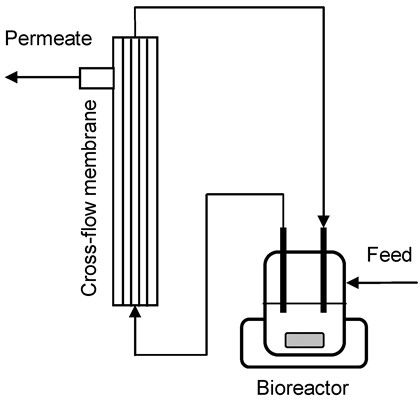
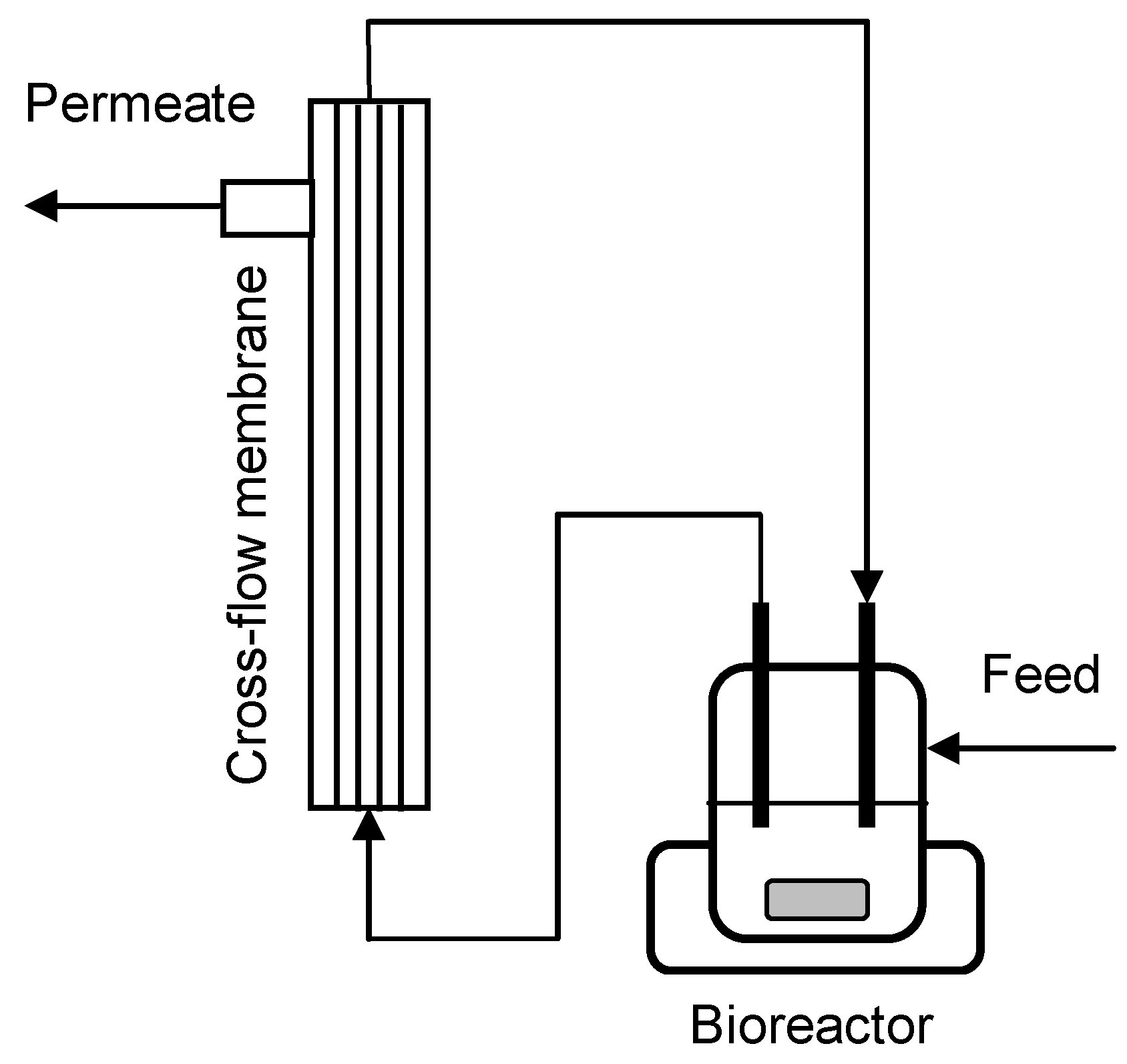
Continuous Ethanol Production with a Membrane Bioreactor at High Acetic Acid Concentrations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yeast Cell Dry Weight during Continuous Cultivations

2.2. Continuous Alcohol Fermentation
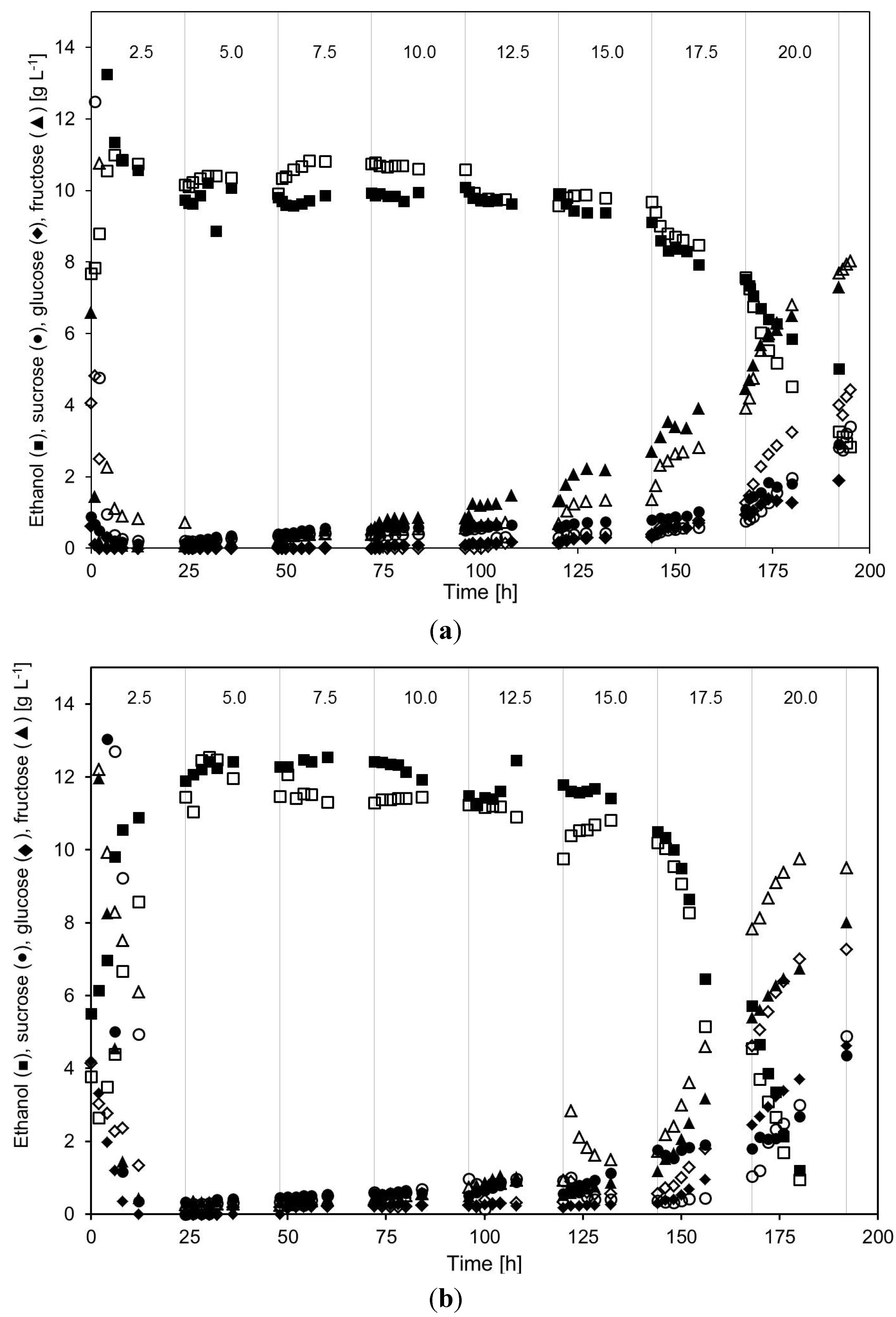
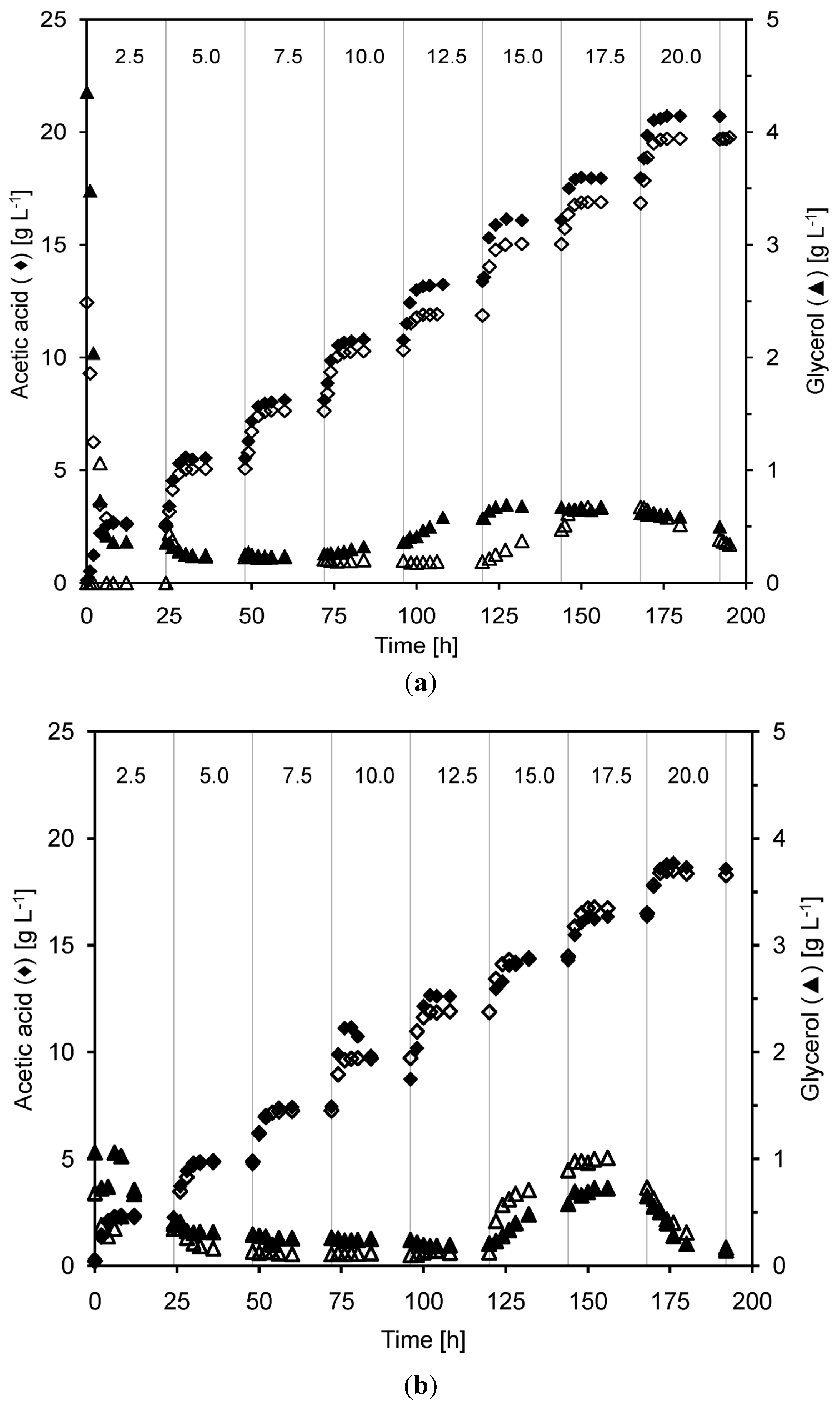
2.3. Product Yields and Ethanol Productivity
| Acetic Acid conc. (g·L−1) | Ethanol (g·L−1) | Sugar a (g·L−1) | Glycerol (g·L−1) | Biomass (g·L−1) | Yp/s (g·g−1) | Ygly/s (g·g−1) | rp (g·L−1·h−1) | qp (g·g−1·h−1) | η (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Undissociated at pH 5 | Cultivation A | ||||||||
| 2.6 | 0.9 | 9.73 | 0.20 | 0.35 | 190.6 | 0.39 | 0.016 | 4.87 | 0.026 | 99.2 |
| 5.5 | 2.0 | 9.81 | 0.62 | 0.26 | 196.6 | 0.40 | 0.012 | 4.91 | 0.025 | 97.6 |
| 8.1 | 3.0 | 9.94 | 1.04 | 0.26 | 183.2 | 0.42 | 0.012 | 4.97 | 0.027 | 95.8 |
| 10.8 | 3.9 | 10.08 | 1.49 | 0.36 | 173.2 | 0.43 | 0.017 | 5.04 | 0.029 | 94.0 |
| 13.4 | 4.9 | 9.91 | 2.04 | 0.57 | 165.2 | 0.44 | 0.028 | 4.96 | 0.030 | 91.8 |
| 16.1 | 5.9 | 9.12 | 3.88 | 0.67 | 156.5 | 0.43 | 0.033 | 4.56 | 0.029 | 84.6 |
| 18.0 | 6.6 | 7.53 | 6.49 | 0.62 | 149.0 | 0.41 | 0.034 | 3.77 | 0.025 | 73.7 |
| 20.7 | 7.6 | 5.01 | 12.09 | 0.50 | 141.6 | 0.38 | 0.032 | 2.51 | 0.018 | 52.2 |
| Total | Undissociated at pH 5 | Cultivation B | ||||||||
| 2.5 | 0.9 | 10.15 | 0.94 | 0.45 | 150.0 | 0.43 | 0.020 | 5.08 | 0.034 | 96.2 |
| 5.1 | 1.9 | 9.91 | 0.55 | 0.23 | 151.9 | 0.40 | 0.010 | 4.96 | 0.033 | 97.8 |
| 7.6 | 2.8 | 10.74 | 0.76 | 0.21 | 142.3 | 0.44 | 0.010 | 5.37 | 0.038 | 97.0 |
| 10.3 | 3.8 | 10.58 | 1.08 | 0.20 | 143.3 | 0.43 | 0.009 | 5.29 | 0.037 | 95.7 |
| 11.9 | 4.3 | 9.76 | 1.15 | 0.19 | 139.0 | 0.43 | 0.009 | 4.88 | 0.035 | 95.1 |
| 15.0 | 5.5 | 9.68 | 2.04 | 0.47 | 137.3 | 0.42 | 0.022 | 4.84 | 0.035 | 91.9 |
| 16.8 | 6.1 | 7.57 | 5.94 | 0.67 | 128.6 | 0.39 | 0.036 | 3.79 | 0.029 | 76.7 |
| 19.7 | 7.2 | 3.27 | 14.51 | 0.39 | 118.6 | 0.31 | 0.035 | 1.64 | 0.014 | 42.1 |
| Acetic Acid conc. (g·L−1) | Ethanol (g·L−1) | Sugar a (g·L−1) | Glycerol (g·L−1) | Biomass (g·L−1) | Yp/s (g·g−1) | Ygly/s (g·g−1) | rp (g·L−1·h−1) | qp (g·g−1·h−1) | η (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Undissociated at pH 5 | Cultivation A | ||||||||
| 2.3 | 0.9 | 10.89 | 0.65 | 0.39 | 14.3 | 0.43 | 0.015 | 5.45 | 0.381 | 0.98 |
| 4.8 | 2.0 | 12.32 | 0.79 | 0.29 | 20.4 | 0.48 | 0.011 | 6.16 | 0.302 | 0.97 |
| 7.4 | 3.0 | 12.29 | 1.25 | 0.26 | 22.3 | 0.49 | 0.010 | 6.15 | 0.276 | 0.95 |
| 10.8 | 3.9 | 11.93 | 1.23 | 0.24 | 23.3 | 0.48 | 0.010 | 5.96 | 0.256 | 0.95 |
| 12.6 | 4.9 | 11.62 | 1.27 | 0.21 | 23.1 | 0.51 | 0.009 | 5.81 | 0.252 | 0.95 |
| 14.3 | 5.9 | 11.43 | 3.25 | 0.58 | 23.3 | 0.50 | 0.025 | 5.71 | 0.246 | 0.88 |
| 16.4 | 6.6 | 6.46 | 9.65 | 0.65 | 21.9 | 0.39 | 0.039 | 3.23 | 0.148 | 0.63 |
| 18.9 | 7.6 | 1.19 | 17.00 | 0.14 | 20.8 | 0.13 | 0.015 | 0.60 | 0.029 | 0.35 |
| Total | Undissociated at pH 5 | Cultivation B | ||||||||
| 2.3 | 0.9 | 8.58 | 0.27 | 0.35 | 11.9 | 0.35 | 0.014 | 4.29 | 0.360 | 0.99 |
| 4.9 | 1.9 | 11.98 | 0.76 | 0.13 | 17.1 | 0.50 | 0.005 | 5.99 | 0.350 | 0.97 |
| 7.3 | 2.8 | 11.31 | 1.23 | 0.11 | 22.0 | 0.48 | 0.005 | 5.66 | 0.257 | 0.95 |
| 9.7 | 3.8 | 11.45 | 1.95 | 0.10 | 24.0 | 0.50 | 0.004 | 5.73 | 0.239 | 0.92 |
| 11.9 | 4.3 | 10.90 | 2.19 | 0.12 | 21.6 | 0.48 | 0.005 | 5.45 | 0.252 | 0.91 |
| 14.5 | 5.5 | 10.82 | 2.66 | 0.89 | 23.6 | 0.49 | 0.040 | 5.41 | 0.229 | 0.89 |
| 16.5 | 6.1 | 5.15 | 13.51 | 0.73 | 27.0 | 0.45 | 0.064 | 2.58 | 0.095 | 0.46 |
| 18.3 | 7.2 | 0.95 | 21.68 | 0.17 | 18.3 | 0.29 | 0.053 | 0.48 | 0.026 | 0.13 |
2.4. Applicability of MBR for 2nd Generation Lignocellulosic Bioethanol Production
3. Materials and Methods
3.1. Microorganism and Medium
3.2. Dry Cell Weight Determination
3.3. Sugar and Extracellular Metabolite Analysis
3.4. Cross-Flow Membrane Unit
3.5. MBR Cultivations
4. Conclusions
- (1)
- In relation to ethanol production, no major differences could be seen in the acetic acid tolerance of the yeast cultivated either at very high or high yeast concentrations. In the employed MBR system, both very high and high cell density yeast cultivations were able to withstand total acetic acid concentrations of up to 15–16 g·L−1 at pH 5.0 without any major impact on the ethanol production. However, at even higher acetic acid concentrations, the ethanol production started to decrease.
- (2)
- The cross-flow membrane showed a very stable permeability even at high yeast cell densities and the rapid dilution rate of 0.5 h−1, corresponding to a permeate outflow of 1.5 L·m−2·h−1 during 196 h. No particular cleaning of the module was required during the cultivation.
- (3)
- Because the cultivations could be performed at a high dilution rate of 0.5 h−1 a high volumetric ethanol productivity of 5.04–6.16 g·L−1·h−1 could be reached at very high yeast concentrations even when only 25 g·L−1 sucrose medium was fed to the MBR. This indicates that the MBR system can be used to reach high ethanol productivity, which is crucial for successful bioethanol production from lignocelluloses.
- (4)
- Very high yeast cell densities, up to 200 g dry weight L−1, could be maintained in the MBR.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, W.H.; Chimphango, A.F.A.; den Haan, R.; Görgens, J.F.; Chirwa, P.W.C. Next-generation cellulosic ethanol technologies and their contribution to a sustainable Africa. Interface Focus 2011, 1, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Ghazoul, J. Biofuels, biodiversity, and people: Understanding the conflicts and finding opportunities. Biol. Conserv. 2008, 141, 2450–2460. [Google Scholar] [CrossRef]
- Abreu-Cavalheiro, A.; Monteiro, G. Solving ethanol production problems with genetically modified yeast strains. Braz. J. Microbiol. 2014, 44, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Grohman, K.; Torget, R.; Himmem, M. Optimization of dilute acid pretreatment of biomass. Biotechnol. Bioeng. Symp. 1985, 15, 59–80. [Google Scholar]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Eklund, R.; Gustafsson, L.; Niklasson, C.; Lidén, G. Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind. Eng. Chem. Res. 1997, 36, 4659–4665. [Google Scholar] [CrossRef]
- Van Zyl, C.; Prior, B.A.; du Preez, J.C. Acetic acid inhibition of d-xylose fermentation by Pichia stipitis. Enzyme Microb. Technol. 1991, 13, 82–86. [Google Scholar] [CrossRef]
- Chen, X.; Shekiro, J.; Elander, R.; Tucker, M. Improved Xylan Hydrolysis of Corn Stover by Deacetylation with High Solids Dilute Acid Pretreatment. Ind. Eng. Chem. Res. 2011, 51, 70–76. [Google Scholar]
- Moreno, A.D.; Tomás-Pejó, E.; Ibarra, D.; Ballesteros, M.; Olsson, L. Fed-batch SSCF using steam-exploded wheat straw at high dry matter consistencies and a xylose-fermenting Saccharomyces cerevisiae strain: Effect of laccase supplementation. Biotechnol. Biofuels 2013, 160, 1–10. [Google Scholar]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Niklasson, C.; Lidén, G. Acetic acid—Friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem Eng. Sci. 1997, 52, 2653–2659. [Google Scholar]
- Zhu, J.Y.; Gleisner, R.; Scott, C.T.; Luo, X.L.; Tian, S. High titer ethanol production from simultaneous enzymatic saccharification and fermentation of aspen at high solids: A comparison between SPORL and dilute acid pretreatments. Bioresour. Technol. 2011, 102, 8921–8929. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Zhang, J.G.; Liu, X.Y.; He, X.P.; Guo, X.N.; Lu, Y.; Zhang, B.R. Improvement of acetic acid tolerance and fermentation performance of Saccharomyces cerevisiae by disruption of the FPS1 aquaglyceroporin gene. Biotechnol. Lett. 2011, 33, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, L.; Santos, A.X.; Riezman, H.; Olsson, L.; Bettiga, M. Lipidomic Profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii Reveals Critical Changes in Lipid Composition in Response to Acetic Acid Stress. PLoS One 2013, 8, 1–12. [Google Scholar]
- Pampulha, M.E.; Loureiro-Dias, M.C. Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acids. Appl. Microbiol. Biotechnol. 1990, 34, 375–380. [Google Scholar]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl. Environ. Microbiol. 2002, 68, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.S.; Lee, Y.Y. Ethanol fermentation of crude acid hydrolyzate of cellulose using high-level yeast inocula. Biotechnol. Bioeng. 1985, 27, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Impact of furfural on rapid ethanol production using a membrane bioreactor. Energies 2013, 6, 1604–1617. [Google Scholar] [CrossRef]
- Talebnia, F.; Taherzadeh, M.J. In situ detoxification and continuous cultivation of dilute-acid hydrolyzate to ethanol by encapsulated S. cerevisiae. J. Biotechnol. 2006, 125, 377–384. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Millati, R.; Niklasson, C. Continuous cultivation of dilute-acid hydrolysates to ethanol by immobilized Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2001, 95, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Galbe, M.; Hahn-Hägerdal, B. Evaluation of cell recycling in continuous fermentation of enzymatic hydrolysates of spruce with Saccharomyces cerevisiae and on-line monitoring of glucose and ethanol. Appl. Microbiol. Biotechnol. 1998, 50, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Melzoch, K.; Rychtera, M.; Markvichov, N.; Pospíchalová, V.; Basařová, G.; Manakov, M. Application of a membrane recycle bioreactor for continuous ethanol production. Appl. Microbiol. Biotechnol. 1991, 34, 469–472. [Google Scholar] [CrossRef]
- Basso, L.C.; de Amorim, H.V.; de Oliveira, A.J.; Lopes, M.L. Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res. 2008, 8, 1155–1163. [Google Scholar] [PubMed]
- Brandberg, T.; Karimi, K.; Taherzadeh, M.J.; Franzén, C.J.; Gustafsson, L. Continuous fermentation of wheat-supplemented lignocellulose hydrolysate with different types of cell retention. Biotechnol. Bioeng. 2007, 98, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Pirt, S. Maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B Biol. Sci. 1965, 163, 224–231. [Google Scholar] [PubMed]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.L.; Botting, C.H.; Antrobus, R.; Coote, P.J. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: Regulating adaptation to citric acid stress. Mol. Cell. Biol. 2004, 24, 3307–3323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; Wang, M.; He, Q.; Zhang, H. The inhibition of Saccharomyces cerevisiae cells by acetic acid quantified by electrochemistry and fluorescence. Bioelectrochemistry 2008, 72, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Sousa, M.J.; Silva, M.T.; Leão, C.L.; Côrte-Real, M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 2001, 147, 2409–2415. [Google Scholar] [PubMed]
- Van Dijken, J.P.; Scheffers, W.A. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Lett. 1986, 32, 199–224. [Google Scholar] [CrossRef]
- Gilbert, A.; Sangurdekar, D.P.; Srienc, F. Rapid strain improvement through optimized evolution in the cytostat. Biotechnol. Bioeng. 2009, 103, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Sur, H.W.; Cui, Z. Experimental study on the enhancement of yeast microfiltration with gas sparging. J. Chem. Technol. Biotechnol. 2001, 76, 477–484. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Continuous Ethanol Production with a Membrane Bioreactor at High Acetic Acid Concentrations. Membranes 2014, 4, 372-387. https://doi.org/10.3390/membranes4030372
Ylitervo P, Franzén CJ, Taherzadeh MJ. Continuous Ethanol Production with a Membrane Bioreactor at High Acetic Acid Concentrations. Membranes. 2014; 4(3):372-387. https://doi.org/10.3390/membranes4030372
Chicago/Turabian StyleYlitervo, Päivi, Carl Johan Franzén, and Mohammad J. Taherzadeh. 2014. "Continuous Ethanol Production with a Membrane Bioreactor at High Acetic Acid Concentrations" Membranes 4, no. 3: 372-387. https://doi.org/10.3390/membranes4030372
APA StyleYlitervo, P., Franzén, C. J., & Taherzadeh, M. J. (2014). Continuous Ethanol Production with a Membrane Bioreactor at High Acetic Acid Concentrations. Membranes, 4(3), 372-387. https://doi.org/10.3390/membranes4030372