ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8 Particles
2.3. Membrane Preparation
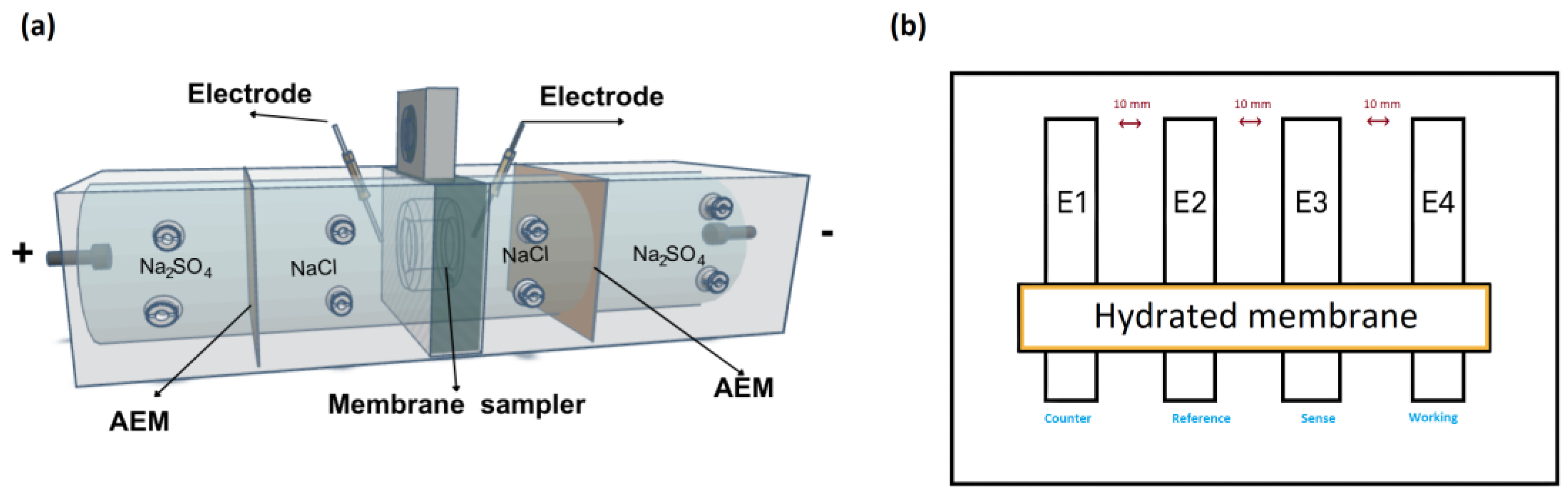
2.4. Membrane Characterization
2.5. Water Uptake Test
2.6. Durability Test
2.7. Surface Electrical Resistance
2.8. Anti-Fouling Analysis
2.9. Impedance Spectroscopy Characterizations
3. Results and Discussion
3.1. Membrane Characterization
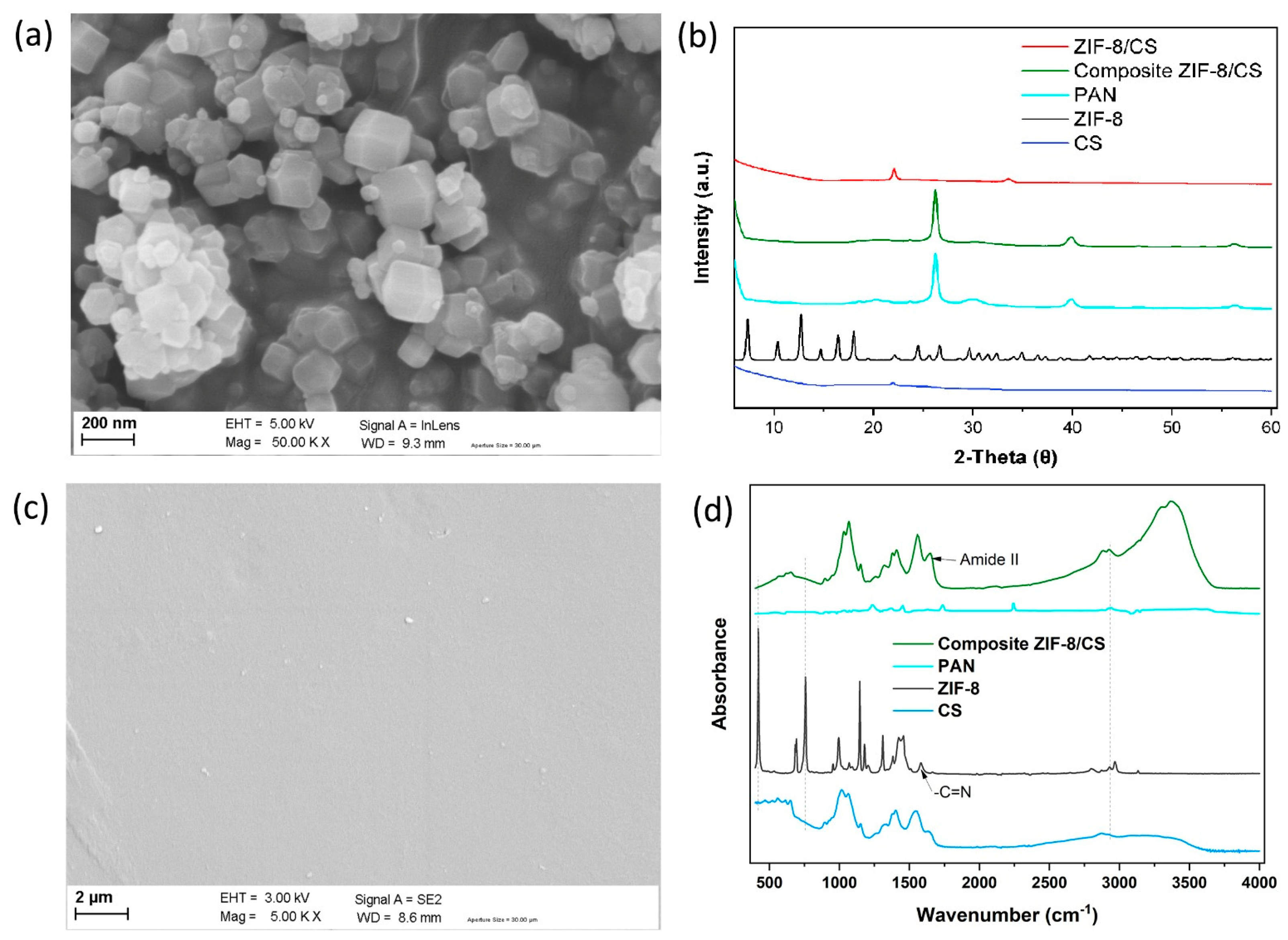
3.1.1. Characterization of ZIF-8
3.1.2. Morphology of the Membranes
3.1.3. XRD Characterizations of ZIF-8/CS Composite Membranes
3.1.4. FTIR Spectroscopy Analysis of Membranes
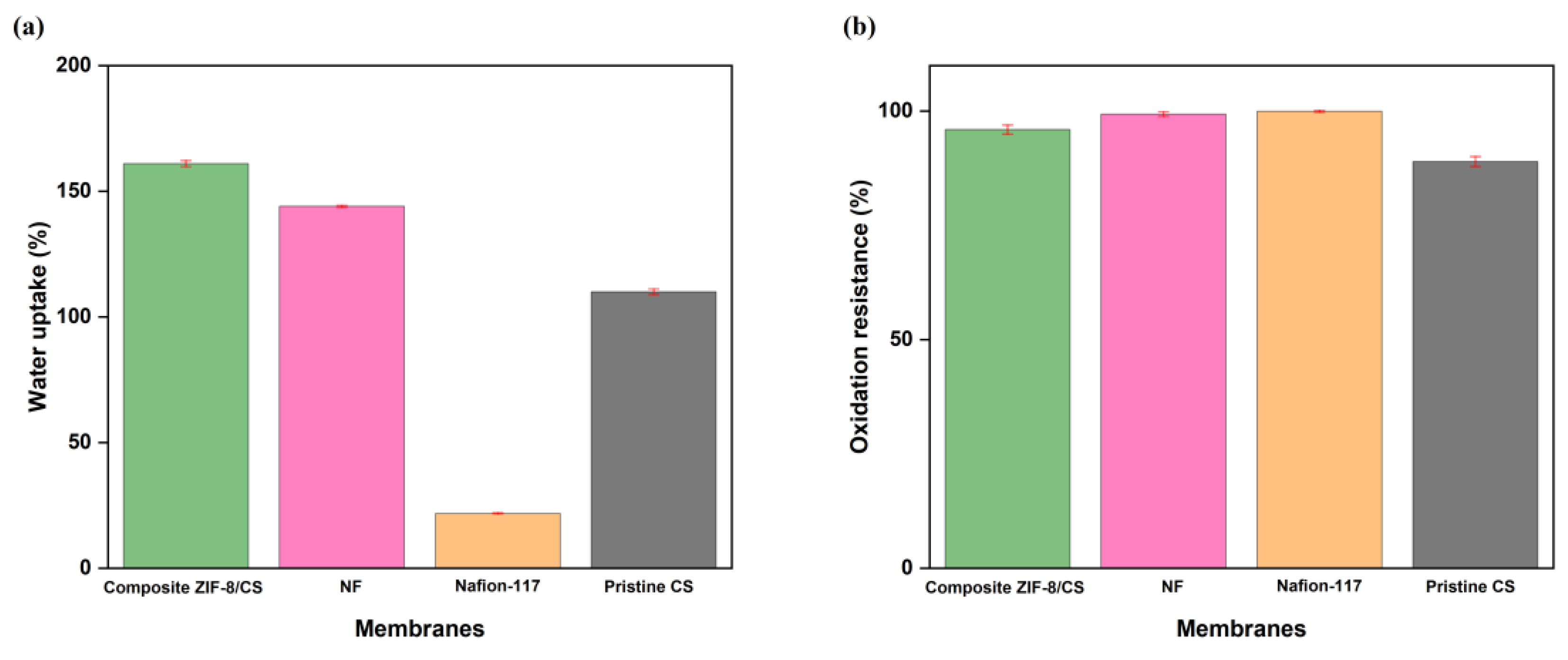
3.2. Water Uptake of the Membrane
3.3. Durability Test Results
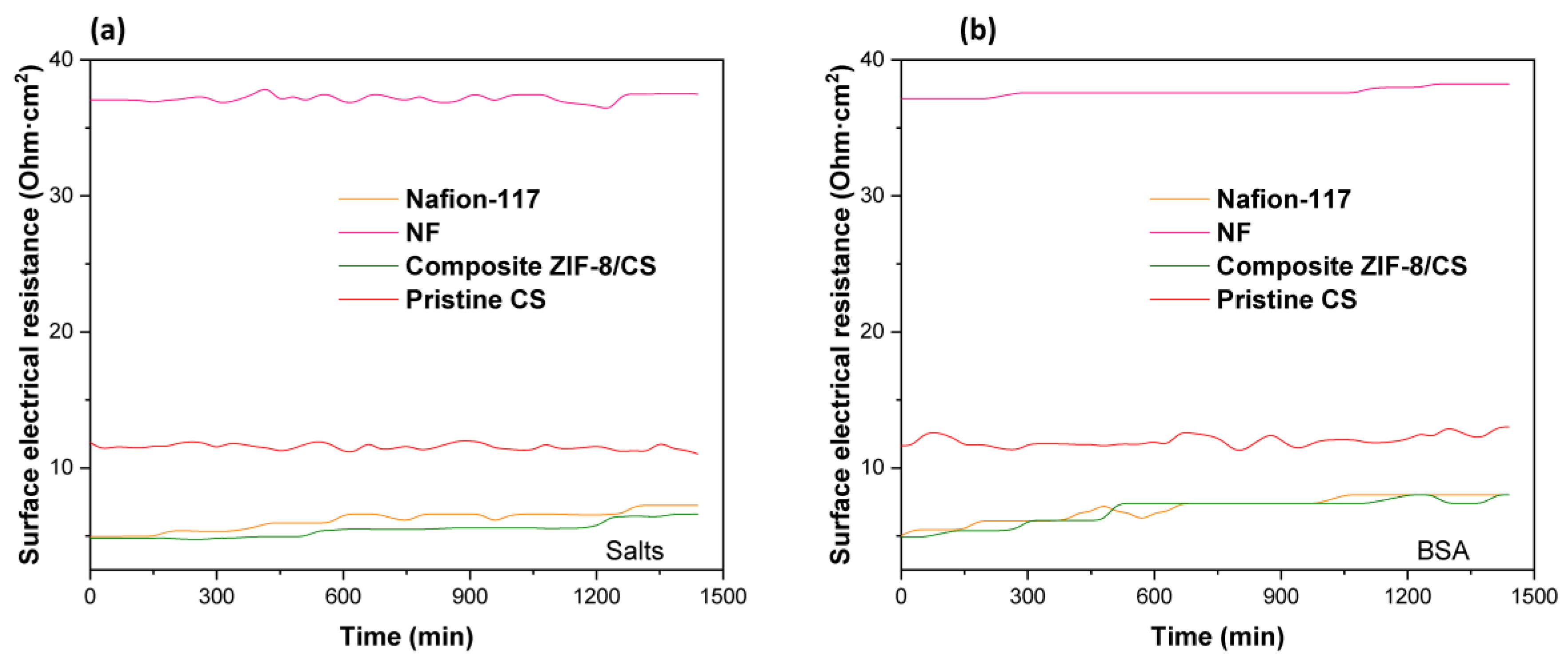
3.4. Surface Electrical Resistance and Anti-Fouling Evaluation
3.5. Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penzy, K.; Muhammad, S.; Shahzad, M.; Hussain, I.; Khan, S.A.; Abbasi, A.M.; Khan, I.; Ahmad, R. Industrial wastewater irrigation increased higher heavy metals uptake and expansins, metacaspases, and cystatin genes expression in Parthenium and maize. Environ. Monit. Assess. 2023, 195, 1430. [Google Scholar] [CrossRef]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Sheikh, M.; Harami, H.R.; Rezakazemi, M.; Cortina, J.L.; Aminabhavi, T.M.; Valderrama, C. Towards a sustainable transformation of municipal wastewater treatment plants into biofactories using advanced NH3-N recovery technologies: A review. Sci. Total Environ. 2023, 904, 166077. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Luo, Y.; Bai, Y.; Fan, J. Bioremediation of phenolic pollutants by algae—Current status and challenges. Bioresour. Technol. 2022, 350, 126930. [Google Scholar] [CrossRef]
- Abbasi, N.; Ahmadi, M.; Naseri, M. Quality and cost analysis of a wastewater treatment plant using GPS-X and CapdetWorks simulation programs. J. Environ. Manag. 2021, 284, 111993. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Cardeña, R.; Cortón, E.; Buitrón, G. Hydrogen production in two-chamber MEC using a low-cost and biodegradable poly(vinyl) alcohol/chitosan membrane. Bioresour. Technol. 2021, 319, 124168. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Uduma, R.C.; Oguzie, K.L.; Chijioke, C.F.; Ogbulie, T.E.; Oguzie, E.E. Bioelectrochemical technologies for simultaneous treatment of dye wastewater and electricity generation: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10415–10434. [Google Scholar] [CrossRef]
- Abbassi, R.; Yadav, A.K. Introduction to microbial fuel cells: Challenges and opportunities. In Integrated Microbial Fuel Cells for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Oliot, M.; Galier, S.; de Balmann, H.R.; Bergel, A. Ion transport in microbial fuel cells: Key roles, theory and critical review. Appl. Energy 2016, 183, 1682–1704. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.; Ajayi, F.F.; Kim, I.S. Biohydrogen production via biocatalyzed electrolysis in acetate-fed bioelectrochemical cells and microbial community analysis. Int. J. Hydrogen Energy 2008, 33, 5184–5192. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, S.; Mahimai, B.M.; Kannaiyan, D.; Deivanayagam, P. Synthesis and fabrication of Cu-trimesic acid MOF anchored sulfonated Poly(2,5-benzimidazole) membranes for PEMFC applications. Int. J. Hydrogen Energy 2023, 48, 36063–36075. [Google Scholar] [CrossRef]
- Jonathan, B.-C.; Kim, M.; Kim, J.R. Bioelectricity Generation Using a Crosslinked Poly(vinyl alcohol) (PVA) and Chitosan (CS) Ion Exchange Membrane in Microbial Fuel Cell. J. Electrochem. Sci. Technol. 2023, 14, 303–310. [Google Scholar] [CrossRef]
- Mukoma, P.; Jooste, B.R.; Vosloo, H.C.M. Synthesis and characterization of cross-linked chitosan membranes for application as alternative proton exchange membrane materials in fuel cells. J. Power Sources 2004, 136, 16–23. [Google Scholar] [CrossRef]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of modified chitosan membrane for microbial fuel cell: Roles of proton carrier site and positive charge. J. Clean. Prod. 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Li, W.; Xu, P.; Wang, Z.; He, Y.; Qin, H.; Zeng, Y.; Li, Y.; Zhang, Z.; Gao, J. MOFs meet membrane: Application in water treatment and separation. Mater. Chem. Front. 2023, 7, 5140–5170. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Xu, X.; Hartanto, Y.; Nikolaeva, D.; He, Z.; Chergaoui, S.; Luis, P. High-performance ZIF-8/biopolymer chitosan mixed-matrix pervaporation membrane for methanol/dimethyl carbonate separation. Sep. Purif. Technol. 2022, 293, 121085. [Google Scholar] [CrossRef]
- Leus, K.; Bogaerts, T.; De Decker, J.; Depauw, H.; Hendrickx, K.; Vrielinck, H.; Van Speybroeck, V.; Van Der Voort, P. Systematic study of the chemical and hydrothermal stability of selected ‘stable’ Metal Organic Frameworks. Microporous Mesoporous Mater. 2016, 226, 110–116. [Google Scholar] [CrossRef]
- Xu, X.; Van Eygen, G.; Hartanto, Y.; Van der Bruggen, B.; Luis, P. Nanostructural modulation of chitosan matrix for facilitating transport of organic molecules across the membrane by pervaporation. J. Memb. Sci. 2024, 706, 122953. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; El-Desouky, E.A.; Soliman, E.A.; El-Naim, G.A. Novel sulphonated poly (vinyl chloride)/poly (2-acrylamido-2-methylpropane sulphonic acid) blends-based polyelectrolyte membranes for direct methanol fuel cells. Polym. Test. 2020, 89, 106604. [Google Scholar] [CrossRef]
- Veiga, I.G.; Moraes, A.M. Study of the swelling and stability properties of chitosan-xanthan membranes. J. Appl. Polym. Sci. 2012, 124 (Suppl. 1), E154–E160. [Google Scholar] [CrossRef]
- Qiao, J.; Saito, M.; Hayamizu, K.; Okada, T. Degradation of Perfluorinated Ionomer Membranes for PEM Fuel Cells during Processing with H2O2. J. Electrochem. Soc. 2006, 153, A967. [Google Scholar] [CrossRef]
- Zhao, D.; Yi, B.L.; Zhang, H.M.; Yu, H.M. MnO2/SiO2-SO3H nanocomposite as hydrogen peroxide scavenger for durability improvement in proton exchange membranes. J. Memb. Sci. 2010, 346, 143–151. [Google Scholar] [CrossRef]
- Schauer, J.; Brožová, L. Heterogeneous ion-exchange membranes based on sulfonated poly(1,4-phenylene sulfide) and linear polyethylene: Preparation, oxidation stability, methanol permeability and electrochemical properties. J. Memb. Sci. 2005, 250, 151–157. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Soria, R.B.; Zhu, J.; Gonza, I.; Van der Bruggen, B.; Luis, P. Effect of (TiO2: ZnO) ratio on the anti-fouling properties of bio-inspired nanofiltration membranes. Sep. Purif. Technol. 2020, 251, 117280. [Google Scholar] [CrossRef]
- Wang, W.; Fu, R.; Liu, Z.; Wang, H. Low-resistance anti-fouling ion exchange membranes fouled by organic foulants in electrodialysis. Desalination 2017, 417, 1–8. [Google Scholar] [CrossRef]
- Madih, K.; El-Shazly, A.H.; Elkady, M.F.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Mehdipour-Ataei, S. Synthesis of a novel poly(amide-imide) (PAI) and preparation and characterization of PAI blended polyethersulfone (PES) membranes. J. Memb. Sci. 2008, 311, 349–359. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, Z.; Ding, H.; Pan, F.; Wang, B.; Yang, J.; Cao, X. Elevated pervaporation performance of polysiloxane membrane using channels and active sites of metal organic framework CuBTC. J. Memb. Sci. 2015, 481, 73–81. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Goh, P.S.; Rana, D.; Matsuura, T. Aqueous room temperature synthesis of zeolitic imidazole framework 8 (ZIF-8) with various concentrations of triethylamine. RSC Adv. 2014, 4, 33292–33300. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroǧlu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Li, H.; Zhan, Y.; Ding, X.; Wang, M.; Wang, X.; Xiao, L. Synthesis of nano-ZIF-8@chitosan microspheres and its rapid removal of p-hydroxybenzoic acid from the agro-industry and preservatives. J. Porous Mater. 2021, 28, 29–38. [Google Scholar] [CrossRef]
- Khajavian, M.; Salehi, E.; Vatanpour, V. Nanofiltration of dye solution using chitosan/poly(vinyl alcohol)/ZIF-8 thin film composite adsorptive membranes with PVDF membrane beneath as support. Carbohydr. Polym. 2020, 247, 116693. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, M.; Salehi, E.; Vatanpour, V. Chitosan/polyvinyl alcohol thin membrane adsorbents modified with zeolitic imidazolate framework (ZIF-8) nanostructures: Batch adsorption and optimization. Sep. Purif. Technol. 2020, 241, 116759. [Google Scholar] [CrossRef]
- Luo, X.; Rojas-Carbonell, S.; Yan, Y.; Kusoglu, A. Structure-transport relationships of poly(aryl piperidinium) anion-exchange membranes: Eeffect of anions and hydration. J. Memb. Sci. 2020, 598, 117680. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Mattsson, B.; Ericson, H.; Torell, L.M.; Sundholm, F. Degradation of a fuel cell membrane, as revealed by micro-Raman spectroscopy. Electrochim. Acta 2000, 45, 1405–1408. [Google Scholar] [CrossRef]
- Hübner, G.; Roduner, E. EPR investigation of HO· radical initiated degradation reactions of sulfonated aromatics as model compounds for fuel cell proton conducting membranes. J. Mater. Chem. 1999, 9, 409–418. [Google Scholar] [CrossRef]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of solution composition on the resistance of ion exchange membranes. J. Memb. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, D.; Wang, Y.; Zhang, Y.; Qi, Y.; Qiu, X.; Wang, Y.X.; Zheng, X. Effect of Na+ on organic fouling depends on Na+ concentration and the property of the foulants. Desalination 2022, 531, 115709. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Schoutteten, K.; D’hAese, A.; Bussche, J.V.D.; Vanhaecke, L.; Thwala, J.; Mamba, B.; Verliefde, A. Role of permeate flux and specific membrane-foulant-solute affinity interactions (∆Gslm) in transport of trace organic solutes through fouled nanofiltration (NF) membranes. J. Memb. Sci. 2016, 518, 203–215. [Google Scholar] [CrossRef]
- Zatadini, R.; Ni’mah, A.F.; Setiawan, Y.; Pradita, A.L.; Pratiwi, D.V.; Syakirina, D.; Insani, M.V.; Naufal, W.M.; Rayhani, W.; Saputra, O.A.; et al. Improvement of Selectivity and Antifouling Properties of Chitosan-modified Polyvinylidene Fluoride (PVDF) Membrane for Protein Filtration. ALCHEMY J. Penelit. Kim. 2023, 19, 210. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhang, Y.; Zhang, Q.; Huo, K.; Han, C. Breaking the trade-off between selectivity and permeability of nanocomposite membrane modified by UIO66@PDA through nonsolvent thermally induced phase separation method. J. Ind. Eng. Chem. 2024, 130, 306–316. [Google Scholar] [CrossRef]
- Choi, M.-J.; Chae, K.-J.; Ajayi, F.F.; Kim, K.-Y.; Yu, H.-W.; Kim, C.-W.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Montalvillo, M.; Silva, V.; Palacio, L.; Calvo, J.I.; Carmona, F.J.; Hernández, A.; Prádanos, P. Charge and dielectric characterization of nanofiltration membranes by impedance spectroscopy. J. Memb. Sci. 2014, 454, 163–173. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research progress of proton exchange membrane failure and mitigation strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef]
- Avramov, S.G.; Lefterova, E.; Penchev, H.; Sinigersky, V.; Slavcheva, E. Comparative study on the proton conductivity of perfluorosulfonic and polybenzimidazole based polymer electrolyte membranes. Bulg. Chem. Commun. 2016, 48, 43–50. [Google Scholar]
- Samsudin, A.M.; Hacker, V. Preparation and characterization of PVA/PDDA/nano-zirconia composite anion exchange membranes for fuel cells. Polymers 2019, 11, 1399. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Fu, G.; Zhang, Z. Simultaneous electricity generation and nitrogen and carbon removal in single-chamber microbial fuel cell for high-salinity wastewater treatment. J. Clean. Prod. 2020, 276, 123203. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene oxide-impregnated PVA-STA composite polymer electrolyte membrane separator for power generation in a single-chambered microbial fuel cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Akel, M. Fabrication of Zr MOF-doped polyvinylidene fluoride membranes and testing in H-type microbial fuel cell. Int. J. Hydrogen Energy 2024, 54, 1264–1272. [Google Scholar] [CrossRef]
- Venkatesan, P.N.; Dharmalingam, S. Characterization and performance study of phase inversed Sulfonated Poly Ether Ether Ketone—Silico tungstic composite membrane as an electrolyte for microbial fuel cell applications. Renew. Energy 2017, 102, 77–86. [Google Scholar] [CrossRef]
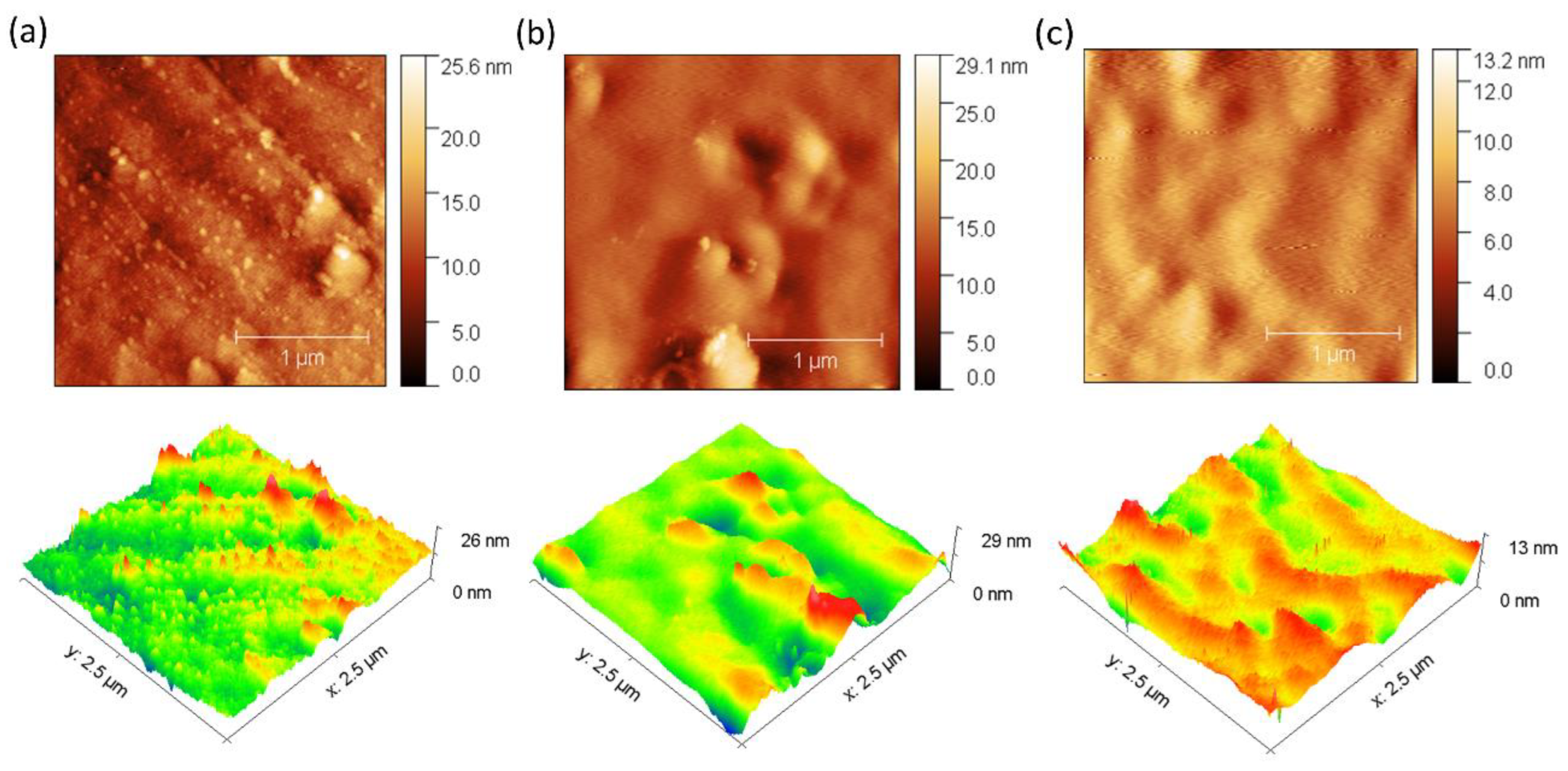
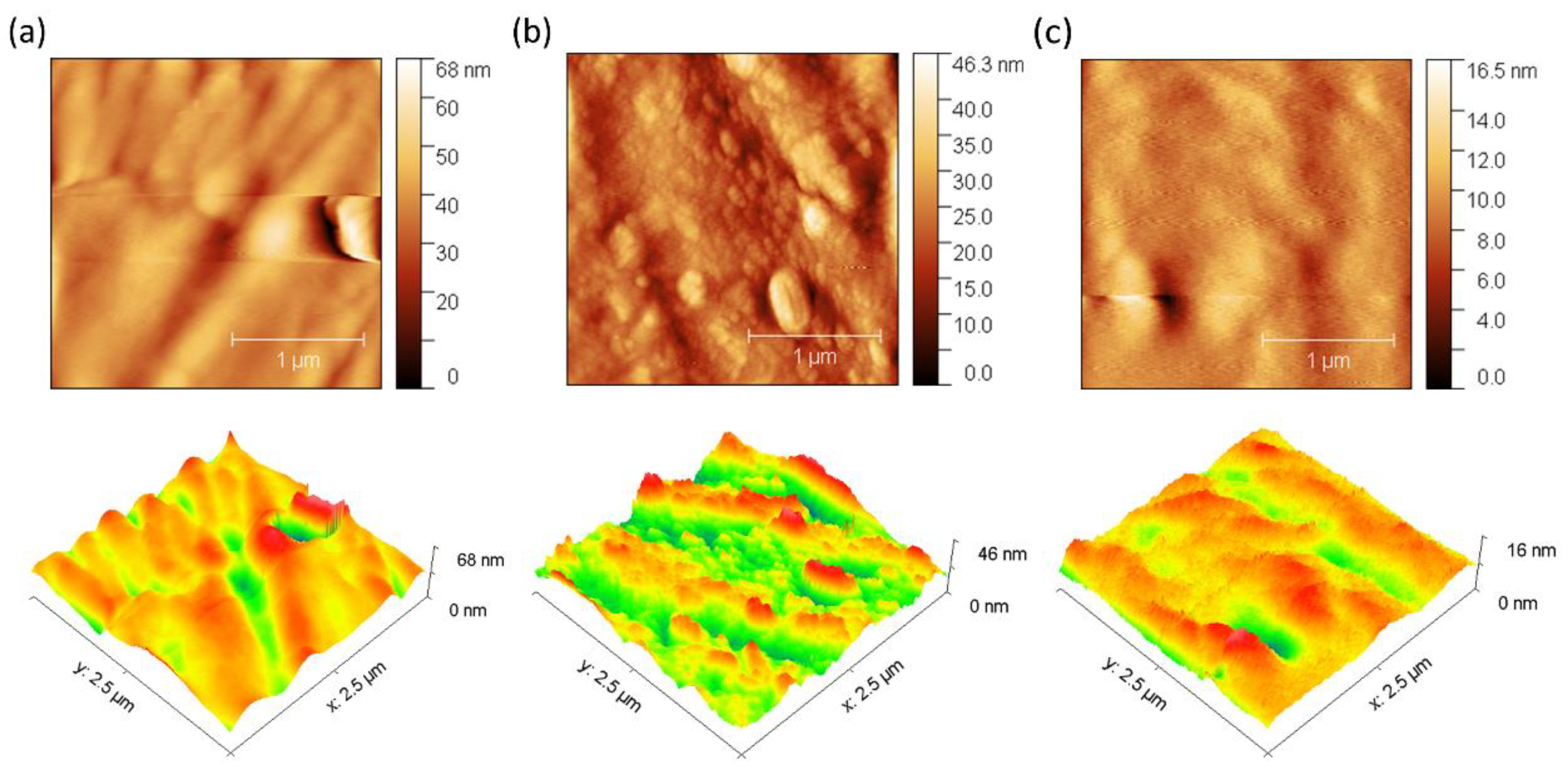
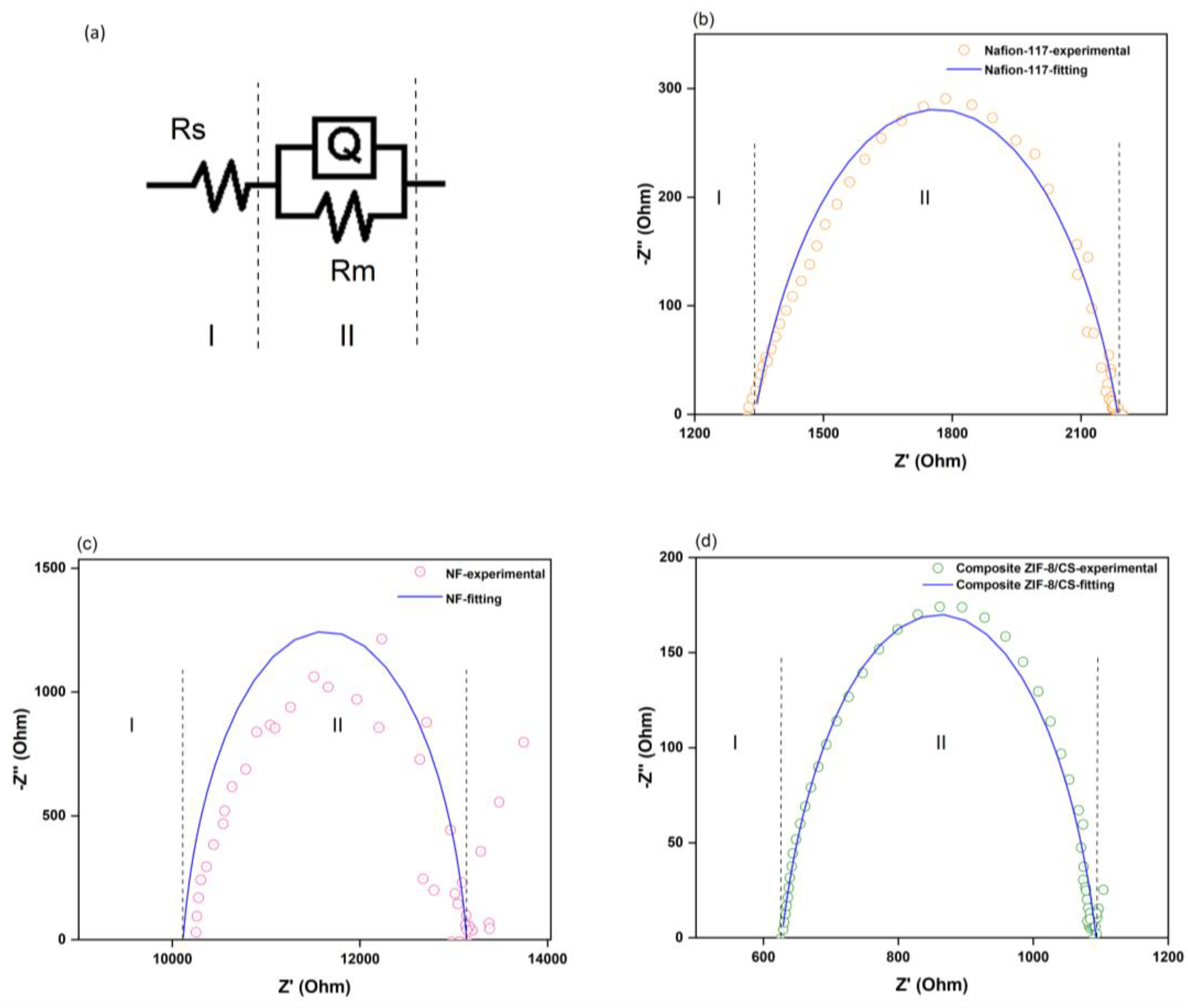
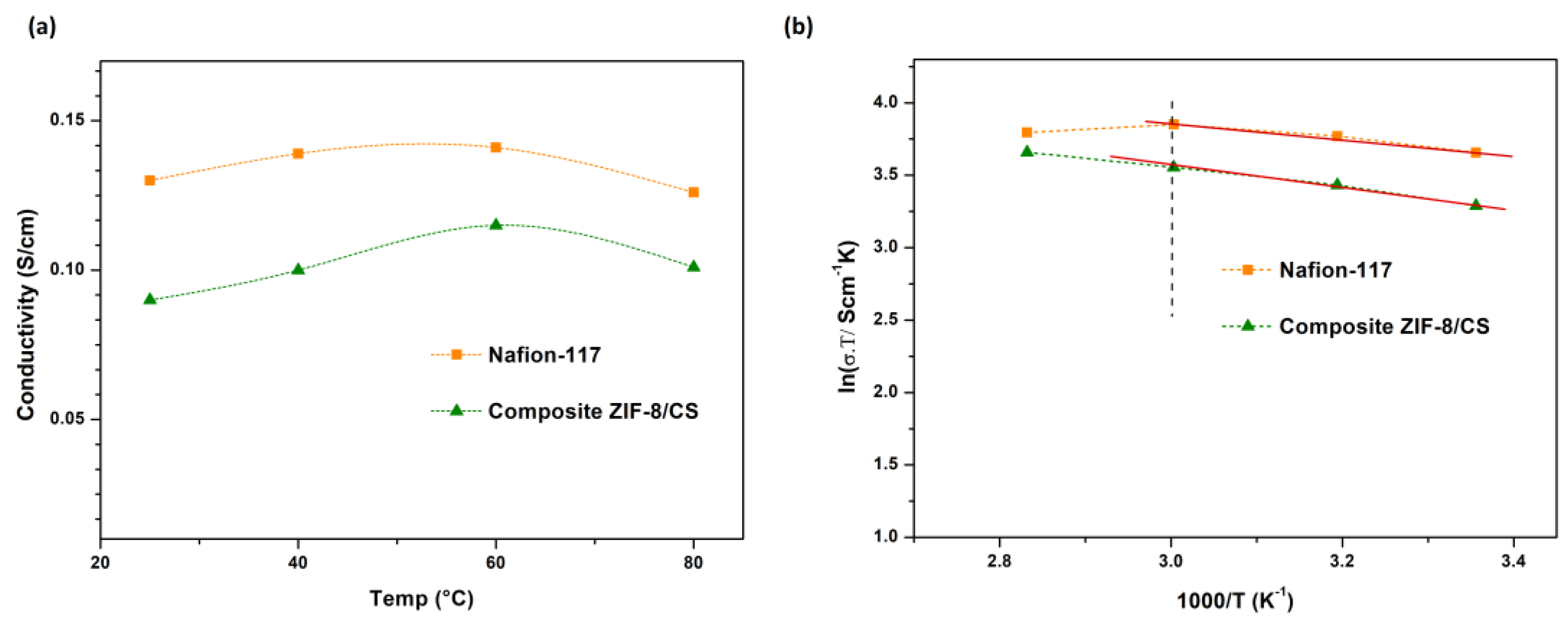
| Parameter | Membranes | ||
|---|---|---|---|
| Nafion-117 | NF | Composite ZIF-8/CS | |
| Mean roughness (Sa, nm) | 1.54 before → 4.30 after | 1.98 before → 4.30 after | 0.85 before → 1.07 after |
| Change in Sa (%) | 179.48 | 117.12 | 25.88 |
| Maximum height (Sz, nm) | 22.10 before → 67.90 after | 29.14 before → 46.35 after | 13.05 before → 16.45 after |
| Change in Sz (%) | 207.30 | 59.01 | 26.02 |
| Parameter | Membranes | ||
|---|---|---|---|
| Nafion-117 | NF | Composite ZIF-8/CS | |
| Membrane resistance (Rm, Ω) | 844.7 | 3023.0 | 465.8 |
| Chi-squared (χ2, Fit) | 1.7 × 10−4 | 6.9 × 10−3 | 8.2 × 10−5 |
| conductivity (σ, Scm−1) | 0.13 | 1.3 × 10−2 | 0.09 |
| Study | Membrane | Thickness (µm) | Water Uptake (%) | Ionic Conductivity (S·cm−1) | Main Advantages |
|---|---|---|---|---|---|
| This work | PAN/Chitosan/ZIF-8 | 120 | 160.00 | 0.09 | Low surface resistance, excellent antifouling properties (BSA and salts), and high oxidative stability |
| [58] | Nafion 117 | 200 | 15.87 | 0.10 | Low surface resistance, good mechanical strength, and high oxidative stability |
| [59] | Chitosan/MWCNT | 240 | 0.7 | – | Biocompatibility, low surface resistance, mechanical and thermal stability |
| [60] | PVA/STA/GO | 112 | – | 0.035 | Mechanical strength, thermal stability, and hydrophilicity |
| [61] | Zr-MOF/PVDF | – | – | – | Improved ion selectivity and proton conductivity from Zr-MOF; PVDF provides chemical and mechanical stability |
| [62] | SPEEK/STA | 190 | 21.28 | 1.54 × 10−3 | High proton conductivity, oxidative stability, and hydrophilicity |
| [37] | Sulfonated PSEBS/ sulfonated SiO2 | 120 | 40 | 3.21 × 10−2 | Excellent proton conductivity and membrane stability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pupiales, H.; Soria, R.B.; Arboleda, D.; Cevallos, C.; Alcívar, C.; Francis, L.; Xu, X.; Luis, P. ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems. Membranes 2025, 15, 282. https://doi.org/10.3390/membranes15090282
Pupiales H, Soria RB, Arboleda D, Cevallos C, Alcívar C, Francis L, Xu X, Luis P. ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems. Membranes. 2025; 15(9):282. https://doi.org/10.3390/membranes15090282
Chicago/Turabian StylePupiales, Henry, Raúl Bahamonde Soria, Daniel Arboleda, Carlos Cevallos, Christian Alcívar, Laurent Francis, Xiao Xu, and Patricia Luis. 2025. "ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems" Membranes 15, no. 9: 282. https://doi.org/10.3390/membranes15090282
APA StylePupiales, H., Soria, R. B., Arboleda, D., Cevallos, C., Alcívar, C., Francis, L., Xu, X., & Luis, P. (2025). ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems. Membranes, 15(9), 282. https://doi.org/10.3390/membranes15090282








