Recent Progress on the Development of Polyetheretherketone Membranes for Water Remediation
Abstract
1. Introduction
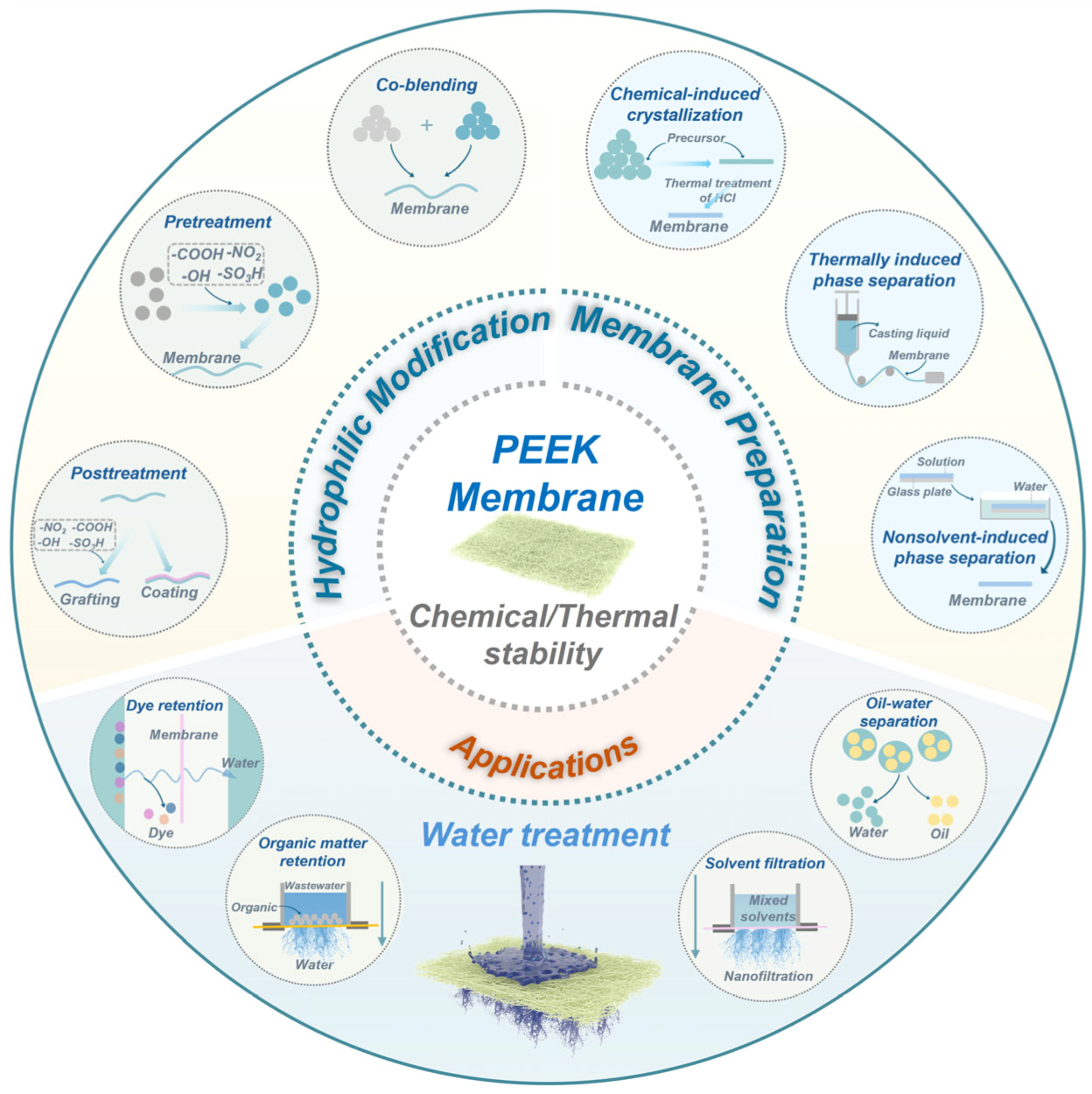
2. Preparation of PEEK Membranes
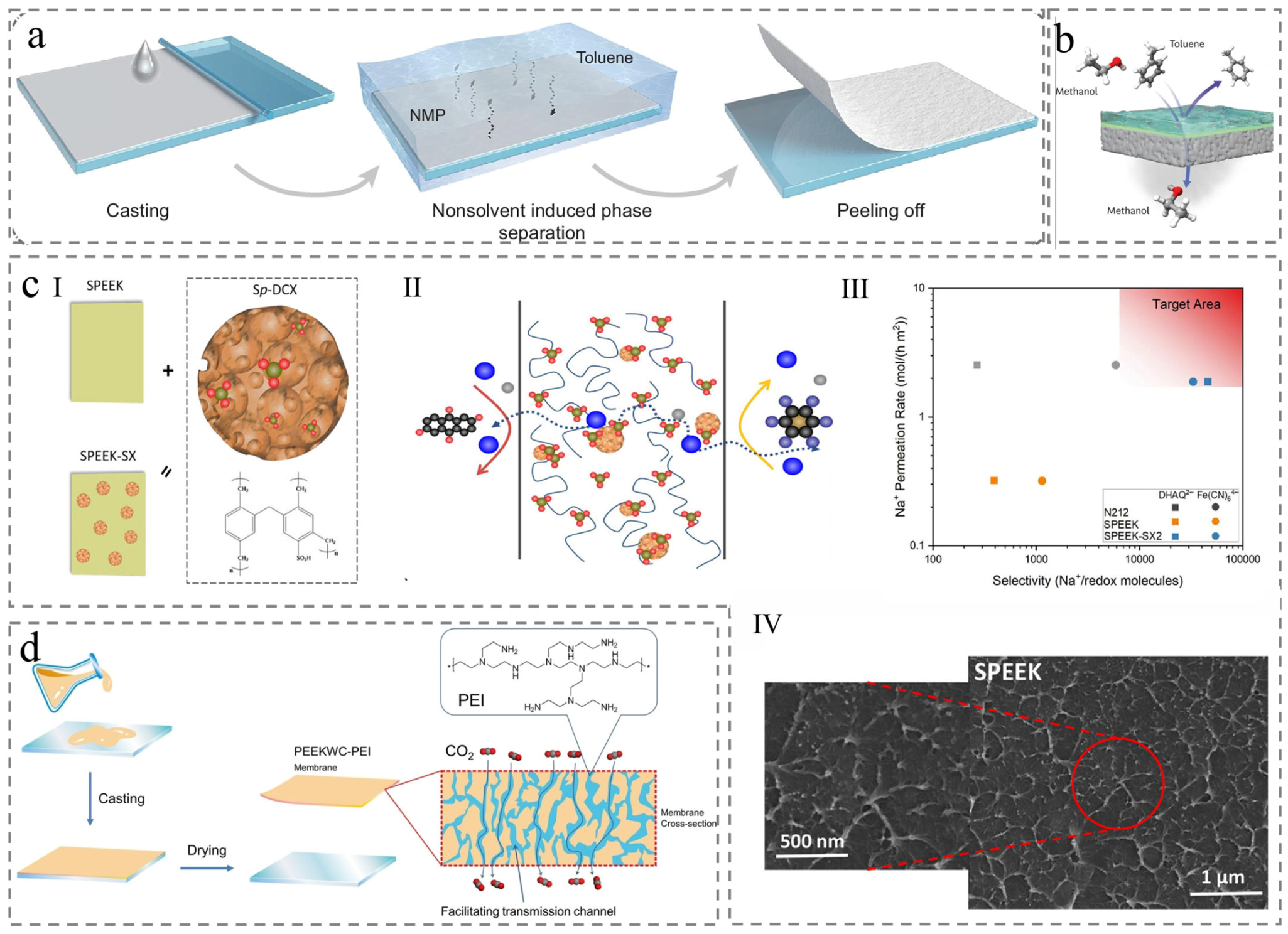
2.1. Nonsolvent-Induced Phase Separation (NIPS)
2.2. Thermally Induced Phase Separation (TIPS)
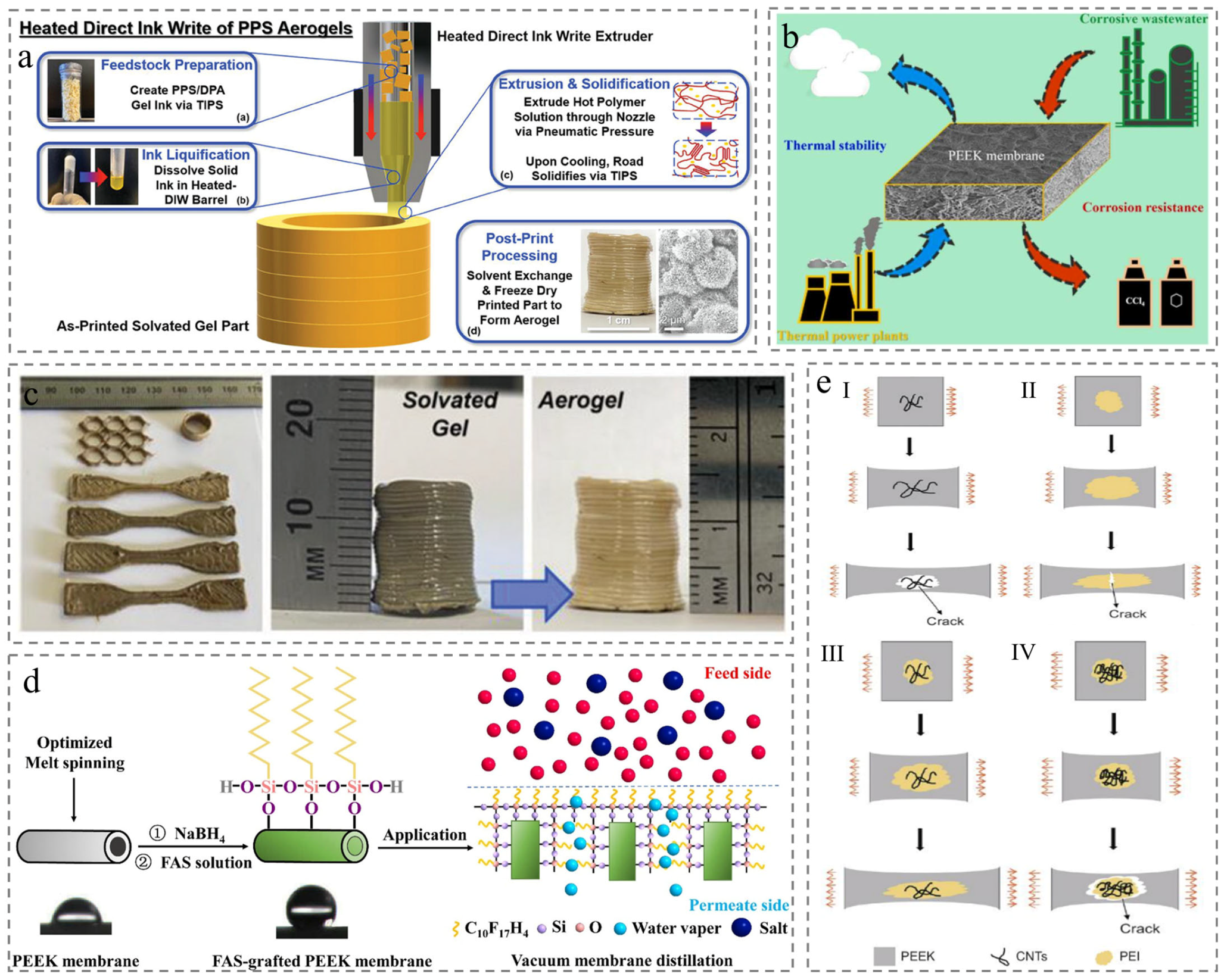
2.3. Chemical-Induced Crystallization (CIC)
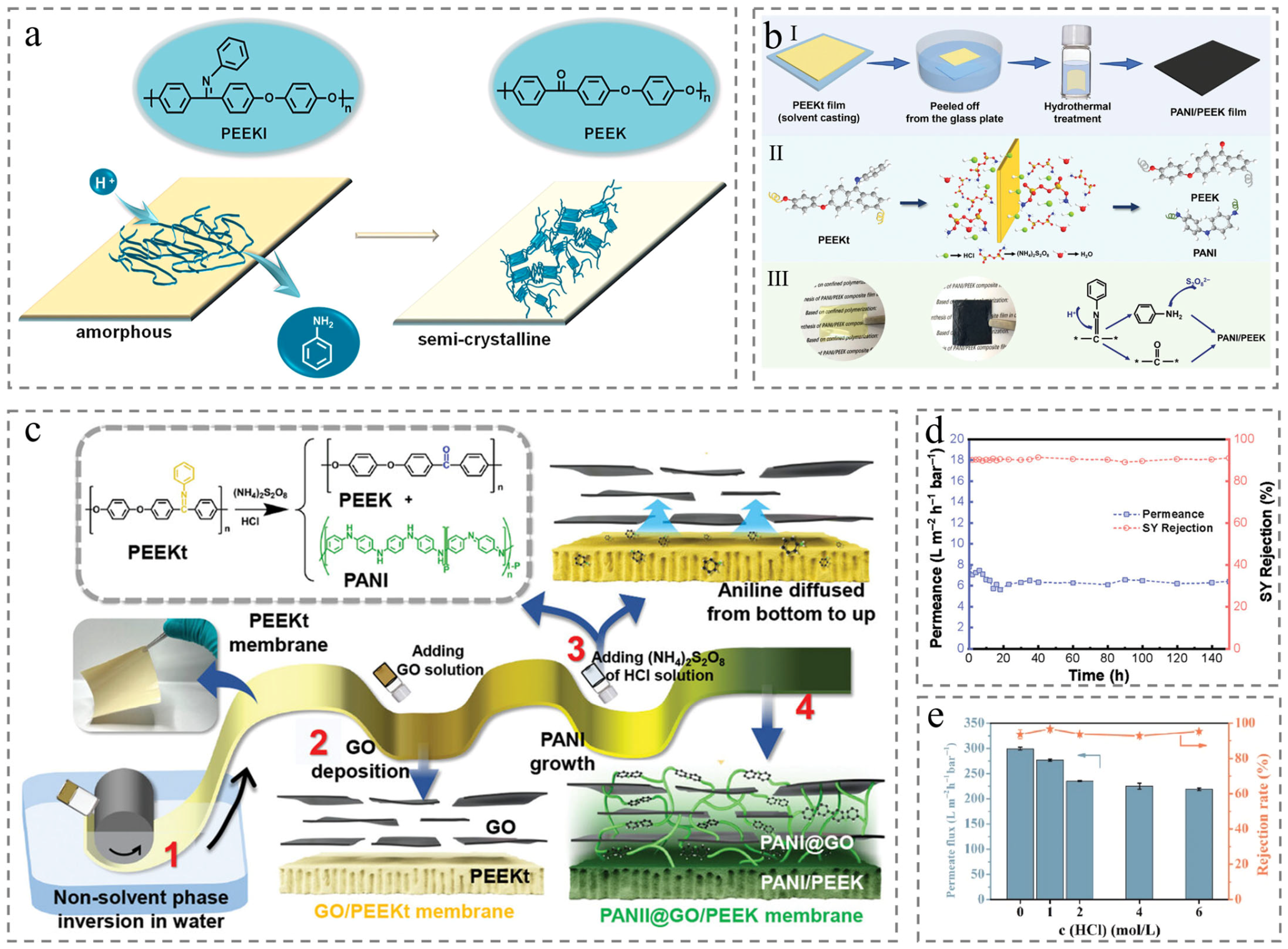
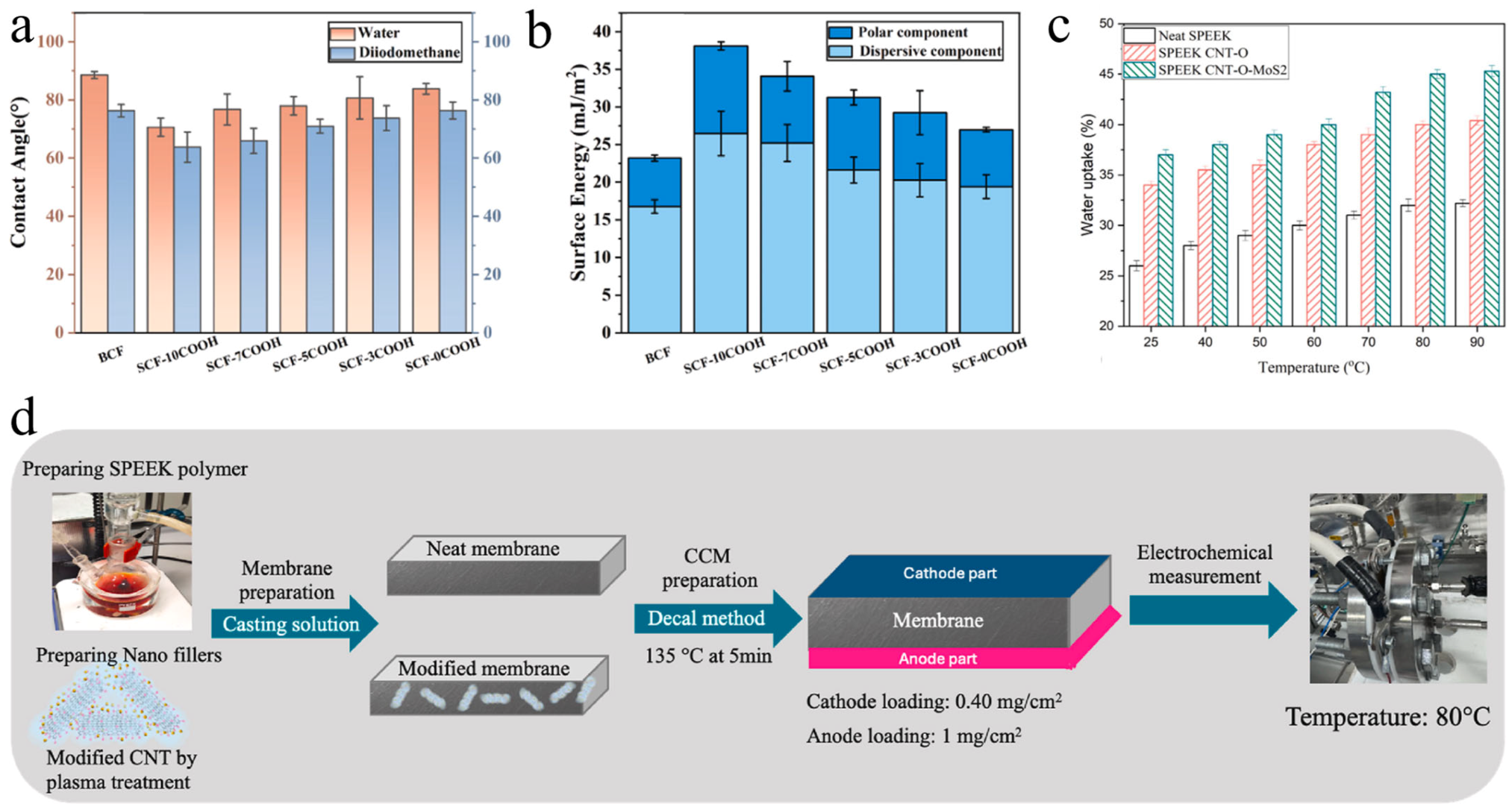
3. Hydrophilic Modifications
3.1. Pretreatment
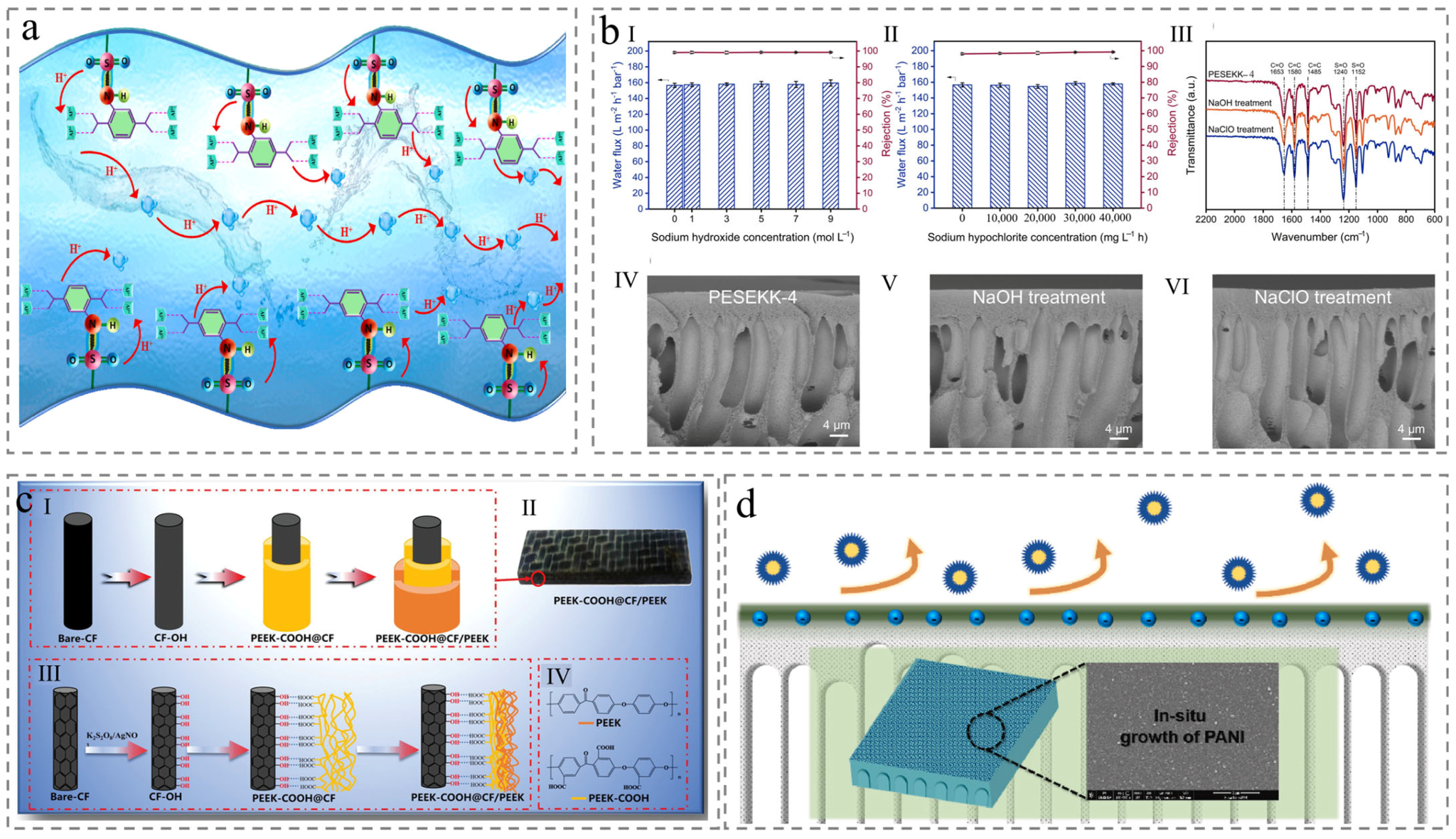
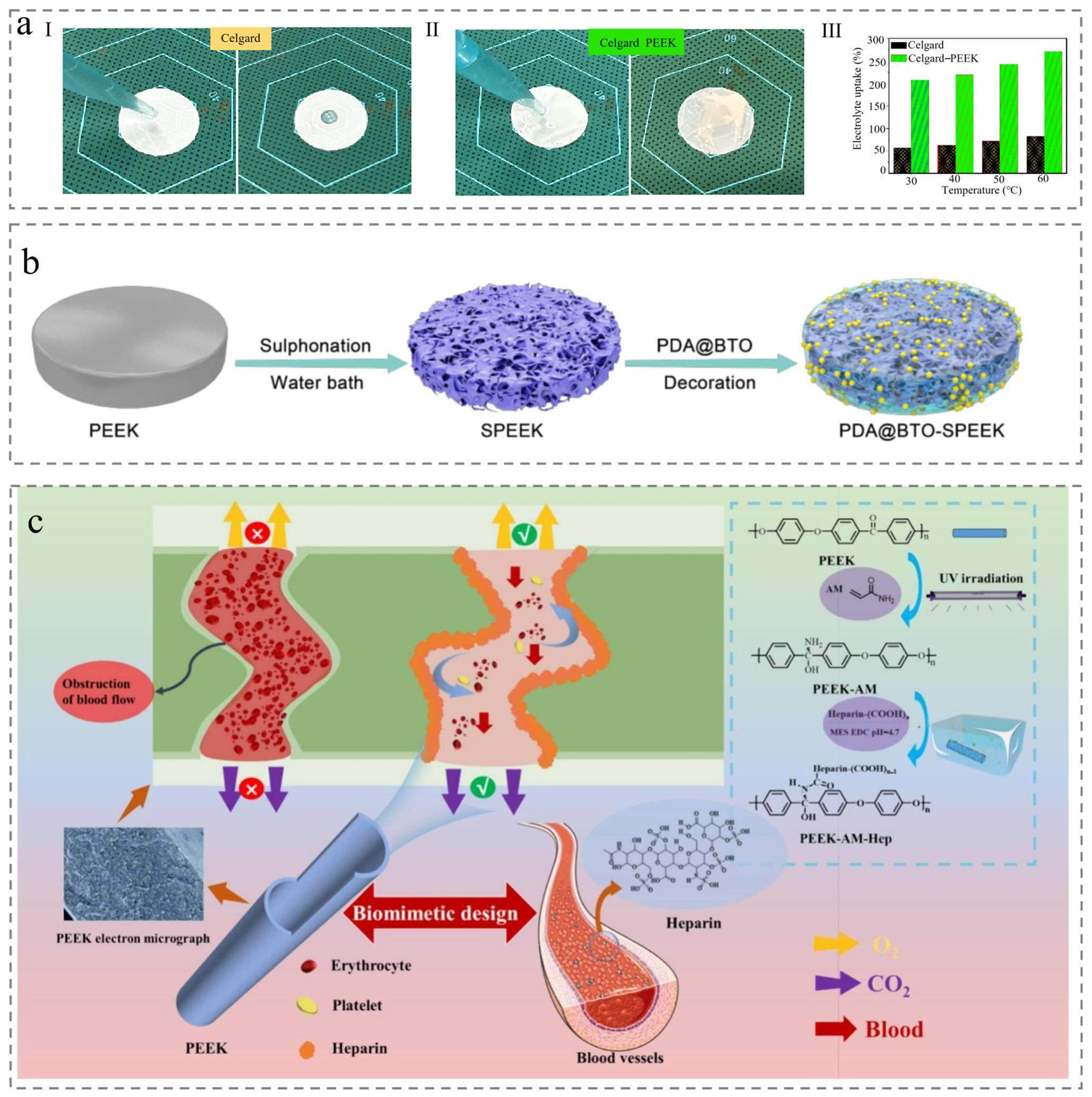
3.2. Co-Blending
3.3. Post-Treatment
| Hydrophilic Modification Methods | Examples | Water Contact Angle | Reference |
|---|---|---|---|
| Pretreatment | Graft sulfonic acid group | From 110° to 73° | [53,54] |
| Grafted carboxyl group | From 111° to 24° | [55] | |
| Incorporating polyaniline | From 109° to 78° | [45] | |
| Co-blending modification | Blend with carboxylated polyimide | From 89° to 70° | [60] |
| Blend with carbon nanotube and MoS2 | From 80° to 65° | [61] | |
| Post-treatment | Grafting ethyl hydroxy acrylate | From 97° to 30° | [63] |
| Grafting polyallylamine | From 91° to 75° | [64] |
4. Applications for Water Remediation
4.1. Synthetic Dyes Removal
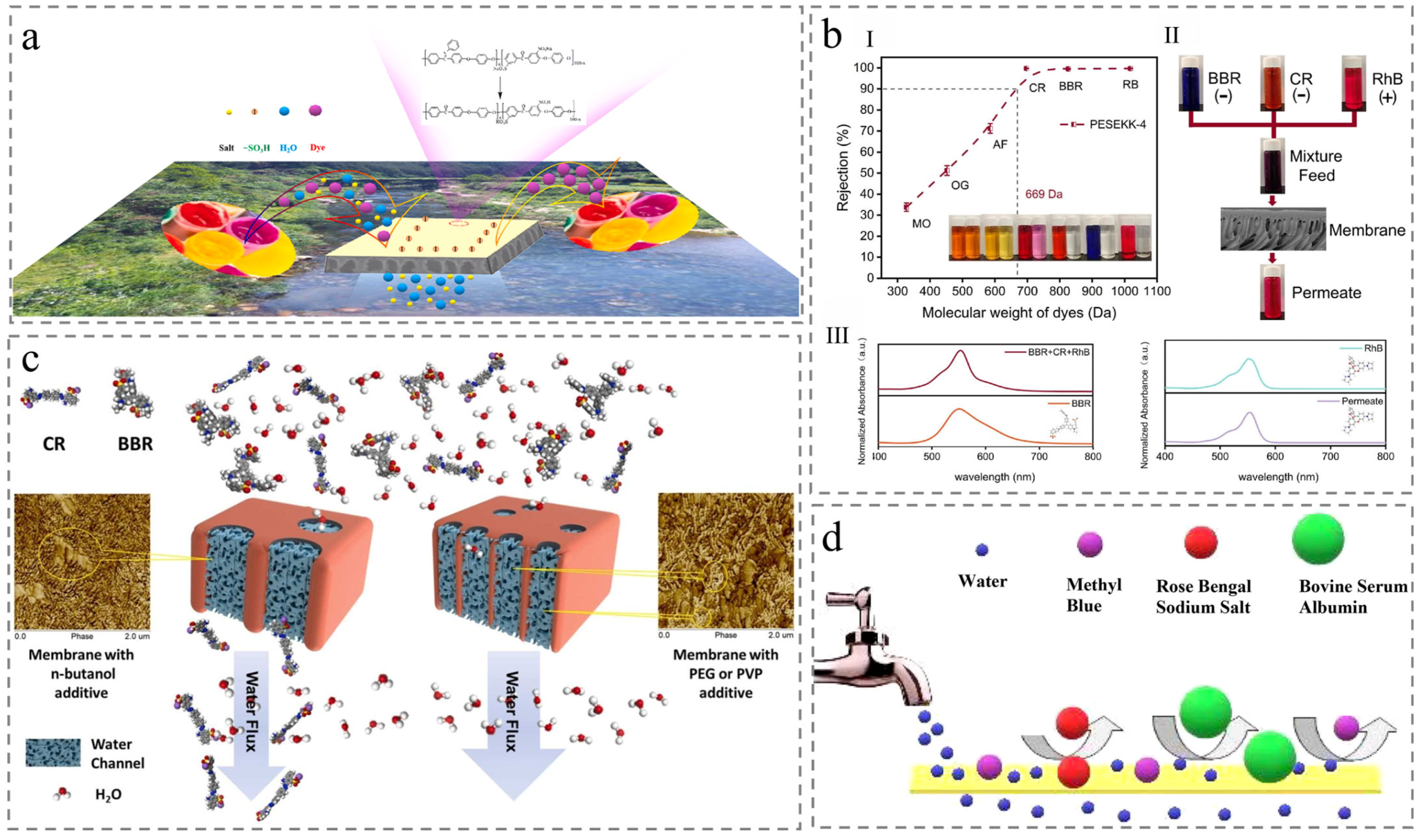
4.2. Organic Solvent Nanofiltration
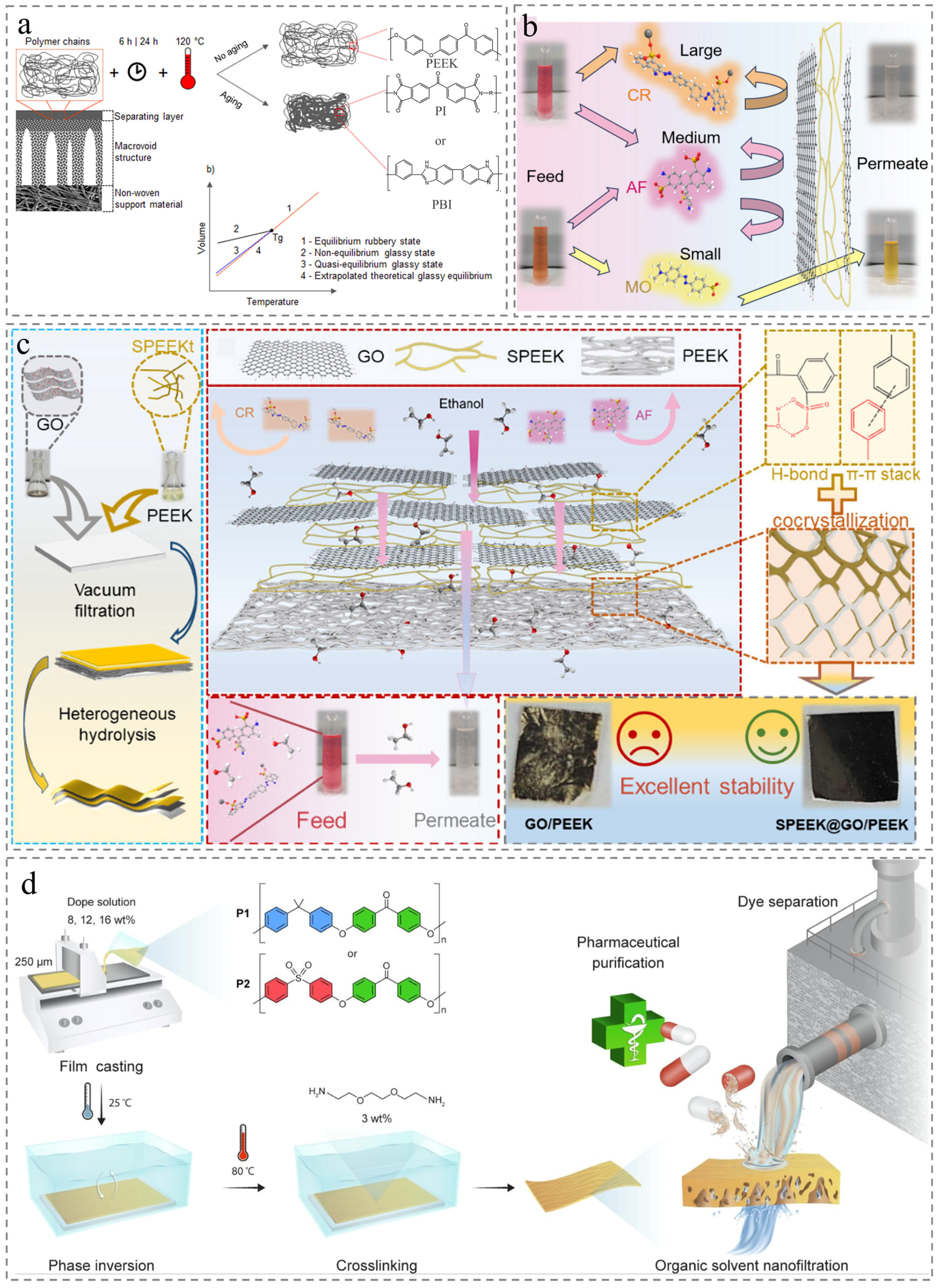
4.3. Natural Organic Matter Removal
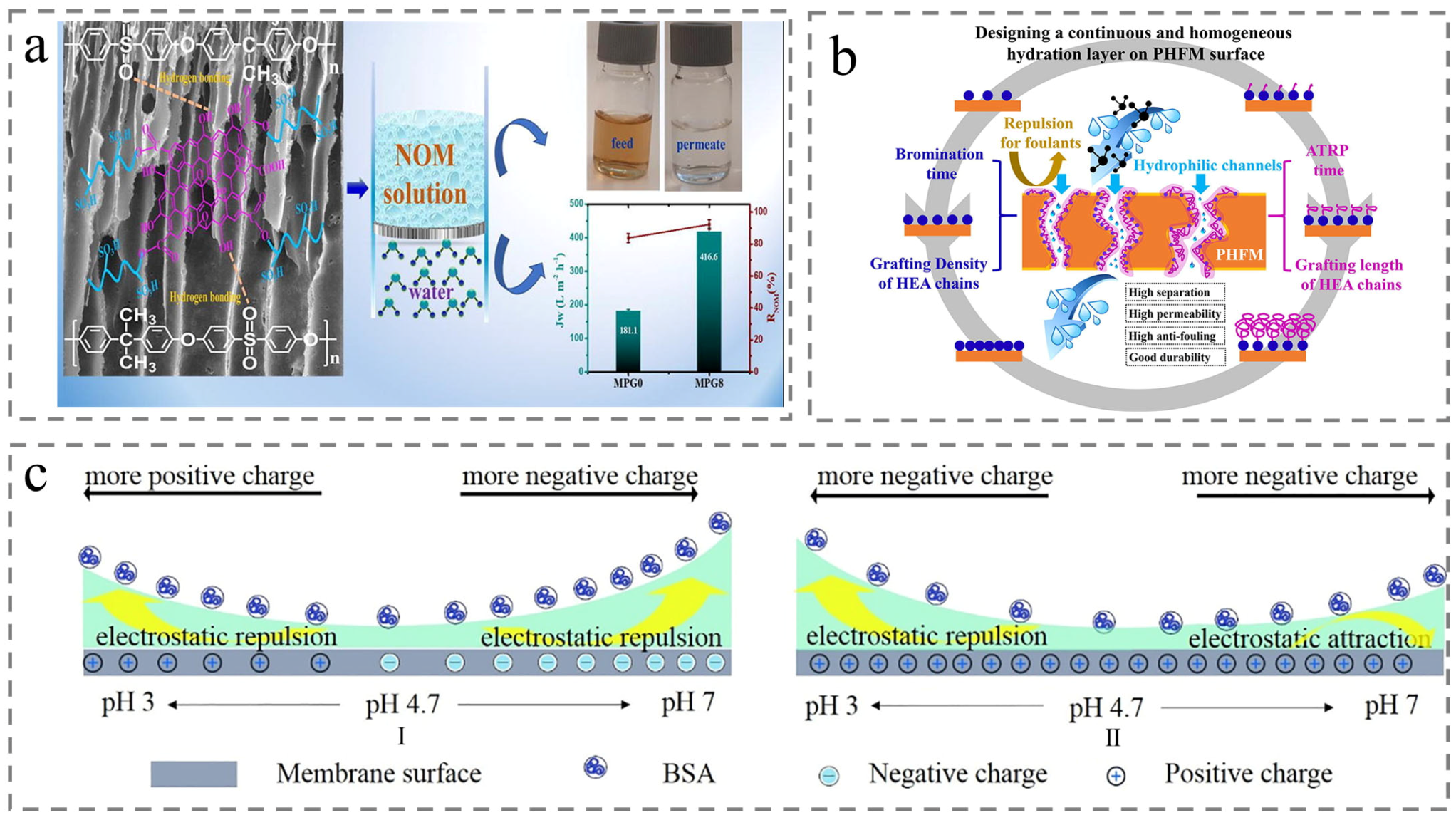
4.4. Oil–Water Separation
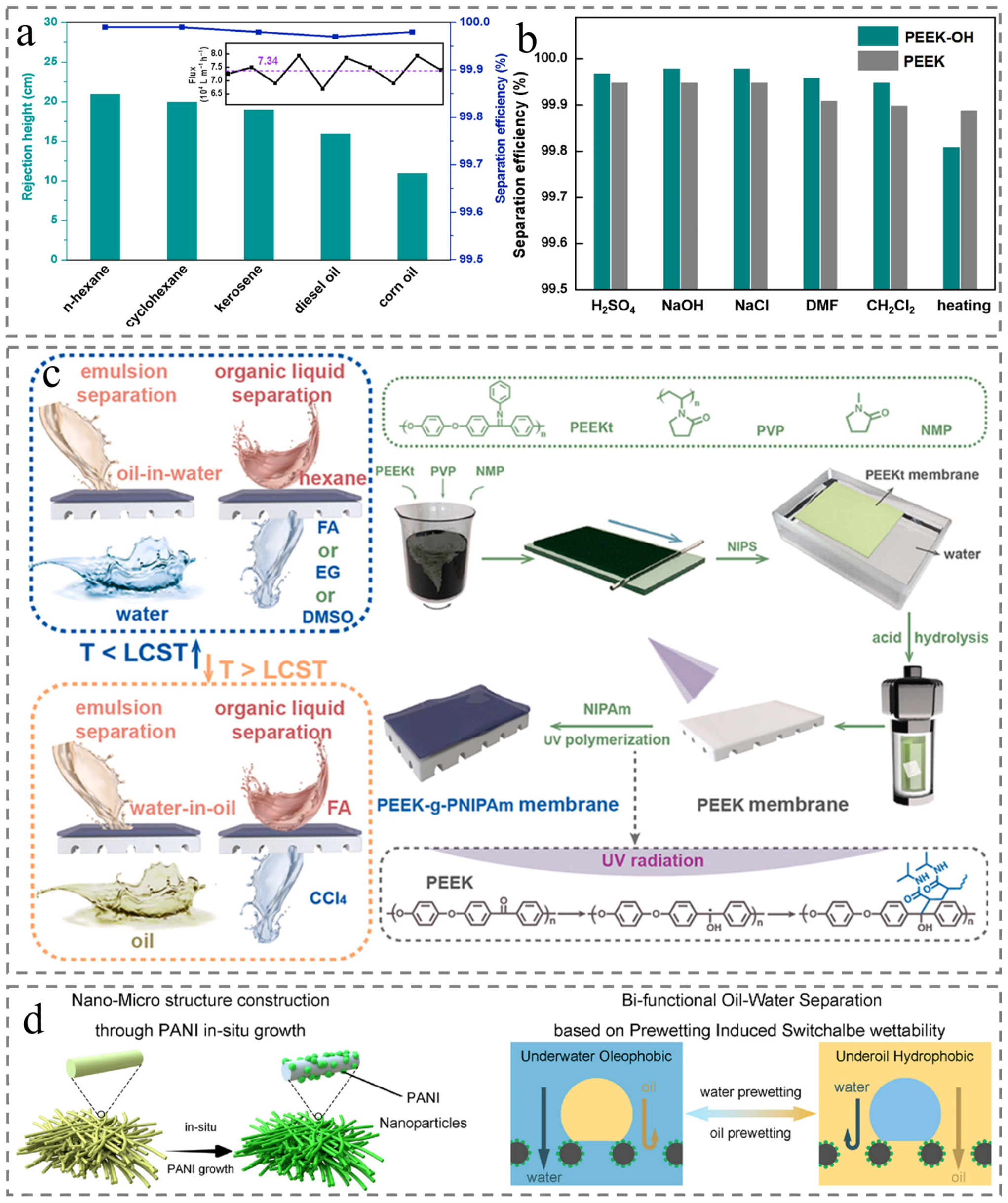
5. Conclusions
6. Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Hou, Y.M.; Shah, P.; Constantoudis, V.; Gogolides, E.; Kappl, M.; Butt, H. A super liquid-repellent hierarchical porous membrane for enhanced membrane distillation. Nat. Commun. 2023, 14, 6886. [Google Scholar] [CrossRef]
- Wang, S.; Gui, L.; Qiao, D.; Tao, M.; Huang, S.; Tian, X. Fluorinated covalent organic framework membranes with robust wetting resistance for durable membrane distillation. Angew. Chem. Int. Ed. Engl. 2025, 64, e202507913. [Google Scholar] [CrossRef]
- Yue, C.Z.; Zheng, W.B.; Wang, Q.Y.; Wang, Z.D.; Li, B.; Zhang, C.M.; Ming, P.W. Challenges in membrane electrode assemblies at elevated temperatures for proton exchange membrane fuel cells: A review. Energy Environ. Sci. 2025, 18, 6934–6982. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Wang, X.H.; Zhou, Y.J.; Qi, J.W.; Yang, Y.; Wang, W.; Li, J.S. Volatile sieving using architecturally designed nanochannel lamellar membranes in membrane desalination. ACS Nano 2025, 19, 5577–5588. [Google Scholar] [CrossRef]
- Guan, S.W.; Chen, S.H.; Zhang, X.M.; Zhang, H.F.; Liu, X.D.; Hou, Z.Y.; Wang, F.; Qian, S.; Zhu, H.Q.; Tan, J.; et al. Metastructure “trap” coating by acoustic confinement effect for antibacterial sonothermal therapy. Adv. Funct. Mater. 2024, 34, 2316093. [Google Scholar] [CrossRef]
- Ren, J.H.; Xu, J.M.; Ju, M.C.; Chen, X.; Zhao, P.Y.; Meng, L.X.; Lei, J.X.; Wang, Z. Long-term durable anion exchange membranes based on imidazole-functionalized poly (ether ether ketone) incorporating cationic metal-organic framework. Adv. Powder Mater. 2022, 1, 100017. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, X.R.; Lu, X.H.; Shi, X.T.; Zhang, J.L.; Guo, H.; He, M.K.; Gu, J.W. Interface strengthening for carbon fiber-reinforced poly (ether-ether-ketone) laminated composites by introducing fluorene-containing branched poly (aryl-ether-ketone). Interdiscip. Mater. 2024, 3, 919–934. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Li, T.Y.; Yan, L.L.; Jiao, Y.; Zhang, Y.J.; Wang, K.; Cheng, Z.J.; Ma, J.; Shao, L. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci. Adv. 2023, 9, eadh8195. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.N.; Liu, Y.X.; Li, J.H.; Sun, Q.; Liu, Z.X.; Tang, J.B.; Zhang, Y.F.; An, M.; Liu, H.; et al. Molecularly engineered rigid ultra-micropore membranes for ultrahigh-power osmotic energy harvesting from high-temperature hypersaline brine. Adv. Mater. 2025, 37, 2505485. [Google Scholar] [CrossRef]
- Guo, X.W.; Brown, R.; Dong, Y. High-performance elastomer with excellent resistance to low temperature, aging and solvent. Adv. Funct. Mater. 2025, 35, 2501171. [Google Scholar] [CrossRef]
- Chen, M.; Qiao, Y.S.; Yu, L.; Wang, W.; Wang, W.T.; Sun, H.F.; Xu, Y.Z.; Bai, J.X.; Zhou, J.; Geng, D.C. A microenvironment responsive polyetheretherketone implant with antibacterial and osteoimmunomodulatory properties facilitates osseointegration. Bioact. Mater. 2025, 43, 273–291. [Google Scholar] [CrossRef]
- Li, Z.K.; Yu, A.F.; Liu, J.T.; Shi, Y.H.; Hu, W.W.; Wang, Z.L.; Zhai, J.Y. Elevating triboelectric polymer charge-carrying capacity via cascaded charge confinement and planting for remarkable performance amplification. Mater. Today 2025, 83, 242–251. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.F.; Li, L. Phase transition and electrical conversion properties of Ge/Sb nano-multilayer films on flexible substrates. NPJ Flex. Electron. 2024, 8, 8. [Google Scholar] [CrossRef]
- Dai, X.K.; Zhou, K.X.; Zhang, L.; Wu, T.Y.; Ye, H.M.; Cao, X.; Han, Y.; Huang, G.Y.; Xu, S.M. Polymer-based solid electrolyte with ultra thermostability exceeding 300 °C for high-temperature lithium-ion batteries in oil drilling industries. Nano Energy 2025, 133, 110475. [Google Scholar] [CrossRef]
- Zhu, J.M.; Xiong, S.X.; Gong, G.H.; Yu, S.Y.; Zhang, D.; Zhang, Z.Q.; Li, S.L.; Hu, Y.X. Electroactive carbon-doped ceramic membranes with controllable macrostructure and microstructure toward efficient dye wastewater treatment. Adv. Funct. Mater. 2025, 11, 2506495. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, J.; Kong, X.Y.; Xin, W.W.; Zhou, T.; Qian, Y.C.; Yang, L.S.; Pang, J.H.; Jiang, L.; Wen, L.P. Robust sulfonated poly (ether ether ketone) nanochannels for high-performance osmotic energy conversion. Natl. Sci. Rev. 2020, 7, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Xiao, G.Y.; Song, Z.G.; Lu, Y.P. The formation process and mechanism of the 3D porous network on the sulfonated PEEK surface. ACS Appl. Mater. Interfaces 2024, 16, 13585–13596. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, S.; Song, Z.G.; Xiao, G.Y.; Lu, Y.P. Alternative to surface sulfonation: Biofunctionalizing PEEK surfaces via 3D porous coatings using the nonsolvent-induced phase separation approach. Chem. Eng. J. 2025, 516, 164025. [Google Scholar] [CrossRef]
- Liang, W.Q.; Ghasemiestahbanati, E.; Eden, N.T.; Acharya, D.; Doherty, C.M.; Majumder, M.; Hill, M.R. Flow battery with remarkably stable performance at high current density: Development of a nonfluorinated separator with concurrent rejection and conductivity. Angew. Chem. Int. Ed. Engl. 2025, 64, e202505383. [Google Scholar] [CrossRef]
- Niccolai, F.; Guazzelli, E.; Koura, Z.E.; Pucher, I.; Martinelli, E. Sulfonated PEEK-based proton exchange membranes for semi-organic AQDS/ bromine redox flow batteries. J. Energy Storage 2025, 130, 117366. [Google Scholar] [CrossRef]
- Pandey, M.; Mairal, A.; Gupta, H.; Kumar, A.; Bhattacharya, S. Rapid surface modification of PEEK by ambient temperature sulfonation for high shelf-life biomedical applications. Surf. Interfaces 2024, 54, 105117. [Google Scholar] [CrossRef]
- Lafi, A.G.A.; Arfan, A.; Alnaama, D.; Hasan, R.; Allaf, T.; Ibrahim, M.; Alssayes, G. Sulfonation of poly (ether ether ketone): An evidence for di-substitution of the repeat unit. J. Polym. Res. 2022, 29, 434. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.L.; Feng, Z.R.; Song, J.; Qiao, Q.; Wu, Y.P.; Ren, X.M. A freezing-tolerant superior proton conductive hydrogel comprised of sulfonated poly (ether-ether-ketone) and poly (vinyl-alcohol) as a quasi-solid-state electrolyte in a proton battery. J. Mater. Chem. C Mater. 2023, 11, 13113–13119. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Esmailinezhad, M.; Drioli, E. Pervaporation separation of meoh/mtbe mixtures with modified peek membrane: Effect of operating conditions. J. Membr. Sci. 2011, 371, 1–9. [Google Scholar] [CrossRef]
- Han, G.L.; Chen, Z.; Cai, L.F.; Zhang, Y.H.; Tian, J.F.; Ma, H.H.; Fang, S.M. Post-synthetic mil-53(al)-so 3 h incorporated sulfonated polyarylethersulfone with cardo (spes-c) membranes for separating methanol and methyl tert-butyl ether mixture. Sep. Purif. Technol. 2019, 220, 268–275. [Google Scholar] [CrossRef]
- Esposito, R.; Di Vincenzo, M.; Gopalsamy, K.; Ganesan, S.; Upadhyaya, L.; Grande, C.; Szekely, G.; Nunes, S.P. Membranes for the pervaporation of solvent azeotropes: From molecular to process design. Chem. Eng. J. 2025, 515, 163728. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Yu, Y.W.; Wang, J.H.; Wang, Y.; Pan, W.H.; Liu, C.W.; Liu, P.; Liang, L.J.; Xu, C.W.; Liu, Y.X. Polyethyleneimine-functionalized phenolphthalein-based cardo poly (ether ether ketone) membrane for CO2 separation. J. Ind. Eng. Chem. 2020, 83, 20–28. [Google Scholar] [CrossRef]
- Gao, R.S.; Zhang, Q.G.; Lv, R.X.; Soyekwo, F.; Zhu, A.M.; Liu, Q.L. Highly efficient polymer-MOF nanocomposite membrane for pervaporation separation of water/methanol/MTBE ternary mixture. Chem. Eng. Res. Des. 2017, 117, 688–697. [Google Scholar] [CrossRef]
- Iulianelli, A.; Clarizia, G.; Gugliuzza, A.; Ebrasu, D.; Bevilacqua, A.; Trotta, F.; Basile, A. Sulfonation of PEEK-WC polymer via chloro-sulfonic acid for potential PEM fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 12688–12695. [Google Scholar] [CrossRef]
- Hendrix, K.; Vaneynde, M.; Koeckelberghs, G.; Vankelecom, I.F.J. Synthesis of modified poly (ether ether ketone) polymer for the preparation of ultrafiltration and nanofiltration membranes via phase inversion. J. Membr. Sci. 2013, 447, 96–106. [Google Scholar] [CrossRef]
- Hendrix, K.; Van Eynde, M.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking of modified poly (ether ether ketone) membranes for use in solvent resistant nanofiltration. J. Membr. Sci. 2013, 447, 212–221. [Google Scholar] [CrossRef]
- Tang, Y.H.; Lin, Y.K.; Ma, W.Z.; Wang, X.L. A review on microporous polyvinylidene fluoride membranes fabricated via thermally induced phase separation for MF/UF application. J. Membr. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Godshall, G.F.; Rau, D.A.; Williams, C.B.; Moore, R.B. Additive manufacturing of poly (phenylene sulfide) aerogels via simultaneous material extrusion and thermally induced phase separation. Adv. Mater. 2024, 36, 2307881. [Google Scholar] [CrossRef]
- Aristizabal, S.L.; Upadhyaya, L.; Falca, G.; Gebreyohannes, A.Y.; Aijaz, M.O.; Karim, M.R.; Nunes, S.P. Acid-free fabrication of polyaryletherketone membranes. J. Membr. Sci. 2022, 660, 120798. [Google Scholar] [CrossRef]
- Cheng, X.; Li, M.; Wang, J.Y.; Yang, J.; Wei, K.; Zhang, Y.H.; Chai, W.Q.; You, J.C. Crystallization-template-induced PEEK membranes for particulate matter capture at high temperature and separation of emulsion containing corrosive component. J. Environ. Chem. Eng. 2022, 10, 107469. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Liu, J.H.; Xu, G.H.; Jiang, H.C.; Jie, X.M.; Cao, Y.M. Preparation of nano-porous poly (ether ether ketone) hollow fiber membrane and its performance for desalination in vacuum membrane distillation. Desalination 2023, 551, 116417. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, G.Y.; Zhang, X.C.; Shu, J.C.; Wang, C.X.; Zhai, Z.; Jie, X.M.; Zhao, S.M.; Zhao, Y.C. Template-assisted fabrication of polyether ether ketone hollow fiber membrane for highly efficient separation of binary dye mixtures. J. Membr. Sci. 2024, 698, 122634. [Google Scholar] [CrossRef]
- Lassus, A.; Therriault, D.; Favis, B.D.; Virgilio, N. Tailoring the morphology in partially and fully miscible mixtures of PEEK and PEI. Polymer 2025, 326, 128315. [Google Scholar] [CrossRef]
- Song, J.P.; Zhao, Y.; Li, X.K.; Xiong, S.; Li, S.; Wang, K. Increasing the toughness while reducing the viscosity of carbon nano-tube/polyether imide/polyether ether ketone nanocomposites. New Carbon. Mater. 2024, 39, 715–728. [Google Scholar] [CrossRef]
- Zhang, C.; You, J.A.; Wang, X.; Hou, R.; Li, X.F.; Sun, Y.X.; Qin, G.R.; Li, S.H.; Zhang, S.B. Design of precursor polymers assists the processing of poly (ether ether ketone) membranes in solvents. Macromolecules 2024, 57, 2446–2457. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.F.; Sun, Y.X.; Li, S.H.; Zhang, S.B. Screening of the biphenyl polyamides nanofilms appropriate for molecular separation in organic solution feed. Sep. Purif. Technol. 2025, 358, 130232. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhou, S.Y.; Qin, G.R.; Guo, J.; Zhang, Q.F.; Li, S.H.; Zhang, S.B. A chemical-induced crystallization strategy to fabricate poly (ether ether ketone) asymmetric membranes for organic solvent nanofiltration. J. Membr. Sci. 2021, 620, 118899. [Google Scholar] [CrossRef]
- Yu, H.T.; Guan, J.Y.; Chen, Y.H.; Sun, Y.X.; Zhou, S.Y.; Zheng, J.F.; Zhang, Q.F.; Li, S.H.; Zhang, S.B. Large-area soluble covalent organic framework oligomer coating for organic solution nanofiltration membranes. Small 2024, 20, 2305613. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Cao, N.; Li, C.H.; Sun, R.Y.; Li, W.Y.; Chen, L.Y.; Sun, Y.R.; Zhang, H.B.; Pang, J.H.; Jiang, Z.H. Micro-nanostructure tuning of PEEK porous membrane surface based on PANI in-situ growth for antifouling ultrafiltration membranes. J. Membr. Sci. 2022, 663, 121058. [Google Scholar] [CrossRef]
- Han, H.G.; Zhang, C.; Yu, H.T.; Liu, Z.; Guo, J.; Zhang, Q.F.; Sun, Y.X.; Li, S.H.; Zhang, S.B. A novel organic solvent nanofiltration membrane prepared via green mild and simple crosslinking process. J. Membr. Sci. 2023, 688, 122151. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Cao, N.; Sun, Z.H.; Li, W.Y.; Sun, Y.R.; Zhang, H.B.; Pang, J.H.; Jiang, Z.H. Based on confined polymerization: In situ synthesis of PANI/peek composite film in one-step. Adv. Sci. 2022, 9, 2103706. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Zhong, J.D.; Sun, R.Y.; Wei, Y.Z.; Sun, Z.H.; Li, W.Y.; Chen, L.C.; Sun, Y.R.; Zhang, H.B.; Pang, J.H.; et al. Insitu integrated fabrication for multi-interface stabilized and highly durable polyaniline@graphene oxide/polyether ether ketone special separation membranes. Adv. Sci. 2023, 10, 2302654. [Google Scholar] [CrossRef]
- Ghalei, B.; Wakimoto, K.; Wu, C.Y.; Isfahani, A.P.; Yamamoto, T.; Sakurai, K.; Higuchi, M.; Chang, B.K.; Kitagawa, S.; Sivaniah, E. Rational tuning of zirconium metal-organic framework membranes for hydrogen purification. Angew. Chem. Int. Ed. Engl. 2019, 58, 19034–19040. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, R.Z.; Jia, T.Z.; Cao, X.L.; Wang, Q.; Cao, J.R.; Li, S.; Shi, Q.X.; Isaacs, L.; Sun, S.P. Voltage-gated membranes incorporating cucurbit[n]uril molecular containers for molecular nanofiltration. J. Am. Chem. Soc. 2022, 144, 6483–6492. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Zhu, Y.Z.; Liu, C.; Lee, K.R.; Hung, W.S.; Wang, Z.Y.; Li, Y.Y.; Elimelech, M.; Jin, J.; Lin, S.H. Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1Å precision separation. Nat. Commun. 2020, 11, 2015. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yu, L.; Chu, T.C.; Niu, H.Y.; Wang, J.; Cai, Y. Constructing chemical stable 4-carboxyl-quinoline linked covalent organic frameworks via doebner reaction for nanofiltration. Nat. Commun. 2022, 13, 2615. [Google Scholar] [CrossRef]
- Guo, Y.M.; Sun, X.; Ding, S.S.; Lu, J.; Wang, H.T.; Zhu, Y.; Jiang, L. Charge-gradient sulfonated poly (ether ether ketone) membrane with enhanced ion selectivity for osmotic energy conversion. ACS Nano 2024, 18, 7161–7169. [Google Scholar] [CrossRef]
- Andra, S.; Gao, K.; Cai, J.J.; Han, J.; Nie, Y.; Lun, H.J.; Bai, Y.; Li, Y.M. Covalent integration of NH2-MIL-53(Al) into speek for enhanced proton exchange membrane performance. J. Power Sources 2025, 638, 236475. [Google Scholar] [CrossRef]
- Ren, T.N.; Zhu, G.M.; Zhang, C.S.; Wang, S.Y. Preparation of CF/PEEK composites with high mechanical performance using peek derivatives as the sizing agent. Macromol. Rapid Commun. 2023, 44, 2200738. [Google Scholar] [CrossRef]
- Li, Z.; Cao, T.T.; Zhang, Y.; Han, Y.; Xu, S.M.; Xu, Z.H. Novel lithium ion battery separator based on hydroxymethyl functionalized poly (ether ether ketone). J. Membr. Sci. 2017, 540, 422–429. [Google Scholar] [CrossRef]
- Weintraub, S.; Gellerman, G.; Bazylevich, A.; Bikson, B. Reduction of mesoporous poly (ether ether ketone) materials, preserving pore size. Polym. Int. 2024, 73, 471–477. [Google Scholar] [CrossRef]
- Zhang, H.X.; Lai, X.; You, J.; Wang, W.; Wu, M.H.; Liu, L.M.; Chen, H.Y.; Luo, Z.S.; Zhu, H.F.; Wang, Y.H.; et al. Facile construction of novel poly (ether sulfone ether ketone ketone) loose nanofiltration membrane for efficient dye/salt separation. Desalination 2025, 601, 118573. [Google Scholar] [CrossRef]
- Ma, H.B.; Wang, M.; Hou, J.W.; Wang, X.L.; Sun, P.C.; Wang, F.F. Strong and tough water-tolerant conductive eutectogels with phase-separated hydrophilic/hydrophobic dual ionic channels. Adv. Mater. 2025, 37, 2500770. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Zhang, K.; Wang, S.; Chen, C.H.; Zhao, X.G.; Zhou, H.W.; Wang, D.M. Construction of “ rigid-and-flexible “ interphase by waterborne carboxylated polyimide sizing agent for interfacial enhancement of carbon fiber/poly ether ether ketone (CF/PEEK) composites. Compos. B Eng. 2025, 298, 112388. [Google Scholar] [CrossRef]
- Namdar, H.; Crespi, E.; Bisello, A.; Speranza, G.; Gottardi, G.; Laidani, N.; Gamba, G.; Testi, M. Performance of catalyst coated sulfonated poly ether ether ketone membrane in PEM water electrolysis: Effects of membrane modification by inclusion of MoS2-coated carbon nanotubes. Int. J. Hydrogen Energy 2025, 130, 324–336. [Google Scholar] [CrossRef]
- Chen, Y.; Song, K.Y.; Li, Z.Y.; Su, Y.; Yu, L.; Chen, B.Y.; Huang, Q.J.; Da, L.T.; Han, Z.G.; Zhou, Y.F.; et al. Antifouling asymmetric block copolymer nanofilms via freestanding interfacial polymerization for efficient and sustainable water purification. Angew. Chem. Int. Ed. Engl. 2024, 63, e202408345. [Google Scholar] [CrossRef]
- Huang, T.J.; Li, J.F.; Chen, Y.; Zhong, T.H.; Liu, P.Q. Improving permeability and antifouling performance of poly (ether ether ketone) membranes by photo-induced graft polymerization. Mater. Today Commun. 2020, 23, 100945. [Google Scholar] [CrossRef]
- Hendrix, K.; Vandoorne, S.; Koeckelberghs, G.; Vankelecom, I.F.J. SRNF membranes for edible oil purification: Introducing free amines in crosslinked PEEK to increase membrane hydrophilicity. Polymer 2014, 55, 1307–1316. [Google Scholar] [CrossRef]
- Li, S.B.; Zhou, S.Y.; Qin, X.P.; Zhang, S.C.; Zhao, X.U.; Wang, K.X.; Liu, P.Q. Heparin-modified polyether ether ketone hollow fiber membrane with improved hemocompatibility and air permeability used for extracorporeal membrane oxygenation. Int. J. Biol. Macromol. 2024, 279, 135481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Zhang, Z.G.; Du, X.W.; Wang, Y.L.; Guo, X.H.; Yu, M.X.; Liu, B.J.; Hu, W.; Shen, L.; Lu, Y.F.; et al. Poly (ether ether ketone) conferred polyolefin separators with high dimensional thermal stability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 37354–37360. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Mehmood, A.; Hussain, A.; Ahmad, M.; Mushtaq, M.A.; Hussain, S.N.; Raza, N.; Cai, X.; Liu, D. Designing dendrite resistive poly (ether-ether-ketone) modified multifunctional Celgard separator for lithium metal batteries: Mechanistic and experimental study. J. Energy Storage 2024, 90, 111717. [Google Scholar] [CrossRef]
- Xu, X.J.; Zuo, J.H.; Zeng, H.J.; Zhao, Y.; Fan, Z.J. Improving osseointegration potential of 3D printed PEEK implants with biomimetic periodontal ligament fiber hydrogel surface modifications. Adv. Funct. Mater. 2024, 34, 2308811. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.Y.; Zhao, S.Y.; Cheng, B. Ultrasound-triggered piezoelectric polyetheretherketone with boosted osteogenesis via regulating Akt/GSK3β/β-catenin pathway. J. Nanobiotechnol. 2024, 22, 539. [Google Scholar] [CrossRef]
- Yameen, B.; Alvarez, M.; Azzaroni, O.; Jonas, U.; Knoll, W. Tailoring of poly (ether ether ketone) surface properties via surface-initiated atom transfer radical polymerization. Langmuir 2009, 25, 6214–6220. [Google Scholar] [CrossRef]
- Yousaf, A.; Farrukh, A.; Oluz, Z.; Tuncel, E.; Duran, H.; Dogan, S.Y.; Tekinay, T.; Rehman, H.U.; Yameen, B. UV-light assisted single step route to functional PEEK surfaces. React. Funct. Polym. 2014, 83, 70–75. [Google Scholar] [CrossRef]
- Weng, Y.T.; Liu, H.W.; Pei, A.; Shi, F.F.; Wang, H.S.; Lin, C.Y.; Huang, S.S.; Su, L.Y.; Hsu, J.P.; Fang, C.C.; et al. An ultrathin ionomer interphase for high efficiency lithium anode in carbonate based electrolyte. Nat. Commun. 2019, 10, 5824. [Google Scholar] [CrossRef]
- Lin, J.Y.; Yu, Z.J.; Chen, T.C.; Huang, J.M.; Chen, L.X.; Li, J.J.; Li, X.W.; Huang, X.L.; Luo, J.Q.; Ang, E.Y.M.; et al. Sub-4 nanometer porous membrane enables highly efficient electrodialytic fractionation of dyes and inorganic salts. Nat. Commun. 2025, 16, 3671. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.C.; Liu, N.; Zhu, A.M.; Liu, Q.L.; Lin, Z.; Zhang, Q.G. Upscaling preparation of poly (biphenyl-trifluoroacetophenone) hollow fiber loose membranes for high-efficiency dye/salt separation. Adv. Funct. Mater. 2025, 35, 2416490. [Google Scholar] [CrossRef]
- Cao, N.; Sun, Y.R.; Wang, J.; Zhang, H.B.; Pang, J.H.; Jiang, Z.H. Strong acid-and solvent-resistant polyether ether ketone separation membranes with adjustable pores. Chem. Eng. J. 2020, 386, 124086. [Google Scholar] [CrossRef]
- Cao, N.; Yue, C.; Lin, Z.Y.; Li, W.Y.; Zhang, H.B.; Pang, J.H.; Jiang, Z.H. Durable and chemical resistant ultra-permeable nanofiltration membrane for the separation of textile wastewater. J. Hazard. Mater. 2021, 414, 125489. [Google Scholar] [CrossRef]
- Yu, Y.W.; Wu, Y.; Li, Z.C.; Wang, Y.; Liu, P.; Wang, L.H.; Liu, C.W.; Wan, Y.; Sun, X.W.; Pan, W.H. Separation performance enhancement of PEEKWC/PEI cross-linked ultrafiltration membranes by poly (ethylene glycol) and polyvinylpyrrolidone as polymeric additives. J. Environ. Chem. Eng. 2024, 12, 111678. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.J.; Li, L.S.; Tang, Z.Y. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2019, 31, 1806090. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Shi, G.M.; Feng, Y.N.; Li, B.F.; Tham, H.M.; Lai, J.; Chun, T. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Ren, D.; Ren, S.P.; Lin, Y.K.; Xu, J.H.; Wang, X.L. Recent developments of organic solvent resistant materials for membrane separations. Chemosphere 2021, 271, 129425. [Google Scholar] [CrossRef] [PubMed]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Membr. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef]
- Burgal, J.D.S.; Peeva, L.; Livingston, A. Negligible ageing in poly (ether-ether-ketone) membranes widens application range for solvent processing. J. Membr. Sci. 2017, 525, 48–56. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.X.; Han, H.G.; Zhang, Q.F.; Li, S.H.; Zhang, S.B. Microporous and functional group co-designed polyesteramide membranes for efficient and broad-spectrum organic solvent nanofiltration. Adv. Membr. 2024, 4, 100098. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Cao, N.; Lin, Z.Y.; Chen, G.N.; Chen, L.Y.; Zhao, B.Q.; Liu, J.D.; Li, W.Y.; Jiang, Z.H.; Pang, J.H. Fabrication of robust go composite membranes through novel polyether ether ketone weaving strategies for organic solvent nanofiltration. Nano Lett. 2025, 25, 6879–6887. [Google Scholar] [CrossRef]
- Alqadhi, N.; Abdellah, M.H.; Nematulloev, S.; Mohammed, O.F.; Abdulhamid, M.A.; Szekely, G. Solution-processable poly(ether-ether-ketone) membranes for organic solvent nanofiltration: From dye separation to pharmaceutical purification. Sep. Purif. Technol. 2024, 328, 125072. [Google Scholar] [CrossRef]
- Alqadhi, N.; Oldal, D.G.; Gopalsamy, K.; Abdulhamid, M.A.; Szekely, G. Poly (ether-ether-ketone) copolymers featuring sulfonyl moieties for organic solvent nanofiltration membranes. Sep. Purif. Technol. 2025, 355, 129628. [Google Scholar] [CrossRef]
- Chen, B.Z.; Zhang, M.; Wang, L.; Li, L.; Han, Q.; Liu, X.; Wang, M.X.; Liu, B.; Jiang, Y.; Wang, Z.Y. Enhanced organic pollutant degradation in a two-dimensional Fe-doped crystalline carbon nitride membrane with near 100% singlet oxygen generation through the Fe-O-N configuration. Appl. Catal. B-Environ. Energy 2025, 363. [Google Scholar] [CrossRef]
- Kumar, M.; Baniowda, H.M.; Sreedhar, N.; Curcio, E.; Arafat, H.A. Fouling resistant, high flux, charge tunable hybrid ultrafiltration membranes using polymer chains grafted graphene oxide for NOM removal. Chem. Eng. J. 2021, 408, 127300. [Google Scholar] [CrossRef]
- Zhang, S.C.; Chen, C.F.; Su, Z.X.; Qin, X.P.; Jiang, M.J.; Liu, P.Q. High efficiency and flexible construction of hydrophilic polymer brushes on polyether ether ketone hollow-fiber membrane surface for improving permeability separation, and anti-fouling performances. Chem. Eng. J. 2023, 454, 140176. [Google Scholar] [CrossRef]
- Jiang, C.H.; Huang, T.J.; Chen, Y.; Su, Z.X.; Yan, X.; Xu, Q.B.; Jiang, M.J.; Liu, P.Q. The effect of grafting monomer charge on the antifouling performance of poly (ether ether ketone) hollow fiber membrane by ultraviolet irradiation polymerization. Polym. Int. 2021, 70, 1057–1064. [Google Scholar] [CrossRef]
- Rehman, A.; Sohail, M.; Baig, N.; Yuan, K.; Abdala, A.; Wahab, M.A. Next-generation stimuli-responsive smart membranes: Developments in oil/ water separation. Adv. Colloid. Interface Sci. 2025, 341, 103487. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.R.; Bi, W.H.; Jiang, Z.H.; Zhang, M.; Pang, J.H. High-strength corrosion resistant membranes for the separation of oil/water mixtures and immiscible oil mixtures based on PEEK. J. Membr. Sci. 2020, 616, 118418. [Google Scholar] [CrossRef]
- Yang, F.; Li, S.; Qi, H.Y.; Sun, W.B.; Li, J.B.; Zhang, M.; Chen, Z.; Pang, J.H.; Jiang, Z.H. Preparation and properties of PEEK-g-PNIPAM separation membranes with thermo-responsiveness for emulsion and organic liquid separation. J. Membr. Sci. 2024, 693, 122322. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Lin, Z.Y.; Chen, G.N.; Zhao, Z.W.; Li, W.Y.; Chen, L.Y.; Sun, Y.R. PEEK-PANI bi-functional oil-water separation membrane based on structural switchable wettability. J. Membr. Sci. 2025, 716, 123501. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.M.; Li, Y.X.; Hu, D.; Feng, X.D.; Li, W.L. Enhancing interface compatibility of UiO-66-NH2 and polyamide by incorporating dopamine into thin film nanocomposite membranes. J. Membr. Sci. 2022, 654, 120565. [Google Scholar] [CrossRef]
- Liu, J.X.; Peng, M.X.; Chen, L.C.; Li, T.; Liu, B.Z.; Zhao, D.S.; Wang, Z.H.; Ma, J.Y.; Chu, H.Q.; Tang, C.Y. Smart membrane with reversible superwetting transition for on-demand antifouling and self-cleaning. Environ. Sci. Technol. 2025, 59, 16056–16065. [Google Scholar] [CrossRef]
- Liang, Y.Y. Role of spacers in osmotic membrane desalination: Advances, challenges, practical and artificial intelligence-driven solutions. Process Saf. Environ. 2025, 201, 107587. [Google Scholar] [CrossRef]
- Osman, A.; Nasr, M.; Farghali, M.; Bakr, S.; Eltaweil, A.; Rashwan, A.; Abd El-Monaem, E. Machine learning for membrane design in energy production, gas separation, and water treatment: A review. Environ. Chem. Lett. 2024, 22, 505–560. [Google Scholar] [CrossRef]
| Polymer | Abbreviation | Molecular Structure |
|---|---|---|
| Poly (etherether ketone) | PEEK | 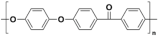 |
| Poly(oxa-p-phenylene-3,3-phtalido-p-phenylenxoxa-p-phenylenoxoxy-p-phenylene) with Cardo group | PEEK-WC | 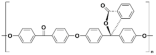 |
| Sulphonated poly (etherether ketone) | SPEEK | 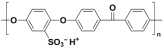 |
| Sulphonated poly(oxa-p-phenylene-3,3-phtalido-p-phenylenxoxa-p-phenylenoxoxy-p-phenylene) with Cardo group | SPEEK-WC | 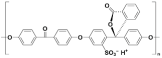 |
| Modified PEEK with diphenolic acid | VAPEEK | 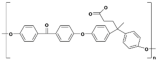 |
| Modified PEEK with tertiarybutylhydroquinone (TBHQ) (instead of hydroquinone) | TBPEEK | 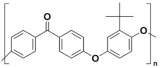 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, L.; Liu, H.; Li, X.; Li, D.; Yan, L.; Cheng, X. Recent Progress on the Development of Polyetheretherketone Membranes for Water Remediation. Membranes 2025, 15, 256. https://doi.org/10.3390/membranes15090256
Zhou J, Wang L, Liu H, Li X, Li D, Yan L, Cheng X. Recent Progress on the Development of Polyetheretherketone Membranes for Water Remediation. Membranes. 2025; 15(9):256. https://doi.org/10.3390/membranes15090256
Chicago/Turabian StyleZhou, Jingwen, Longjun Wang, Hong Liu, Xinhao Li, Dalong Li, Linlin Yan, and Xiquan Cheng. 2025. "Recent Progress on the Development of Polyetheretherketone Membranes for Water Remediation" Membranes 15, no. 9: 256. https://doi.org/10.3390/membranes15090256
APA StyleZhou, J., Wang, L., Liu, H., Li, X., Li, D., Yan, L., & Cheng, X. (2025). Recent Progress on the Development of Polyetheretherketone Membranes for Water Remediation. Membranes, 15(9), 256. https://doi.org/10.3390/membranes15090256