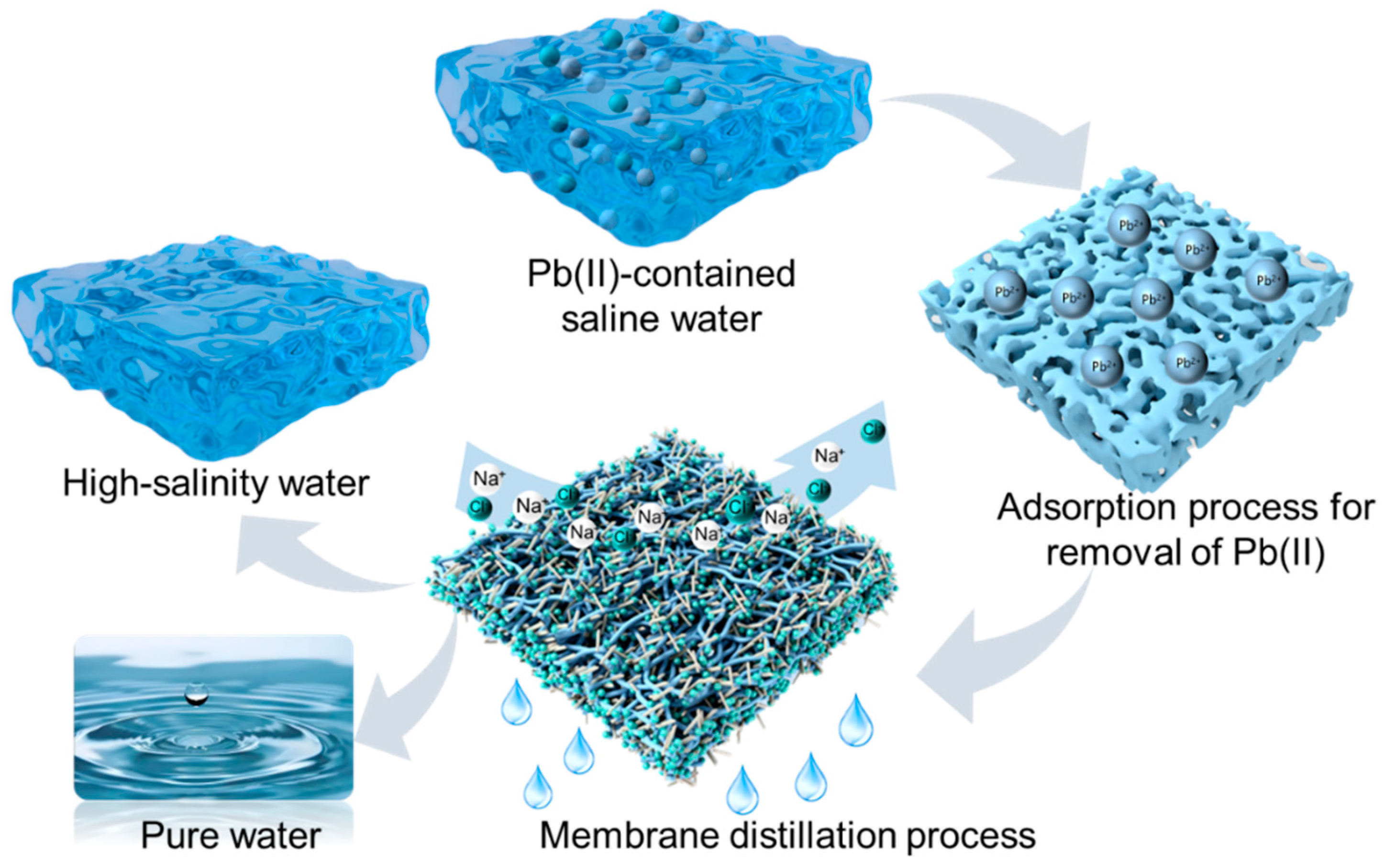
Synergistic Adsorption–Membrane Distillation for Heavy Metal Extraction and Water Reclamation from Saline Waste Streams
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of FeOOH Nanopowders
2.3. Fabrication of FeOOH@PVDF Membrane
2.4. Characterization of the Adsorption Membrane
2.5. Adsorption Characterization of the Simulated Waste Water
2.6. MD Characterization of the Simulated Waste Water
3. Results and Discussion
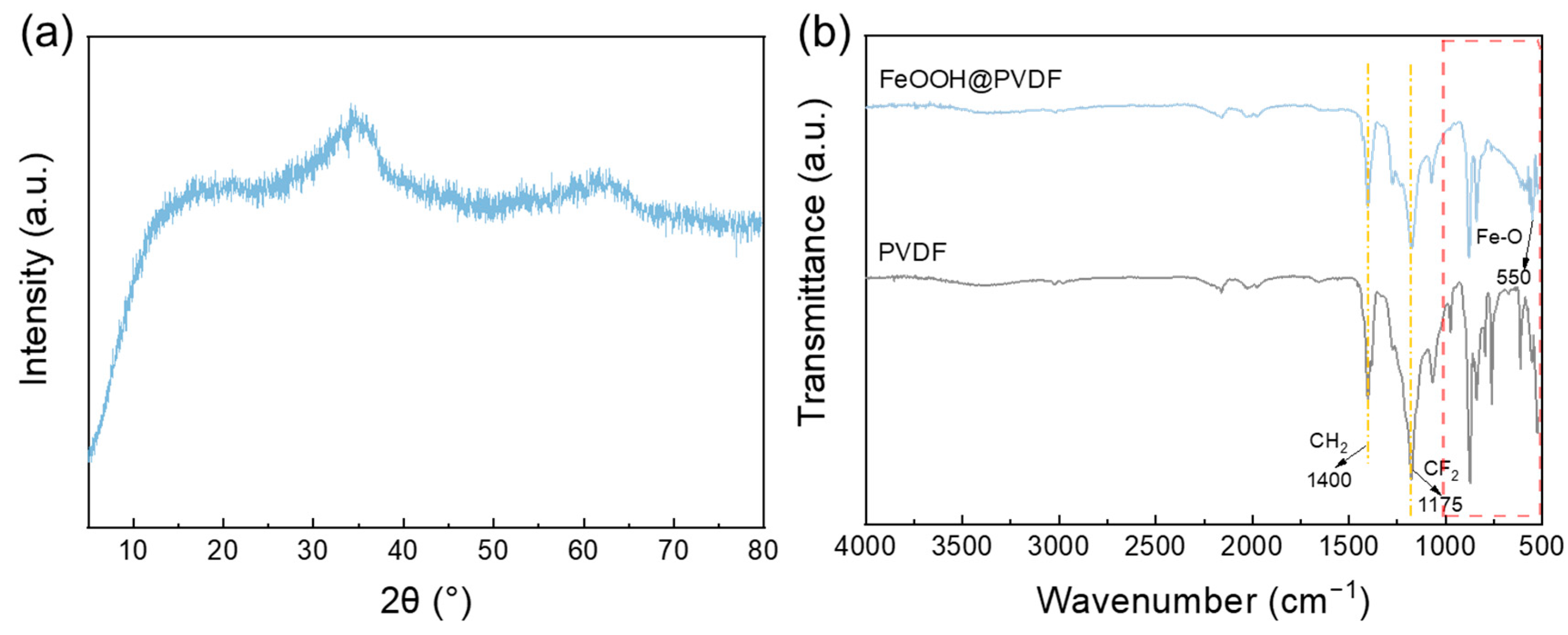
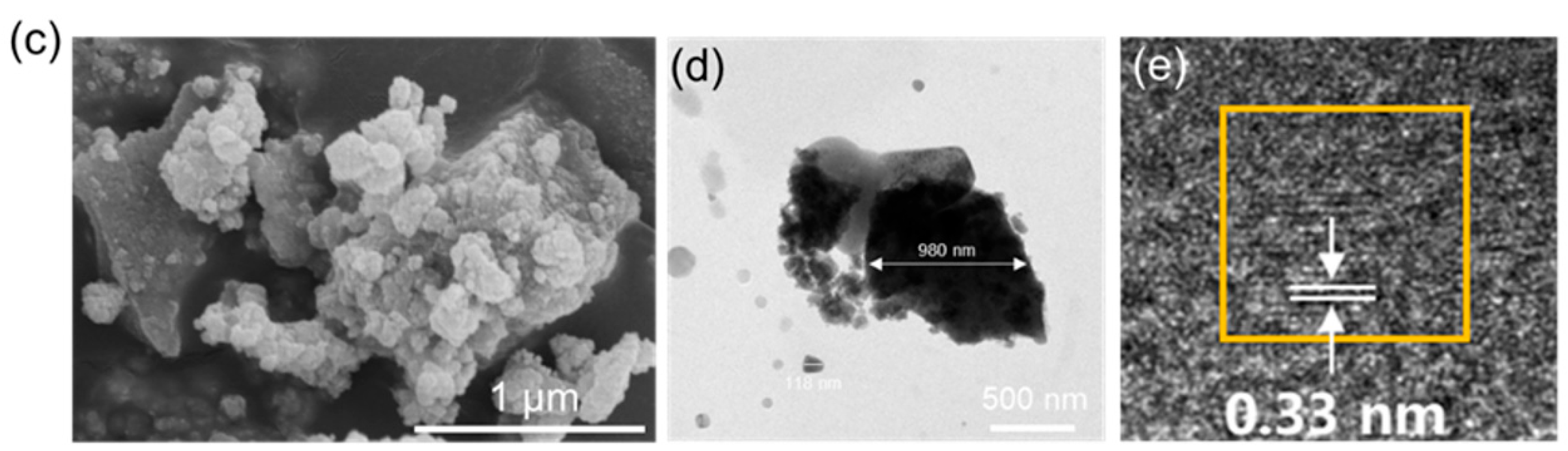
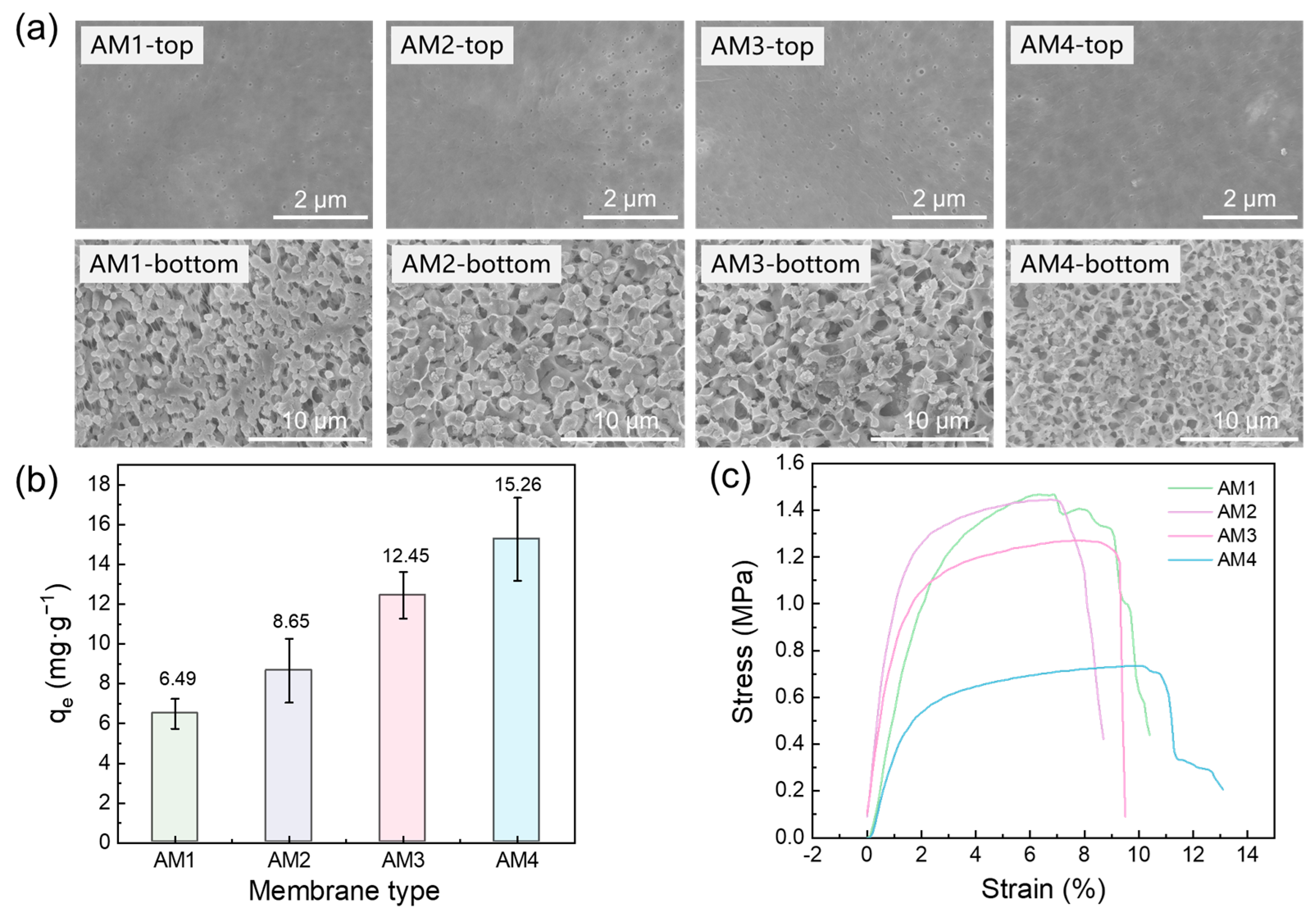
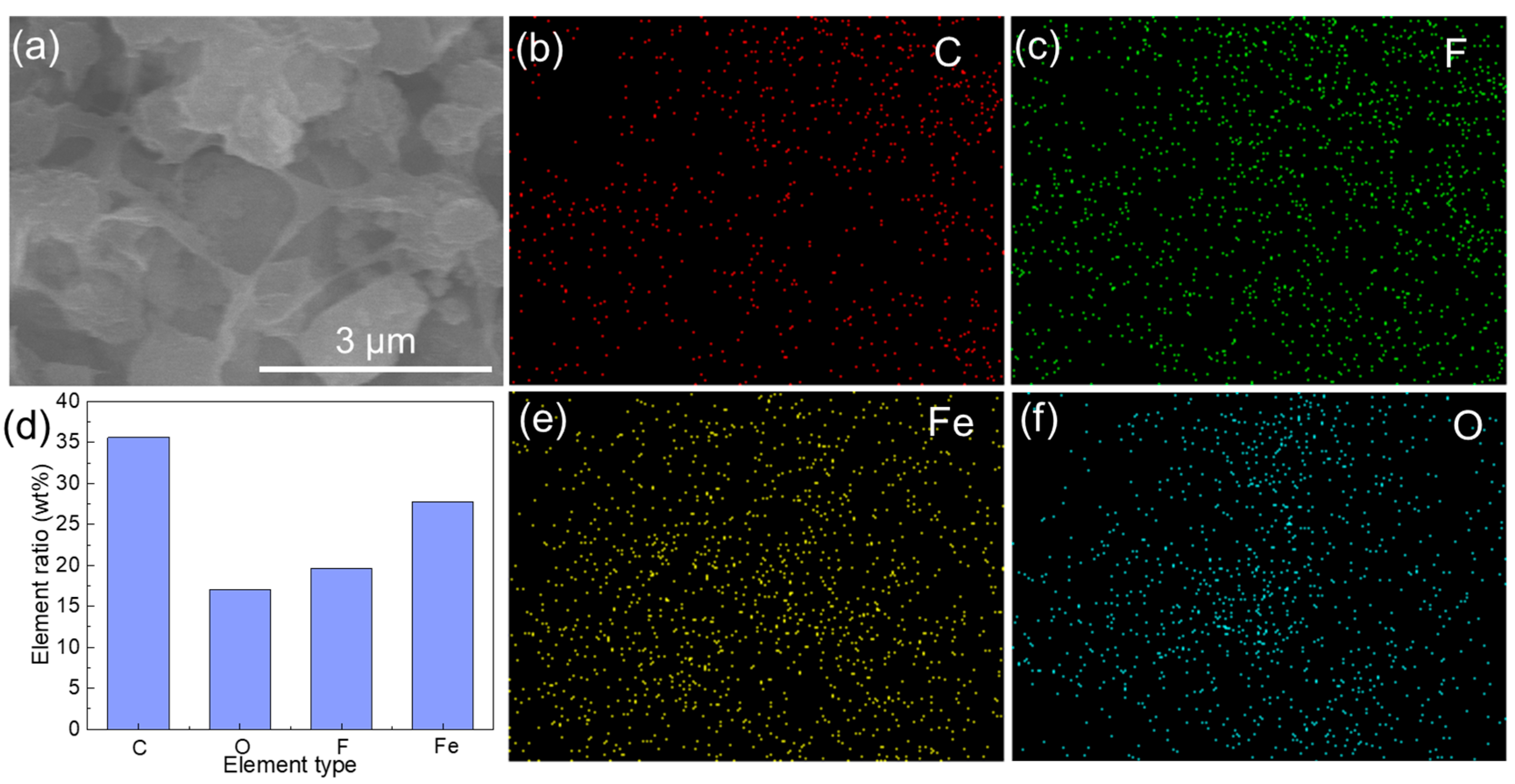
3.1. Characterization of FeOOH Powders and Membranes
3.2. Adsorption Process in the Pb(II)-Contained Saline Water
3.2.1. Effect of Solution pH
3.2.2. Effect of FeOOH Content
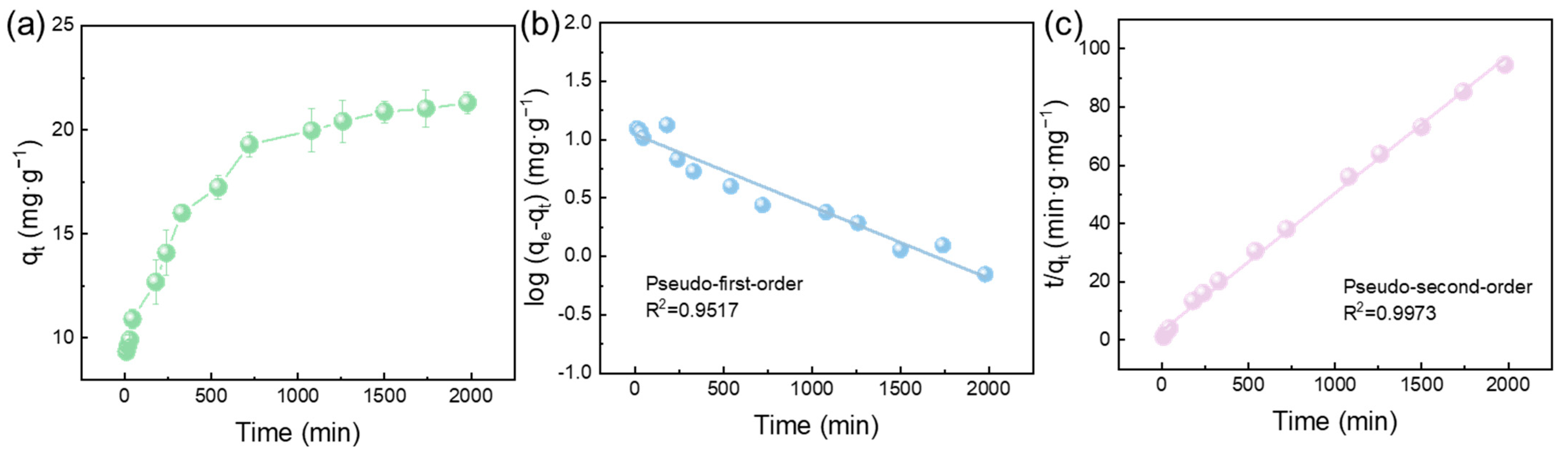
3.2.3. Effect of Adsorption Time
3.2.4. Effect of Initial Pb(II) Concentrations
3.2.5. Effect of Adsorption Temperature
3.2.6. Effect of Ionic Strength
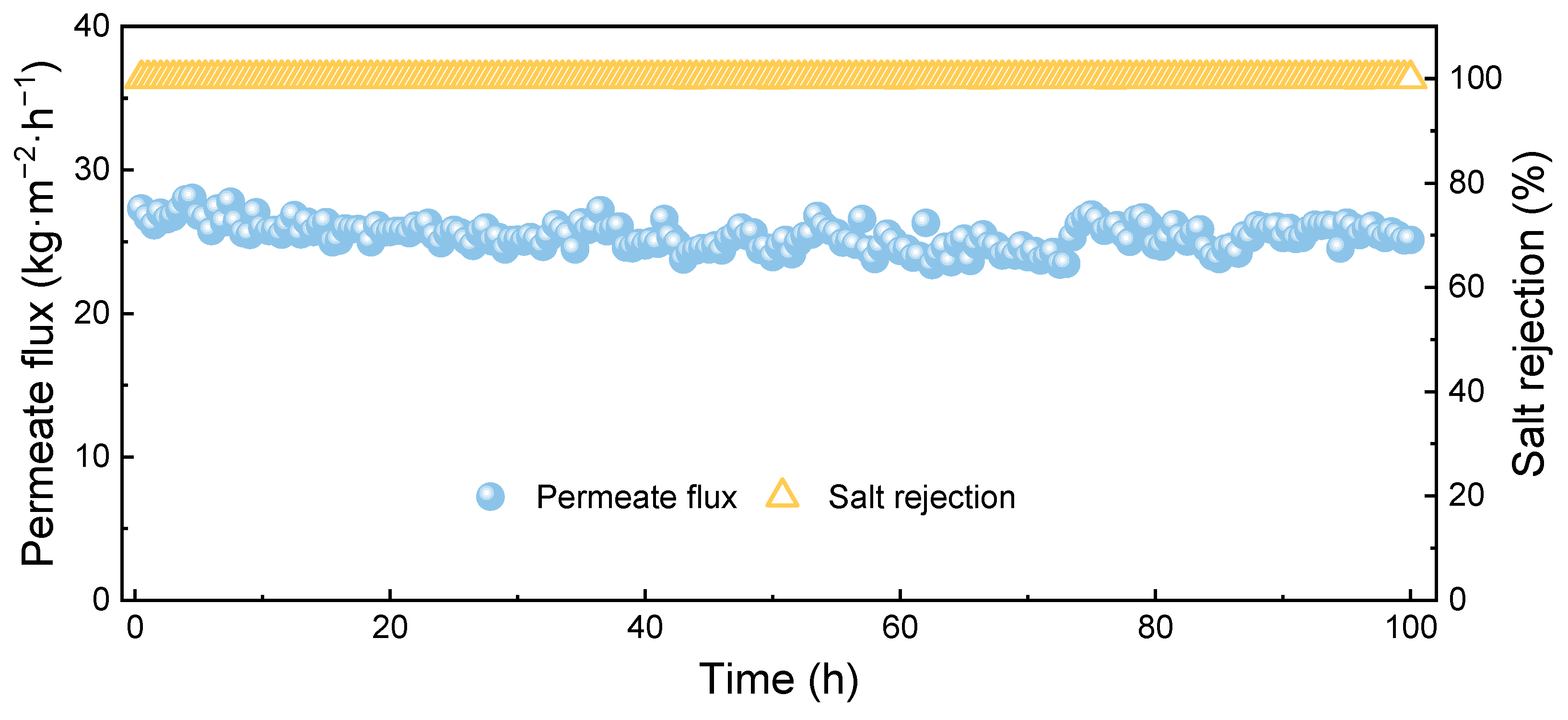
3.3. Membrane Distillation Process for the Heavy-Metal Eemoved Saline Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gogoi, P.; Bora, P.; Hazarika, S. Electroconductive membrane for water treatment: A new paradigm for accessing clean water. Desalination 2025, 601, 118567. [Google Scholar] [CrossRef]
- Yun, B.; Shim, J.; Moon, J.; Lee, S.; Jeong, K.; Cho, K.H. Towards autonomous operation of two-stage reverse osmosis water treatment system with multi-agent reinforcement learning. Desalination 2025, 609, 118870. [Google Scholar] [CrossRef]
- Dansawad, P.; Li, Y.; Li, Y.; Zhang, J.; You, S.; Li, W.; Yi, S. Machine learning toward improving the performance of membrane-based wastewater treatment: A review. Adv. Membr. 2023, 3, 100072. [Google Scholar] [CrossRef]
- Lin, B.; Deng, X.; Chen, J.; Low, Z.-X.; Zhong, Z.; Xing, W. Integration of oxidation processes and ceramic membrane filtration for advanced water treatment: A review of foulant-membrane interactions. Adv. Membr. 2025, 5, 100138. [Google Scholar] [CrossRef]
- Liu, E.; Fan, C.; Zhao, M.; Jiang, S.; Wang, Z.; Jin, Z.; Bei, K.; Zheng, X.; Wu, S.; Zeng, Q. Effects of heavy metals on denitrification processes in water treatment: A review. Sep. Purif. Technol. 2022, 299, 121793. [Google Scholar] [CrossRef]
- Na Nagara, V.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of heavy metals from stormwater runoff using granulated drinking water treatment residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Li, J. Adsorption of Cd(II) and Pb(II) by Mg-modified straw biochar. Desalination Water Treat. 2023, 292, 131–140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Ji, C. Preparation of CoFe2O4-CMC and its adsorption mechanism study toward Pb(II). Chem. Phys. Lett. 2024, 853, 141528. [Google Scholar] [CrossRef]
- Aziz, A.; Agamuthu, P.; Fauziah, S.; Ahmed, A. Removal of bisphenol A and 2,4-Di-tert-butylphenol from landfill leachate using plant- based coagulant. Waste Manag. Res. J. Sustain. Circ. Econ. 2018, 36, 975–984. [Google Scholar] [CrossRef]
- Özcan, A.C.; Gürel, L. Kalsine Karadeniz midye kabukları kullanılarak kimyasal çöktürmeyle Kurşun, Nikel ve Bakır giderimi. J. Fac. Eng. Arch. Gazi Univ. 2022, 38, 743–752. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in biological methods for the sequestration of heavy metals from water bodies: A review. Environ. Toxicol. Pharmacol. 2022, 94, 103927. [Google Scholar] [CrossRef]
- Nie, G.; Liu, X.; Li, X.; Meng, C.; Wang, W.; Zou, D. Efficient phosphate removal and recovery by using nanosized La(III) oxides anchored on aminated biomass waste. Sep. Purif. Technol. 2022, 305, 122513. [Google Scholar] [CrossRef]
- Vesali-Naseh, M.; Naseh, M.R.V.; Ameri, P. Adsorption of Pb (II) ions from aqueous solutions using carbon nanotubes: A systematic review. J. Clean. Prod. 2021, 291, 125917. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Zhao, Q.; Huang, M.; Ouyang, X. Comparative adsorption of heavy metal ions in wastewater on monolayer molybdenum disulfide. Green Energy Environ. 2021, 6, 751–758. [Google Scholar] [CrossRef]
- Kruisdijk, E.; Goedhart, R.; van Halem, D. Biological arsenite oxidation on iron-based adsorbents in groundwater filters. Water Res. 2024, 262, 122128. [Google Scholar] [CrossRef]
- Sharma, M.; Dhiware, P.; Laddha, H.; Janu, V.C.; Gupta, R. Harnessing magnetically separable iron based adsorbents for enhanced uranium adsorption. Coord. Chem. Rev. 2024, 508, 215766. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Wu, Z.; Wang, Z.; Meng, F.; Wu, Z.; Lin, Z.; Li, X. Impact of silicate on the microstructure of β-FeOOH and its adsorption of As. Sep. Purif. Technol. 2024, 354, 129221. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Liu, H.; Wang, W.; Yang, T. α-FeOOH-Modified Sn/N-Codoped TiO2 Bifunctional Composites for As(III) Removal through Photocatalytic Oxidation and Simultaneous Adsorption. Langmuir 2024, 40, 15252–15262. [Google Scholar] [CrossRef]
- Qin, L.; Dong, G.; Nie, Y.; Fakhrullin, R.; Zhang, B.; Zhang, Y. Progress in design of halloysite nanotubes-polymer nanocomposite membranes and their applications. Adv. Membr. 2024, 4, 100091. [Google Scholar] [CrossRef]
- Hadi, M.K.; Su, L.; Li, Y.; Ismail, A.; Sangaraju, S.; Ran, F. Tethering hydrophilic macromolecules onto inorganic nanoparticles via RAFT toward biocompatible polyethersulfone membrane. Adv. Membr. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Zou, D.; Nie, D.; Wang, W.; Xu, Q.; Nie, G. Fabrication of a MoS2@PAN composite membrane for efficient removal of toxic Cr(VI). Desalination 2024, 600, 118460. [Google Scholar] [CrossRef]
- Aftab, B.; Yin, G.; Maqbool, T.; Hur, J.; Wang, J. Enhanced landfill leachate treatment performance by adsorption-assisted membrane distillation. Water Res. 2023, 250, 121036. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Amhamed, A.O.A.; Yuliwati, E.; Puteh, M.H.; Kurniawan, T.A.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Robust omniphobic ceramic hollow fibre membrane with leaf-like copper oxide hierarchical structure by membrane distillation. Desalination 2023, 564, 116816. [Google Scholar] [CrossRef]
- Ali, A.; Shirazi, M.M.A.; Nthunya, L.; Castro-Muñoz, R.; Ismail, N.; Tavajohi, N.; Zaragoza, G.; Quist-Jensen, C.A. Progress in module design for membrane distillation. Desalination 2024, 581, 117584. [Google Scholar] [CrossRef]
- Jankowski, W.; Kujawski, W.; Kujawa, J. Spicing up membrane distillation: Enhancing PVDF membrane performance with cinnamic acid. Desalination 2024, 575, 117304. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F. Mitigating near-surface polarizations in membrane distillation via membrane surface decoration. Desalination 2024, 579, 117507. [Google Scholar] [CrossRef]
- Xu, J.; Xia, L.; Liu, J.; Guan, K.; Luo, P.; Matsuyama, H.; Zou, D.; Zhong, Z. Fabrication of omniphobic PVDF membrane with SiO2–FeOOH hierarchical structures for robust membrane distillation. J. Membr. Sci. 2024, 713, 123252. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, H.; Lei, X.; Lian, Q.; Roy, A.; Doucet, D.; Yan, H.; Zappi, M.E.; Gang, D.D. A comparative study for phosphate adsorption on amorphous FeOOH and goethite (α-FeOOH): An investigation of relationship between the surface chemistry and structure. Environ. Res. 2021, 199, 111223. [Google Scholar] [CrossRef]
- Eren, E.; Gumus, H. Characterization of the structural properties and Pb(II) adsorption behavior of iron oxide coated sepiolite. Desalination 2011, 273, 276–284. [Google Scholar] [CrossRef]





| Label | FeOOH (g) | PVDF (g) | DMF (g) |
|---|---|---|---|
| AM1 | 4.500 | 3.000 | 16.50 |
| AM2 | 4.875 | 2.625 | 16.50 |
| AM3 | 5.250 | 2.250 | 16.50 |
| AM4 | 5.626 | 1.875 | 16.50 |
| Pb(Ⅱ) C0 (mg·L−1) | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|
| qe, exp (mg·g−1) | qe, cal (mg·g−1) | k1 (min−1) | qe, cal (mg·g−1) | k2 (g·mg−1·min−1) | |||
| 25 | 21.639 | 11.10 | 0.0014 | 0.9517 | 21.133 | 0.000711 | 0.9973 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | KF [(mg·g−1) (L·mg−1)(1/n)] | n | ||
| 29.68 | 0.1392 | 0.9765 | 5.61 | 2.21 | 0.7448 |
| Temperature (K) | ΔGθ (kJ·mol−1) | ΔHθ (kJ·mol−1) | ΔSθ (kJ·mol−1·K−1) |
|---|---|---|---|
| 298 | −2.70 | 34.36 | 0.1246 |
| 308 | −4.00 | ||
| 318 | −5.44 | ||
| 328 | −6.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, J.; Liu, M.-L.; Nie, G.; Zou, D. Synergistic Adsorption–Membrane Distillation for Heavy Metal Extraction and Water Reclamation from Saline Waste Streams. Membranes 2025, 15, 271. https://doi.org/10.3390/membranes15090271
Xu J, Liu J, Liu M-L, Nie G, Zou D. Synergistic Adsorption–Membrane Distillation for Heavy Metal Extraction and Water Reclamation from Saline Waste Streams. Membranes. 2025; 15(9):271. https://doi.org/10.3390/membranes15090271
Chicago/Turabian StyleXu, Jie, Jinxin Liu, Mei-Ling Liu, Guangze Nie, and Dong Zou. 2025. "Synergistic Adsorption–Membrane Distillation for Heavy Metal Extraction and Water Reclamation from Saline Waste Streams" Membranes 15, no. 9: 271. https://doi.org/10.3390/membranes15090271
APA StyleXu, J., Liu, J., Liu, M.-L., Nie, G., & Zou, D. (2025). Synergistic Adsorption–Membrane Distillation for Heavy Metal Extraction and Water Reclamation from Saline Waste Streams. Membranes, 15(9), 271. https://doi.org/10.3390/membranes15090271







