Zero Liquid Discharge of High-Salinity Produced Water via Integrated Membrane Distillation and Crystallization: Experimental Study and Techno-Economic Analysis
Abstract
1. Introduction
2. Methods
2.1. Materials and Characterization
2.1.1. Materials
2.1.2. Membrane Modules Preparation
2.2. Direct Contact Membrane Distillation and Crystallization (DCMD-Cr)
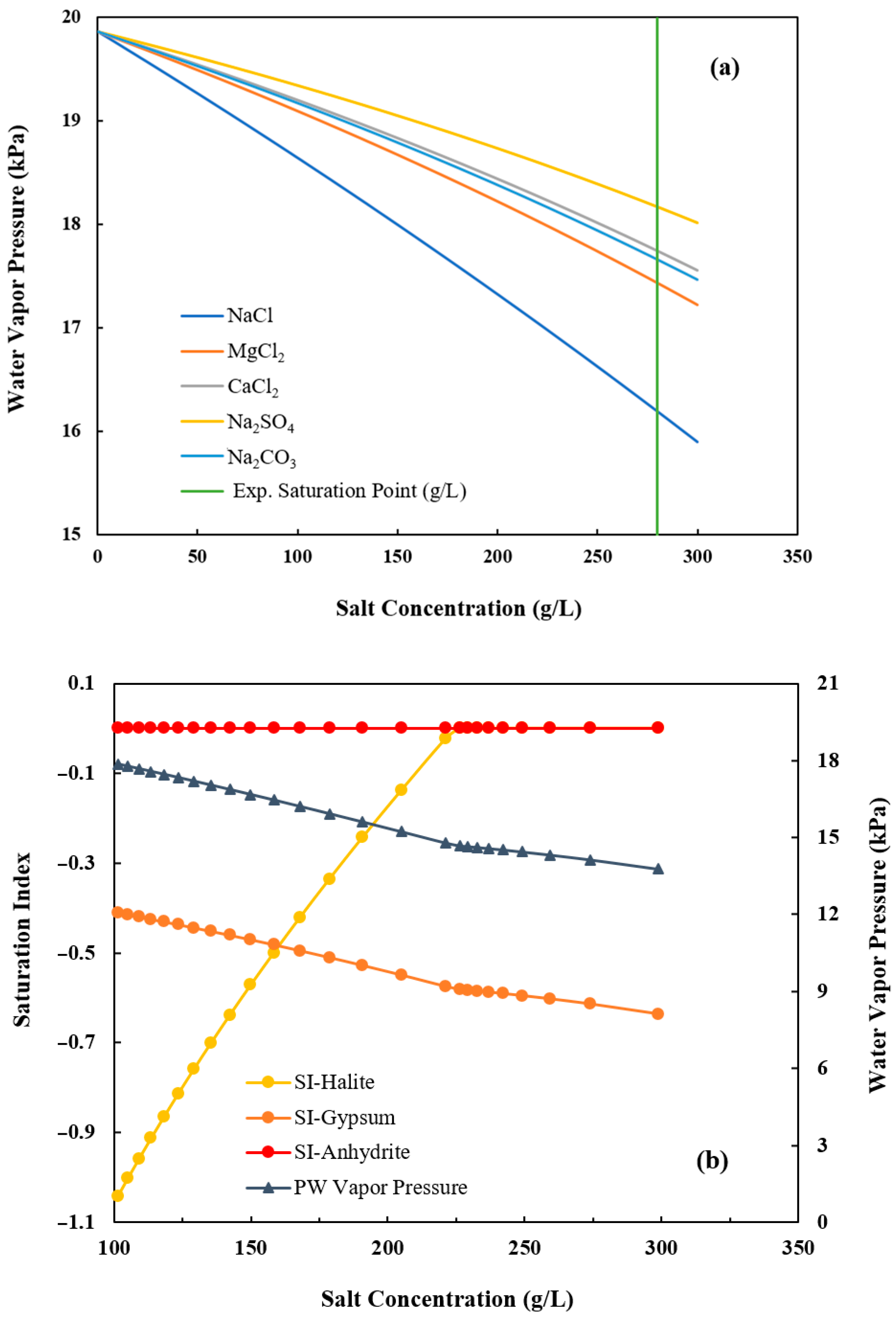
2.3. PHREEQC Modeling
2.4. Techno-Economic Analysis
3. Results and Discussion
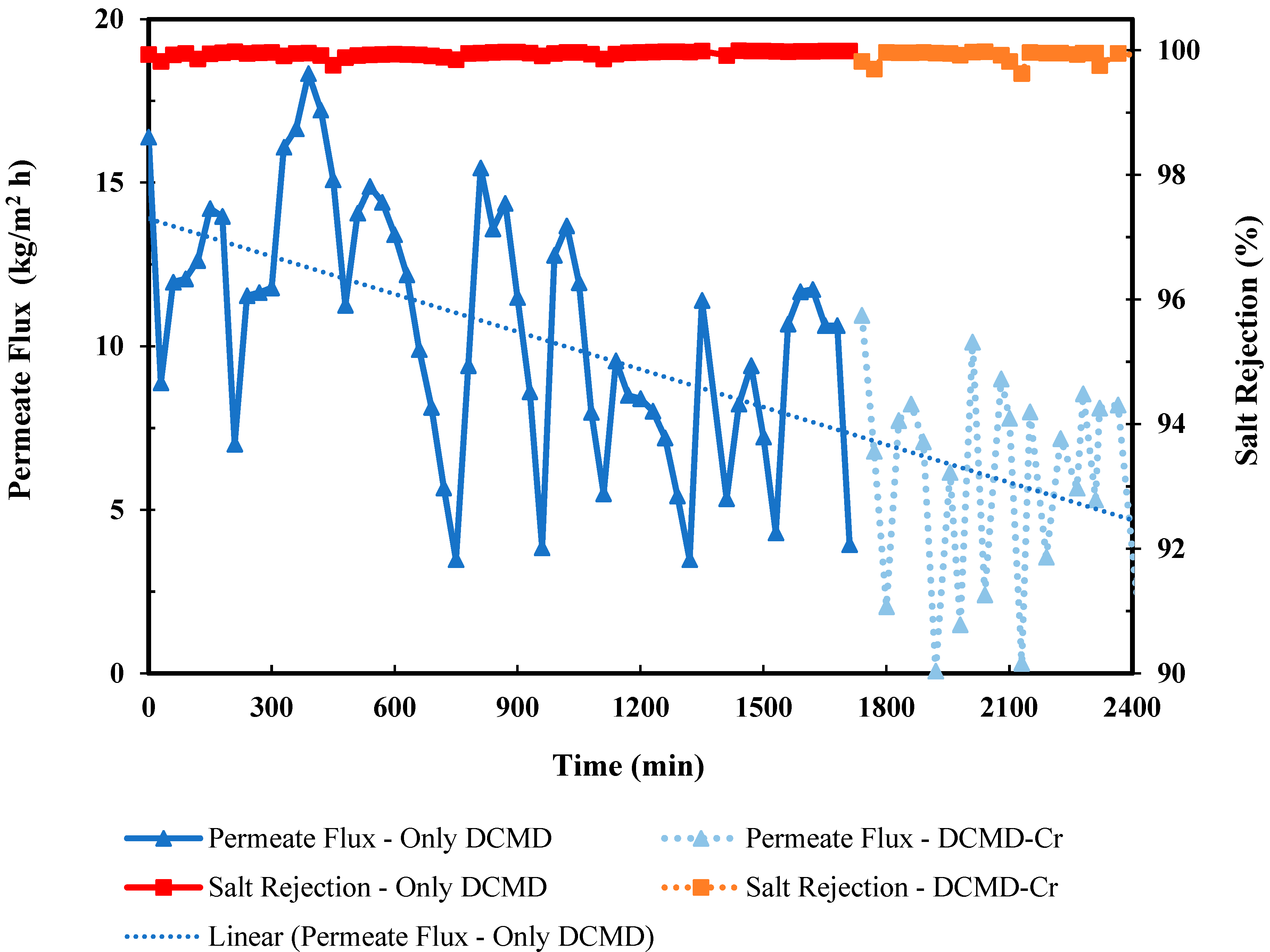
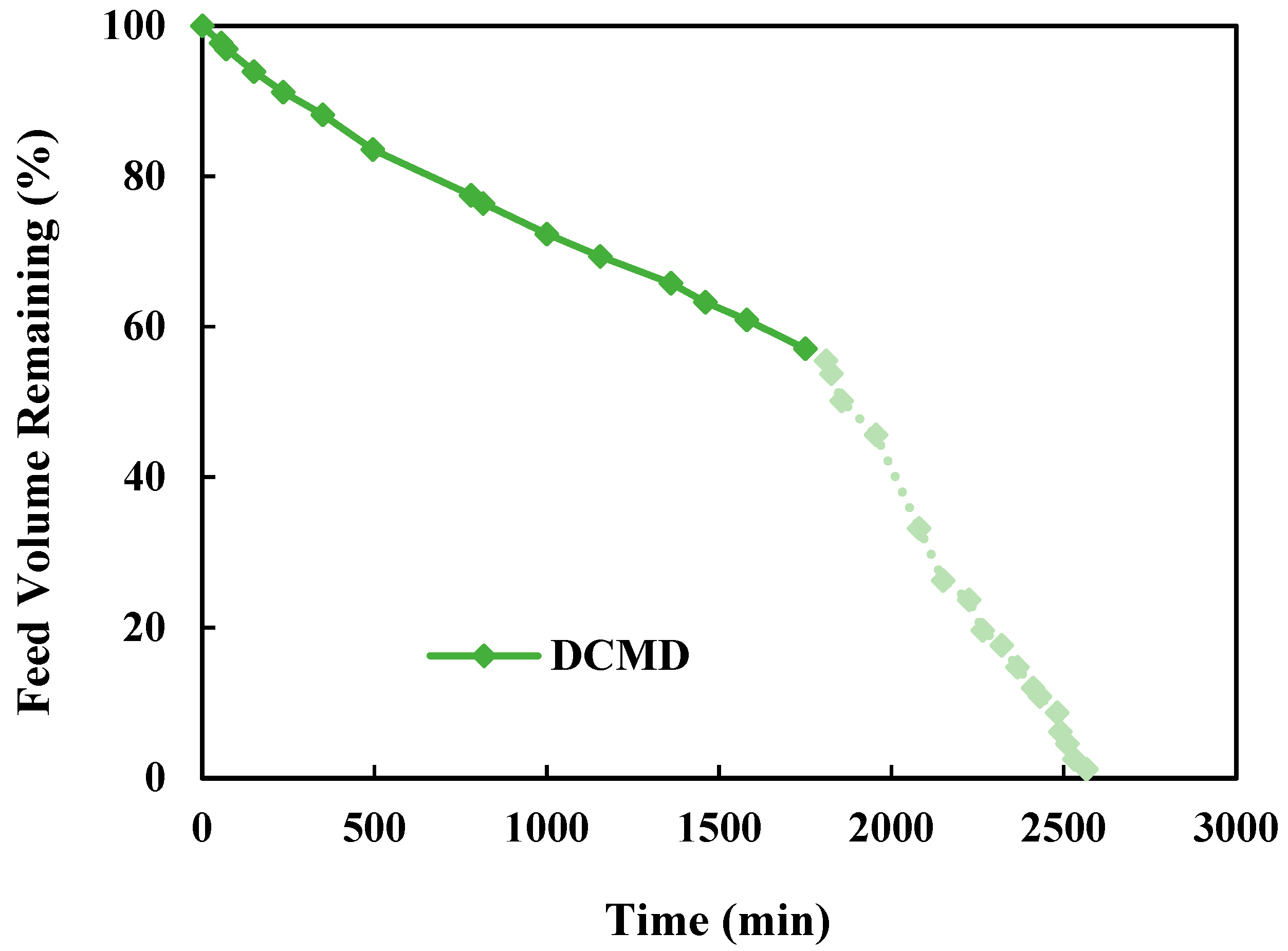
3.1. DCMD-Cr Performance Evaluation
3.1.1. Permeate Water Flux and Salt Rejection
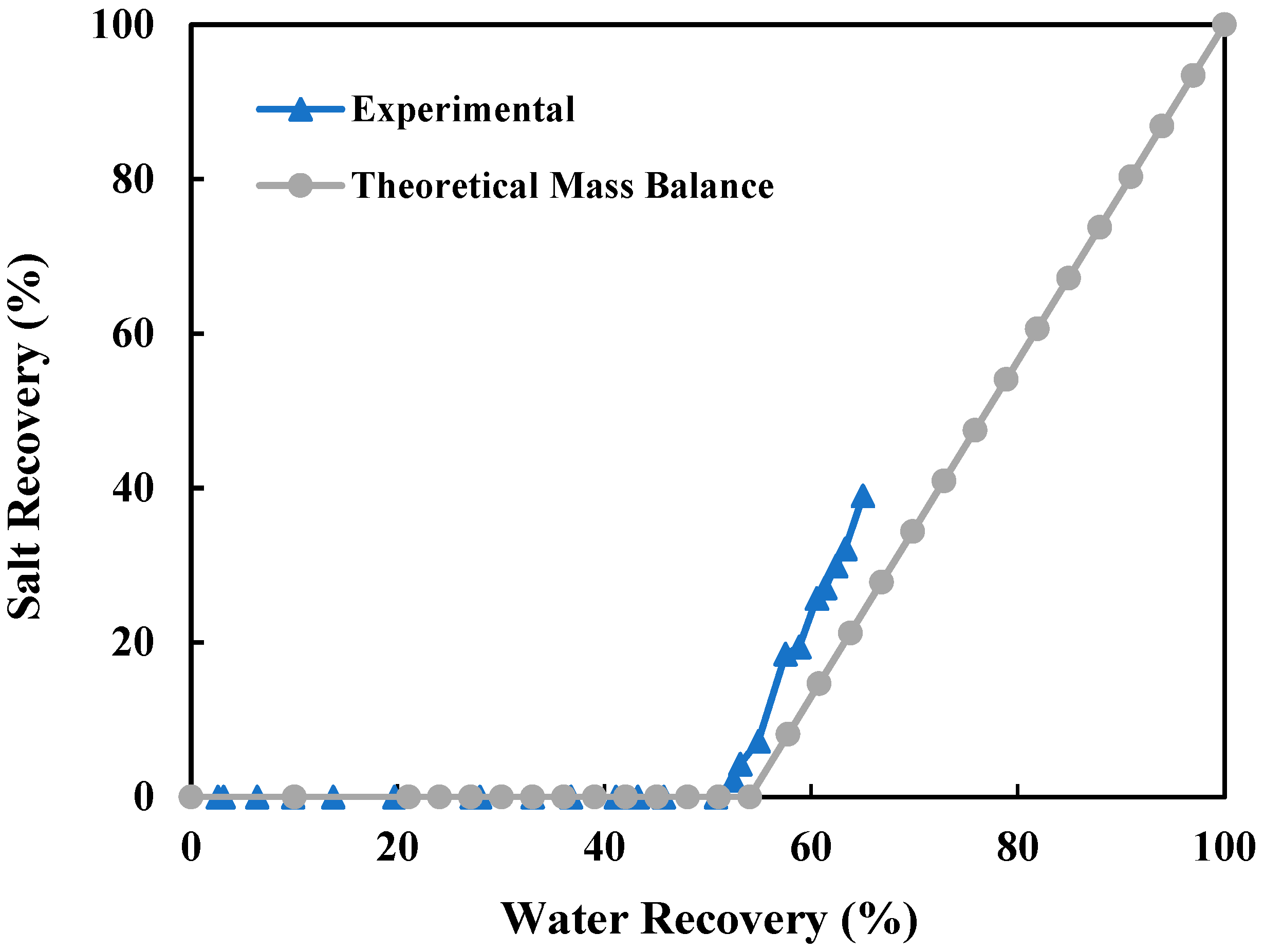
3.1.2. Overall Water and Salt Recovery
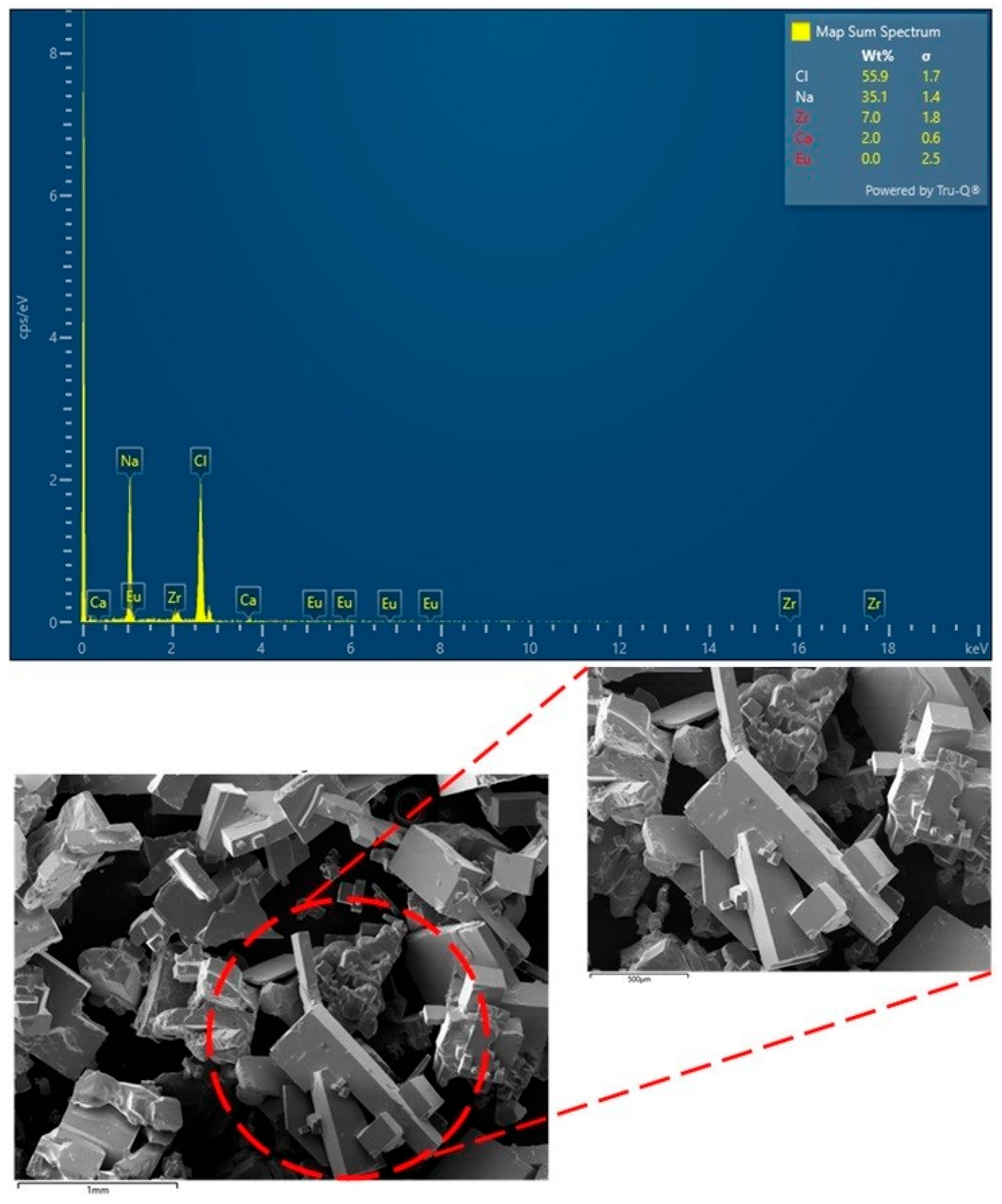
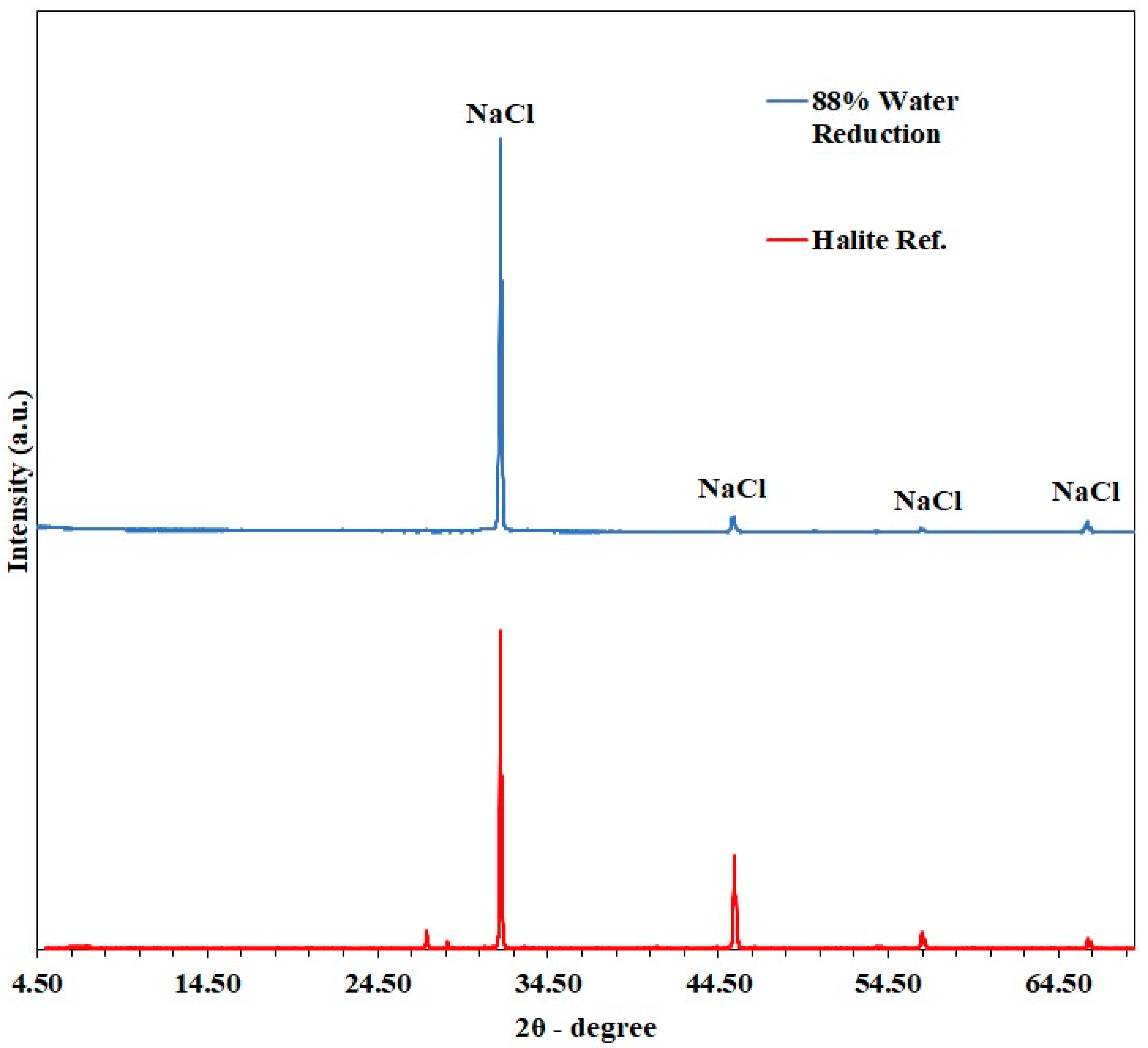
3.1.3. Salt Characterization
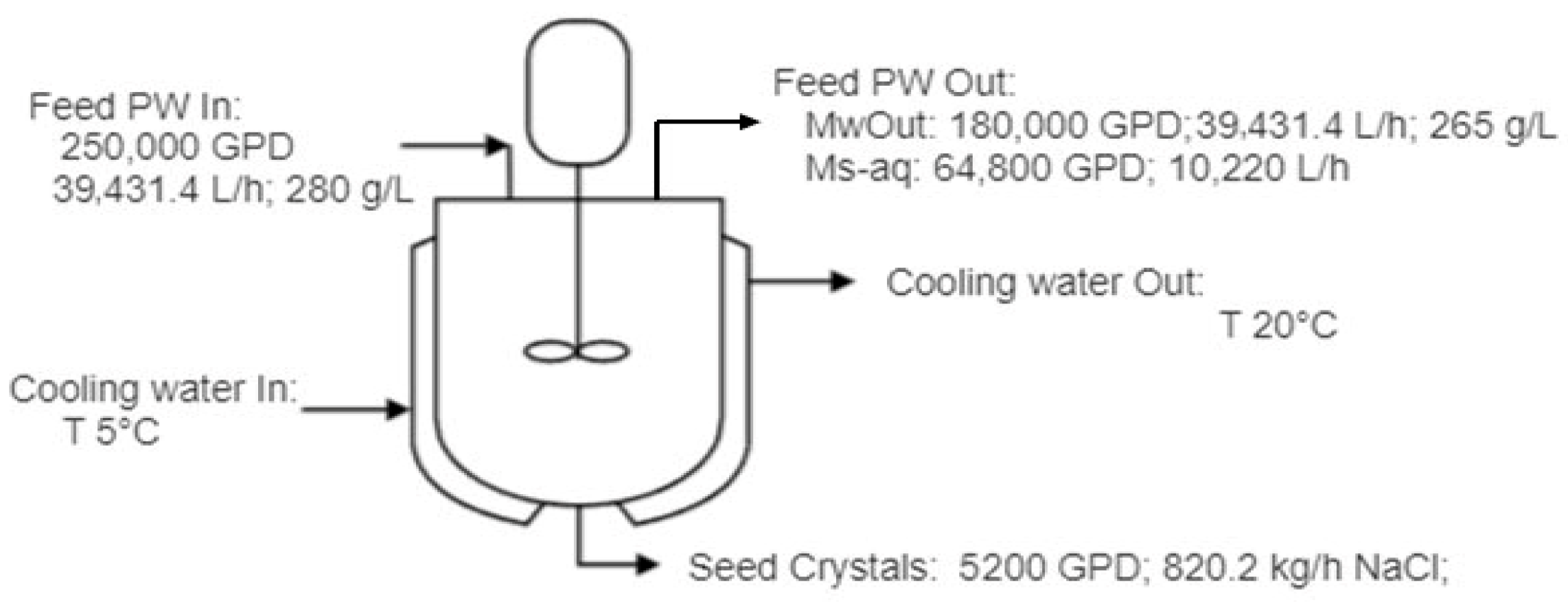
3.2. Optimization and Scale-Up
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Salmi, M.; Laqbaqbi, M.; Al-Obaidani, S.; Al-Maamari, R.S.; Khayet, M.; Al-Abri, M. Application of membrane distillation for the treatment of oil field produced water. Desalination 2020, 494, 114678. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Hong, S. Recovery of water and minerals from shale gas produced water by membrane distillation crystallization. Water Res. 2018, 129, 447–459. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Yogarathinam, L.T.; Zulhairun, A.K.; Ismail, A.F. Current advances in membrane technologies for produced water desalination. Desalination 2020, 493, 114643. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J. Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery—Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar] [CrossRef]
- Hou, D.; Ding, C.; Fu, C.; Wang, D.; Zhao, C.; Wang, J. Electrospun nanofibrous omniphobic membrane for anti-surfactant-wetting membrane distillation desalination. Desalination 2019, 468, 114068. [Google Scholar] [CrossRef]
- El-badawy, T.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.; Adam, M.R.; Tai, Z.S.; Ravi, J.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; et al. Progress in treatment of oilfield produced water using membrane distillation and potentials for beneficial re-use. Sep. Purif. Technol. 2021, 278, 119494. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, J.; Chung, T.-S. Integration of membrane distillation (MD) and solid hollow fiber cooling crystallization (SHFCC) systems for simultaneous production of water and salt crystals. J. Membr. Sci. 2018, 564, 905–915. [Google Scholar] [CrossRef]
- Zarkadas, D.M.; Sirkar, K.K. Solid Hollow Fiber Cooling Crystallization. Ind. Eng. Chem. Res. 2004, 43, 7163–7180. [Google Scholar] [CrossRef]
- Charcosset, C.; Kieffer, R.; Mangin, D.; Puel, F. Coupling between Membrane Processes and Crystallization Operations. Ind. Eng. Chem. Res. 2010, 49, 5489–5495. [Google Scholar] [CrossRef]
- Das, P.; Dutta, S.; Singh, K.K. Insights into membrane crystallization: A sustainable tool for value added product recovery from effluent streams. Sep. Purif. Technol. 2021, 257, 117666. [Google Scholar] [CrossRef]
- Balis, E.; Griffin, J.C.; Hiibel, S.R. Membrane Distillation-Crystallization for inland desalination brine treatment. Sep. Purif. Technol. 2022, 290, 120788. [Google Scholar] [CrossRef]
- Creusen, R.; van Medevoort, J.; Roelands, M.; van Renesse van Duivenbode, A.; Hanemaaijer, J.H.; van Leerdam, R. Integrated membrane distillation–crystallization: Process design and cost estimations for seawater treatment and fluxes of single salt solutions. Desalination 2013, 323, 8–16. [Google Scholar] [CrossRef]
- Shin, Y.; Sohn, J. Mechanisms for scale formation in simultaneous membrane distillation crystallization: Effect of flow rate. J. Ind. Eng. Chem. 2016, 35, 318–324. [Google Scholar] [CrossRef]
- Lou, X.Y.; Xu, Z.; Bai, A.P.; Resina-Gallego, M.; Ji, Z.G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes 2020, 10, 19. [Google Scholar] [CrossRef]
- Zhang, X.; Koirala, R.; Pramanik, B.; Fan, L.; Zhang, Y.; Date, A.; Jegatheesan, V. Performance of membrane distillation assisted crystallization and crystal characteristics for resource recovery from desalination brine. Desalination 2024, 574, 117244. [Google Scholar] [CrossRef]
- Ji, X.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Mondal, S.; Macedonio, F.; Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 2016, 505, 167–173. [Google Scholar] [CrossRef]
- Nathoo, J.; Randall, D.G. Thermodynamic modelling of a membrane distillation crystallisation process for the treatment of mining wastewater. Water Sci. Technol. 2015, 73, 557–563. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Jia, F.; Li, J.; Wang, J. Recovery of boric acid from the simulated radioactive wastewater by vacuum membrane distillation crystallization. Ann. Nucl. Energy 2017, 110, 1148–1155. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Sørensen, J.M.; Svenstrup, A.; Scarpa, L.; Carlsen, T.S.; Jensen, H.C.; Wybrandt, L.; Christensen, M.L. Membrane crystallization for phosphorus recovery and ammonia stripping from reject water from sludge dewatering process. Desalination 2018, 440, 156–160. [Google Scholar] [CrossRef]
- Shi, W.; Li, T.; Tian, Y.; Li, H.; Fan, M.; Zhang, H.; Qin, X. An innovative hollow fiber vacuum membrane distillation-crystallization (VMDC) coupling process for dye house effluent separation to reclaim fresh water and salts. J. Clean. Prod. 2022, 337, 130586. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.-S. Development of simultaneous membrane distillation–crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Tun, C.M.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membrane distillation crystallization of concentrated salts—Flux and crystal formation. J. Membr. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Nariyoshi, Y.N.; Pantoja, C.E.; Seckler, M.M. Evaluation of sodium chloride crystallization in membrane distillation crystallization applied to water desalination. Braz. J. Chem. Eng. 2016, 33, 675–690. [Google Scholar] [CrossRef][Green Version]
- Ruiz Salmón, I.; Luis, P. Membrane crystallization via membrane distillation. Chem. Eng. Process. Process Intensif. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Seriche, G.; Barros, L.; González, C.; Romero, J.; Ruby-Figueroa, R.; Santoro, S.; Curcio, E.; Estay, H. Water recovery assessment from hypersaline lithium-rich brines using Membrane Distillation-Crystallization. Desalination 2022, 537, 115887. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, M.; Hu, X.; Chang, H.; Yan, Z.; Liang, Y.; Meng, Y.; Liang, H. Evaluation of the performance of ultrasound-assisted membrane distillation crystallization process for water and sodium chloride recovery in hypersaline solution. Desalination 2022, 531, 115727. [Google Scholar] [CrossRef]
- Saud, A.; Ali, A.; Quist-Jensen, C.A. Membrane distillation crystallization’s parametric analysis for magnesium sulphate crystallization from simulated nanofiltration brine. Sep. Purif. Technol. 2025, 376, 133852. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization control via membrane distillation-crystallization: A review. Desalination 2021, 519, 115315. [Google Scholar] [CrossRef]
- Hsieh, I.M.; Lin, B.; Mahbub, H.; Carter, Z.; Jebur, M.; Cao, Y.; Brownlow, J.; Wickramasinghe, R.; Malmali, M. Field demonstration of intensified membrane distillation for treating oilfield produced waters from unconventional wells. Desalination 2023, 564, 116771. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes 2015, 5, 772–792. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous recovery and crystallization control of saline organic wastewater by membrane distillation crystallization. AIChE J. 2017, 63, 2187–2197. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Tung, K.-L.; Ward, J.D. Design and economic analysis of membrane-assisted crystallization processes. J. Taiwan Inst. Chem. Eng. 2017, 81, 159–169. [Google Scholar] [CrossRef]
- von Eiff, D.; Wong, P.W.; Gao, Y.; Jeong, S.; An, A.K. Technical and economic analysis of an advanced multi-stage flash crystallizer for the treatment of concentrated brine. Desalination 2021, 503, 114925. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Ruiz Salmón, I.; Luis, P. Economic evaluation of salt recovery from wastewater via membrane distillation-crystallization. Sep. Purif. Technol. 2020, 235, 116075. [Google Scholar] [CrossRef]
- Ali, A.; Hvid Jacobsen, J.; Casper Jensen, H.; Lykkegaard Christensen, M.; Quist-Jensen, C.A. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Appl. Sci. 2019, 9, 287. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, X.; Gusnawan, P.; Zhang, G.; Yu, J. Crosslinked PVDF based hydrophilic-hydrophobic dual-layer hollow fiber membranes for direct contact membrane distillation desalination: From the seawater to oilfield produced water. J. Membr. Sci. 2021, 619, 118802. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PhreeqC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Report 6 A43; U.S. Geological Survey: Denver, CO, USA, 2013.
- Global Water Intelligence. Zero Liquid Discharge—Maximum Recovery and Why It Finally Makes Sense; Global Water Intelligence: Oxford, UK, 2019. [Google Scholar]
- Ettouney, H.M.; El-Dessouky, H.T.; Faibish, R.S.; Gowin, P.J. Evaluating the Economics of Desalination. Chem. Eng. Prog. 2002, 98, 32–39. [Google Scholar]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. A techno-economic assessment of membrane distillation for treatment of Marcellus shale produced water. Desalination 2017, 416, 24–34. [Google Scholar] [CrossRef]


| MDC | Crystallization | Membrane Characteristics | Feed | Permeate | Results | References | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Temp. (°C) | Material | Pore Size (µm) | Type | Temp. (°C) | TDS (ppm) | Flow Rate | Type | Temp. (°C) | TDS (ppm) | Flow Rate | Permeate Flux | ZLD | Type of Salt Recovered | Total Cost | ||
| DCMD-Cr | Cooling | 20 | PVDF- HF | 0.27 | Oilfield PW | 60 | 156,700 | 100 rpm | DI | 20 | - | 40 rpm | 1.5–7.5 kg/m2h | ✓ | NaCl | 1.41 0.64 (w/waste heat) USD/barrel | This study |
| DCMD-Cr | Evaporation | - | PP and PVDF -HF | 0.2, 0.23 | Oilfield PW | 35 45 55 | 248,000 | 150–250 mL/min | DI | 10 | - | 70 mL/min | 1–3.4 L/m2h | ✕ | NaCl | (w/salt sale) 1.52, 1.24 USD/m3 | [37] |
| DCMD-Cr | Evaporation and Cooling | 40 | PTFE-FS | 0.22 | Synthetic SGPW | 60 | 150,000 | CFV 0.25 m/s | DI | 20 | - | CFV 0.25 m/s | - | ✓ | BaCl2, CaCO3, NaCl | [38] | |
| DCMD-Cr | Cooling | 10 | PP-HF | 0.17 | Oilfield PW | 55–75 | 116,800 | - | DI | - | - | - | 1.5–2.5 kg/m2h | ✕ | NaCl | - | [39] |
| DCMD-Cr | Cooling | 15 | PP-HF | 0.2 | PW | 38 | 240,000 | 200 L/h | DI | 25 | - | - | 2.5 L/m2h | ✕ | - | - | [11] |
| DCMD-Cr | Evaporation and Cooling | 30–50 | PP-HF | 0.22 | SGPW | 60 | 30,000 | 1.2–3.6 L/min | DI | 20 | - | CFV 0.1–0.3 m/s | 2.5 kg/m2h | ✓ | NaCl CaCO3 | - | [2] |
| OMD-Cr | Evaporative | - | PTFE-PES | - | CaCO3 NaCl Solution | 77–80 64–78 | 70–72 70–76 | 4–8 L/min | DI | 70–72 70–76 | - | 4–8 L/min | 6–16 1–30 kg/m2h | ✓ | CaCO3 NaCl | 1.09/m3 | [17] |
| VCMD-Cr | Cooling | 20 | PP-HF | 0.18 | Radioactive wastewater | 20–70 | 500–110,000 | 41.8 L/h | Vacuum | - | - | 0.97 atm | 6.2–5.5 kg/m2h | ✕ | Boric Acid | - | [25] |
| DCMD-Cr | Cooling | - | PP-HF | 0.2 | Anodizing wastewater | 38–56 | 887,000 | 21.6 L/h | DI | 15 | - | 0.8–1.7 m/s | 1.29–3.86 kg/m2h | ✓ | Na2SO4 | - | [43] |
| DCMD-Cr | Evaporative and Seeding | - | PP | 0.2 | Sludge dewatering reject | 45–65 | 1391– 2345 | 20 L/h | - | - | - | - | 0.0013–0.0105 kg/m2h | ✕ | Phosphorous | - | [26] |
| DCMD-Cr | Evaporation | 60 | PVDF | 0.22 | Seawater | 60 | 65,000 | 200–1250 mL/min | DI | 20 | - | - | 3.8 kg/m2h | ✕ | - | - | [18] |
| Property | Species | Original PW Values |
|---|---|---|
| Cations (ppm) | Sodium Na+ | 49,958 |
| Potassium K+ | 893 | |
| Magnesium Mg2+ | 1132 | |
| Calcium Ca2+ | 10,724 | |
| Ammonium NH4+ | 1128 | |
| Lithium Li+ | 28 | |
| Anions (ppm) | Chloride Cl− | 96,560 |
| Bromide Br− | 1094 | |
| Sulfate, SO42− | 544 | |
| Alkalinity (ppm) | HCO3− | 98 |
| Total Dissolved Solids (TDS) (ppm) | 156,700 | |
| pH | 7.24 |
| Properties | Value | Units |
|---|---|---|
| Porosity | 80.6 ± 0.4 | % |
| Thickness | 135 ± 2 | µm |
| Diameter Outer | 1088 ± 2 | µm |
| Diameter Inner | 767 ± 2 | µm |
| Pore Size | 0.27 ± 0.02 | µm |
| Bubble Point Pore Size | 0.40 ± 0.02 | µm |
| Configuration | Hollow Fiber (HF) | - |
| Module Length | 22 | cm |
| Number of Fibers in the Module | 6 | - |
| Value | Units | |
|---|---|---|
| Plant Capacity | 500,000 (78,846) | GPD (kg/h) |
| Plant Lifetime | 30 | Year |
| Plant Availability | 90 | % |
| Produced Water, Initial TDS | 100,000 | mg/L |
| Produced Water, Concentrate TDS | 280,000 | mg/L |
| Recovered Product Water | <50 | mg/L |
| Water Recovery | 90 | % |
| Membrane Cost | 20 | USD/m2 |
| Membrane Replacement | 20 | %/year |
| Pre-Treatment | 80 | USD/m3/day |
| Electricity Cost | 0.069 | USD/kWh |
| Steam Price | 0.008 | USD/kg |
| Scenario | Capital Cost (USD/bbl.) | Operating Cost (USD/bbl.) | Total Cost (USD/bbl.) | ||
|---|---|---|---|---|---|
| MD | Crystallization | MD | Crystallization | ||
| (1) | 0.08 | 0.04 | 0.79 | 0.50 | 1.41 |
| (2) | 0.08 | 0.04 | 0.02 | 0.50 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, G.T.; He, Z.; Kessie, J.; Yu, J. Zero Liquid Discharge of High-Salinity Produced Water via Integrated Membrane Distillation and Crystallization: Experimental Study and Techno-Economic Analysis. Membranes 2025, 15, 281. https://doi.org/10.3390/membranes15090281
Fernandez GT, He Z, Kessie J, Yu J. Zero Liquid Discharge of High-Salinity Produced Water via Integrated Membrane Distillation and Crystallization: Experimental Study and Techno-Economic Analysis. Membranes. 2025; 15(9):281. https://doi.org/10.3390/membranes15090281
Chicago/Turabian StyleFernandez, Gabriela Torres, Zongjie He, Jeremiah Kessie, and Jianjia Yu. 2025. "Zero Liquid Discharge of High-Salinity Produced Water via Integrated Membrane Distillation and Crystallization: Experimental Study and Techno-Economic Analysis" Membranes 15, no. 9: 281. https://doi.org/10.3390/membranes15090281
APA StyleFernandez, G. T., He, Z., Kessie, J., & Yu, J. (2025). Zero Liquid Discharge of High-Salinity Produced Water via Integrated Membrane Distillation and Crystallization: Experimental Study and Techno-Economic Analysis. Membranes, 15(9), 281. https://doi.org/10.3390/membranes15090281





