Optimization of the Quality of Reclaimed Water from Urban Wastewater Treatment in Arid Region: A Zero Liquid Discharge Pilot Study Using Membrane and Thermal Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Inflow Characteristics
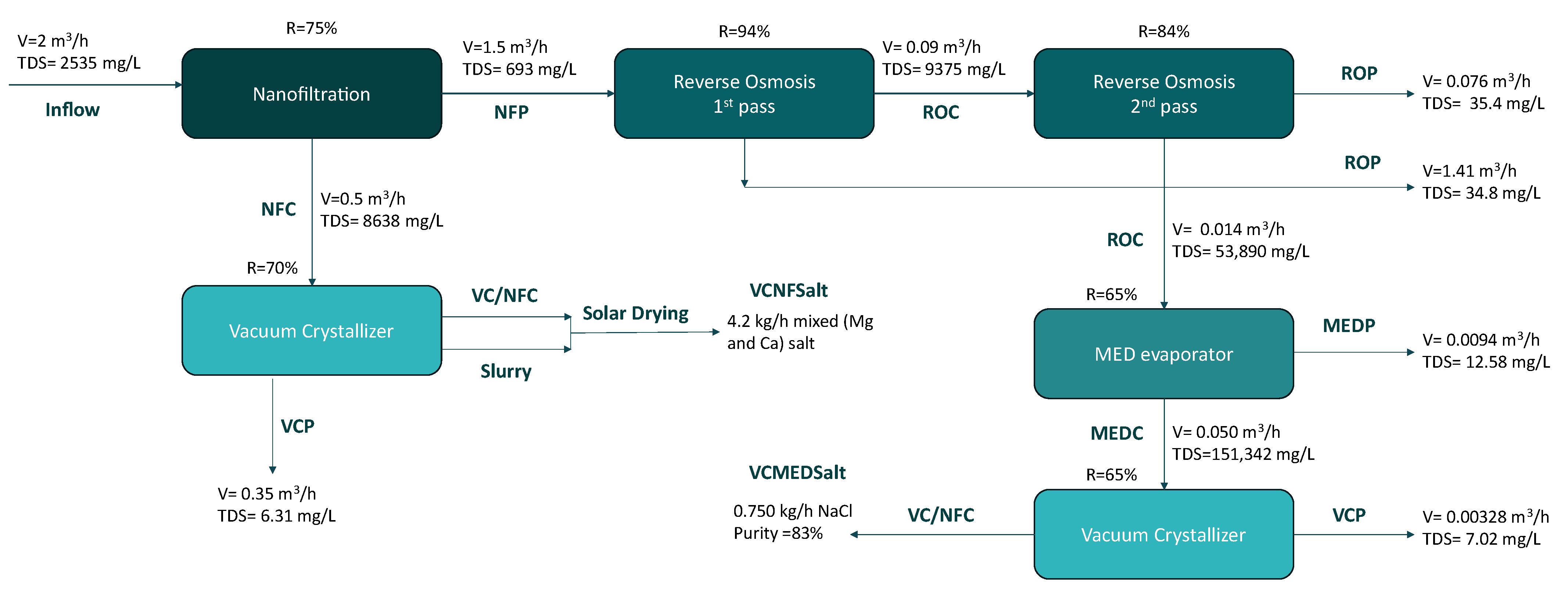
2.2. Description of the ZLD Pilot Design
2.3. Description of the ZLD Pilot Design
2.3.1. Membrane Technologies/Equipment and System Description
Nanofiltration Unit
Reverse Osmosis Unit
2.3.2. Thermal Technologies/Equipment and System Description
Multi Effect Distillation (MED) Evaporator
Vacuum Crystallizer (VC)
2.4. Configurations for Calculations
2.4.1. Membrane Units
2.4.2. Thermal Units
3. Results
3.1. Results of Each Operated Technology
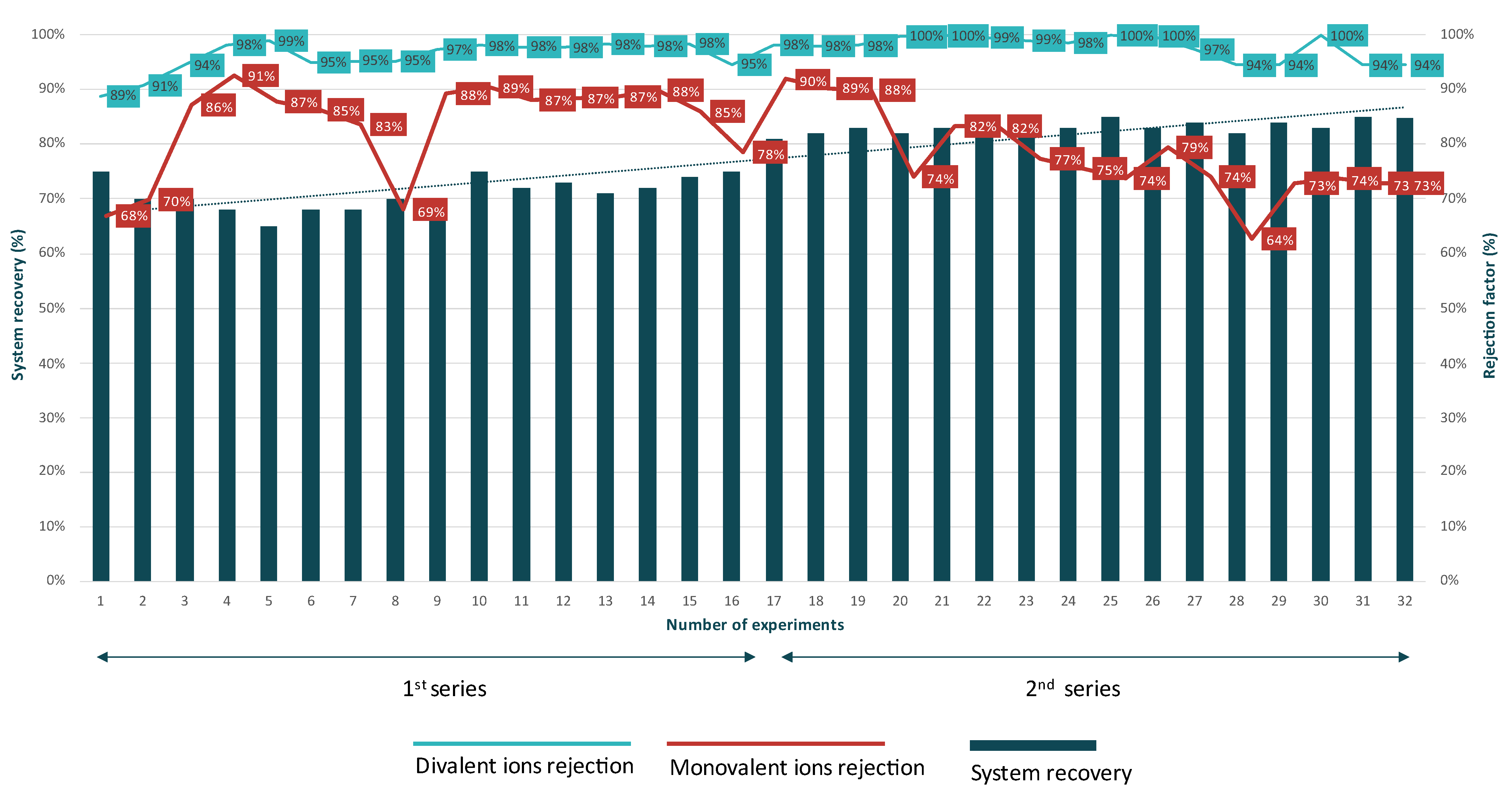
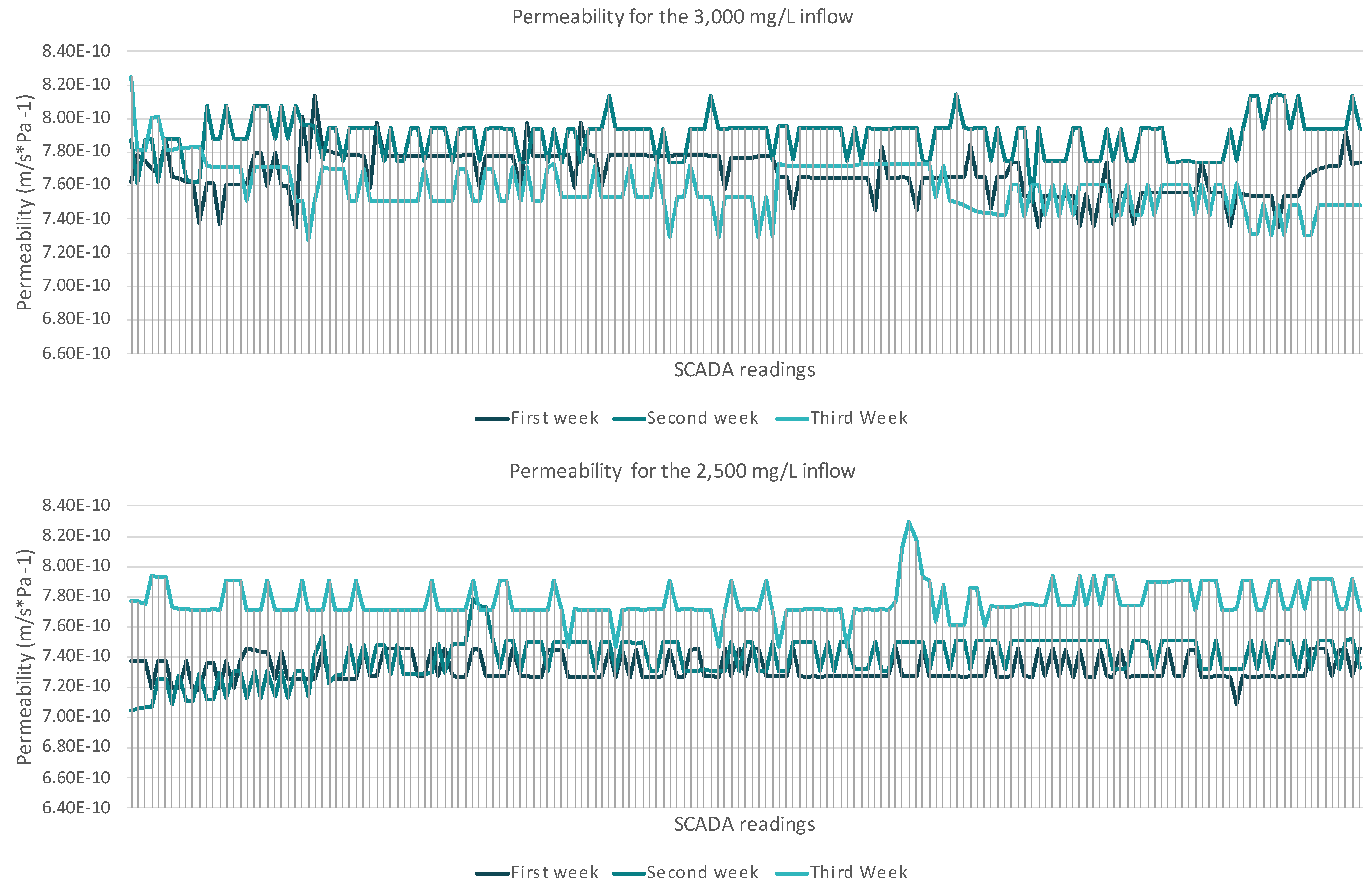
3.1.1. Nanofiltration Unit
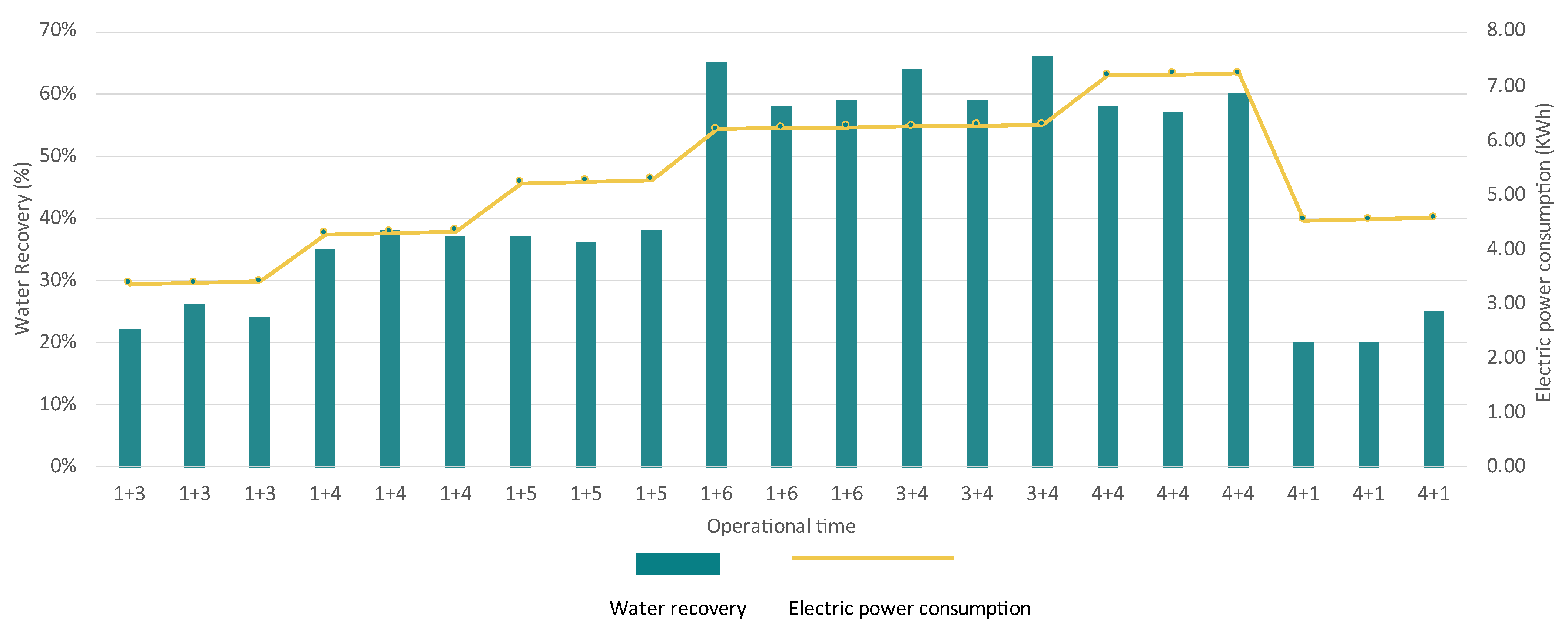
3.1.2. Reverse Osmosis Unit
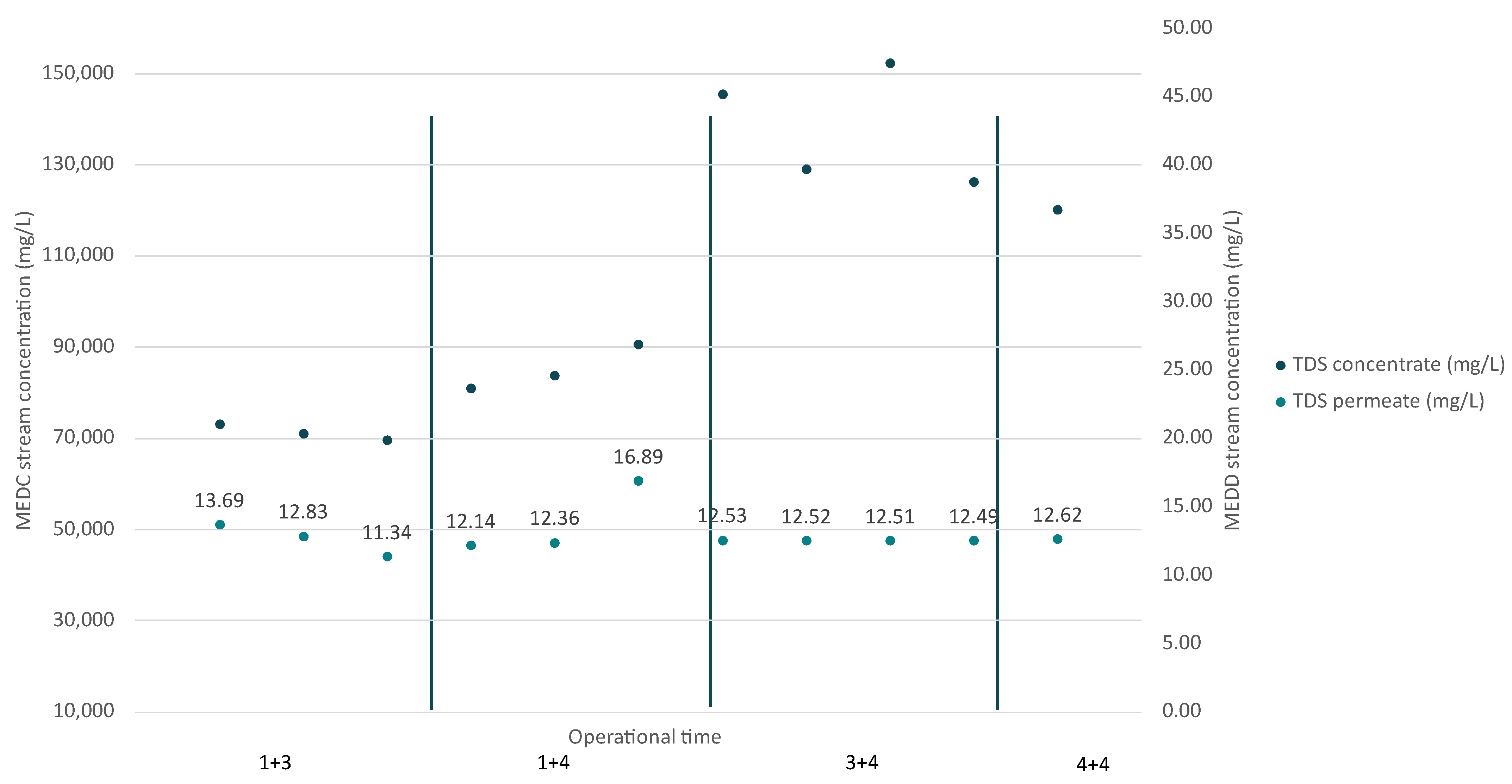
3.1.3. MED Evaporator Unit
3.1.4. Vacuum Crystallizer Unit
4. Discussion
4.1. Discussion of ZLD System Results
4.1.1. Membrane Units
4.1.2. Thermal Units
4.2. Overall Evaluation of the ZLD System
4.2.1. Chemical Use During the Operation
4.2.2. Recovered Water from the Pilot Unit
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO. Water Reuse: Opportunities and Challenges. 2023. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000388948 (accessed on 2 May 2025).
- Gleick, P.H. The World’s Water Volume 7: The Biennial Report on Freshwater Resources; Island Press: Washington, DC, USA, 2011. [Google Scholar]
- World Water Council. Annual Report 2014. 2014. Available online: https://www.worldwatercouncil.org/sites/default/files/Official_docs/WWC_Annual_Report_2014.pdf (accessed on 2 May 2025).
- Global Market Insights. Water Recycle and Reuse Market Size & Share, Forecast 2034. 2025. Available online: https://www.gminsights.com/industry-analysis/water-recycle-and-reuse-market (accessed on 2 May 2025).
- Cheng, H.; Hu, Y.; Zhao, J. Meeting China’s water shortage crisis: Current practices and challenges. Environ. Sci. Technol. 2009, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Metcalf and Eddy Inc.; Asano, T.; Burton, F.L.; Leverenz, H.; Tsuchihashi, R.; Tchobanoglous, G. Water Reuse; McGraw-Hill Professional Publishing: New York, NY, USA, 2007; pp. 6–15. [Google Scholar]
- Xu, M.; Bai, X.; Pei, L.; Pan, H. A research on application of water treatment technology for reclaimed water irrigation. Int. J. Hydrogen Energy 2016, 41, 15930–15937. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-based processes used in municipal wastewater treatment for water reuse: State-of-the-art and performance analysis. Membranes 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Areosa, I.; Martins, T.A.; Lourinho, R.; Batista, M.; Brito, A.G.; Amaral, L. Treated wastewater reuse for irrigation: A feasibility study in Portugal. Sci. Total Environ. 2024, 954, 176698. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Bagińska, S.; Malinowski, P. Assessing the feasibility of recovering water from treated municipal wastewater. J. Ecol. Eng. 2024, 25, 119–130. [Google Scholar] [CrossRef]
- Ramm, K.; Smol, M. Water reuse—Analysis of the possibility of using reclaimed water depending on the quality class in the European countries. Sustainability 2023, 15, 12781. [Google Scholar] [CrossRef]
- Eurostat. Sustainable Development Indicators: Water Exploitation Index. Available online: https://ec.europa.eu/eurostat/databrowser/view/sdg_06_60/default/map?lang=en (accessed on 2 May 2025).
- Water Development Department. Water Balance. Ministry of Agriculture, Rural Development and Environment, Cyprus. Available online: https://www.moa.gov.cy/moa/wdd/wdd.nsf/page10_en/page10_en?opendocument (accessed on 2 May 2025).
- Water Development Department. Recycled Water. Ministry of Agriculture, Rural Development and Environment, Cyprus. Available online: https://www.moa.gov.cy/moa/wdd/wdd.nsf/page24_en/page24_en?opendocument (accessed on 2 May 2025).
- Chen, C.; Guo, L.; Yang, Y.; Oguma, K.; Hou, L. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021, 801, 149678. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, Y.; Shen, C. Ecological and environmental effects of reclaimed water (RW) irrigation and recharge on soil environment and groundwater quality: A systematic review. Pol. J. Environ. Stud. 2024, 33, 6025–6036. [Google Scholar] [CrossRef]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Pitt, T.; Petrie, P.R. Impact of long-term recycled water irrigation on crop yield and soil chemical properties. Agric. Water Manag. 2020, 237, 106167. [Google Scholar] [CrossRef]
- Badr, E.S.A.; Tawfik, R.T.; Alomran, M.S. An assessment of irrigation water quality with respect to the reuse of treated wastewater in Al-Ahsa Oasis, Saudi Arabia. Water 2023, 15, 2488. [Google Scholar] [CrossRef]
- Mounzer, O.; Pedrero, F.; Bayona, J.M.; Nicolás, E.; Alarcón, J.J. Transient soil salinity under the combined effect of reclaimed water and regulated deficit drip irrigation of Mandarin trees. Agric. Water Manag. 2013, 120, 23–29. [Google Scholar] [CrossRef]
- Leogrande, R.; Pedrero, F.; Nicolás, E.; Vitti, C.; Lacolla, G.; Stellacci, A.M. Reclaimed water use in agriculture: Effects on soil chemical and biological properties in a long-term irrigated citrus farm. Agronomy 2022, 12, 1317. [Google Scholar] [CrossRef]
- Oron, G.; Gillerman, L.; Bick, A.; Buriakovsky, N.; Manor, Y.; Ben-Yitshak, E.; Hagin, J. A two-stage membrane treatment of secondary effluent for unrestricted reuse and sustainable agricultural production. Desalination 2006, 187, 335–345. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Attia, N.F.; Al-Ghusain, I.A. Performance evaluation of a wastewater reclamation plant using ultrafiltration and reverse osmosis. Desalin. Water Treat. 2015, 54, 2928–2938. [Google Scholar] [CrossRef]
- Kim, I.S.; Oh, B.S.; Yoon, S.; Shon, H.; Lee, S.; Hong, S. Wastewater reclamation. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Tong, T.; Xu, L.; Horseman, T.; Westerhoff, P.; Xu, P.; Yao, Y.; Zhang, X.; Alghanayem, R.; Lin, S. Brine management with zero and minimal liquid discharge. Nat. Rev. Clean Technol. 2025, 1, 185–200. [Google Scholar] [CrossRef]
- Wu, J.; Hoek, E.M.V. Current opportunities and challenges in membrane-based brine management. Curr. Opin. Chem. Eng. 2025, 47, 101079. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 2016, 13, 6846–6855. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane Technologies for Sustainable Wastewater Treatment: Advances, Challenges, and Applications in Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) Systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef]
- Radha; Sharma, N.; Prakash, S.; Kumari, N.; Sharma, D.; Laller, R.; Puri, S. From Challenges to Opportunities: Exploring Minimum Liquid Discharge and Zero Liquid Discharge Strategies for Wastewater Management and Resource Recovery. In Role of Science and Technology for Sustainable Future: Volume 2—Applied Sciences and Technologies; Springer Nature: Singapore, 2025; pp. 371–394. [Google Scholar]
- Chen, W.; Lu, S.; Pan, N.; Wang, Y.; Wu, L. Impact of reclaimed water irrigation on soil health in urban green areas. Chemosphere 2015, 119, 654–661. [Google Scholar] [CrossRef]
- Martínez-Alvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. Seawater desalination for crop irrigation—Current status and perspectives. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 461–492. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Guidelines for Water Reuse; EPA/625/R-04/108; U.S. Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Abdelkader, B.A.; Antar, M.A.; Khan, Z. Nanofiltration as a pretreatment step in seawater desalination: A review. Arab. J. Sci. Eng. 2018, 43, 4413–4432. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Shahonya, I.; Nangolo, F.; Erinosho, M.; Angula, E. Scaling and fouling of reverse osmosis (RO) membrane: Technical review. In Advances in Material Science and Engineering: Selected Articles from ICMMPE 2020; Springer: Singapore, 2021; pp. 41–47. [Google Scholar]
- DuPont Water Solutions. FilmTec™ NF90-4040 Element Product Data Sheet; DuPont: Wilmington, DE, USA, 2021; Available online: https://www.lenntech.com/Data-sheets/Dow-Filmtec-NF90-4040.pdf (accessed on 30 May 2025).
- Lenntech. (n.d.). DOW FILMTEC™ LC HR-4040 Element: Product Data Sheet. Available online: https://www.lenntech.com/Data-sheets/Dow-Filmtec-LC-HR-4040.pdf (accessed on 9 June 2025).
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef]
- Jim, K.M.; Kim, K.J.; Jang, Y.N. Effect of supersaturation on the particle size of ammonium sulfate in semibatch evaporative crystallization. Ind. Eng. Chem. Res. 2013, 52, 11151–11158. [Google Scholar] [CrossRef]
- Tahir, F.; Al-Ghamdi, S.G. Integrated MED and HDH desalination systems for an energy-efficient zero liquid discharge (ZLD) system. Energy Rep. 2022, 8, 29–34. [Google Scholar] [CrossRef]
- Panagopoulos, A. Process simulation and techno-economic assessment of a zero liquid discharge/multi-effect desalination/thermal vapor compression (ZLD/MED/TVC) system. Int. J. Energy Res. 2020, 44, 473–495. [Google Scholar] [CrossRef]
- Pala, J.K.; Moulik, S.; Ghosh, A.K.; Kamesh, R.; Roy, A. A zero liquid discharge strategy with MSF coupled with crystallizer. In Sustainable Water Treatment: Advances and Technological Interventions; Moulik, S., Mullick, A., Roy, A., Eds.; Scrivener Publishing LLC: Salem, MA, USA, 2022; pp. 487–515. [Google Scholar]
- Scelfo, G.; Trezzi, A.; Vassallo, F.; Cipollina, A.; Landi, V.; Xenogianni, C.; Tamburini, A.; Xevgenos, D.; Micale, G. Demonstration of ultra-high-water recovery and brine concentration in a prototype evaporation unit: Towards zero liquid discharge desalination. Sep. Purif. Technol. 2025, 354, 129427. [Google Scholar] [CrossRef]
- Schwantes, R.; Chavan, K.; Winter, D.; Felsmann, C.; Pfafferott, J. Techno-economic comparison of membrane distillation and MVC in a zero liquid discharge application. Desalination 2018, 428, 50–68. [Google Scholar] [CrossRef]
- DOW Water Solutions. Filmtec™ Reverse Osmosis Membranes. Technical Manual; Form 609-00071-399; DOW Water Solutions: Midland, MI, USA, 2010; pp. 1–180. [Google Scholar]
- Figueira, M.; Rodríguez-Jiménez, D.; López, J.; Reig, M.; Cortina, J.L.; Valderrama, C. Experimental and Economic Evaluation of Nanofiltration as a Pre-Treatment for Added-Value Elements Recovery from Seawater Desalination Brines. Desalination 2023, 549, 116321. [Google Scholar] [CrossRef]
- Du, J.R.; Zhang, X.; Feng, X.; Wu, Y.; Cheng, F.; Ali, M.E.A. Desalination of high salinity brackish water by an NF–RO hybrid system. Desalination 2020, 491, 114445. [Google Scholar] [CrossRef]
- Altaee, A.; Hilal, N. High recovery rate NF–FO–RO hybrid system for inland brackish water treatment. Desalination 2015, 363, 19–25. [Google Scholar] [CrossRef]
- Hudaib, B.; Hajarat, R.; Al-Zoubi, H.; Omar, W. Brackish water treatment by ceramic TiO2 low-pressure nanofiltration membranes. Glob. Nest J. 2023, 25, 138–147. [Google Scholar]
- Yammine, S.; Rabagliato, R.; Vitrac, X.; Peuchot, M.M.; Ghidossi, R. The use of nanofiltration membranes for the fractionation of polyphenols from grape pomace extracts. Oeno One 2019, 53, 11–26. [Google Scholar] [CrossRef]
- Ali, M.E. Nanofiltration process for enhanced treatment of RO brine discharge. Membranes 2021, 11, 212. [Google Scholar] [CrossRef]
- Park, M.; Park, J.; Lee, E.; Khim, J.; Cho, J. Application of nanofiltration pretreatment to remove divalent ions for economical seawater reverse osmosis desalination. Desalin. Water Treat. 2016, 57, 20661–20670. [Google Scholar] [CrossRef]
- Elazhar, F.; Elazhar, M.; El-Ghzizel, S.; Tahaikt, M.; Zait, M.; Dhiba, D.; Taky, M. Nanofiltration–reverse osmosis hybrid process for hardness removal in brackish water with higher recovery rate and minimization of brine discharges. Process Saf. Environ. Prot. 2021, 153, 376–383. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, R.; Rajput, V.D.; Minkina, T.; Agarwal, S.; Garg, M.C. A systematic approach towards optimization of brackish groundwater treatment using nanofiltration (NF) and reverse osmosis (RO) hybrid membrane filtration system. Chemosphere 2022, 303, 135230. [Google Scholar] [CrossRef]
- Ortega-Delgado, B.; Ruiz-Aguirre, A.; Zaragoza, G. Detailed thermodynamic analysis and modeling of forward feed multi-effect distillation systems. Desalin. Water Treat. 2017, 97, 94–108. [Google Scholar] [CrossRef]
- Xevgenos, D.; Moustakas, K.; Malamis, S.; Loizidou, M. An overview on the management of brine solutions. Desalin. Water Treat. 2016, 57, 2100–2114. [Google Scholar]
- Drioli, E.; Curcio, E.; Di Profio, G.; Fontananova, E. Brines from industrial water recycling: New ways to solve an old problem. Desalination 2020, 495, 114659. [Google Scholar]
- Atieh, M.A.; Arribas, L.; Zhou, Y.; Hilal, N. A review on the management and treatment of brine solutions. Desalination 2020, 491, 114530. [Google Scholar]
- Cipollina, A.; Misseri, A.; Galia, O.; Scialdone, G.; D’Alì Staiti, G.; Micale, G. Integrated production of fresh water, sea salt and magnesium from sea water. Desalin. Water Treat. 2012, 49, 390–403. [Google Scholar] [CrossRef]
- Poirier, K.; Lotfi, M.; Garg, K.; Patchigolla, K.; Anthony, E.J.; Faisal, N.H.; Al Mhanna, N. A Comprehensive Review of Pre- and Post-Treatment Approaches to Achieve Sustainable Desalination for Different Water Streams. Desalination 2023, 566, 116944. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot demonstration of the NH3/CO2 forward osmosis desalination process on high salinity brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Gohil, P.P.; Desai, H.; Kumar, A.; Kumar, R. Current status and advancement in thermal and membrane-based hybrid seawater desalination technologies. Water 2023, 15, 2274. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Panagopoulos, A. Assessing the energy footprint of desalination technologies and minimal/zero liquid discharge (MLD/ZLD) systems for sustainable water protection via renewable energy integration. Energies 2025, 18, 962. [Google Scholar] [CrossRef]
- Puretech Environmental. (n.d.). Evaporators. Available online: https://puretechenvironmental.com/technology/evaporators/ (accessed on 3 June 2025).
- Elginoz, N.; Papadaskalopoulou, C.; Harris, S. Using life cycle assessment at an early stage of design and development of zero discharge brine treatment and recovery. Water Resour. Ind. 2022, 28, 100184. [Google Scholar] [CrossRef]
- Panagopoulos, A. Beneficiation of saline effluents from seawater desalination plants: Fostering the zero liquid discharge (ZLD) approach—A techno-economic evaluation. J. Environ. Chem. Eng. 2021, 9, 105338. [Google Scholar] [CrossRef]
- Song, Y. Environmental Impacts of Zero Liquid Discharge Technologies. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2020. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/154870/Song,%20Yichuan%20Thesis.pdf?sequence=1 (accessed on 20 June 2025).
- Avramidi, M.; Klempetsani, S.; Kyriazi, M.; Gzyl, G.; Skalny, A.; Turek, M.; Loizidou, M. Innovative solution for the recovery of clean water and high-purity minerals from the coal mine sector. Desalination 2025, 586, 119121. [Google Scholar] [CrossRef]





| Element | Symbol | Unit | Value (Average/AVE) | Standard Deviation (SD) |
|---|---|---|---|---|
| Sodium | Na | mg/L | 490 | 20 |
| Potassium | K | mg/L | 43 | 2 |
| Magnesium | Mg | mg/L | 61 | 4 |
| Calcium | Ca | mg/L | 149 | 12 |
| Chloride | Cl | mg/L | 890 | 36 |
| Sulphates | SO4 | mg/L | 500 | 40 |
| Tot. Phosphorous | P | mg/L | 1.52 | 0 |
| Tot. Nitrogen | N | mg/L | 10.4 | 0 |
| Elec. conductivity | EC | mS/cm | 4.2 | 1.2 |
| pH | pH | 7.9 | 1 | |
| Biochemical Oxygen Demand | BOD5 | mg/L | <1 | 0 |
| Chemical Oxygen Demand | COD | mg/L | 20 | 12 |
| Tot. Susp. Solids | TSS | mg/L | 7 | 9 |
| NF Membranes/NF 90-4040 | RO Membranes/LC HR 4040 | |
|---|---|---|
| Materials | Polyamide thin-film composite | Polyamide thin-film composite |
| Permeate flow rate/membrane | 7.6 m3/d | 11 m3/day |
| pH range | 2–11 | 1–13 |
| Max operating pressure | 41 bar | 41 bar |
| Salt rejection/membrane | >90% | >99.7% |
| Physicochemical Parameters | Apparatus | Method |
|---|---|---|
| EX SITU | ||
| Na+, K+, Mg2+, Ca2+, Cl− | Ion chromatograph, Thermo Dionex ICS-5000 | ASTM D6919-17 |
| SO42− | Ion chromatograph, Thermo Dionex ICS-5000 | APHA-AWWA-WEF Method 4110 |
| TDS | Calculation (sum of ionic concentrations) | - |
| IN SITU | ||
| pH | Elmetron EPX-1t pH electrode or equivalent; Elmetron EPX-4UB or equivalent; Elmetron CX-401 or equivalent | EPA Method 150.1 |
| BOD | BOD bottles (300 mL), incubator, DO meter | Standard Methods for the Examination of Water and Wastewater, 5210 B |
| Conductivity (mS/cm) | Elmetron ECF-1t; flow sensor | SESDPROC-106-R6 |
| Hours of Operation | TDS (mg/L) of Distillate Water | Electric Power Consumption (KWh) |
|---|---|---|
| 8 | 12.1 | 3.84 |
| 10.9 | 3.46 | |
| 12.2 | 3.72 | |
| 9 | 11.1 | 5.07 |
| 10.8 | 4.71 | |
| 10.2 | 2.52 | |
| 12.5 | 4.26 | |
| 3.6 | 4.22 | |
| 10 | 12.9 | 5.12 |
| 10.1 | 4.67 | |
| 12.4 | 4.66 | |
| 10.1 | 5.43 |
| MED Unit | Crystallizer Unit | ||||
|---|---|---|---|---|---|
| Element | Symbol | RO Concentrate (After Second Pass) (AVE) | MED Concentrate (AVE) | VC/MEDC (AVE) | VC/NFC (AVE) |
| Value | Value | Value | Value | ||
| Potassium | K+ | 1822 | 5101 | 14,282 | 605 |
| Sodium | Na+ | 12,381 | 34,276 | 94,893 | 5970 |
| Calcium | Ca2+ | 1239 | 3517 | 9987 | 6259 |
| Magnesium | Mg2+ | 158 | 437 | 1209 | 1056 |
| Chloride | Cl− | 38,233 | 107,854 | 304,257 | 9913 |
| Sulphates | SO42− | 59 | 157 | 145 | 4542 |
| pH | pH | 6.8 | 6.6 | 7.2 | 7.1 |
| Concentration | TDS | 53,897 | 151,349 | 424,779 | 28,351 |
| Volume | m3/h | 0.0144 | 0.00504 | 0.001764 | 0.15 |
| NaCl purity | % | 81 | 38 | ||
| System Water Recovery | % | 65 | 65 | 70 | |
| Unit | Operational Parameters | Inlet Unit Volumes (m3/h) | Unit Energy Consumption (kWh/m3 Unit Inlet) | Energy Consumption per Unit of 2 m3 ZLD Inflow (kWh) |
|---|---|---|---|---|
| NF |
| 2 | 1 | 2 |
| 1st pass RO |
| 1.5 | 0.8 | 1.2 |
| 2nd pass RO | 0.09 | 2 | 0.18 | |
| MED |
| 0.014 | 60 | 0.84 |
| VC/MEDC |
| 0.005 | 40 | 0.2 |
| VC/NFC |
| 0.5 | 40 | 20 |
| Parameters | Limits from Regulation (EU) 2020/741 (Class A) | UWWTPs ≥ 2000 p.e (Applied in Cyprus) | UWWTPs ≤ 2000 p.e. (Applied in Cyprus) | ||||
|---|---|---|---|---|---|---|---|
| All Crops and Green Areas (a) | Vegetables Eaten Cooked (b) | Products for Human Consumption and Green Areas with Limited Access to the Public | Crops for Animal Feed | Industrial Plants | |||
| BOD5 mg/L | 10 | 10 | 10 | 10 | 25 | 25 | 25 |
| COD mg/L | 70 | 70 | 70 | 125 | 125 | 125 | |
| Suspended Solids mg/L | 10 | 10 | 10 | 10 | 35 | 35 | 35 |
| E. coli/100 mL | 5 | - | 5 | 50 | 200 | 200 | 200 |
| Tot. Nitrogen mg/L | - | 15 | - | - | - | - | - |
| Tot. Phosphorus mg/L | - | 10 | - | - | - | - | - |
| FOG mg/L | - | - | 5 | 5 | 5 | 5 | 5 |
| pH | - | 6.5–8.5 | 6.5–8.5 | 6.5–8.5 | 6.5–8.5 | 6.5–8.5 | 6.5–8.5 |
| Conductivity μS/cm | - | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 |
| Cl (mg/L) | - | 300 | 300 | 300 | 300 | 300 | 300 |
| B (mg/L) | - | - | 1 | 1 | 1 | 1 | 1 |
| Residual Chlorine mg/L | - | - | 2 | 2 | 2 | 2 | 2 |
| RO 1st Pass | RO 2nd Pass | MED Evaporator | VC/MEDc | VC/NFc | Mixed Recovered Water | UWWTPs ≥ 2000 p.e (Limits Applied in Cyprus) | |
|---|---|---|---|---|---|---|---|
| Ion | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| K+ | 2.1 | 2.1 | 0.75 | 0.38 | 0.45 | 1.80 | |
| Na+ | 18.3 | 18.8 | 1.63 | 0.92 | 0.98 | 14.95 | |
| Ca2+ | 0.7 | 0.6 | 1.20 | 1.48 | 1.20 | 0.79 | |
| Mg2+ | 0.3 | 0.4 | 0.13 | 0.89 | 0.79 | 0.42 | |
| Cl− | 12.7 | 12.8 | 8 | 2.94 | 2.87 | 10.77 | 300 |
| SO42− | 0.7 | 0.6 | 0.85 | 0.38 | 0.26 | 0.59 | |
| Tot. Phosphorous | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 15 |
| Tot. Nitrogen | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 10 |
| TDS | 34.8 | 35.4 | 12.6 | 7.02 | 6.6 | 29.3 | |
| BOD5 | <1 | <1 | <1 | <1 | <1 | <1 | 10 |
| COD | n.d | n.d | n.d | n.d | n.d | n.d | 70 |
| pH | 5.9 | 6.1 | 5.7 | 6.3 | 6.3 | 6.7 | 6.5–8.5 |
| Suspended Solids mg/L | 0.5 | 0.1 | n.d | n.d | n.d | 0.2 | 10 |
| Conductivity μS/cm | 35 < EC < 50 | 35 < EC < 50 | 7 < EC < 10 | 10 < EC < 20 | 11 < EC < 18 | 47 | 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidi, M.; Loizou, C.; Kyriazi, M.; Malamis, D.; Kalli, K.; Hadjicharalambous, A.; Kollia, C. Optimization of the Quality of Reclaimed Water from Urban Wastewater Treatment in Arid Region: A Zero Liquid Discharge Pilot Study Using Membrane and Thermal Technologies. Membranes 2025, 15, 199. https://doi.org/10.3390/membranes15070199
Avramidi M, Loizou C, Kyriazi M, Malamis D, Kalli K, Hadjicharalambous A, Kollia C. Optimization of the Quality of Reclaimed Water from Urban Wastewater Treatment in Arid Region: A Zero Liquid Discharge Pilot Study Using Membrane and Thermal Technologies. Membranes. 2025; 15(7):199. https://doi.org/10.3390/membranes15070199
Chicago/Turabian StyleAvramidi, Maria, Constantinos Loizou, Maria Kyriazi, Dimitris Malamis, Katerina Kalli, Angelos Hadjicharalambous, and Constantina Kollia. 2025. "Optimization of the Quality of Reclaimed Water from Urban Wastewater Treatment in Arid Region: A Zero Liquid Discharge Pilot Study Using Membrane and Thermal Technologies" Membranes 15, no. 7: 199. https://doi.org/10.3390/membranes15070199
APA StyleAvramidi, M., Loizou, C., Kyriazi, M., Malamis, D., Kalli, K., Hadjicharalambous, A., & Kollia, C. (2025). Optimization of the Quality of Reclaimed Water from Urban Wastewater Treatment in Arid Region: A Zero Liquid Discharge Pilot Study Using Membrane and Thermal Technologies. Membranes, 15(7), 199. https://doi.org/10.3390/membranes15070199








