Silicalite Nanosheet Laminated Membranes: Effects of Layered Structure on the Performance in Pervaporation Desalination
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Material Characterizations
2.3. SNS and SNP Synthesis
2.4. Membrane Fabrication on Alumina and PVDF Substrates
2.5. Membrane Performance for PV of Concentrated Brines
3. Results and Discussion
3.1. SNP Preparation
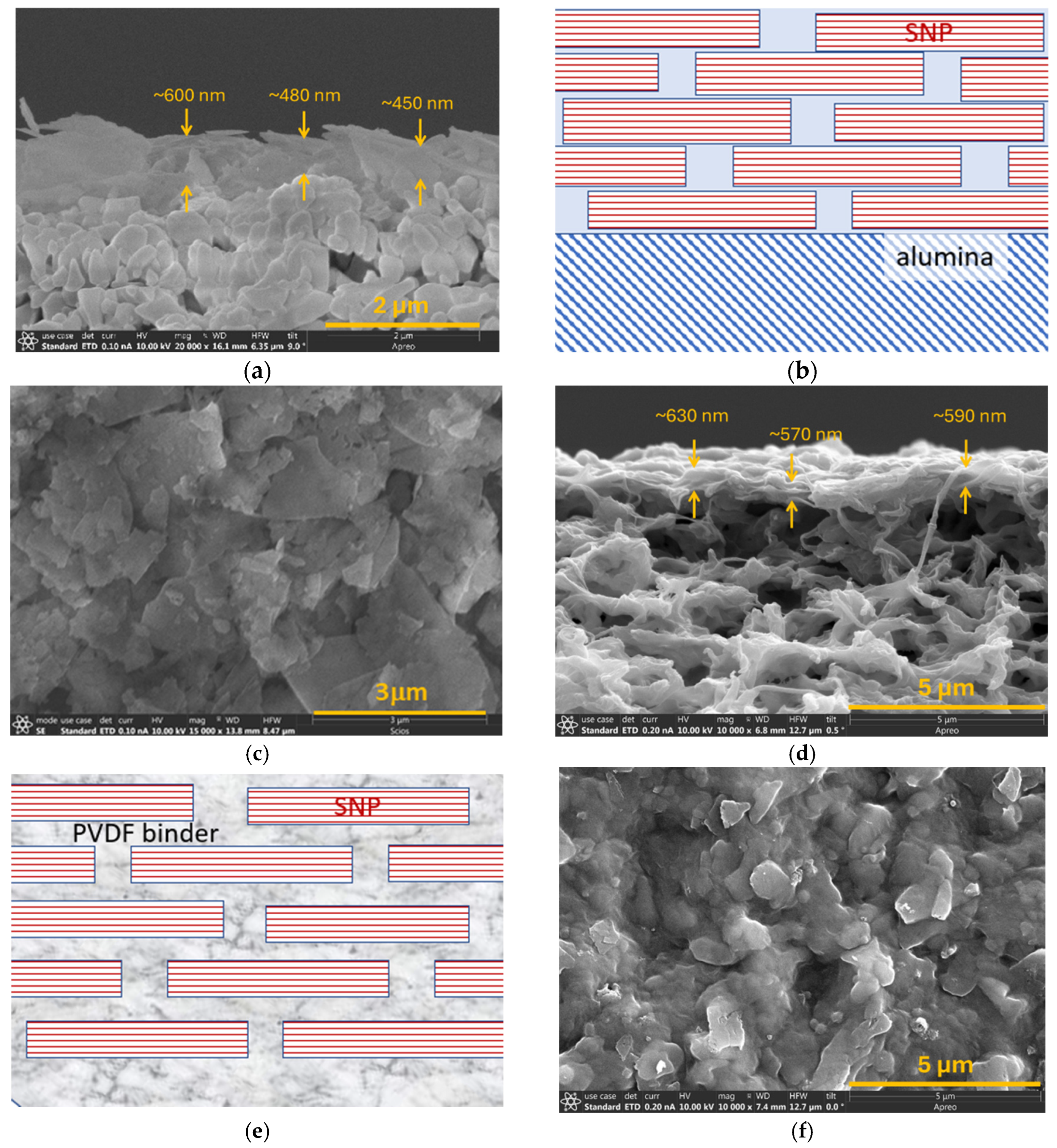
3.2. SNP Laminated Membranes
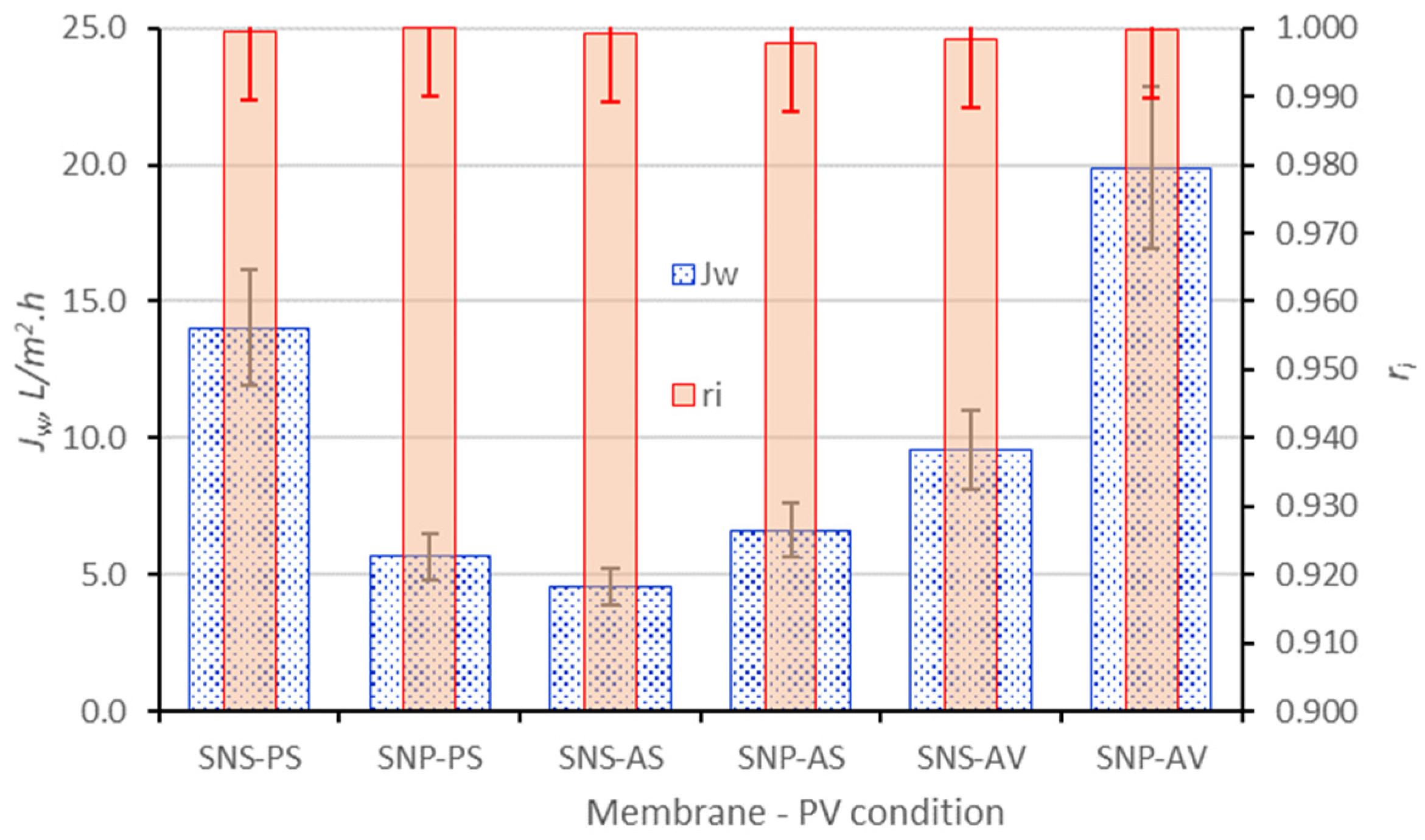
3.3. PV on SNP Laminated Membranes
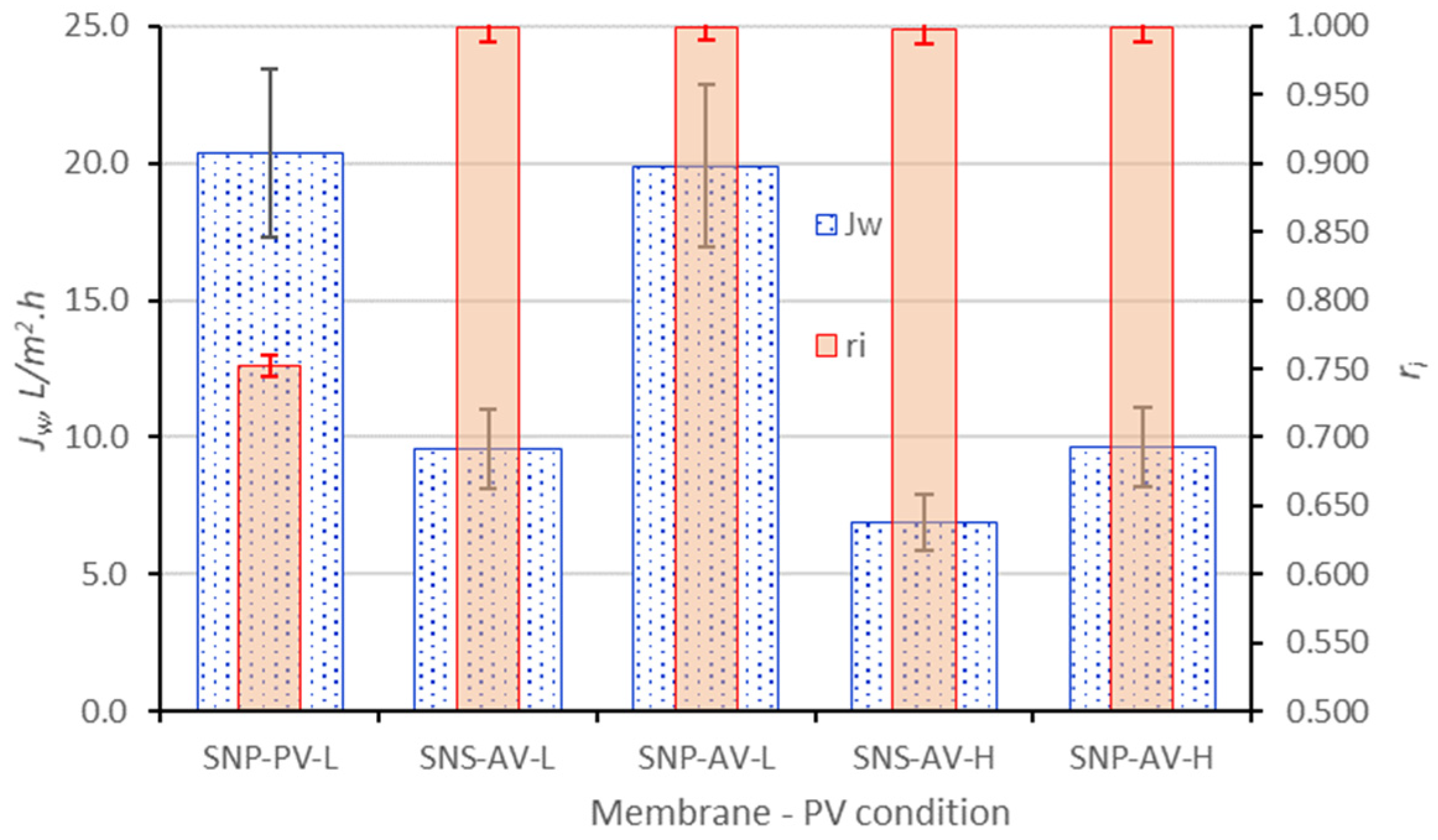
3.4. PV for High TDS Brines
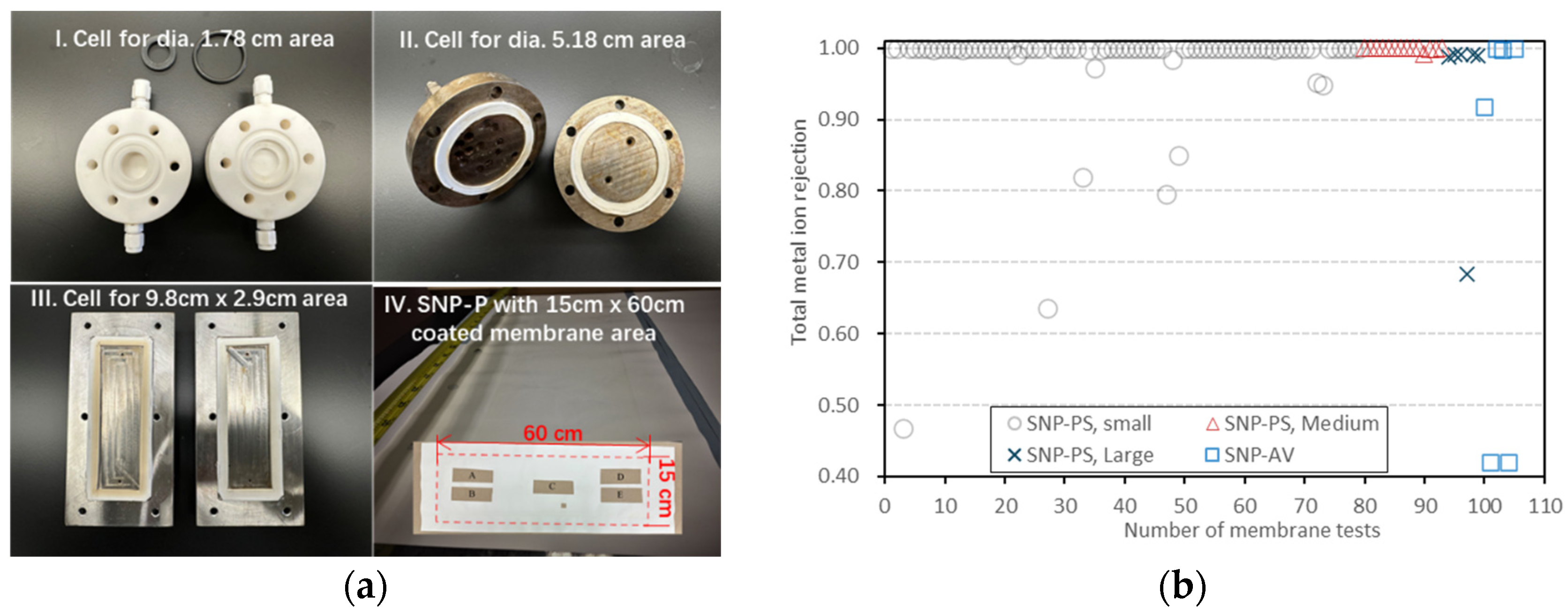
3.5. Membrane Reproducibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul Razak, N.A.; Mat Shayuti, M.S.; Othman, N.H.; Marpani, F.; Alias, N.H.; Shahruddin, M.Z.; Abd Rahman, N.; Heng, S.L.; Lai, S.O.; Ismail, A.F.; et al. A mini review on the potential of zeolite membrane for pervaporation. Chem. Eng. Commun. 2025, 212, 793–818. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Wang, J.; Raza, W.; Liu, G.; Lu, J.; Zhang, Y. Microwave synthesis of NaA zeolite membranes on coarse macroporous α-Al2O3 tubes for desalination. Microporous Mesoporous Mater. 2020, 306, 110360. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Huang, A. Seeding-free synthesis of zeolite FAU membrane for seawater desalination by pervaporation. Microporous Mesoporous Mater. 2016, 234, 377–383. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, X.; Sun, L.; Rong, H.; Zhu, G. A zeolite-like aluminophosphate membrane with molecular-sieving property for water desalination. Chem. Sci. 2018, 9, 2533–2539. [Google Scholar] [CrossRef]
- Yu, L.; Al-Jariry, N.; Serikbayeva, T.; Hedlund, J. Ultra-thin zeolite CHA and FAU membranes for desalination by pervaporation. Sep. Purif. Technol. 2022, 294, 121177. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Nenoff, T.M.; Lee, R. Desalination by reverse osmosis using MFI zeolite membranes. J. Membr. Sci. 2004, 243, 401–404. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Production of ultra pure water by desalination of seawater using a hydroxy sodalite membrane. J. Membr. Sci. 2010, 356, 52–57. [Google Scholar] [CrossRef]
- Donato, L.; Garofalo, A.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep. Purif. Technol. 2020, 237, 116376. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, P.; Cao, B.; Lau, C.H.; Xue, Y.; Zhang, R.; Li, P. Fabricating thin-film composite membranes for pervaporation desalination via photo-crosslinking. Desalination 2021, 512, 115128. [Google Scholar] [CrossRef]
- Swenson, P.; Tanchuk, B.; Gupta, A.; An, W.; Kuznicki, S.M. Pervaporative desalination of water using natural zeolite membranes. Desalination 2012, 285, 68–72. [Google Scholar] [CrossRef]
- Drobek, M.; Yacou, C.; Motuzas, J.; Julbe, A.; Ding, L.; Diniz da Costa, J.C. Long term pervaporation desalination of tubular MFI zeolite membranes. J. Membr. Sci. 2012, 415–416, 816–823. [Google Scholar] [CrossRef]
- Duke, M.C.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.S.; Diniz da Costa, J.C. Seawater desalination performance of MFI type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Kim, D.; Ghosh, S.; Akter, N.; Kraetz, A.; Duan, X.; Gwak, G.; Rangnekar, N.; Johnson, J.R.; Narasimharao, K.; Malik, M.A.; et al. Twin-free, directly synthesized MFI nanosheets with improved thickness uniformity and their use in membrane fabrication. Sci. Adv. 2022, 8, eabm8162. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zeng, S.; Xu, Z.; Arvanitis, A.; Yang, S.; Gu, X.; Dong, J. Ultrathin ZSM-5 zeolite nanosheet laminated membrane for high-flux desalination of concentrated brines. Sci. Adv. 2018, 4, eaau8634. [Google Scholar] [CrossRef]
- Cao, Z.; Iskhakova, L.; Sun, X.; Tang, Z.; Dong, J. ZSM-5 Zeolite Nanosheet-Based Membranes on Porous Polyvinylidene Fluoride for High-Flux Desalination. ACS Appl. Nano Mater. 2021, 4, 2895–2902. [Google Scholar] [CrossRef]
- Cao, Z.; Iskhakova, L.; Sun, X.; Dong, J. A Study on the Synthesis and Proton Transport Behavior of Multilayered ZSM-5 Zeolite Nanosheet Membranes Laminated on Polymer Substrates. Membranes 2023, 13, 305. [Google Scholar] [CrossRef]
- Xue, Q.; Zhu, J.; Meng, W.; Zhang, K. Effect of mxene nanosheet dispersed phases on the fabrication of polyamide nanofiltration membranes. ACS Appl. Eng. Mater. 2023, 1, 679–689. [Google Scholar] [CrossRef]
- Cao, Z.; Iskhakova, L.; Sun, X.; Anjikar, N.D.; Yang, S.; Dong, J. Self-seeded growth of very large open-structured zeolite nanosheet assemblies with extraordinary micropore accessibility. Microporous Mesoporous Mater. 2022, 336, 111854. [Google Scholar] [CrossRef]
- Iskhakova, L.; Cao, Z.; Sun, X.; Gabski, J.; Dong, J. Preactivated zeolite nanosheet plate-tiled membrane on porous PVDF film: Synthesis and study of proton-selective ion conduction. J. Membr. Sci. 2023, 669, 121328. [Google Scholar] [CrossRef]
- Kaplan, R.; Mamrosh, D.; Salih, H.H.; Dastgheib, S.A. Assessment of desalination technologies for treatment of a highly saline brine from a potential CO2 storage site. Desalination 2017, 404, 87–101. [Google Scholar] [CrossRef]
- Cha-umpong, W.; Li, Q.; Razmjou, A.; Chen, V. Concentrating brine for lithium recovery using GO composite pervaporation membranes. Desalination 2021, 500, 114894. [Google Scholar] [CrossRef]
- Garofalo, A.; Carnevale, M.C.; Donato, L.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Scale-up of MFI zeolite membranes for desalination by vacuum membrane distillation. Desalination 2016, 397, 205–212. [Google Scholar] [CrossRef]
- Garofalo, A.; Donato, L.; Drioli, E.; Criscuoli, A.; Carnevale, M.C.; Alharbi, O.; Aljlil, S.A.; Algieri, C. Supported MFI zeolite membranes by cross flow filtration for water treatment. Sep. Purif. Technol. 2014, 137, 28–35. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, H.; Sun, L.; Zhang, P.; Yang, Y.; Jiang, L.; Wu, S.; Zhu, G.; Zou, X. Fabrication and evaluation of effective zeolite membranes for water desalination. Desalination 2021, 504, 114974. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using NaA zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Membr. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]


| Membranes | Thickness (μm) | Water Flux (L/m2·h) | Salt Rejection | Salt Concentration (g/L) | T (°C) | Ref. |
|---|---|---|---|---|---|---|
| MFI (ZSM-5) | 0.65 | 11 | 99.9% | 260 | 75 | [16] |
| MFI (ZSM-5) | 0.41 | 3.5 | 99.9% | 280 | 80 | [15] |
| MFI (ZSM-5) | – | 0.72 | >99% | 38 | 80 | [12] |
| MFI (ZSM-5) | 3.3 | 11.5 | 96% | 3 | 75 | [11] |
| MFI (Silicalite-1) | – | 7 | 99% | 10 | 60 | [24] |
| MFI (Silicalite-1) | 5 | 13.8 | 99.8% | 10 | 60 | [23] |
| MFI (Silicalite-1) | 5 | 3.7 | 94.6% | 75 | 60 | [23] |
| MFI (Silicalite-1) | 5 | 20.6 | 99.9% | 10 | 70 | [8] |
| MFI (Silicalite-1) | 5 | 12.2 | 96.9% | 70 | 70 | [8] |
| MFI (Silicalite-1) | 7.08 | 1.22 | 99.8% | 30 | 80 | [25] |
| LTA (NaA) | 3.75 | 1 | 99.44% | 30 | 25 | [25] |
| LTA (NaA) | ~9 | 1.9 | 99.9% | 10 | 69 | [26] |
| FAU | 2 | 4 | >99% | 35 | 75 | [3] |
| FAU | 0.5 | 18 | 99.7% | 30 | 70 | [5] |
| CHA | 0.6 | 13 | 99.9% | 30 | 70 | [5] |
| AEI (AlPO4-18) | 15 | 2.14 | 99.7% | 30 | 25 | [4] |
| SOD (Sodalite) | 1 | 3.5 | >99.99% | 10 | 200 | [7] |
| HEU (Clinoptilolite) | – | 2.5 | 95.8% | 0.1 | 93 | [10] |
| MFI (SNP-A) | 0.5 | 19.9 | 99.9% | 70 | 73 | This work |
| MFI (SNP-A) | 0.5 | 9.56 | 99.9% | 260 | 73 | This work |
| MFI (SNP-P) | 0.6 | 14 | >99.9% | 70 | 73 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sun, X.; Sharma, Y.; Iskhakova, L.; Cao, Z.; Dong, J. Silicalite Nanosheet Laminated Membranes: Effects of Layered Structure on the Performance in Pervaporation Desalination. Membranes 2026, 16, 32. https://doi.org/10.3390/membranes16010032
Sun X, Sharma Y, Iskhakova L, Cao Z, Dong J. Silicalite Nanosheet Laminated Membranes: Effects of Layered Structure on the Performance in Pervaporation Desalination. Membranes. 2026; 16(1):32. https://doi.org/10.3390/membranes16010032
Chicago/Turabian StyleSun, Xinhui, Yukta Sharma, Landysh Iskhakova, Zishu Cao, and Junhang Dong. 2026. "Silicalite Nanosheet Laminated Membranes: Effects of Layered Structure on the Performance in Pervaporation Desalination" Membranes 16, no. 1: 32. https://doi.org/10.3390/membranes16010032
APA StyleSun, X., Sharma, Y., Iskhakova, L., Cao, Z., & Dong, J. (2026). Silicalite Nanosheet Laminated Membranes: Effects of Layered Structure on the Performance in Pervaporation Desalination. Membranes, 16(1), 32. https://doi.org/10.3390/membranes16010032




