A Crown Ether-Based Covalent Organic Polymer Composite Membrane and Its Application in Molecular Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterizations
2.2. Synthesis of COPs
2.3. Fabrication of the COP-Based Composite Membrane
2.4. Dye Adsorption Tests
2.5. Filtration Performance Evaluation
3. Results and Discussion
3.1. Membrane Characterization
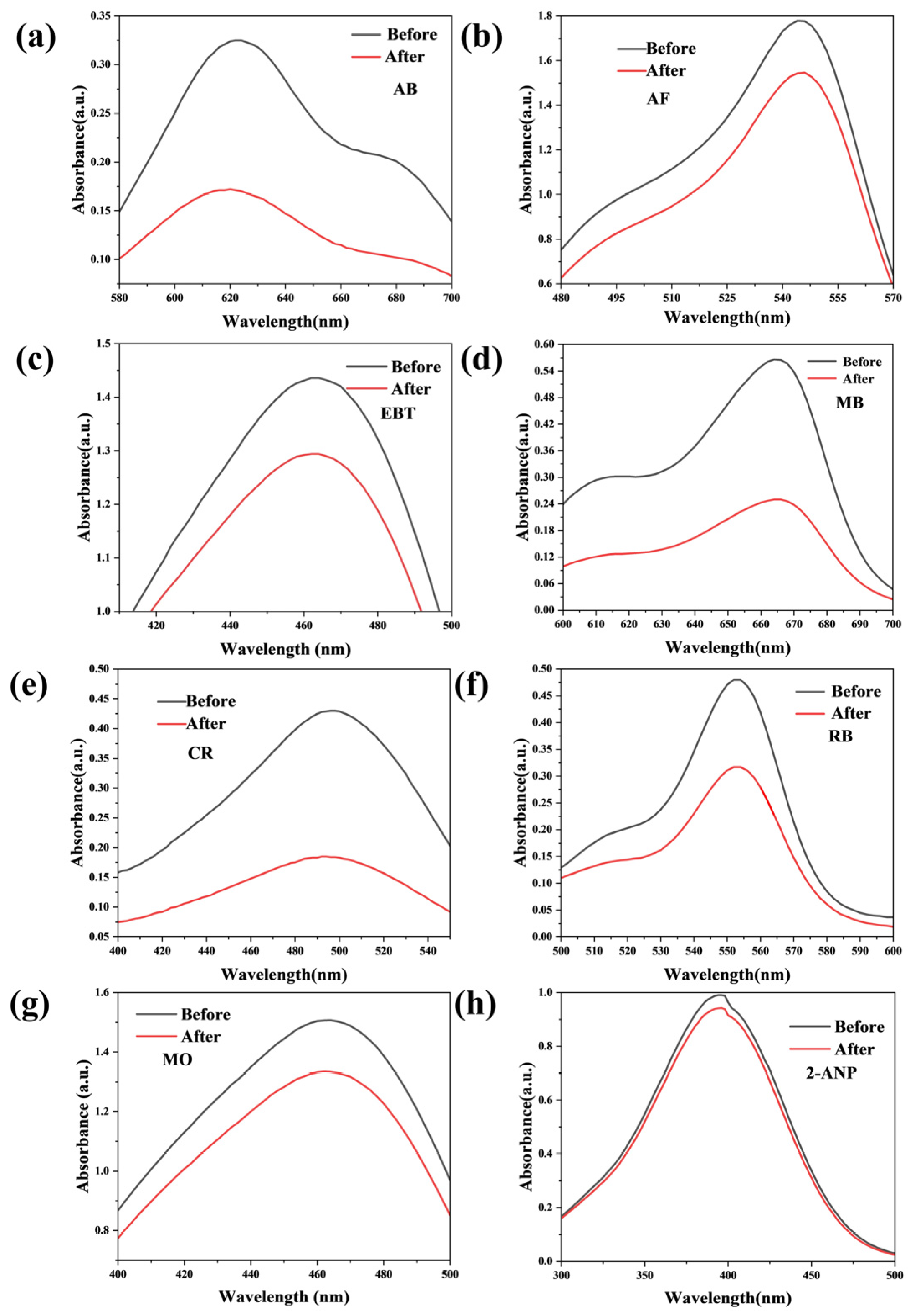
3.2. Molecular Sieving Performance of the Membranes
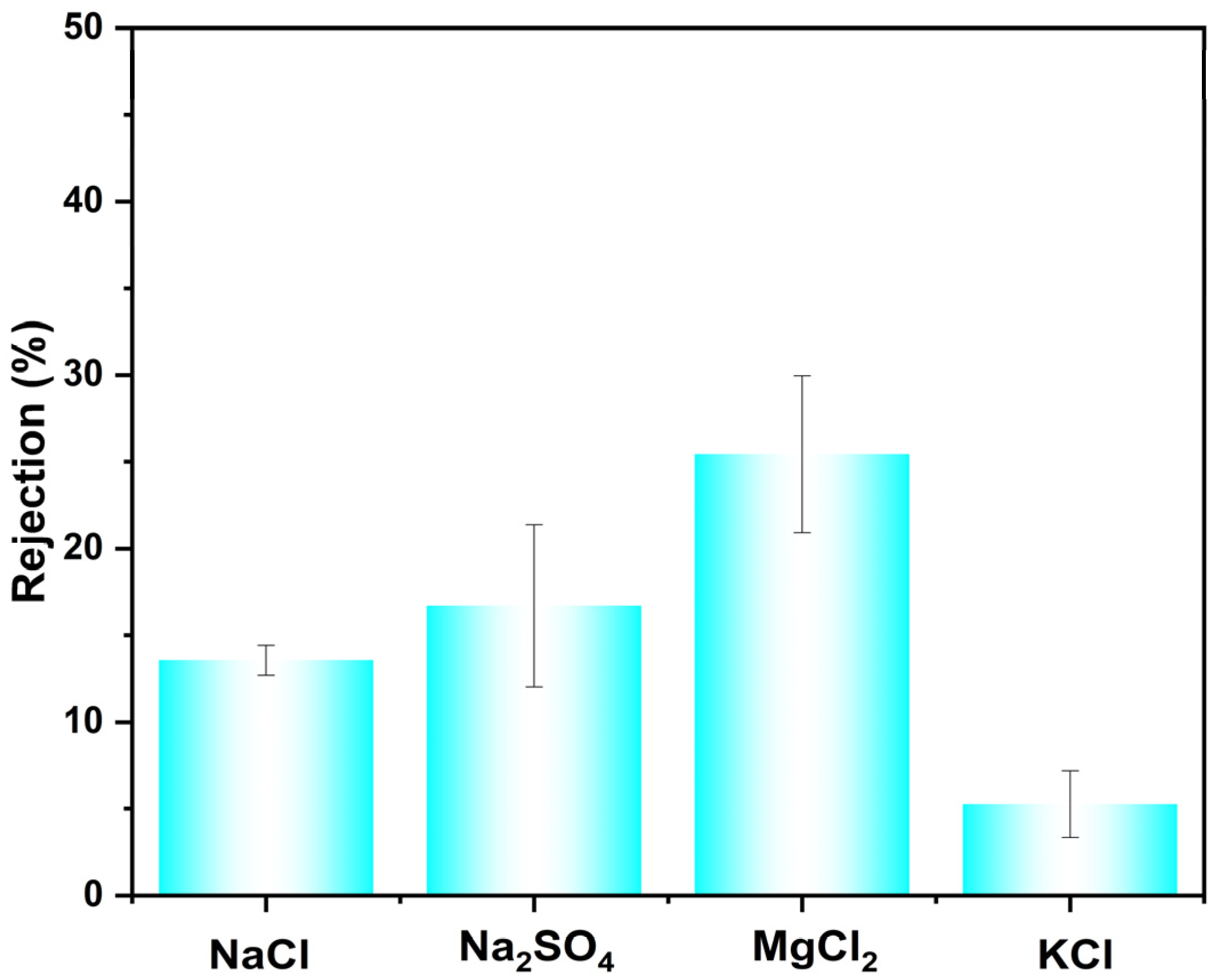
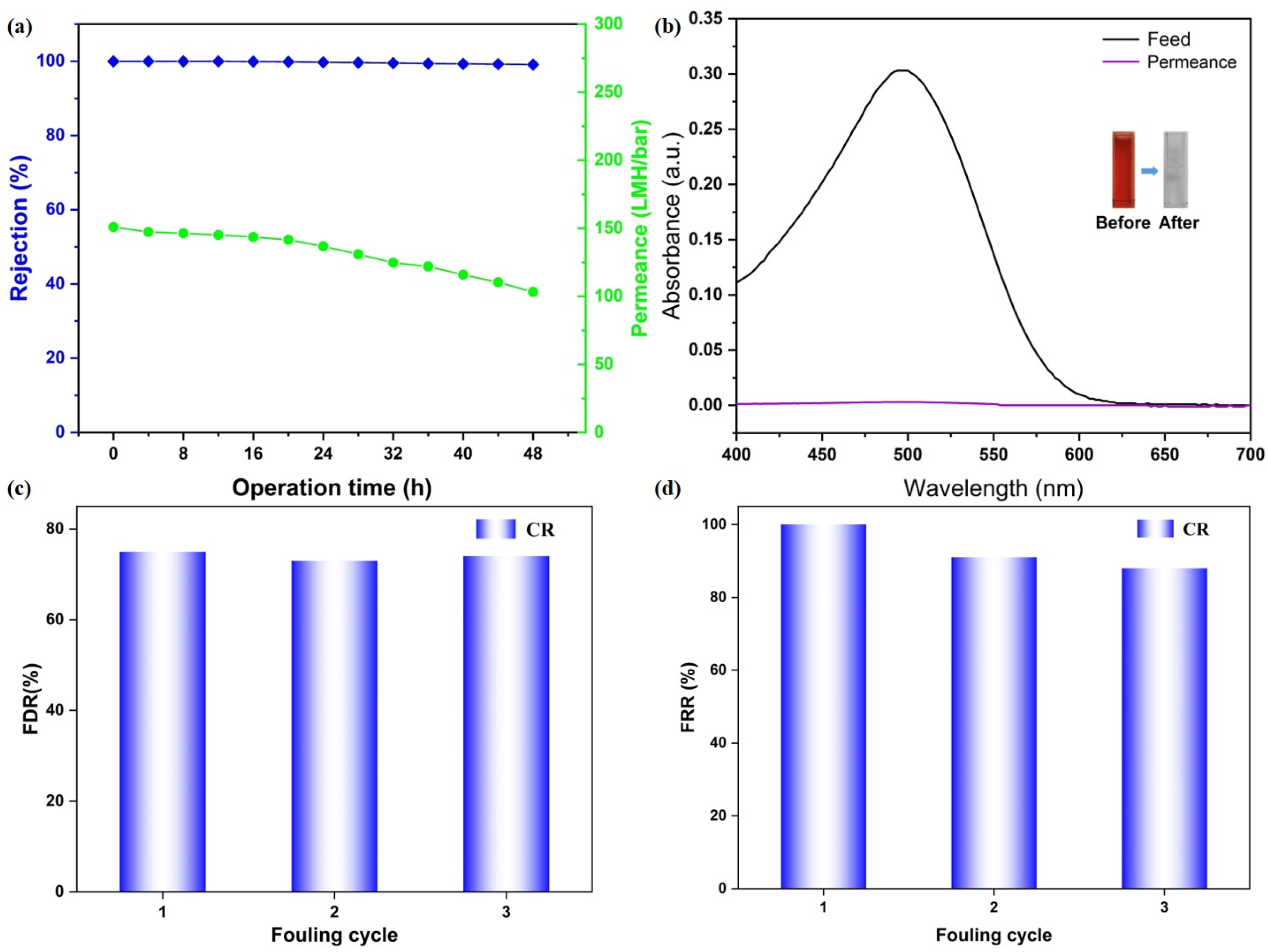
3.3. Rejection Performance Toward the Inorganic Salt and the Long-Term Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COP | Covalent organic polymer |
| PA | Polyamide |
| IP | Interfacial polymerization |
| NF | Nanofiltration |
| TFC | Thin-film composite |
| LMH/bar | L m−2 h−1 bar−1 |
| SPES | Sulfonated polyethersulfone |
| DCM | Dichloromethane |
| SEM | Scanning electron microscopy |
| MMMs | Mixed-matrix membranes |
| FT-IR | Fourier transform infrared |
| TGA | Thermogravimetric analysis |
| PXRD | Powder X-ray diffraction |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| NLDFT | Nonlocal density functional theory |
| DBC | Dibenzo-18-crown-6-ether |
| TMC | Trimesoyl chloride |
| MPD | m-Phenylenediamine |
| CR | Congo red |
| AF | Acid fuchsin |
| RhB | RhodamineB |
| MB | Methylene blue |
| EBT | Eriochrome BlackT |
| MO | Methyl orange |
| AB | Alcian blue 8GX |
| CM-DBC· | Composite membrane based on COP-DBC |
| CM-TBDBC | Composite membrane based on COP-TBDBC |
| 2-ANP | 2-Amino-5-nitrophenol |
| FRR | Flux recovery ratio |
| FDR | Flux decline ratio |
Appendix A

References
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Lau, W.-J.; Ismail, A. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–331. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Geens, J.; Vandecasteele, C. Influence of membrane morphology on the performance of sulfonated polyethersulfone (SPES) membranes. Sep. Purif. Technol. 2001, 22, 519–528. [Google Scholar] [CrossRef]
- Hong, S.U.; Bruening, M.L. Separation of amino acid mixtures using multilayer polyelectrolyte nanofiltration membranes. J. Membr. Sci. 2006, 280, 1–5. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Characterization and prediction of nanofiltration membrane performance—A general assessment. Chem. Eng. Res. Des. 1998, 76, 885–893. [Google Scholar] [CrossRef]
- Wang, X.L.; Tsuru, T.; Nakao, S.; Kimura, S. The electrostatic and steric-hindrance model for the transport of charged solutes through nanofiltration membranes. J. Membr. Sci. 1997, 135, 19–32. [Google Scholar] [CrossRef]
- Szoke, S.; Patzay, G.; Weiser, L. Characteristics of thin-film nanofiltration membranes at various pH-values. Desalination 2003, 151, 123–129. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Exploitation of intrinsic microporosity in polymer-based materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed 2017, 56, 9292–9316. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed-Matrix Membranes for Improved Molecular Separation. Adv. Mater 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Molecular Separation. Front. Chem. Sci. Eng. 2018, 12, 481–496. [Google Scholar]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 2019, 141, 1807–1822. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, M.; Strauss, I.; Wang, X.; Wang, Y.; Wang, H.; Weidler, P.G.; Gliemann, H.; Heissler, S.; Woll, C.; et al. Covalent organic framework membranes for efficient CO2/CH4 Separation. Chem. Mater. 2017, 29, 7194–7200. [Google Scholar]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabres i Xamena, F.X.; Gascon, J. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Tian, B.; Feng, Y.; Luo, C.; Wang, J.; Chen, Z.; Hu, J. A hierarchically acylhydrazone-linked COFs composite membrane for iodine interception and organic solvent forward osmosis. Sep. Purif. Technol. 2025, 378, 134662. [Google Scholar] [CrossRef]
- Babujohn, N.A.; Eluri, A.; Nabeela, V.P. One-pot synthesis of crystalline covalent organic polymers with tunable pores for the removal of gold and toxic organic pollutants. Chem. Eng. J. 2023, 464, 142459. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, R.; Chen, T.; Wang, G.; Liu, S.; Chen, Z.; Hu, J. Phenanthroline-based microporous organic materials for removal of Cu(II) from aqueous solution and reutilization of spent adsorbent as catalysts. Water Sci. Technol. 2022, 86, 496–510. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Zhang, T.; Ge, B.; Niu, Q.J.; Sun, H. Rapid preparation of extremely highly permeable covalent organic polymers nanofiltration membranes for alcohol recovery via interfacial polymerization. Adv. Membr. 2024, 4, 100107. [Google Scholar] [CrossRef]
- Zhao, B.; Luo, C.; Feng, Y.; Wang, B.; Chen, Z.; Hu, J. A pH-responsive covalent organic framework membrane based on flexible building blocks for superhigh-flux dye/salt separation. Desalination 2025, 606, 118783. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-flux membranes based on the covalent organic framework COF-LZU1 for selective dye separation by nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, Y.; Wu, J.; Wang, Y. Beyond monomer design: Multi-dimensional control of interfacial polymerization via sulfonated diamine and crown ether synergy for precision dye/Salt Separation. J. Membr. Sci. 2026, 740, 124924. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, N.; Wang, D.; Zhang, J.; Li, Y.; Wang, Z. Polyethylenimine-mica nanosheets/cellulose nanofibers stacked loose nanofiltration membrane for effective dye/salt separation. Desalination 2023, 551, 116410. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, P.; Huang, W.; Yu, S.; Liu, M.; Gao, C. High-flux composite hollow fiber nanofiltration membranes fabricated through layer-by-layer deposition of oppositely charged crosslinked polyelectrolytes for dye removal. J. Membr. Sci. 2015, 492, 312–321. [Google Scholar] [CrossRef]
- Shevate, R.; Shaffer, D.L. Large-Area 2D Covalent Organic Framework Membranes with Tunable Single-Digit Nanopores for Predictable Mass Transport. ACS Nano 2022, 16, 2407–2418. [Google Scholar] [CrossRef]
- Lu, X.; Gao, X.; Hou, X.; Chen, B.; Han, P.; Wang, T.; Meng, F.; Fu, Y. Fabrication of Prussian blue mixed matrix membranes based on the synchronous growth for water treatment. Chem. Eng. J. 2026, 528, 172346. [Google Scholar] [CrossRef]
- Hao, S.; Wen, J.; Li, S.; Wang, J.; Jia, Z. Preparation of COF-LZU1/PAN membranes by an evaporation/casting method for separation of dyes. J. Mater. Sci. 2020, 55, 14817–14828. [Google Scholar] [CrossRef]




| COP-DBC Dosage (mg) * | P (LMH/bar) ** | CR Rejection (%) | P (LMH/bar) *** | AF Rejection (%) |
|---|---|---|---|---|
| 0.5 | 254.2 | 97.6 | 580.3 | 58.2 |
| 1.0 | 184.2 | 98.8 | 547.1 | 63.9 |
| 1.5 | 154.7 | 99.5 | 536.0 | 71.8 |
| 2.0 | 143.7 | 99.9 | 375.8 | 86.9 |
| 2.5 | 132.6 | 99.9 | 193.4 | 89.3 |
| Variate | P (LMH/bar) | AF Rejection (%) | |
|---|---|---|---|
| Concentration of MPD (wt%) | 2.0 | 127.1 | 98.4 |
| 3.5 | 386.8 | 86.6 | |
| 5.0 | 690.8 | 64.2 | |
| Concentration of TMC (wt%) | 0.15 | 138.2 | 95.5 |
| 0.25 | 381.3 | 87.3 | |
| 0.35 | 165.8 | 92.3 | |
| Time of IP (s) | 4 | 652.1 | 72.0 |
| 6 | 364.7 | 87.9 | |
| 8 | 116.1 | 94.5 | |
| 10 | -- * | -- * | |
| Mixture | P (LMH/bar) | Selectivity (α) |
|---|---|---|
| CR/KCl | 138.2 | 186.8 |
| CR/NaCl | 116.1 | 128.5 |
| AF/KCl | 337.1 | 5.72 |
| AF/NaCl | 290.1 | 3.93 |
| Membranes | Water Permeance (LMH/bar) | CR Rejection (%) | KCl Rejection (%) | α | References |
|---|---|---|---|---|---|
| Tubular COF-LZU1 | 30.0 | 98.6 | 3.0 | 69.3 | [35] |
| CE18/DSDA-TMC | 132.0 | 99.3 | 1.4 | 140.9 | [36] |
| PEI-mica/CNFs | 62.2 | 98.9 | 7.1 | 84.5 | [37] |
| PEI/CMCNa/PP NF | 14.2 | 99.4 | 46.8 | 88.7 | [38] |
| TpPa-Py | 174.0 | 99.0 | 3.8 | 96.2 | [39] |
| PB/TA/PPM | 80.8–135.4 | 98.8 | 5.1 | 79.1 | [40] |
| COF-LZU1/PAN | 70.0–50.0 | 96.7 | 6.5 | 28.3 | [41] |
| CM-DBC * | 138.2 | 99.5 | 6.6 | 186.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chen, Y.; Shi, W.; Liu, M.; Huang, Z.; Hu, J.; Chen, Z. A Crown Ether-Based Covalent Organic Polymer Composite Membrane and Its Application in Molecular Separation. Membranes 2026, 16, 56. https://doi.org/10.3390/membranes16020056
Chen Y, Shi W, Liu M, Huang Z, Hu J, Chen Z. A Crown Ether-Based Covalent Organic Polymer Composite Membrane and Its Application in Molecular Separation. Membranes. 2026; 16(2):56. https://doi.org/10.3390/membranes16020056
Chicago/Turabian StyleChen, Yike, Wenju Shi, Meitong Liu, Zhihong Huang, Jianshe Hu, and Zhangpei Chen. 2026. "A Crown Ether-Based Covalent Organic Polymer Composite Membrane and Its Application in Molecular Separation" Membranes 16, no. 2: 56. https://doi.org/10.3390/membranes16020056
APA StyleChen, Y., Shi, W., Liu, M., Huang, Z., Hu, J., & Chen, Z. (2026). A Crown Ether-Based Covalent Organic Polymer Composite Membrane and Its Application in Molecular Separation. Membranes, 16(2), 56. https://doi.org/10.3390/membranes16020056






