Influence of Membrane Salt Rejection Properties on Cake-Enhanced Concentration Polarization Effects During Colloidal Fouling of Nanofiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane and Foulant Characterization
2.3. Fouling and Salt Rejection Experiments
2.4. Modeling Fouling Effects on NaCl Rejection
2.4.1. Development of the Solution-Diffusion (SD) Model
2.4.2. Development of the Cake-Enhanced Concentration Polarization (CECP) Model
3. Results and Discussion
3.1. Foulant and Membrane Properties
3.2. Membrane Fouling Propensity
3.3. Effect of Fouling on Salt (NaCl) Rejection
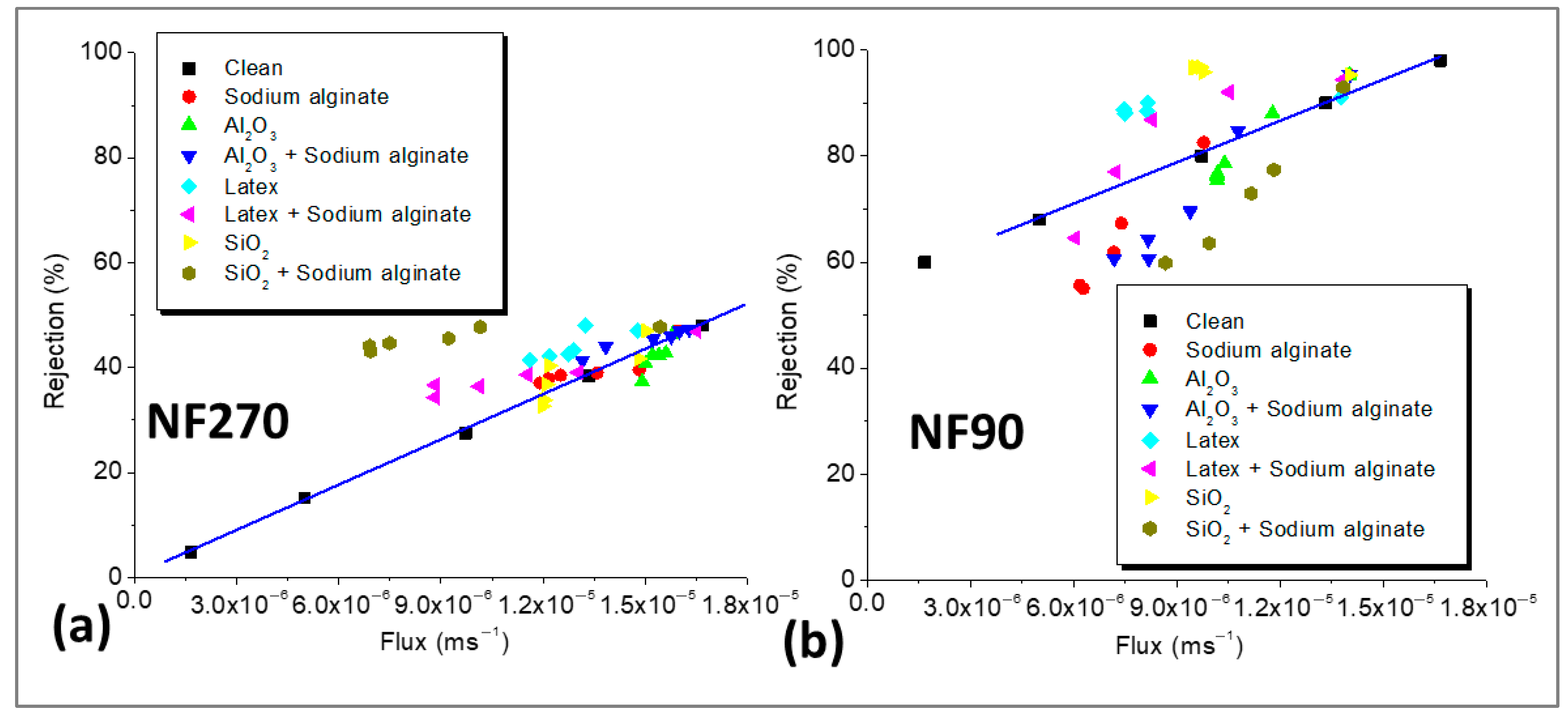
3.4. Verification of Cake-Enhanced Concentration Polarization (CEC) Effects on Salt Rejection
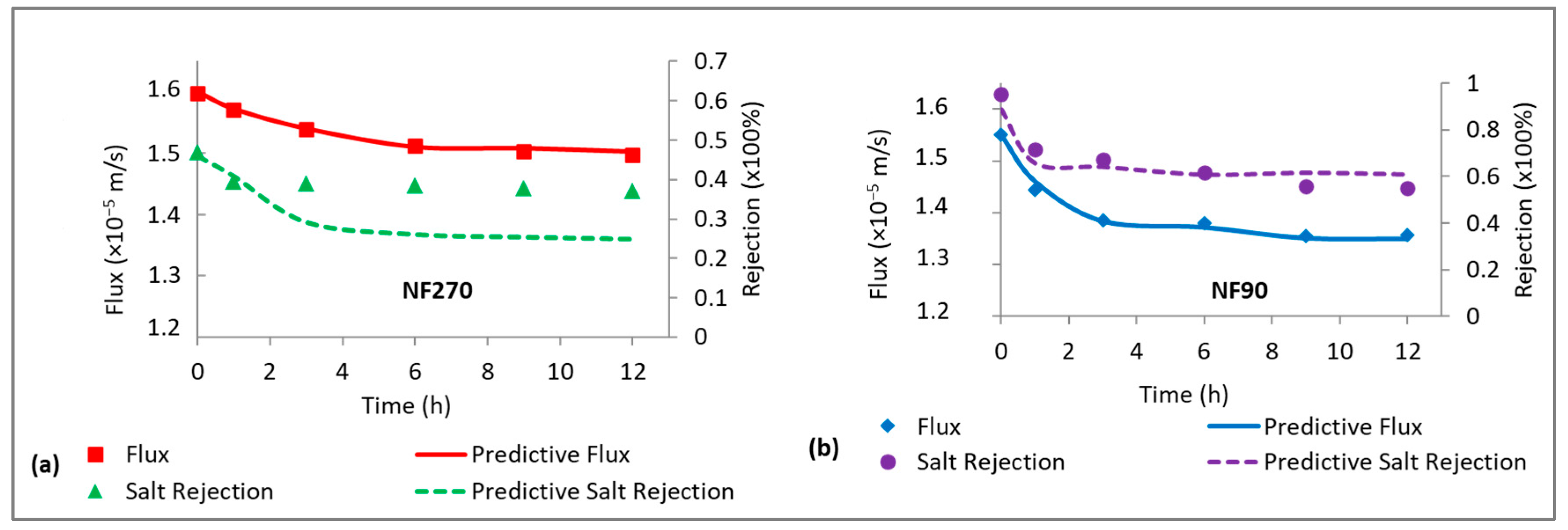
3.5. Evidence of CECP Effects in Low-Salt-Rejecting Membranes (NF270)
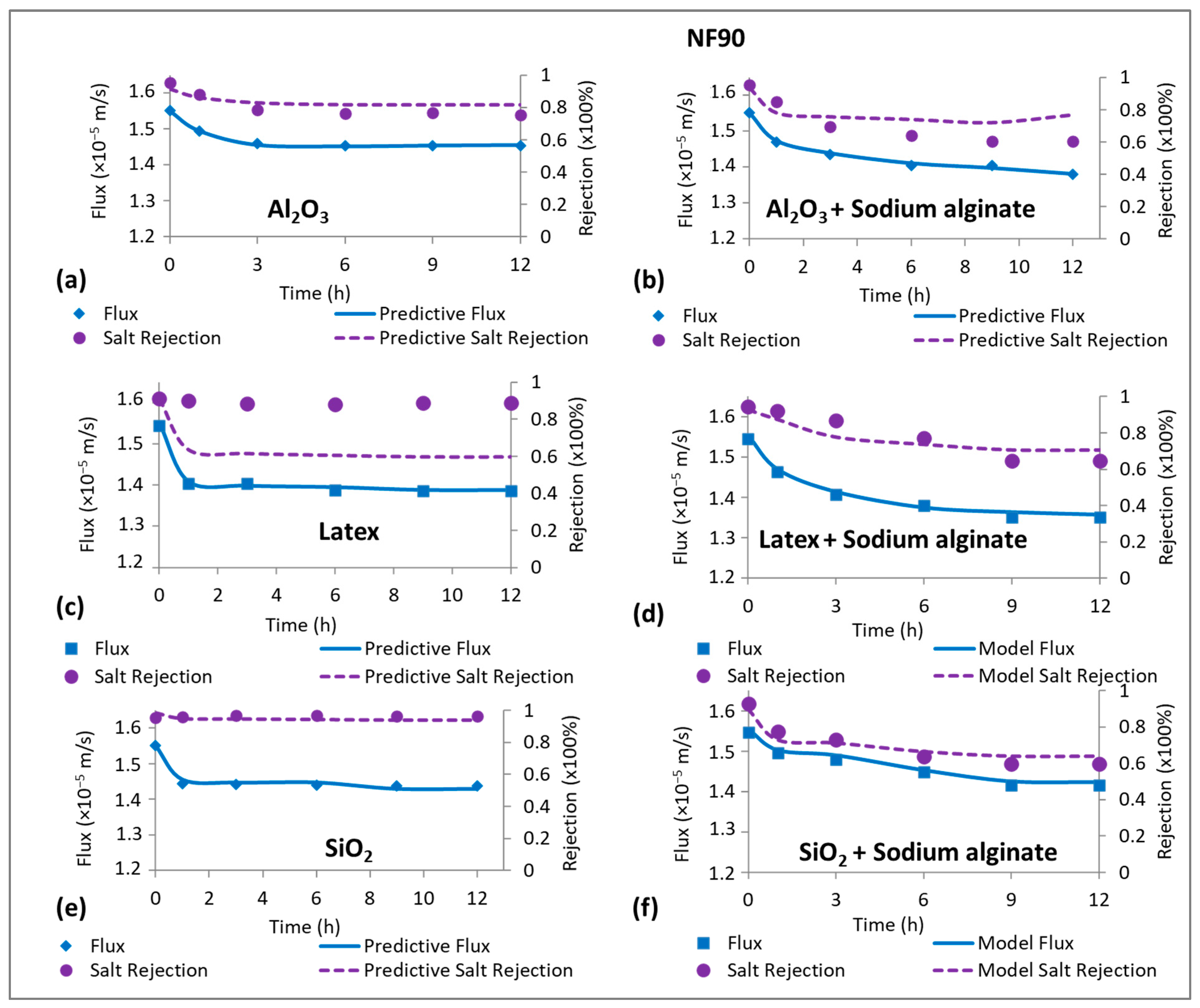
3.6. Evidence of CECP Effects in High-Salt-Rejecting Membranes (NF90)
3.7. Evidence of Declining Flux Effects on Salt Rejection
3.8. Membrane Autopsy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF | Nanofiltration |
| RO | Reverse Osmosis |
| CP | Concentration Polarization |
| CECP | Cake-Enhanced Concentration Polarization |
| SD | Solution-Diffusion |
References
- Wang, T.; Wang, H.; Ren, L.; Zhou, H.; Dai, R.; Wu, Z.; Wang, Z. Selective Ion Permeation Enables Reduced Combined Gypsum-Organic Fouling of Loose Nanofiltration Membranes. Desalination 2024, 580, 117567. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Coleman, P.J.; Espendiller, C. Mechanisms Underlying the Effects of Membrane Fouling on the Nanofiltration of Trace Organic Contaminants. Desalination 2010, 250, 682–687. [Google Scholar] [CrossRef]
- Ren, L.F.; Zhang, S.; Ma, Z.; Qiu, Y.; Ying, D.; Jia, J.; Shao, J.; He, Y. Antibiotics Separation from Saline Wastewater by Nanofiltration Membrane Based on Tannic Acid-Ferric Ions Coordination Complexes. Desalination 2022, 541, 116034. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Thwala, J.M.; Mamba, B.B.; Haese, A.D.; D’Haese, A.; Verliefde, A.R. Factors Governing Combined Fouling by Organic and Colloidal Foulants in Cross-Flow Nanofiltration. J. Membr. Sci. 2015, 491, 53–62. [Google Scholar] [CrossRef]
- Lin, D.; Lai, C.; Wang, X.; Wang, Z.; Kuang, K.; Wang, Z.; Du, X.; Liu, L. Enhanced Membrane Fouling by Microplastics during Nanofiltration of Secondary Effluent Considering Secretion, Interaction and Deposition of Extracellular Polymeric Substances. Sci. Total Environ. 2024, 906, 167110. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, O.T.; Nthunya, L.N.; Motsa, M.M.; Morifi, E.; Richards, H.; Mamba, B.B. Fouling of High Pressure-Driven NF and RO Membranes in Desalination Processes: Mechanisms and Implications on Salt Rejection. Chem. Eng. Res. Des. 2023, 199, 268–295. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Q.; Jiang, Y.; Yu, L.; Song, N.; Zhao, D.; Sui, L.; Dong, L. Antifouling and Antibacterial Bioactive Metabolites of Marine Fungus (Terrein)/Polyamide Thin-Film Composite Reverse Osmosis Membranes for Desalination Applications. Desalination 2024, 572, 117140. [Google Scholar] [CrossRef]
- Azaïs, A.; Mendret, J.; Petit, E.; Brosillon, S. Evidence of Solute-Solute Interactions and Cake Enhanced Concentration Polarization during Removal of Pharmaceuticals from Urban Wastewater by Nanofiltration. Water Res. 2016, 104, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Lau, W.J.; Yao Koe, A.Z.; Lim, W.J.; Ooi, B.S. Simultaneous Phosphate Recovery and Sodium Removal from Brackish Aquaculture Effluent via Diafiltration-Nanofiltration Process. Chemosphere 2022, 307, 136104. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Simon, A.; Alturki, A.A.; Bilitewski, B.; Price, W.E.; Nghiem, L.D. Effects of Fouling and Scaling on the Retention of Trace Organic Contaminants by a Nanofiltration Membrane: The Role of Cake-Enhanced Concentration Polarisation. Sep. Purif. Technol. 2010, 73, 256–263. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, Y.; Yang, C.; Venema, P.; Rietveld, L.C.; Heijman, S.G.J. Concentration Polarizations of PEG and Silica Colloids in Ceramic Nanofiltration. Desalination 2024, 583, 117722. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Licon, E.; Valderrama, C.; Gibert, O.; Cortina, J.L. Evaluating the Integration of Nanofiltration Membranes in Advanced Water Reclamation Schemes Using Synthetic Solutions: From Phosphorous Removal to Phosphorous Circularity. Sep. Purif. Technol. 2022, 290, 120914. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, H.; Park, E.; Han, J.I. Modeling of Forward Osmosis for Microalgae Harvesting. J. Membr. Sci. 2022, 642, 119910. [Google Scholar] [CrossRef]
- Lin, D.; Lai, C.; Shen, X.; Wang, Z.; Xu, D.; Nie, J.; Song, W.; Du, X.; Liu, L. Electro-Coagulation Pretreatment for Improving Nanofiltration Membrane Performance during Reclamation of Microplastic-Contaminated Secondary Effluent: Unexpectedly Enhanced Membrane Fouling and Mechanism Analysis by MD-DFT Simulation. Chem. Eng. J. 2024, 498, 155779. [Google Scholar] [CrossRef]
- Lin, D.; Xu, D.; Fang, K.; Wang, Z.; Du, X.; Ke, Q.; Liang, H.; Ulbricht, M. How in Situ Fabricated Polyzwitterionic Hydrogel Coating on Nanofiltration and Ultrafiltration Membranes Influences Mass Transfer Processes and Fouling Development. J. Membr. Sci. 2024, 711, 123174. [Google Scholar] [CrossRef]
- Ali, S.; Wang, R.; Huang, H.; Yin, S.; Feng, X. Per- and Polyfluoroalkyl Substance Separation by NF and RO Membranes: A Critical Evaluation of Advances and Future Perspectives. Environ. Sci. Water Res. Technol. 2024, 10, 1994–2012. [Google Scholar] [CrossRef]
- Tomczak, W. The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths. Membranes 2022, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, O.T.; Motsa, M.M.; Nkambule, T.I.; Mamba, B.B. Rejection of Trace Organic Compounds by Membrane Processes: Mechanisms, Challenges, and Opportunities. Rev. Chem. Eng. 2022, 39, 875–910. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Dykstra, J.E.; Porada, S.; Biesheuvel, P.M.; Elimelech, M. Salt and Water Transport in Reverse Osmosis Membranes: Beyond the Solution-Diffusion Model. Environ. Sci. Technol. 2021, 55, 16665–16675. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, T.O.; Hoek, E.M.V.; Mamba, B.B.; Verliefde, A.R.D. Influence of Organic, Colloidal and Combined Fouling on NF Rejection of NaCl and Carbamazepine: Role of Solute–Foulant–Membrane Interactions and Cake-Enhanced Concentration Polarisation. J. Membr. Sci. 2014, 471, 35–46. [Google Scholar] [CrossRef]
- Abuelgasim, H.; Nasri, N.; Futterlieb, M.; Souissi, R.; Souissi, F.; Panglisch, S.; Elsherbiny, I.M.A. Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes. Membranes 2025, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Verliefde, A.; Van der Meeren, P.; Van der Bruggen, B. Solution-Diffusion Processes. In Encyclopedia of Membrane Science and Technology; Hoek, E.M.V., Tarabara, V.V., Eds.; Wiley and Sons: Hoboken, NJ, USA; New York, NY, USA, 2013; p. 4013. ISBN 9781118522318. [Google Scholar]
- Hoek, E.M.V.; Kim, A.S.; Elimelech, M. Influence of Crossflow Membrane Filter Geometry and Shear Rate on Colloidal Fouling in Reverse Osmosis and Nanofiltration Separations. Environ. Eng. Sci. 2002, 19, 357–372. [Google Scholar] [CrossRef]
- Hoek, E.M.; Elimelech, M. Cake-Enhanced Concentration Polarization: A New Fouling Mechanism for Salt-Rejecting Membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Sutzkover, I.; Hasson, D.; Semiat, R. Simple Technique for Measuring the Concentration Polarization Level in a Reverse Osmosis System. Desalination 2000, 131, 117–127. [Google Scholar] [CrossRef]
- Schock, G.; Miquel, A. Mass Transfer and Pressure Loss in Spiral Wound Modules. Desalination 1987, 64, 339–352. [Google Scholar] [CrossRef]
- Boudreau, B.P. The Diffusive Tortuosity of Fine-Grained Unlithified Sediments. Geochim. Cosmochim. Acta 1996, 66, 3139–3142. [Google Scholar] [CrossRef]
- Jin, X.; Jawor, A.; Kim, S.; Hoek, E.M.V. Effects of Feed Water Temperature on Separation Performance and Organic Fouling of Brackish Water RO Membranes. Desalination 2009, 238, 346–359. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Schoutteten, K.V.K.M.; D’Haese, A.; Van den Bussche, J.; Vanhaecke, L.; Thwala, J.M.M.; Mamba, B.B.B.; Verliefde, A.R.D. Role of Permeate Flux and Specific Membrane-Foulant-Solute Affinity Interactions (∆Gslm) in Transport of Trace Organic Solutes through Fouled Nanofiltration (NF) Membranes. J. Membr. Sci. 2016, 518, 203–215. [Google Scholar] [CrossRef]
- Lin, Y.L.; Zheng, N.Y.; Hsu, Y.J. Enhancing Membrane Separation Performance in the Conditions of Different Water Electrical Conductivity and Fouling Types via Surface Grafting Modification of a Nanofiltration Membrane, NF90. Environ. Res. 2023, 239, 117346. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Marts, M.; Drewes, J.E. The Effect of Organic Membrane Fouling on the Properties and Rejection Characteristics of Nanofiltration Membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Heiranian, M.; Fan, H.; Song, L.; Li, Y.; Elimelech, M. Water Transport in Reverse Osmosis Membranes Is Governed by Pore Flow, Not a Solution-Diffusion Mechanism. Sci. Adv. 2023, 9, eadf8488. [Google Scholar] [CrossRef] [PubMed]





| Foulant Solution | Foulant (mg/L) | NaCl (mM) | |||
|---|---|---|---|---|---|
| Sodium Alginate | Latex | Al2O3 | SiO2 | ||
| Sodium alginate | 30 | 0 | 0 | 0 | 10 |
| Latex | 0 | 50 | 0 | 0 | 10 |
| Latex + Sodium alginate | 30 | 50 | 0 | 0 | 10 |
| Al2O3 | 0 | 0 | 50 | 0 | 10 |
| Al2O3 + Sodium alginate | 30 | 0 | 50 | 0 | 10 |
| SiO2 | 0 | 0 | 0 | 50 | 10 |
| SiO2 + Sodium alginate | 30 | 0 | 0 | 50 | 10 |
| Model Foulant | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Sodium alginate | 122.3 ± 5.2 | −34.2 ± 2.3 |
| Latex | 158.5 ± 5.2 | −41.8 ± 3.2 |
| Al2O3 | 161.5 ± 2.8 | 38.2 ± 1.5 |
| SiO2 | 135.4 ± 4.2 | −28.8 ± 2.1 |
| Parameter | Membrane | |
|---|---|---|
| NF270 | NF90 | |
| Contact angle (°) | 39 ± 2 | 50 ± 3 |
| Pure water permeability (Lm−2 h−1 bar−1) | 13 ± 2 | 7.1 ± 3 |
| MgSO4 rejection (%) | 96 ± 3 | 98 ± 2 |
| NaCl rejection (%) | 45 ± 4 | 96 ± 3 |
| Zeta potential (mV) | −15 ± 3.2 | −18 ± 1.4 |
| Membrane resistance (m−1) | 3.8 ± 1.1 × 1013 | 4.1 ± 0.8 × 1013 |
| NF270 | NF90 | |||||
|---|---|---|---|---|---|---|
| (µm) | (×1012 1/m) | (µm) | (×1012 1/m) | |||
| Sodium Alginate | 2.2 | 89.0 | 4.96 | 10.4 | 123.5 | 17.578 |
| Al2O3 | 3.8 | 9.0 | 0.91 | 4.6 | 43.5 | 4.41 |
| Al2O3 + Sodium alginate | 3.4 | 28.0 | 2.84 | 12.9 | 103.5 | 14.53 |
| Latex | 2.4 | 34.0 | 2.43 | 4.5 | 98.6 | 5.92 |
| Latex + Sodium alginate | 4.6 | 94.0 | 6.48 | 10.8 | 148.6 | 6.77 |
| SiO2 | 2.9 | 24.0 | 3.46 | 4.6 | 61.7 | 5.27 |
| SiO2 + Sodium alginate | 2.9 | 106.2 | 6.79 | 9.2 | 111.9 | 4.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlangu, O.T.; Mamba, B.B. Influence of Membrane Salt Rejection Properties on Cake-Enhanced Concentration Polarization Effects During Colloidal Fouling of Nanofiltration Membranes. Membranes 2025, 15, 215. https://doi.org/10.3390/membranes15070215
Mahlangu OT, Mamba BB. Influence of Membrane Salt Rejection Properties on Cake-Enhanced Concentration Polarization Effects During Colloidal Fouling of Nanofiltration Membranes. Membranes. 2025; 15(7):215. https://doi.org/10.3390/membranes15070215
Chicago/Turabian StyleMahlangu, Oranso Themba, and Bhekie Brilliance Mamba. 2025. "Influence of Membrane Salt Rejection Properties on Cake-Enhanced Concentration Polarization Effects During Colloidal Fouling of Nanofiltration Membranes" Membranes 15, no. 7: 215. https://doi.org/10.3390/membranes15070215
APA StyleMahlangu, O. T., & Mamba, B. B. (2025). Influence of Membrane Salt Rejection Properties on Cake-Enhanced Concentration Polarization Effects During Colloidal Fouling of Nanofiltration Membranes. Membranes, 15(7), 215. https://doi.org/10.3390/membranes15070215







