogenous proteins in terms of their trafficking and oligomerization, the suggested capability of flotillins to induce membrane buds needs to be dissected in further studies. However, this study elegantly shows that flotillins assemble into microdomains, a property which is based on their propensity to form oligomers, as has later also been observed by us and others [12,21,26].

Table 1.
Overview of the literature on flotillins in cellular sorting and endocytosis.
| Sorting Process | References |
|---|---|
| Flotillin assisted endocytosis | [37,41,50,57,58] |
| Polarized sorting | [59,60,61,62,63,64,65] |
| Exosomes | [27,44,46,66] |
| Endosomal sorting | [42,67,68,69] |
| Flotillin oligomerization | [11,12,26,32] |
| Flotillin dependent endocytosis | [20,21] |
4. Flotillin Dependent Cargo Trafficking and Sorting: Beyond Endocytosis
4.1. Flotillins in Sorting Events within Endosomes
In recent years, a number of publications have indicated a role for flotillins in endosomal sorting processes and in the formation of exosomal vesicles in endosomes. Generation and release of exosomes frequently occurs within multivesicular bodies (MVBs), which then fuse with the plasma membrane and thereby release their intraluminal vesicles (ILV) as exosomes to the extracellular space [70,71]. Several publications have suggested a role for lipid microdomains and flotillins in exosome generation [27,44,46,72]. The tetraspanin CD63 and Alix are commonly considered as proteins enriched in exosomes and can thus be used as markers to study exosome release [66]. Strauss and colleagues found that cholesterol treatment of oligodendrocytes resulted in an increased exosome release and these exosomes were enriched in flotillin-2, Alix and EGFP-CD63 [27]. In contrast, Baietti et al. found that Alix and CD63, together with syntenin, reside in flotillin-negative exosomes released from MCF-7 cells [73]. According to Phuyal and colleagues, depletion of neither flotillin-1 nor flotillin-2 influenced the number of exosomes released, whereas the sorting of caveolin-1 and annexin A2 to exosomes was impaired [46]. Thus, flotillins might participate in the sorting of specific proteins towards ILVs that are destined to generate exosomes that do not contain Alix and CD63. However, detailed mechanistic insights into how flotillins sort cargo for exosomal release is still missing.
Other studies have implicated a role for flotillins in cargo recycling. Saslowsky et al. observed that flotillins participate in the sorting of the cholera toxin-GM1 complex from endosomes via the TGN to the ER in zebrafish [67]. Interestingly, depletion of both flotillins rendered the fish resistant to intoxication with cholera toxin. Similar results were observed in mammalian COS-1 cells, in which cholera toxin requires flotillins to exert its cytotoxic effects. Interestingly, the averted toxicity of cholera toxin in flotillin depleted cells was shown not to be due to a reduced binding of cholera toxin to the plasma membrane GM1 or a defect in endocytosis, but rather due to a defect in the transport of cholera toxin from the plasma membrane to the ER [67]. Since neither the binding of cholera toxin to GM1 at the plasma membrane nor its endocytosis were affected by depletion of flotillins, the authors indicated that flotillins might play a role in endosomal sorting of cargo towards the ER or TGN. In line with these findings, Pust and coworkers analyzed the role of flotillins in the cellular transport of ricin and Shiga toxin [68]. Again, the endocytic uptake of both toxins was not affected by depletion of flotillins, whereas the retrograde transport of the toxins towards the TGN and ER was impaired and caused an accumulation of both toxins, thus increasing their toxicity [68].
Well in line with the above findings, we have recently described a novel role for flotillins in the endosomal sorting of the β-secretase BACE1 [69]. Flotillin-1 binds to a di-leucine sorting motif in the cytoplasmic tail of BACE1 and thereby competes with the adapter protein Golgi-localized, gamma adaptin ear-containing, ADP ribosylation factor binding protein 2 (GGA2) for the binding to BACE1 tail. Previous studies have shown that GGA proteins are important for both the retrograde trafficking and recycling of BACE1 towards Golgi and plasma membrane and for sorting to lysosomes for degradation [25,74,75,76,77,78]. Depletion of flotillins leads to an accumulation of BACE1 in endosomes, which in turn increased the amyloidogenic processing of the Alzheimer amyloid precursor protein (APP) [69]. Our study showed for the first time a direct binding of flotillins to a canonical sorting motif in a transmembrane cargo protein. Based on our data, flotillins might thus participate in cargo sorting towards recycling. This is in line with recent findings by Solis and colleagues, who found that overexpressed flotillins associated with tubulovesicular recycling compartments positive for Rab11a, sorting nexin-4 and EH domain containing-1 in A431 cells [42] and that depletion of flotillins affected the recycling of the transferrin receptor and E-cadherin [42,79]. Thus, a novel and highly intriguing role for flotillins in the regulation of cargo sorting events within endosomes appears to be emerging.
4.2. An Indirect Role of Flotillins in Endocytosis: Pre-Endocytic Clustering at the Plasma Membrane
Recent findings have casted some doubt on the direct role of flotillins in the endocytic uptake of some cargo molecules, as flotillins were shown to specifically cluster cargo molecules, such as APP, the dopamine transporter (DAT) and the epidermal growth factor receptor (EGFR), at the plasma membrane prior to endocytosis by means of CME [37,57,80]. Figure 1 summarizes the potential role of flotillins in the endocytosis of cargo proteins. Depletion of flotillin-2, but not of flotillin-1, impairs APP endocytosis in neuroblastoma cells and in primary hippocampal neurons [57]. Strikingly, using STED microscopy, Schneider and colleagues showed that APP requires flotillin-2 for the formation of pre-endocytic clusters at the plasma membrane that are necessary for a proper endocytic uptake of APP [57]. Since APP is a classical cargo protein of CME [81,82,83], the authors suggested that APP is internalized by a specialized CME pathway that is regulated by flotillin-2, but the details of the mechanism how flotillins affect CME still await further characterization.
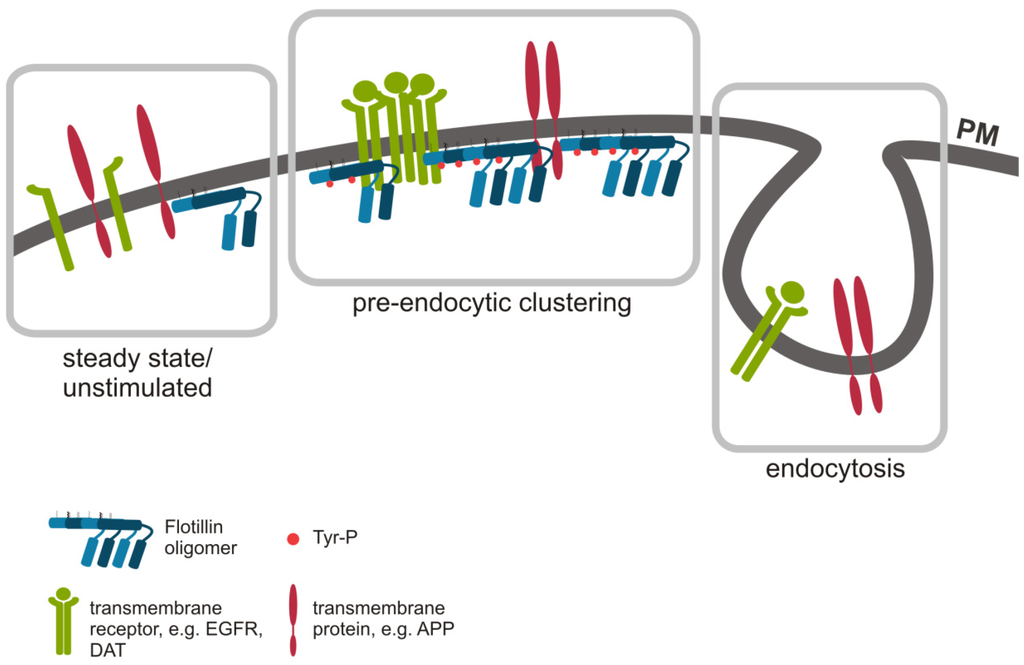
Figure 1.
Flotillin assisted endocytosis. Flotillin microdomains are dynamic, and upon certain stimuli, flotillins form higher order oligomers that can recruit transmembrane proteins, such as EGFR, DAT and APP, into flotillin rafts for pre-endocytic cluster formation (middle). Cargo can then be internalized via endocytosis without a direct involvement of flotillins, e.g., by clathrin mediated endocytosis (right). In resting or growth factor deprived cells, flotillins and the cargo may not reside in the same microdomains (left).
The role of flotillins in the endocytosis of the dopamine transporter DAT has recently been set under debate. While Cremona and co-workers suggested an essential role for flotillins during DAT endocytosis, which goes along with a phosphorylation of flotillin-1 on Ser 315 by protein kinase C (PKC), Sorkina and colleagues established that flotillins actually are necessary for the decreased mobility and clustering of the transporter in the plasma membrane prior to its CME mediated uptake [58,84]. In line with this, we have observed that flotillin-1 influences the clustering of EGFR upon EGF stimulation at the plasma membrane, but not EGFR endocytosis [37]. Even though EGFR has been suggested to utilize both CME or CIE pathways, depending on the ligand dosage [85,86], we did not observe any effect on EGFR uptake upon depletion of flotillin-1 [37], nor did we see a colocalization of flotillins and EGFR in early endocytic vesicles [12,37]. Thus, it appears that although APP, DAT and EGFR are internalized by CME, they all depend on flotillins for preassembly prior to endocytosis. However, a direct molecular connection between flotillin microdomains and clathrin coated structures at the plasma membrane is currently missing, and there is a very limited degree of overlap of clathrin with flotillins at the plasma membrane [20]. Interestingly, there is some evidence suggesting that clathrin coated structures may assemble in plasma membrane microdomains [87], but there are no data as yet if these structures contain flotillins. It is highly unlikely that flotillins function as essential components of CME or coated pit assembly, since endocytosis of clathrin dependent cargo, e.g., transferrin receptor, is not generally impaired upon flotillin depletion (Our unpublished findings). However, it is possible that there may be a specific subset of cargo that is endocytosed by means of CME that takes place from microdomains that contain flotillins.
Interestingly, flotillins contain cholesterol recognition/interaction amino acid consensus (CRAC) motifs and have been suggested to bind cholesterol ([27,88]; our unpublished findings). The transmembrane NPC1L1 protein mediates cellular cholesterol uptake and cycles between the plasma membrane and recycling endosomes [50,89]. Upon its endocytic uptake, NPC1L1 utilizes a CME pathway [89]. Interestingly, by co-immunoprecipitation and FRET analysis, flotillins were shown to associate with NPC1L1 and to be required for a cholesterol induced uptake of NPC1L1 [50]. Furthermore, the presence of NPC1L1 in flotillin microdomains is in line with the binding of flotillins to cholesterol ([50,88]; our unpublished findings) and their scaffolding microdomain activity. Strikingly, Ge et al. suggest that flotillins mediate the recruitment of clathrin and its adaptor protein AP-2 to NPC1L1 and thereby facilitate the uptake of NPC1L1 [50,89]. Therefore, flotillins, due to their propensity to form oligomers and to bind cholesterol, might contribute to microdomain scaffolding in the pre-assembly or clustering of cargo proteins destined for endocytosis. Similar observations have been published by Abrami et al. [90,91,92] who found that the anthrax toxin is endocytosed by CME, but depends on lipid microdomains for the clustering during CCP assembly [90], as has also been shown for the tetanus neurotoxin [93]. Thus, one could assume a general role for lipid microdomains in the pre-endocytic clustering and assembly of cargo molecules destined for endocytosis, independent of the final endocytosis pathway used.
5. Flotillins as Endocytic Cargo during Signaling
So far, most studies addressing flotillins and endocytosis have neglected the possibility that flotillins themselves might be cargo molecules for a CIE pathway. Adding to the controversy as to whether flotillins establish their own endocytosis pathway or only assist in cargo clustering for endocytosis, Glebov and co-workers could not exclude that flotillin-1 might itself be a cargo molecule that is recognized by a CIE pathway [20]. Most studies so far have addressed only steady state endocytic pathways [20,21]. However, it has been conclusively shown that flotillins participate in growth factor signaling and that flotillin microdomains increase in size and translocate to endosomes upon EGF stimulation [12,32,33,37]. These two pathways, the steady state uptake and the growth factor induced translocation, might represent two different routes that flotillins utilize for their internalization. This is also supported by recent findings on dynamin dependency of flotillin uptake. Inhibition of dynamin GTPase activity showed that EGF mediated flotillin uptake requires dynamin activity [22], whereas uptake of some of the suggested flotillin cargo molecules appears to be dynamin independent (reviewed in [23]). However, flotillin uptake – irrespective of whether as a cargo molecule or as a structural endocytic component – depends on cholesterol [50,57]. Dissection of the role of dynamin in the cellular trafficking of flotillins is complicated by the fact that expression of dominant-negative dynamin mutants (K44A, T65A or R399A) impairs the recycling of flotillins from endosomes to the plasma membrane, which also, somewhat surprisingly, appears to require clathrin [22]. However, both dynamin and clathrin have been shown to participate in sorting events in endosomes that mediate recycling of cargo towards the plasma membrane [94,95], making their role in the recycling of flotillins plausible.
6. A New Era: From Flotillin Dependent to Flotillin Assisted Endocytosis
Due to the findings showing that flotillin depletion reduces the uptake of some proteins from the plasma membrane, the term “flotillin dependent endocytosis” has been established. However, this term implies that flotillins are essential mechanistic components of a specific endocytic pathway that is at least severely impaired in their absence (as with “clathrin dependent”). In the case of clathrin and dynamin, the dependency is well established, and the use of the word “dependent” well justified. However, as the evidence for a similar, essential mechanistic role for flotillins and the details of the nature of the endocytic carriers are currently lacking, we suggest that the term “flotillin assisted endocytosis” should rather be used. In our opinion, the word “assisted” describes a process that is facilitated by flotillins (e.g., by cargo sequestering prior to endocytosis) but is not strictly and mechanistically dependent on flotillins as structural component. Thus, as long as the essential nature of flotillins in the endocytosis of a specific set of cargo that is endocytosed by means of the said pathway has not been shown, we feel that “flotillin assisted endocytosis” is currently more adequate.
7. Conclusions: Flotillins in Membrane Trafficking–Getting the Bigger Picture
The above findings show that although flotillins undoubtedly regulate various membrane trafficking events of numerous cargo proteins, the exact step that involves flotillins needs to be carefully dissected to avoid wrong conclusions. In Figure 2, we have summarized the various trafficking steps in which flotillins have been suggested to play a functional role. To define and substantiate the molecular details of an endocytic pathway that depends on flotillins, further evidence needs to be gathered. For example, several studies showed that flotillins colocalize with their putative cargo, e.g., CD59, in early endosomes. Strikingly, most cargo molecules, irrespective of their internalization route, merge in early or sorting endosomes, and therefore, a colocalization in early endosomes does not provide a final proof that the two proteins arrived via the same endocytic uptake route. To visualize that two specific proteins take the same internalization pathway, photo-activatable tags in combination with live imaging should be used. On the other hand, such analysis is always based on ectopic expression of tagged proteins, which may not be identical with the endogenous ones. As already indicated by Glebov et al., isolation of flotillin positive endocytic vesicles with a consecutive mass spectrometric analysis would help characterize the nature of these vesicles and facilitate the identification of structural as well as accessory proteins. The isolation of flotillin positive vesicles could be done in two ways, in order to distinguish between flotillin assisted endocytosis and endocytosis of flotillins as cargo: (1) As described by Glebov et al. using the fluid phase uptake of ferrofluid to analyze vesicles generated by steady-state uptake; and (2) using ferrofluid coupled to EGF to analyze the growth factor induced translocation of flotillins and the respective carrier vesicle composition. To address the question whether flotillins serve as structural components of endocytosis, it would be necessary to not only observe flotillin containing regions with electron microscopy but also to understand the protein structure of flotillins and to define how flotillins might induce invaginations and buds at the plasma membrane.

Figure 2.
Function of flotillins in cellular cargo sorting processes. Flotillin microdomains have been described at the plasma membrane, in early, late and recycling endosomes as well as in exosomes. At the plasma membrane, flotillins assist transmembrane cargo proteins during cluster formation prior to endocytosis. In endosomes, flotillins appear to be involved in cargo sorting towards recycling to the plasma membrane, retrograde transport to the Golgi and ER or to intraluminal vesicles of multivesicular bodies, which then fuse with the plasma membrane and release the internal vesicles as exosomes.
In the future, another important issue will be to dissect the details of flotillin function in endosomal sorting. This will require the identification of cargo molecules—proteins as well as lipids and toxins—that are sorted within the endosomal system by means of flotillin microdomains. Some of the potential cargos have been identified by us and others [67,68,69], but the next challenge will be to identify the accessory proteins and adaptors involved in flotillin mediated sorting. So far, none have been characterized for flotillin assisted endocytic pathways, whereas our findings strongly suggest that the adaptors of the GGA family might be involved in endosomal cargo sorting by flotillins [69]. It will also be important to dissect how flotillins interact with the cargo and the accessory proteins/coats during their sorting function.
Acknowledgments
The research in our laboratory is supported by the state of Hessia (LOEWE Research Focus “Non-neuronal cholinergic Systems”), by the von Behring-Röntgen Foundation (Grant 59-0012), by the Deutsche Forschungsgemeinschaft (DFG, grant Ti291/6-2) and by the Rare Trait Hope Fund to RT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors Contributions
Melanie Meister and Ritva Tikkanen wrote the paper, Melanie Meister made the figures. Both authors read and approved with the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Brown, D.; Waneck, G.L. Glycosyl-phosphatidylinositol-anchored membrane proteins. J. Am. Soc. Nephrol. 1992, 3, 895–906. [Google Scholar]
- Fra, A.M.; Williamson, E.; Simons, K.; Parton, R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994, 269, 30745–30748. [Google Scholar]
- Sargiacomo, M.; Sudol, M.; Tang, Z.; Lisanti, M.P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J. Cell Biol. 1993, 122, 789–807. [Google Scholar] [CrossRef]
- Resh, M.D. Myristylation and palmitylation of Src family members: The fats of the matter. Cell 1994, 76, 411–413. [Google Scholar] [CrossRef]
- Casey, P.J. Protein lipidation in cell signaling. Science 1995, 268, 221–225. [Google Scholar]
- Levental, I.; Grzybek, M.; Simons, K. Greasing their way: Lipid modifications determine protein association with membrane rafts. Biochemistry 2010, 49, 6305–6316. [Google Scholar] [CrossRef]
- Dietrich, C.; Volovyk, Z.N.; Levi, M.; Thompson, N.L.; Jacobson, K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc. Natl. Acad. Sci. USA 2001, 98, 10642–10647. [Google Scholar]
- Neumann-Giesen, C.; Falkenbach, B.; Beicht, P.; Claasen, S.; Luers, G.; Stuermer, C.A.; Herzog, V.; Tikkanen, R. Membrane and raft association of reggie-1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 2004, 378, 509–518. [Google Scholar] [CrossRef]
- Babuke, T.; Ruonala, M.; Meister, M.; Amaddii, M.; Genzler, C.; Esposito, A.; Tikkanen, R. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 2009, 21, 1287–1297. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Porat-Shliom, N.; Cohen, L.A. Clathrin-independent endocytosis: A unique platform for cell signaling and PM remodeling. Cell Signal. 2009, 21, 1–6. [Google Scholar]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Sandvig, K.; Pust, S.; Skotland, T.; van Deurs, B. Clathrin-independent endocytosis: Mechanisms and function. Curr. Opin. Cell Biol. 2011, 23, 413–420. [Google Scholar] [CrossRef]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef]
- Henley, J.R.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998, 141, 85–99. [Google Scholar] [CrossRef]
- Oh, P.; McIntosh, D.P.; Schnitzer, J.E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998, 141, 101–114. [Google Scholar] [CrossRef]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef]
- Frick, M.; Bright, N.A.; Riento, K.; Bray, A.; Merrified, C.; Nichols, B.J. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr. Biol. 2007, 17, 1151–1156. [Google Scholar] [CrossRef]
- Meister, M.; Zuk, A.; Tikkanen, R. Role of dynamin and clathrin in the cellular trafficking of flotillins. FEBS J. 2014, 281, 2956–2976. [Google Scholar] [CrossRef]
- Otto, G.P.; Nichols, B.J. The roles of flotillin microdomains—Endocytosis and beyond. J. Cell Sci. 2012, 124, 3933–3940. [Google Scholar] [CrossRef]
- Morrow, I.C.; Rea, S.; Martin, S.; Prior, I.A.; Prohaska, R.; Hancock, J.F.; James, D.E.; Parton, R.G. Flotillin-1/reggie-2 traffics to surface raft domains via a novel golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J. Biol. Chem. 2002, 277, 48834–48841. [Google Scholar] [CrossRef]
- Li, Y.; Martin, B.R.; Cravatt, B.F.; Hofmann, S.L. DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. J. Biol. Chem. 2012, 287, 523–530. [Google Scholar] [CrossRef]
- Solis, G.P.; Hoegg, M.; Munderloh, C.; Schrock, Y.; Malaga-Trillo, E.; Rivera-Milla, E.; Stuermer, C.A. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. BioChem. J. 2007, 403, 313–322. [Google Scholar] [CrossRef]
- Strauss, K.; Goebel, C.; Runz, H.; Mobius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar]
- Bickel, P.E.; Scherer, P.E.; Schnitzer, J.E.; Oh, P.; Lisanti, M.P.; Lodish, H.F. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 1997, 272, 13793–13802. [Google Scholar]
- Volonte, D.; Galbiati, F.; Li, S.; Nishiyama, K.; Okamoto, T.; Lisanti, M.P. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 1999, 274, 12702–12709. [Google Scholar]
- Fernow, I.; Icking, A.; Tikkanen, R. Reggie-1 and reggie-2 localize in non-caveolar rafts in epithelial cells: Cellular localization is not dependent on the expression of caveolin proteins. Eur. J. Cell Biol. 2007, 86, 345–352. [Google Scholar] [CrossRef]
- Liu, J.; Deyoung, S.M.; Zhang, M.; Dold, L.H.; Saltiel, A.R. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 16125–16134. [Google Scholar] [CrossRef]
- Neumann-Giesen, C.; Fernow, I.; Amaddii, M.; Tikkanen, R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 2007, 120, 395–406. [Google Scholar] [CrossRef]
- Riento, K.; Frick, M.; Schafer, I.; Nichols, B.J. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 2009, 122, 912–918. [Google Scholar]
- Kurrle, N.; John, B.; Meister, M.; Tikkanen, R. Function of Flotillins in Receptor Tyrosine Kinase Signaling and Endocytosis: Role of Tyrosine Phosphorylation and Oligomerization. In Protein Phosphorylation in Human Health; Huang, C., Ed.; InTech Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Edgar, A.J.; Polak, J.M. Flotillin-1: Gene structure: CDNA cloning from human lung and the identification of alternative polyadenylation signals. Int. J. BioChem. Cell Biol. 2001, 33, 53–64. [Google Scholar]
- Rivera-Milla, E.; Stuermer, C.A.; Malaga-Trillo, E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: Convergent evolution of the SPFH domain. Cell Mol. Life Sci. 2006, 63, 343–357. [Google Scholar]
- Amaddii, M.; Meister, M.; Banning, A.; Tomasovic, A.; Mooz, J.; Rajalingam, K.; Tikkanen, R. Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling. J. Biol. Chem. 2012, 287, 7265–7278. [Google Scholar]
- Banning, A.; Regenbrecht, C.R.; Tikkanen, R. Increased activity of mitogen activated protein kinase pathway in flotillin-2 knockout mouse model. Cell Signal. 2014, 26, 198–207. [Google Scholar]
- Banning, A.; Kurrle, N.; Meister, M.; Tikkanen, R. Flotillins in receptor tyrosine kinase signaling and cancer. Cells 2014, 3, 129–149. [Google Scholar]
- Meister, M.; Tomasovic, A.; Banning, A.; Tikkanen, R. Mitogen-Activated Protein (MAP) Kinase Scaffolding Proteins: A Recount. Int. J. Mol. Sci. 2013, 14, 4854–4884. [Google Scholar] [CrossRef]
- Stuermer, C.A.; Lang, D.M.; Kirsch, F.; Wiechers, M.; Deininger, S.O.; Plattner, H. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol. Biol. Cell 2001, 12, 3031–3045. [Google Scholar] [CrossRef]
- Solis, G.P.; Hulsbusch, N.; Radon, Y.; Katanaev, V.L.; Plattner, H.; Stuermer, C.A. Reggies/flotillins interact with Rab11a and SNX4 at the tubulovesicular recycling compartment and function in transferrin receptor and E-cadherin trafficking. Mol. Biol. Cell 2013, 24, 2689–2702. [Google Scholar]
- Gagescu, R.; Demaurex, N.; Parton, R.G.; Hunziker, W.; Huber, L.A.; Gruenberg, J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell 2000, 11, 2775–2791. [Google Scholar]
- de Gassart, A.; Geminard, C.; Fevrier, B.; Raposo, G.; Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 2003, 102, 4336–4344. [Google Scholar] [CrossRef]
- Dermine, J.F.; Duclos, S.; Garin, J.; St-Louis, F.; Rea, S.; Parton, R.G.; Desjardins, M. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 2001, 276, 18507–18512. [Google Scholar]
- Phuyal, S.; Hessvik, N.P.; Skotland, T.; Sandvig, K.; Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014, 281, 2214–2227. [Google Scholar]
- Tomasovic, A.; Traub, S.; Tikkanen, R. Molecular networks in FGF signaling: Flotillin-1 and cbl-associated protein compete for the binding to fibroblast growth factor receptor substrate 2. PLoS ONE 2012, 7, e29739. [Google Scholar] [CrossRef]
- Vercauteren, D.; Piest, M.; van der Aa, L.J.; Al Soraj, M.; Jones, A.T.; Engbersen, J.F.; de Smedt, S.C.; Braeckmans, K. Flotillin-dependent endocytosis and a phagocytosis-like mechanism for cellular internalization of disulfide-based poly(amido amine)/DNA polyplexes. Biomaterials 2011, 32, 3072–3084. [Google Scholar]
- Payne, C.K.; Jones, S.A.; Chen, C.; Zhuang, X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic 2007, 8, 389–401. [Google Scholar]
- Ge, L.; Qi, W.; Wang, L.J.; Miao, H.H.; Qu, Y.X.; Li, B.L.; Song, B.L. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 551–556. [Google Scholar]
- Ford, M.G.; Pearse, B.M.; Higgins, M.K.; Vallis, Y.; Owen, D.J.; Gibson, A.; Hopkins, C.R.; Evans, P.R.; McMahon, H.T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 2001, 291, 1051–1055. [Google Scholar] [CrossRef]
- Al Soraj, M.; He, L.; Peynshaert, K.; Cousaert, J.; Vercauteren, D.; Braeckmans, K.; de Smedt, S.C.; Jones, A.T. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J. Control. Release 2012, 161, 132–141. [Google Scholar]
- Hansen, G.H.; Dalskov, S.M.; Rasmussen, C.R.; Immerdal, L.; Niels-Christiansen, L.L.; Danielsen, E.M. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 2005, 44, 873–882. [Google Scholar]
- Nichols, B.J. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr. Biol. 2003, 13, 686–690. [Google Scholar]
- Kusumi, A.; Koyama-Honda, I.; Suzuki, K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 2004, 5, 213–230. [Google Scholar] [CrossRef]
- Brameshuber, M.; Weghuber, J.; Ruprecht, V.; Gombos, I.; Horvath, I.; Vigh, L.; Eckerstorfer, P.; Kiss, E.; Stockinger, H.; Schutz, G.J. Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J. Biol. Chem. 2010, 285, 41765–41771. [Google Scholar] [CrossRef]
- Schneider, A.; Rajendran, L.; Honsho, M.; Gralle, M.; Donnert, G.; Wouters, F.; Hell, S.W.; Simons, M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. NeuroSci. 2008, 28, 2874–2882. [Google Scholar]
- Cremona, M.L.; Matthies, H.J.; Pau, K.; Bowton, E.; Speed, N.; Lute, B.J.; Anderson, M.; Sen, N.; Robertson, S.D.; Vaughan, R.A.; et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. NeuroSci. 2011, 14, 469–477. [Google Scholar] [CrossRef]
- Gorgens, A.; Beckmann, J.; Ludwig, A.K.; Mollmann, M.; Durig, J.; Horn, P.A.; Rajendran, L.; Giebel, B. Lipid raft redistribution and morphological cell polarization are separable processes providing a basis for hematopoietic stem and progenitor cell migration. Int. J. BioChem. Cell Biol. 2012, 44, 1121–1132. [Google Scholar] [CrossRef]
- Rajendran, L.; Beckmann, J.; Magenau, A.; Boneberg, E.M.; Gaus, K.; Viola, A.; Giebel, B.; Illges, H. Flotillins are involved in the polarization of primitive and mature hematopoietic cells. PLoS ONE 2009, 4, e8290. [Google Scholar]
- Rajendran, L.; Masilamani, M.; Solomon, S.; Tikkanen, R.; Stuermer, C.A.; Plattner, H.; Illges, H. Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 8241–8246. [Google Scholar]
- Langhorst, M.F.; Reuter, A.; Luxenhofer, G.; Boneberg, E.M.; Legler, D.F.; Plattner, H.; Stuermer, C.A. Preformed reggie/flotillin caps: Stable priming platforms for macrodomain assembly in T cells. FASEB J. 2006, 20, 711–713. [Google Scholar]
- Affentranger, S.; Martinelli, S.; Hahn, J.; Rossy, J.; Niggli, V. Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes. BMC Cell Biol. 2011, 12, 28. [Google Scholar] [CrossRef]
- Rossy, J.; Schlicht, D.; Engelhardt, B.; Niggli, V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS ONE 2009, 4, e5403. [Google Scholar]
- Ludwig, A.; Otto, G.P.; Riento, K.; Hams, E.; Fallon, P.G.; Nichols, B.J. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J. Cell Biol. 2010, 191, 771–781. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar]
- Saslowsky, D.E.; Cho, J.A.; Chinnapen, H.; Massol, R.H.; Chinnapen, D.J.; Wagner, J.S.; de Luca, H.E.; Kam, W.; Paw, B.H.; Lencer, W.I. Intoxication of zebrafish and mammalian cells by cholera toxin depends on the flotillin/reggie proteins but not Derlin-1 or -2. J. Clin. Invest. 2010, 120, 4399–4409. [Google Scholar] [CrossRef]
- Pust, S.; Dyve, A.B.; Torgersen, M.L.; van Deurs, B.; Sandvig, K. Interplay between toxin transport and flotillin localization. PLoS ONE 2010, 5, e8844. [Google Scholar]
- John, B.A.; Meister, M.; Banning, A.; Tikkanen, R. Flotillins bind to the dileucine sorting motif of beta-site amyloid precursor protein-cleaving enzyme 1 and influence its endosomal sorting. FEBS J. 2014, 281, 2074–2087. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Staubach, S.; Razawi, H.; Hanisch, F.G. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 2009, 9, 2820–2835. [Google Scholar]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; Zimmermann, P.; David, G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell. Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- He, X.; Chang, W.P.; Koelsch, G.; Tang, J. Memapsin 2 (beta-secretase) cytosolic domain binds to the VHS domains of GGA1 and GGA2: Implications on the endocytosis mechanism of memapsin 2. FEBS Lett. 2002, 524, 183–187. [Google Scholar] [CrossRef]
- Pastorino, L.; Ikin, A.F.; Nairn, A.C.; Pursnani, A.; Buxbaum, J.D. The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total A(beta). Mol. Cell NeuroSci. 2002, 19, 175–185. [Google Scholar]
- Walter, J.; Fluhrer, R.; Hartung, B.; Willem, M.; Kaether, C.; Capell, A.; Lammich, S.; Multhaup, G.; Haass, C. Phosphorylation regulates intracellular trafficking of beta-secretase. J. Biol. Chem. 2001, 276, 14634–14641. [Google Scholar] [CrossRef]
- Kang, E.L.; Cameron, A.N.; Piazza, F.; Walker, K.R.; Tesco, G. Ubiquitin regulates GGA3-mediated degradation of BACE1. J. Biol. Chem. 2010, 285, 24108–24119. [Google Scholar]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef]
- Solis, G.P.; Schrock, Y.; Hulsbusch, N.; Wiechers, M.; Plattner, H.; Stuermer, C.A. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol. Biol. Cell 2012, 23, 1812–1825. [Google Scholar]
- Sorkina, T.; Caltagarone, J.; Sorkin, A. Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis. Traffic 2013, 14, 709–724. [Google Scholar]
- Koo, E.H.; Squazzo, S.L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 1994, 269, 17386–17389. [Google Scholar]
- Koo, E.H.; Squazzo, S.L.; Selkoe, D.J.; Koo, C.H. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J. Cell Sci. 1996, 109, 991–998. [Google Scholar]
- Perez, R.G.; Soriano, S.; Hayes, J.D.; Ostaszewski, B.; Xia, W.; Selkoe, D.J.; Chen, X.; Stokin, G.B.; Koo, E.H. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J. Biol. Chem. 1999, 274, 18851–18856. [Google Scholar] [CrossRef]
- Sorkina, T.; Hoover, B.R.; Zahniser, N.R.; Sorkin, A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 2005, 6, 157–170. [Google Scholar] [CrossRef]
- Sigismund, S.; Argenzio, E.; Tosoni, D.; Cavallaro, E.; Polo, S.; di Fiore, P.P. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell 2008, 15, 209–219. [Google Scholar]
- Sigismund, S.; Woelk, T.; Puri, C.; Maspero, E.; Tacchetti, C.; Transidico, P.; di Fiore, P.P.; Polo, S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 2005, 102, 2760–2765. [Google Scholar] [CrossRef]
- Puri, C.; Tosoni, D.; Comai, R.; Rabellino, A.; Segat, D.; Caneva, F.; Luzzi, P.; di Fiore, P.P.; Tacchetti, C. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell 2005, 16, 2704–2718. [Google Scholar] [CrossRef]
- Roitbak, T.; Ward, C.J.; Harris, P.C.; Bacallao, R.; Ness, S.A.; Wandinger-Ness, A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol. Biol. Cell 2004, 15, 1334–1346. [Google Scholar]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef]
- Abrami, L.; Bischofberger, M.; Kunz, B.; Groux, R.; van der Goot, F.G. Endocytosis of the anthraxtoxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 2010, 6, e1000792. [Google Scholar] [CrossRef]
- Abrami, L.; Kunz, B.; van der Goot, F.G. Anthrax toxin triggers the activation of src-like kinases to mediate its own uptake. Proc. Natl. Acad. Sci. USA 2010, 107, 1420–1424. [Google Scholar] [CrossRef]
- Abrami, L.; Liu, S.; Cosson, P.; Leppla, S.H.; van der Goot, F.G. Anthrax toxin triggers endocytosisof its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 2003, 160, 321–328. [Google Scholar] [CrossRef]
- Deinhardt, K.; Berninghausen, O.; Willison, H.J.; Hopkins, C.R.; Schiavo, G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J. Cell Biol. 2006, 174, 459–471. [Google Scholar] [CrossRef]
- Van Dam, E.M.; Stoorvogel, W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 2002, 13, 169–182. [Google Scholar] [CrossRef]
- Van Dam, E.M.; Ten Broeke, T.; Jansen, K.; Spijkers, P.; Stoorvogel, W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 2002, 277, 48876–48883. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).