Optimization and Scale-Up of a Two-Level Electrodialysis Process for the Concentration of Lithium Chloride with High Energy Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
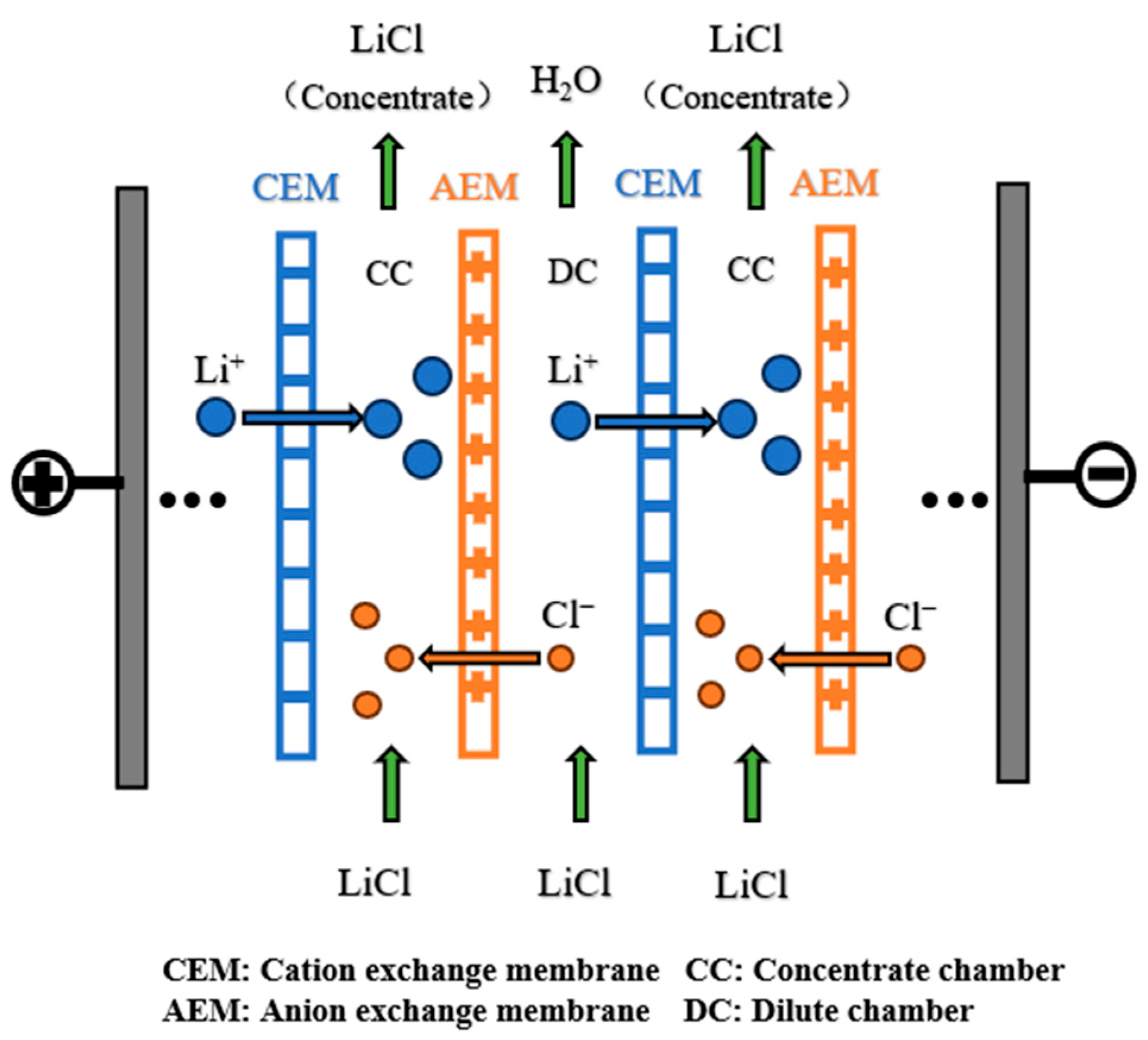
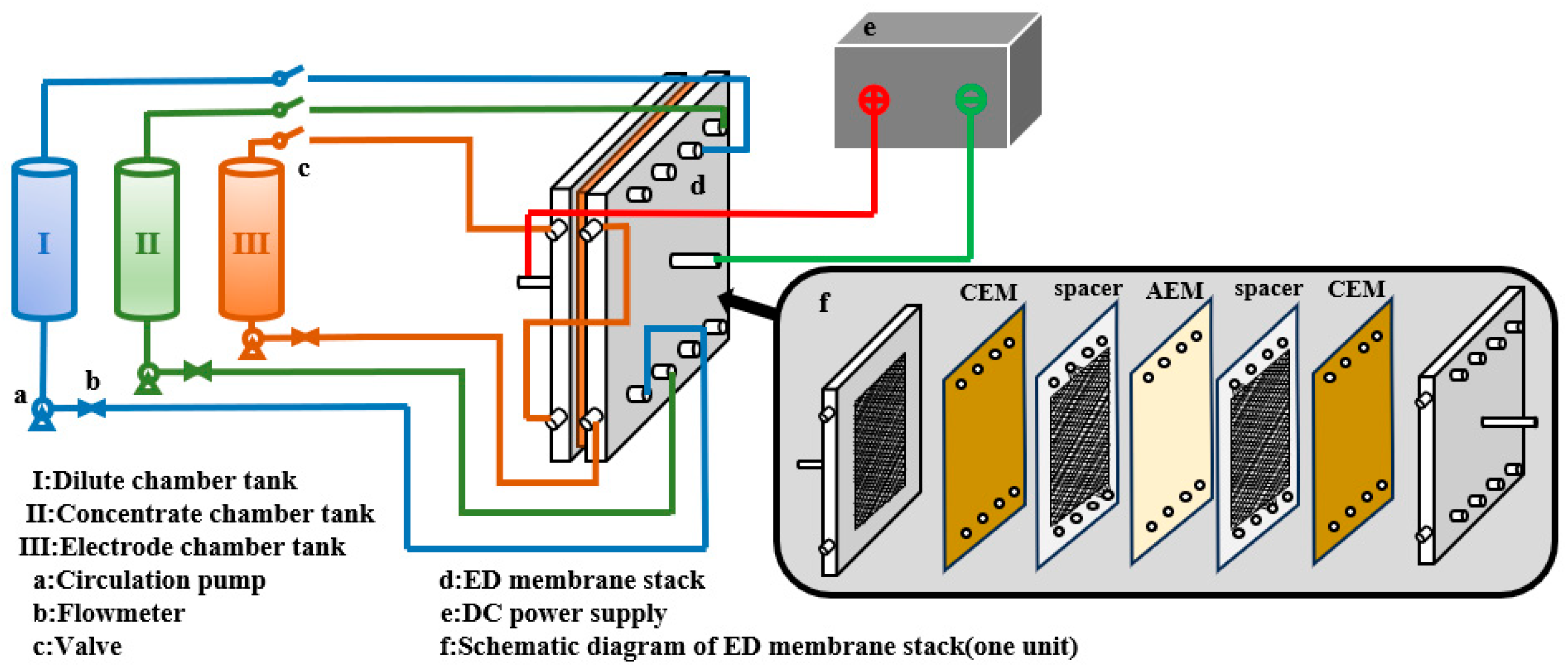
2.2. Experimental Principles and Device
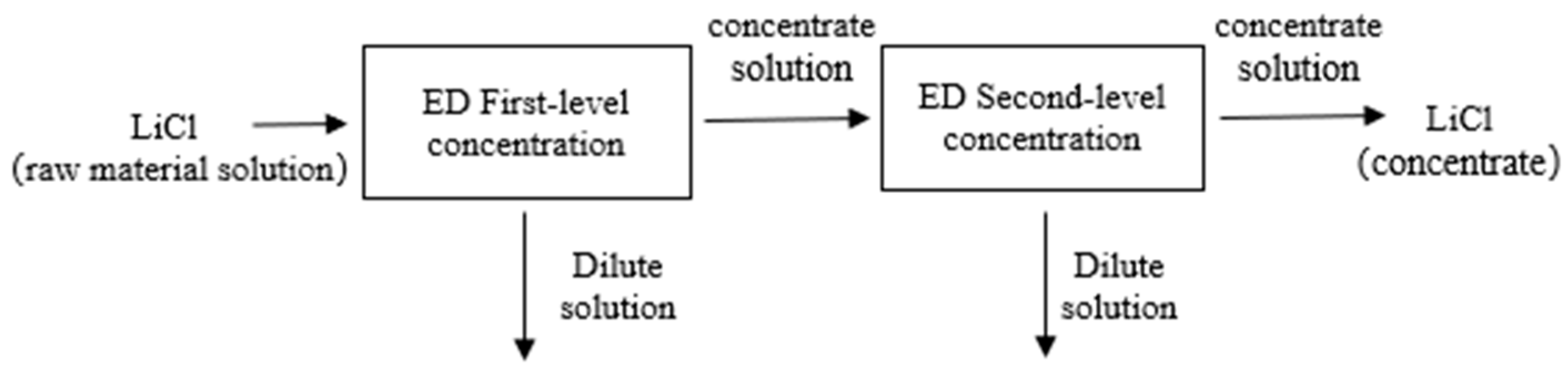
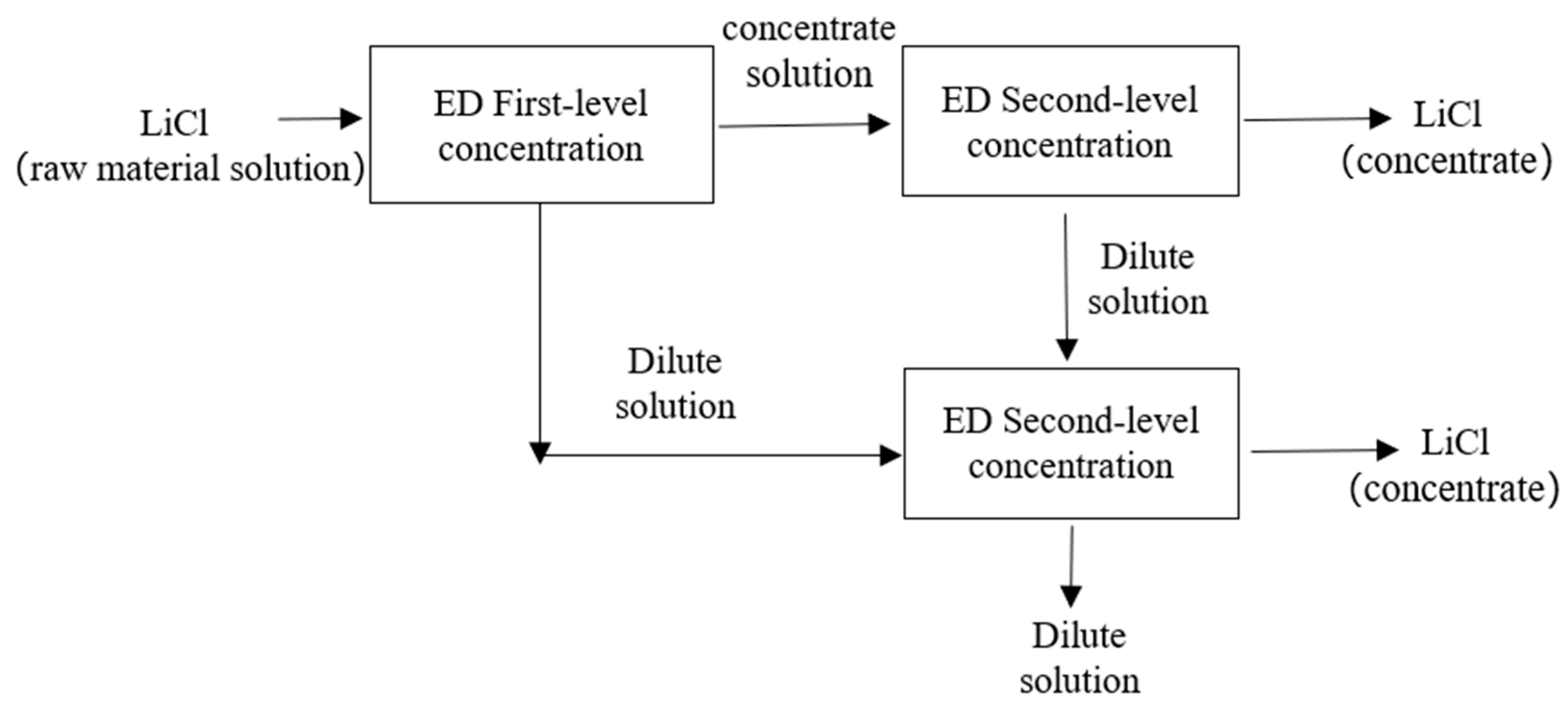
2.3. Experimental Procedure
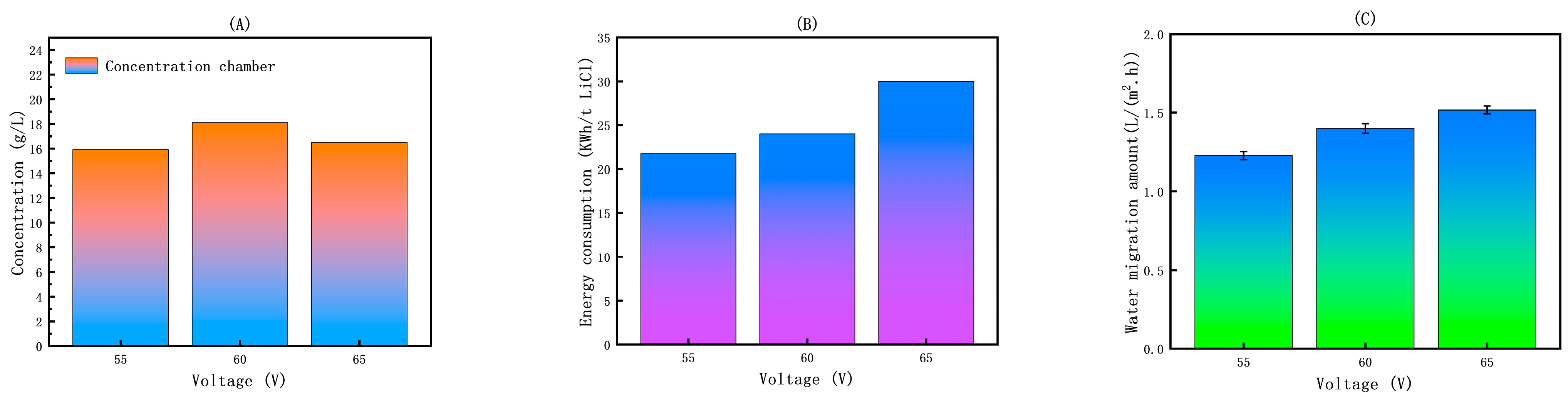
2.3.1. Selection of Voltage
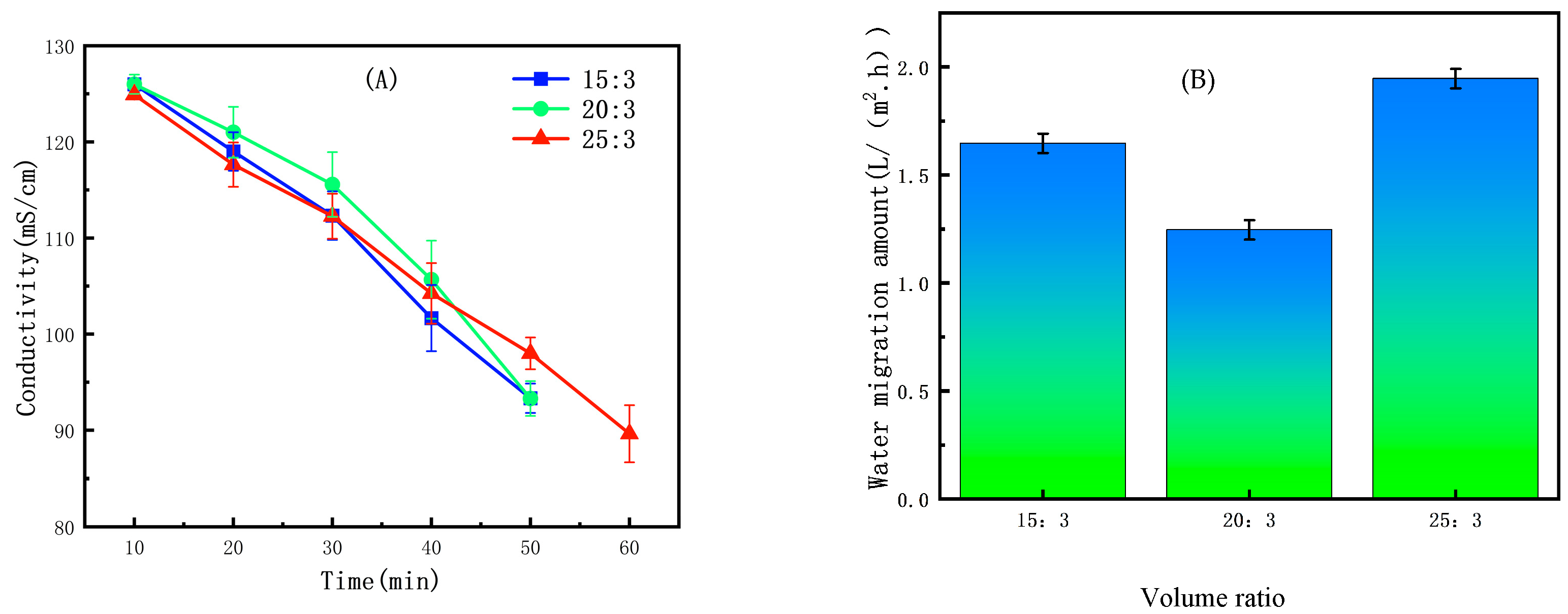
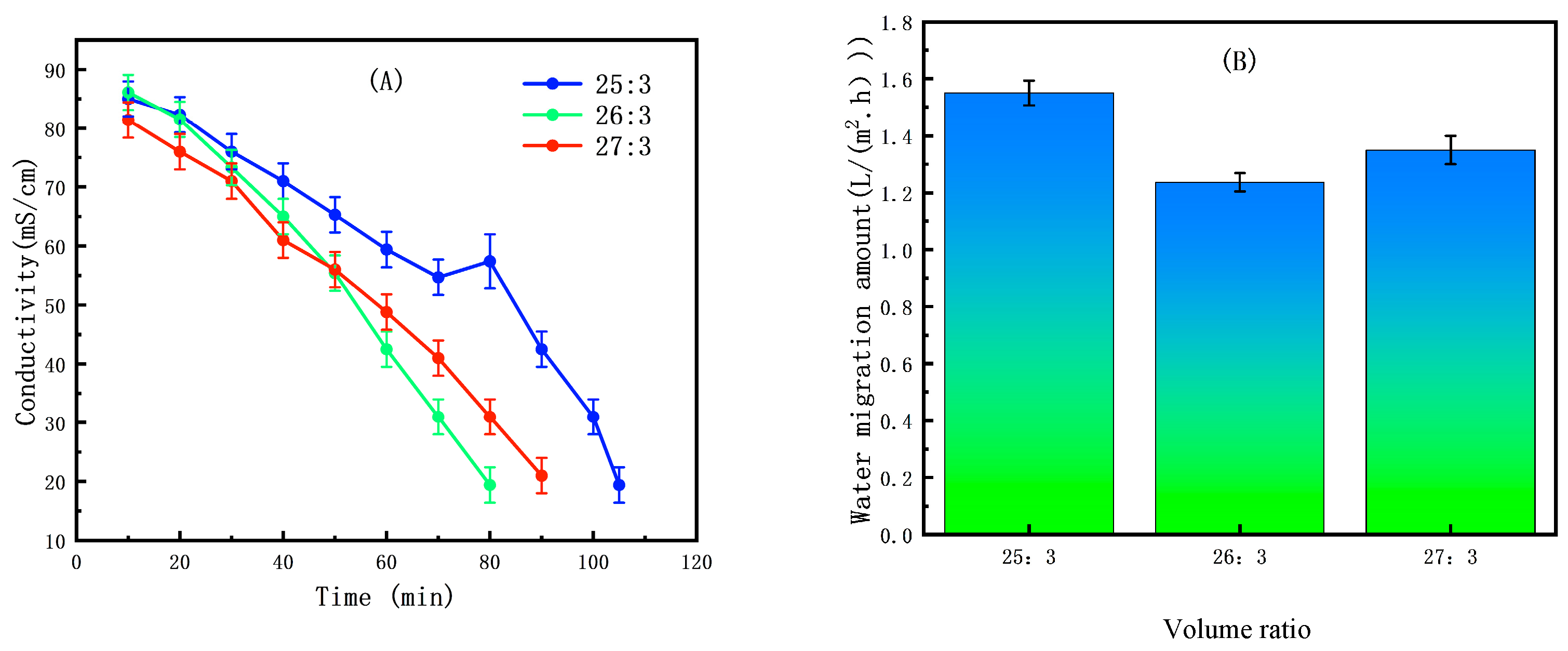
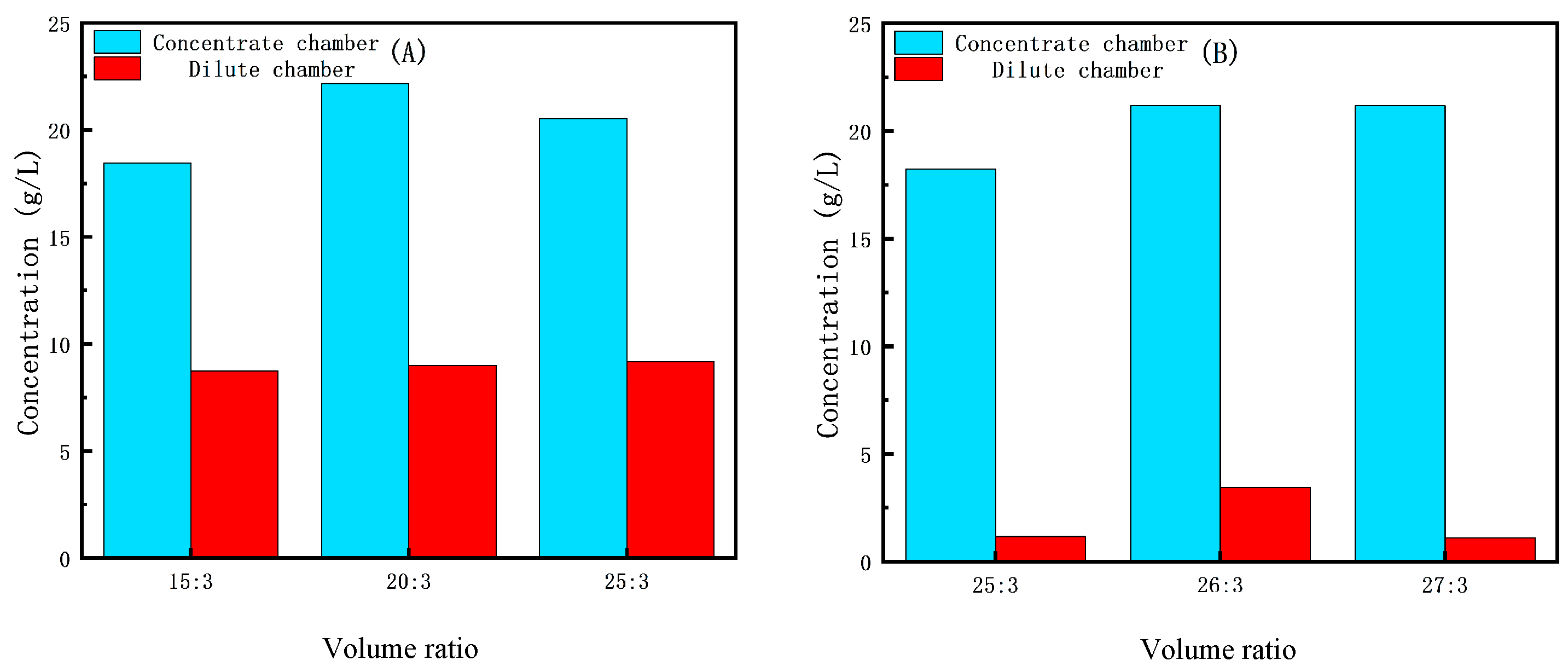
2.3.2. Selection of Volume Ratio
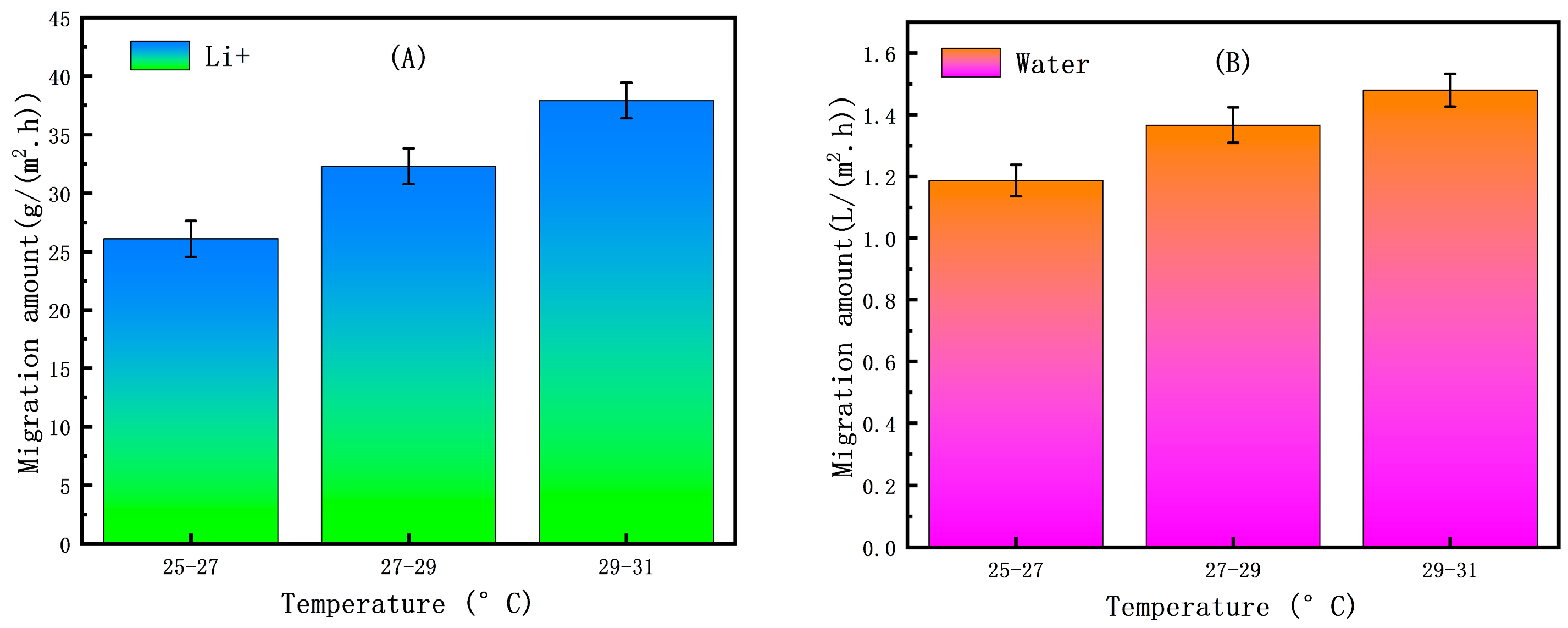
2.3.3. Selection of Temperature
2.4. Analysis Method
- Ion Concentration
- 2.
- Water Migration Amount
- 3.
- Ion Migration Amount
- 4.
- Energy Consumption
3. Results and Discussion
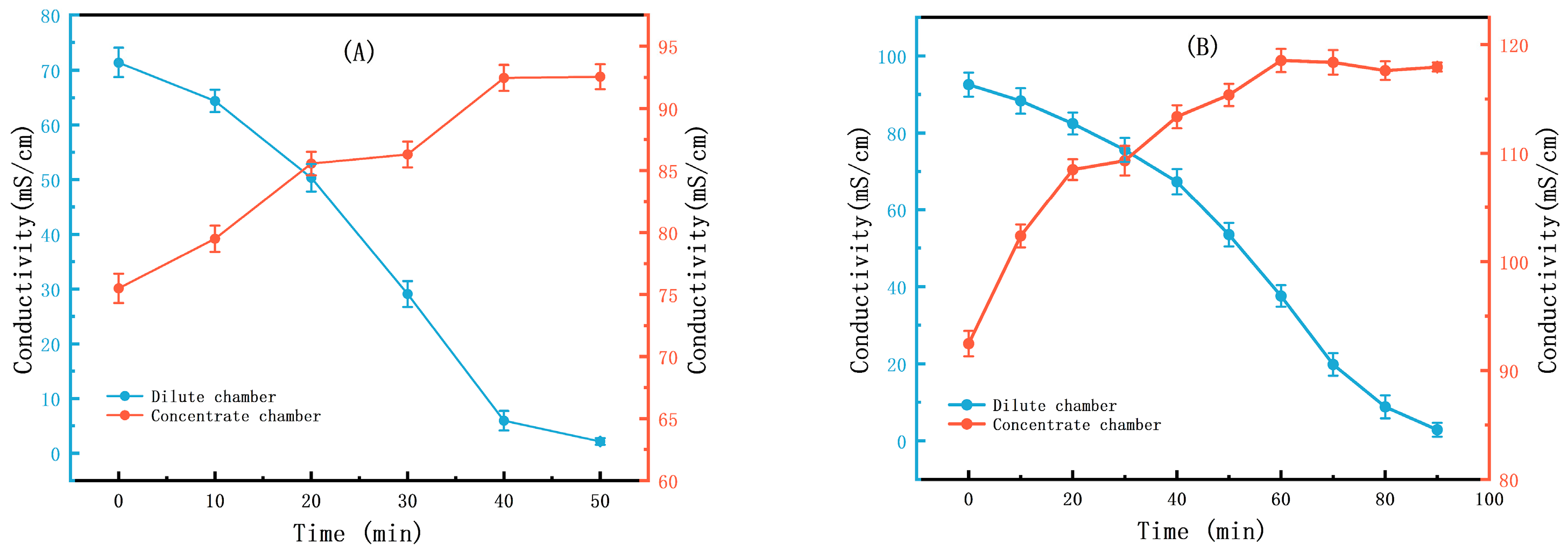
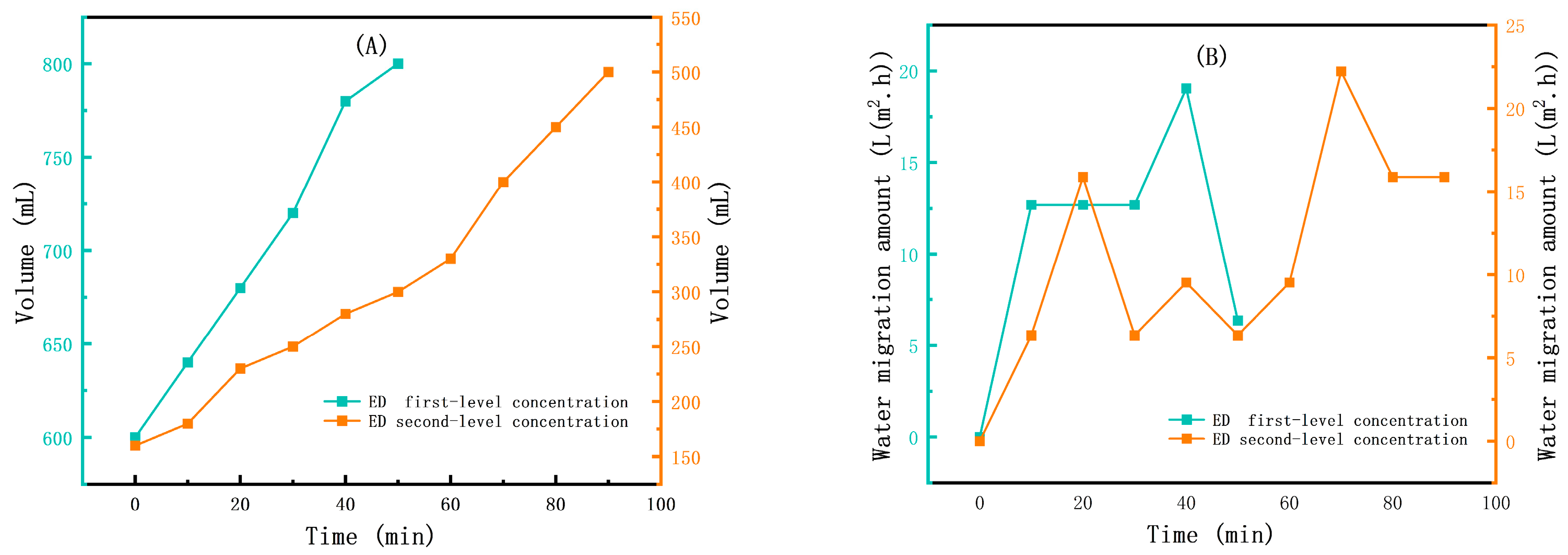
3.1. The Results of the Small-Scale Experiment
3.2. The Results of the Scaling-Up Experiment
3.2.1. The Effect of Voltage on the ED Concentration Process
3.2.2. The Effect of Initial Volume Ratio on the ED Concentration Process
3.2.3. The Effect of Temperature on the ED Concentration Process
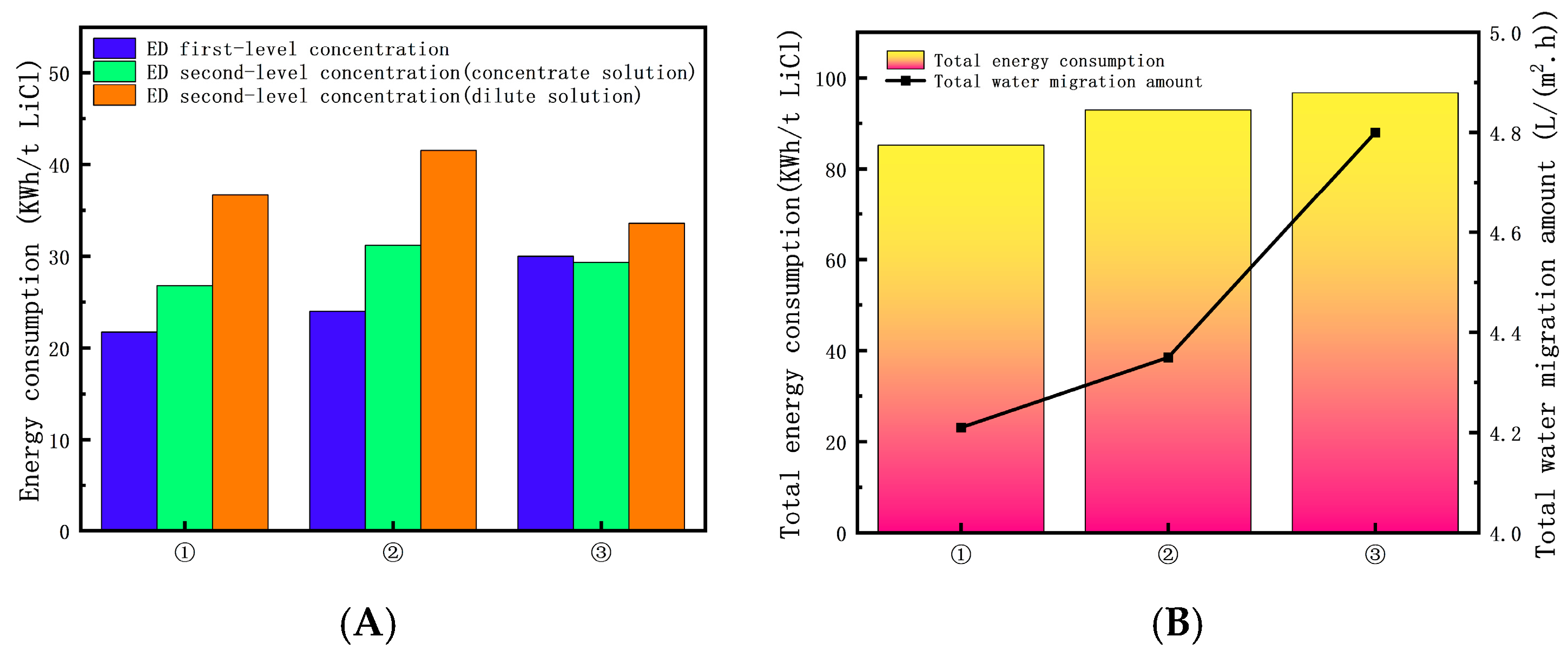
3.2.4. Analysis of Total Energy Consumption for Optimal Process Condition
- (1)
- The reaction temperature control ranges are 29–31 °C. The ED first-level concentration initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 20 L:20 L. The ED second-level concentration (concentrate solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 20 L:3 L. The ED second-level concentration (dilute solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 27 L:3 L.
- (2)
- The reaction temperature control ranges are 27–29 °C. The ED first-level concentration initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 20 L:20 L. The ED second-level concentration (concentrate solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 15 L:3 L. The ED second-level concentration (dilute solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 25 L:3 L.
- (3)
- The reaction temperature control ranges are 25–27 °C. The ED first-level concentration initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 20 L:20 L. The ED second-level concentration (concentrate solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 25 L:3 L. The ED second-level concentration (dilute solution) initial solution volume ratios between the dilute solution tank and the concentrate solution tank are set to 26 L:3 L.
3.3. Preliminary Economic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CEM | Cation exchange membrane |
| AEM | Anion exchange membrane |
| CMT | Cation exchange membrane model |
| AMT | Anion exchange membrane model |
| MVR | Mechanical vapor recompression |
| ED | Electrodialysis |
| CC | Concentrate chamber |
| DC | Dilute chamber |
References
- Mishra, B.; Olson, D.L. Corrosion of Refractory Alloys in Molten Lithium and Lithium Chloride. Miner. Process. Extr. Metall. Rev. 2002, 22, 369–388. [Google Scholar] [CrossRef]
- Ulaganathan, K.; Sinnaeruvadi, K. Metallurgical and Bio-corrosion Attributes of Thermo-mechanically Processed α-Magnesium-Lithium Alloy for Bio-implant Application. J. Oper. Manag. 2023, 75, 2338–2350. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.-T. Preface to the Special Issue: Anode Materials for Rechargeable Batteries. Acta Metall. Sin. Engl. Lett. 2021, 34, 289–290. [Google Scholar] [CrossRef]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.S.; Hammond, P.T.; Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Su, C.C.; Amine, R.; He, M.; Chen, Z.; Amine, K. Novel Analytical Method in Revealing the Solution Struc ture of Lithium-Ion Battery Electrolytes. ECS Meet. Abstr. 2017, MA2017-02, 258. [Google Scholar] [CrossRef]
- Volkmann, C.; Bschor, T.; Köhler, S. Lithium Treatment Over the Lifespan in Bipolar Disorders. Front. Psychiatry 2020, 11, 377. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Vázquez, G.H. Commentary: Lithium treatment for bipolar disorder. Bipolar Disord. 2021, 23, 93–94. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Ali, E.; Orfi, J.; AlAnsary, H.; Lee, J.-G.; Alpatova, A.; Ghaffour, N. Integration of multi effect ev aporation and membrane distillation desalination processes for enhanced performance and recovery ratios. Desalination 2020, 493, 114619. [Google Scholar] [CrossRef]
- Shen, J.; Tan, N.; Li, Z.; Zhang, J. Analysis of a novel double-effect split mechanical vapor recompre ssion systems for wastewater concentration. Appl. Therm. Eng. 2022, 216, 119019. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, K.-R.; Kang, H.-W.; Park, H.S. Reactive-crystallization method for purificatio n of LiCl salt waste. J. Radioanal. Nucl. Chem. 2020, 325, 485–492. [Google Scholar] [CrossRef]
- Nezungai, C.D.; Majozi, T. Optimum synthesis of an electrodialysis framework with a background process—I: A novel electrodialysis model. Chem. Eng. Sci. 2016, 147, 180–188. [Google Scholar] [CrossRef]
- Shi, J.; Gong, L.; Zhang, T.; Sun, S. Study of the Seawater Desalination Performance by Electrodialysis. Membranes 2022, 12, 767. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Gao, T.; Conforti, K.M.; Tian, H.; Bazant, M.Z. Small-scale desalination of seawater by shock electrodialysis. Desalination 2020, 476, 114219. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Karimi, L.; Ghassemi, A.; Zamani Sabzi, H. Quantitative studies of electrodialysis performance. Desalination 2018, 445, 159–169. [Google Scholar] [CrossRef]
- Matsuda, T.; Ogawa, S.; Onoue, Y. Demineralization of sea water by electrodialysis. Desalination 1967, 3, 295–303. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Electrodialysis with Bipolar Membranes for Valorization of Brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
- Chen, X.; Ruan, X.; Kentish, S.E.; Li, G.; Xu, T.; Chen, G.Q. Production of lithium hydroxide by electrodialysis with bipolar membranes. Sep. Purif. Technol. 2021, 274, 119026. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Wenten, I.G.; Siagian, U.W.R. Advancements in Bipolar Membrane Electrodialysis Techniques for Carbon Capture. Langmuir 2024, 40, 9362–9384. [Google Scholar] [CrossRef] [PubMed]
- Monat, L.; Zhang, W.; Jarošíková, A.; Haung, H.; Bernstein, R.; Nir, O. Circular Process for Phosphoric Acid Plant Wastewater Facilitated by Selective Electrodialysis. ACS Sustain. Chem. Eng. 2022, 10, 11567–11576. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Yaroshchuk, A.; Bruening, M.L. Selective concentration of multivalent cations via electrodialysis through tandem membranes. J. Membr. Sci. 2025, 725, 123992. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Zhang, L.; Xie, L.; Zhang, W. Electrodialysis Metathesis for the Production of Potassium Phosphate. Membranes 2025, 15, 136. [Google Scholar] [CrossRef]
- Camacho, L.M.; Fox, J.A.; Ajedegba, J.O. Optimization of electrodialysis metathesis (EDM) desalination using factorial design methodology. Desalination 2017, 403, 136–143. [Google Scholar] [CrossRef]
- Katz, W.E. The electrodialysis reversal (EDR) process. Desalination 1979, 28, 31–40. [Google Scholar] [CrossRef]
- Fubao, Y. Study on electrodialysis reversal (EDR) process. Desalination 1985, 56, 315–324. [Google Scholar] [CrossRef]
- Valero, F.; Arbós, R. Desalination of brackish river water using Electrodialysis Reversal (EDR): Control of the THMs formation in the Barcelona (NE Spain) area. Desalination 2010, 253, 170–174. [Google Scholar] [CrossRef]
- Ortiz, J.M.; Sotoca, J.A.; Expósito, E.; Gallud, F.; García-García, V.; Montiel, V.; Aldaz, A. Brackish water desalination by electrodialysis: Batch recirculation operation modeling. J. Membr. Sci. 2005, 252, 65–75. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Treatment of sea water using electrodialysis: Current efficiency evaluation. Desalination 2009, 249, 279–285. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wu, L.; Wang, Y.; Xu, T. Electrodialytic concentrating lithium salt from primary resource. Desalination 2018, 425, 30–36. [Google Scholar] [CrossRef]
- Yan, H.; Xu, C.; Li, W.; Wang, Y.; Xu, T. Electrodialysis To Concentrate Waste Ionic Liquids: Optimization of Operating Parameters. Ind. Eng. Chem. Res. 2016, 55, 2144–2152. [Google Scholar] [CrossRef]
- Xing, H.; Yang, J.Y. Effect of Electrodialysis on Liquid Separation and Concentration of Lithium Ion Intermediate Products 501. Chem. Eng. Manag. 2022, 8, 143–146. [Google Scholar]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical Modeling of the Effect of Pulsed Electric Field on the Specific Permselectivity of Ion-Exchange Membranes. Membranes 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Altin, S.; Oztekin, E.; Altin, A. Comparison of electrodialysis and reverse electrodialysis processes in the removal of Cu(II) from dilute solutions. Korean J. Chem. Eng. 2017, 34, 2218–2224. [Google Scholar] [CrossRef]
- Ben Sik Ali, M.; Mnif, A.; Hamrouni, B. Modelling of the limiting current density of an electrodialysis process by response surface methodology. Ionics 2018, 24, 617–628. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, J.H.; Park, K.Y. Characteristics of heavy metal separation and determination of limiting current density in a pilot-scale electrodialysis process for plating wastewater treatment. Sci. Total Environ. 2021, 757, 143762. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.; Gorobchenko, A.; Solonchenko, K.; Nikonenko, V. Effect of current density on anion-exchange membrane scaling during electrodialysis of phosphate-containing solution: Experimental study and predictive simulation. Desalination 2025, 600, 118487. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Huang, S.; Cao, Z.; Lu, L.; Zhang, X. Study on mass transfer performance and membrane resistance in concentration of high salinity solutions by electrodialysis. Sep. Purif. Technol. 2022, 281, 119907. [Google Scholar] [CrossRef]
- Wei, G.; Wang, M.; Lin, C.; Xu, C.; Gao, J. Optimizing Operational Parameters for Lithium Hydroxide Production via Bipolar Membrane Electrodialysis. Separations 2024, 11, 146. [Google Scholar] [CrossRef]
- Benneker, A.M.; Rijnaarts, T.; Lammertink, R.G.H.; Wood, J.A. Effect of temperature gradients in (reverse) electrodialysis in the Ohmic regime. J. Membr. Sci. E 2018, 548, 421–428. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, P.P.; Raj, S.K.; Kumari, J.; Rathore, M.S.; Kulshrestha, V. Effect of environmental temperature and applied potential on water desalination performance using electrodialysis. Mater. Today Chem. 2021, 20, 100484. [Google Scholar] [CrossRef]
- Jia, Y.W.; Chen, G.Q.; Kentish, S.E. Investigating the effect of temperature and concentration on the performance of reverse electrodialysis systems. Desalination 2024, 592, 118184. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Bi, J.; Guo, X.; Wang, S.; Jie, L.; Shi, Y.; Zhao, Y. Process intensification of the electrodialysis metathesis for calcium bromide preparation: Effects of key influencing factors and phenomena of water migration. Desalination 2024, 585, 117734. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q.; Li, Y.; Wang, Y.; Xu, T. Water electro-transport with hydrated cations in electrodialysis. Desalination 2015, 365, 204–212. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Li, M. Electrodialysis for lithium recovery from brine: Process configurations, techno-economic analysis, challenges and opportunities with artificial intelligence. J. Environ. Chem. Eng. 2025, 13, 118309. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Membr. Sci. 2019, 570, 245–257. [Google Scholar] [CrossRef]
- Zhu, M.; He, F.; Feng, L.; Chi, Y.; Li, Y.-Y.; Tian, B. Comparison of bipolar membrane electrodialysis, electrodialysis metathesis, and bipolar membrane electrodialysis multifunction for the conversion of waste Na2SO4: Process performance and economic analysis. J. Environ. Manag. 2024, 370, 122513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Patel, S.K.; Elimelech, M. Correlation equation for evaluating energy consumption and process performance of brackish water desalination by electrodialysis. Desalination 2021, 510, 115089. [Google Scholar] [CrossRef]
| Parameters | Mass Fraction (%) | pH | Conductivity (mS/cm) |
|---|---|---|---|
| LiCl raw material solution | 7.68 | 7.06 | 75.6 |
| Membrane | Thickness (μm) | Surface Area Resistance (Ω·cm2) | Ion-Exchange Capacity (meq/g) | Temperature (°C) |
|---|---|---|---|---|
| CMT | 120–130 | 3 a | 1.1 | ≤35 |
| AMT | 120–130 | 5 b | 0.8 | ≤35 |
| Operating Conditions | |
|---|---|
| Number of repeating units | 60 |
| Actual area of single membrane (m2) | 0.125 |
| Total effective area of membrane (m2) | 4.275 |
| Flow rate of each chamber (m3/h) | 1 |
| Feed conductivity (mS/cm) | 75.3 |
| Voltage (V) | 60 |
| fixed investment costs | |
| CMT cation exchange membrane (USD/m2) | 120 |
| AMT anion exchange membrane (USD/m2) | 120 |
| Membrane cost [48,49] (USD) | 1815 |
| Membrane stack cost (USD) a | 2722.5 |
| Equipment cost (USD) b | 4083.75 |
| Total fixed investment cost (USD/year) c | 1317.3 |
| Energy consumption costs | |
| ED energy consumption(kWh/t LiCl) | 85.22 |
| Equipment energy consumption (kWh/t LiCl) | 22.97 |
| Electricity cost (USD/kWh) | 0.1 |
| Energy consumption cost (USD/t LiCl) | 8.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, J.; Yu, L.; Shen, J. Optimization and Scale-Up of a Two-Level Electrodialysis Process for the Concentration of Lithium Chloride with High Energy Efficiency. Membranes 2025, 15, 283. https://doi.org/10.3390/membranes15090283
Zhang Y, Wang J, Yu L, Shen J. Optimization and Scale-Up of a Two-Level Electrodialysis Process for the Concentration of Lithium Chloride with High Energy Efficiency. Membranes. 2025; 15(9):283. https://doi.org/10.3390/membranes15090283
Chicago/Turabian StyleZhang, Yu, Jikuan Wang, Liangyu Yu, and Jiangnan Shen. 2025. "Optimization and Scale-Up of a Two-Level Electrodialysis Process for the Concentration of Lithium Chloride with High Energy Efficiency" Membranes 15, no. 9: 283. https://doi.org/10.3390/membranes15090283
APA StyleZhang, Y., Wang, J., Yu, L., & Shen, J. (2025). Optimization and Scale-Up of a Two-Level Electrodialysis Process for the Concentration of Lithium Chloride with High Energy Efficiency. Membranes, 15(9), 283. https://doi.org/10.3390/membranes15090283







