Abstract
Superparamagnetic magnetite nanoparticles (Fe3O4) have garnered considerable interest due to their unique magnetic properties and potential for integration into multifunctional biomaterials. In particular, their incorporation into bacterial cellulose (BC) matrices offers a promising route for developing sustainable and high-performance magnetic composites. Numerous studies have explored BC-magnetite systems; however, innovations combining ex situ coprecipitation synthesis within BC matrices, tailored reagent molar ratios, stirring protocols, and purification processes remain limited. This study aimed to optimize the ex situ coprecipitation method for synthesizing superparamagnetic magnetite nanoparticles embedded in BC membranes, focusing on enhancing particle stability and crystallinity. BC membranes containing varying concentrations of magnetite (40%, 50%, 60%, and 70%) were characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and vibrating sample magnetometry (VSM). The resulting magnetic BC membranes demonstrated homogenous dispersion of nanoparticles, improved crystallite size (6.96 nm), and enhanced magnetic saturation (Ms) (50.4 emu/g), compared to previously reported methods. The adoption and synergistic optimization of synthesis parameters—unique to this study—conferred greater control over the physicochemical and magnetic properties of the composites. These findings position the optimized BC-magnetite nanocomposites as highly promising candidates for advanced applications, including electromagnetic interference (EMI) shielding, electronic devices, gas sensors, MRI contrast agents, and targeted drug delivery systems.
1. Introduction
Bacterial cellulose (BC) is a biopolymer produced by the fermentation of bacteria and microbial consortia. In addition to featuring nanometric-scale fibers and high hydrogel-forming ability, they can be easily produced without dependence on seasonal factors, large cultivation areas, or long and costly purification processes [1,2,3]. BC properties, such as the ability to absorb hydrophilic compounds when wet, biocompatibility, and strength, position it as a material with significant biotechnological potential for various applications, such as sensors, drug delivery systems, packaging, medical dressings, etc. [4,5]. Owing to all these characteristics and its production through renewable sources (corn steep liquor, tea, fruit residue. etc.), in numerous studies, BC-based materials have been widely used as matrices for producing hybrid and composite materials useful in fields such as medicine, pharmaceuticals, food, electronics, textiles, packaging, and environmental remediation [6,7,8,9].
New BC-based biomaterials with magnetic properties have shown promise as a biotechnological material in various studies, due to a wide range of synthetic routes and dopants for their production [10,11,12]. Additionally, there is a broad spectrum of applications, enabling these materials to be adapted to different manufacturing conditions of temperature, availability of reagents, and equipment to meet diverse demands [13,14,15].
Literature provides several examples of the application of magnetic bacterial cellulose-based materials, which have demonstrated excellent results. In the study by Maruthupandy et al. [16], for example, a composite of bacterial cellulose (BC) with graphene and magnetite was developed, showing strong antimicrobial activity against pathogenic bacteria such as E. coli and P. mirabilis. Similarly, in the work of Chaabane et al. [17], a drug delivery system based on magnetic BC demonstrated antimicrobial activity and exhibited antitumor effects in mice, effectively suppressing cancer development. In Chen et al. [18], a bacterial cellulose material with magnetite exhibited high efficiency in filtering an effluent containing an artificial coloring, Congo Red. In the field of sensors, Chen et al. [19] developed a flexible, wearable sensor composed of BC and cobalt ferrite, capable of monitoring motion. Fan et al. [20] created a material containing bacterial cellulose and magnetite with high shielding efficiency against electromagnetic waves. These examples highlight the variety and broad application potential of magnetic BC in diverse fields.
Magnetite (Fe3O4), which is one of the most commonly used dopants in producing these biomaterials, can exhibit either ferromagnetic or superparamagnetic characteristics, depending on its size [21,22,23,24,25,26]. Coprecipitation syntheses, both in situ and ex situ, are commonly employed for the production and incorporation of magnetite and other magnetic particles [27]. This methodology is widely used thanks to its simplicity and efficiency. However, adopting specific parameters influences the final size of particles and, consequently, their magnetic properties [28].
Souza et al. [29], who conducted a study on coprecipitation routes for in situ and ex-situ magnetite nanoparticles in BC membranes, observed that the method of incorporating magnetite influences the final size of the nanoparticles, the arrangement among BC fibers, and the magnetic properties. The results obtained were satisfactory; however, several studies suggest that some parameters could be optimized to obtain more stable superparamagnetic nanoparticles [30,31,32,33].
Based on the above, the aim of this study was to produce hybrid membranes of BC with superparamagnetic magnetite nanoparticles by enhancing the efficiency of the ex situ coprecipitation synthesis method.
2. Materials and Methods
2.1. Magnetic Biomembrane Production
2.1.1. Microorganism Maintenance
The bacterium Komagataeibacter hansenii UCP1619, utilized for biocellulose (BC) production, was obtained from the Culture Bank of the Catholic University of Pernambuco (UNICAP), Recife, Brazil. The microorganism was preserved in HS medium, originally described by Hestrin and Schramm [34] and modified by Hungund and Gupta [35]. The medium consisted of 2.0% (w/v) glucose, 0.5% (w/v) yeast extract, 0.5% (w/v) peptone, 0.27% (w/v) disodium hydrogen phosphate (Na2HPO4), and 0.15% (w/v) citric acid (C6H8O7).
2.1.2. Preparation of Pre-Inoculum
The procedure was carried out according to the methods described by Costa et al. [36] and Galdino et al. [37]. The bacterium was first activated and inoculated into HS agar medium and then incubated at 30 °C for 48 h. The activated cells were subsequently used to prepare the pre-inoculum, which was cultivated in liquid HS medium for 48 h at 30 °C.
2.1.3. Biomembrane Production
For the fermentative production of BC, the production medium described by Costa et al. [36] and Galdino et al. [37] was used, a culture medium enriched with an agro-industrial residue, corn steep liquor, which enhances the productivity of BC membranes, reduces production costs, and provides an environmentally friendly use for a residue that would otherwise be discarded in nature. The medium consisted of 1.5% glucose, 2.5% corn steep liquor, 0.27% Na2HPO4, and 0.15% citric acid. After adjustment of the pH to 5, 3% of the pre-inoculum was added. BC biofilms were produced using a static fermentation method at 30 °C over a 10-day growth period. After production, the BC films were extracted from the fermented broth, cleaned, purified by immersion in a 4% NaOH solution for 2 h, and thoroughly washed with deionized water.
2.1.4. Production of Magnetite Nanoparticles
The synthesis of magnetite via coprecipitation was optimized based on parameters found in literature, which favor the production of stable and superparamagnetic particles. The molar ratio of Fe2+ to Fe3+ was adjusted from 1:2 to 1:2.1, in accordance with studies by Goss [30], Jiang et al. [31], Eom et al. [32], and Shahid and Choi [33], which revealed that an increase in Fe3+ ions promotes the formation of magnetite while preventing the formation of maghemite. For this purpose, solutions of 3.329 mol/L FeCl3 and 1.96 mol/L FeCl2 were mixed in distilled water and vigorously stirred for 10 min. Adopting vigorous stirring led, in fact, to the increased reactivity of Fe2+ ions, thereby producing Fe3O4 particles with enhanced magnetic properties [38,39,40]. Subsequently, 100 g of NH4OH was slowly added, which turned the solution black, confirming a high percentage of magnetite formation by a qualitative observation. A process similar to that reported by Jiang et al. [31] was employed to purify and stabilize the synthesized particles. An ammonium oleate surfactant (composed of 15 g of oleic acid, 20 mL of distilled water, and 10 mL of NH4OH) was added to the product, forming micelles around the particles to protect them, and then an aqueous solution of hydrochloric acid (1:2 vol) was added to neutralize NH4OH and precipitate Fe3O4. The particles were finally collected with the aid of a magnet, washed with distilled water and isopropanol, and dried on a hot plate at 40 °C, thus preserving their magnetic properties. The Fe3O4 produced was named “MAG N”.
2.1.5. Incorporation of Magnetite Nanoparticles into Biocellulose Membranes
Prior to incorporation, BC was processed using a processor, model New B (Mallory, São Paulo, Brazil), with a power output of 1000 W and a rotational speed of 16,750 rpm, resulting in a biocellulose paste. The use of a high-power, high-speed processor facilitated the processing of bacterial cellulose (BC) and contributed to the uniform dispersion of nanoparticles throughout the matrix. Subsequently, the nanoparticles were incorporated into the BC paste using the same equipment at magnetite concentrations of 40%, 50%, 60%, and 70%, generating the samples labeled BC40MAG, BC50MAG, BC60MAG, and BC70MAG, respectively.
The samples were molded in Petri dishes and dried in an oven at 40 °C for 24 h.
2.2. Physicochemical and Structural Characterizations
2.2.1. X-Ray Diffraction (XRD)
XRD analysis was employed to identify the phase composition of the samples using an XRD-7000 diffractometer (Shimadzu, Kyoto, Japan). Measurements were conducted on powdered samples mounted on an aluminum support, covering a 2θ range from 10° to 80°, utilizing Cu Kα radiation (λ = 1.54056 Å) at a scanning rate of 0.5°/min in continuous mode. The crystallite sizes were determined by applying the Scherrer equation, following the methodology outlined by Burton et al. [41] and Mascolo et al. [42]. The crystallinity was calculated according to the methodology of Ruland and Vonk, as described in the study by Cheah and Masaharu [43].
2.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy
The identification of functional groups in the samples was carried out by FTIR spectroscopy using an IRTracer 100 spectrometer (Shimadzu, Kyoto, Japan). The spectra were recorded over a wavenumber range of 400 to 4000 cm−1, with a resolution of 4 cm−1 and an average of 128 scans.
2.2.3. Scanning Electron Microscopy (SEM)
For SEM analysis, the samples were coated with a thin layer of gold using a sputtering technique. The imaging was performed at room temperature using a Mira 3 microscope (Tescan, Kohoutovice, Czech Republic). Micrographs were obtained at both micrometer and nanometer scales.
2.2.4. Vibrating Sample Magnetometry (VSM)
Magnetization as a function of applied magnetic field (M vs. H) was measured at 300 K using a high-sensitivity magnetometer VSM (MicroSense model 3482-70 Electromagnet, Hamm, Germany). The magnetic field was applied within the range of −20 to 20 kOe, with the instrument operating at a sensitivity of 10−5 emu/g. The sample consisted of superparamagnetic magnetite (Fe3O4) nanoparticles, with a known density of 5.2 g/cm3. All experimental magnetization data were expressed in emu/g. The magnetic behavior of the superparamagnetic nanoparticles was analyzed using the Langevin function, a reliable method for estimating the size of Fe3O4 particles [44] that accurately describes non-interacting, single-domain particles with randomly oriented magnetic moments under an applied magnetic field. The Langevin model is given by Equation (1):
where M(H) is the magnetization as a function of the applied field (emu/cm3), Ms is the saturation magnetization (emu/cm3), μ is the average magnetic moment per nanoparticle (emu), H is the applied magnetic field (Oe), kB is the Boltzmann constant (1.3806 × 10−16 erg/K), and T is the absolute temperature (300 K). Since the experimental data were obtained in mass magnetization units (emu/g), the data were converted to volumetric magnetization (emu/cm3) using the density of the magnetite via the following equation:
where Mv(H) is the volumetric magnetization (emu/cm3), Mm(H) is the mass magnetization (emu/g), and ρ is the density of the magnetite (5.2 g/cm3). The average magnetic moment per nanoparticle was related to its volume, assuming spherical geometry, as follows:
where Msv is the volumetric saturation magnetization (emu/cm3), V is the nanoparticle volume (cm3), and d is the average nanoparticle diameter (cm). For magnetite, the typical bulk saturation magnetization was considered as Msv ≈ 480 emu/cm3 [45].
The experimental mass magnetization data (emu/g) were converted to volumetric units (emu/cm3) using the known density of magnetite. Nonlinear least-squares fitting was applied to fit the experimental data to the Langevin function. The adjustable parameters were the saturation magnetization Msm and the average magnetic moment per nanoparticle μ. The fitting procedure was implemented using a computational environment, OriginLab. Upon determination of μ, the average nanoparticle diameter d was calculated assuming spherical shape, via the following equation:
All variables were appropriately converted to ensure dimensional consistency. The fitted volumetric saturation magnetization Msv was then reconverted to mass magnetization units (emu/g) to allow direct comparison with experimental data, using the following equation:
where Msm is the saturation mass magnetization (emu/g). The quality of the fitting was assessed through the coefficient of determination (R2R2) and residual analysis. The estimated average nanoparticle diameter was compared with values reported in literature for magnetite nanoparticles exhibiting superparamagnetic behavior. The obtained saturation magnetization was also compared with theoretical expectations for nanoscale magnetite, considering possible surface and finite-size effects [46].
3. Results and Discussion
The BC biomembranes exhibited a uniform visual appearance, with a dark coloration corresponding to the amount of magnetite added. Figure 1 shows the aspect of the materials obtained.
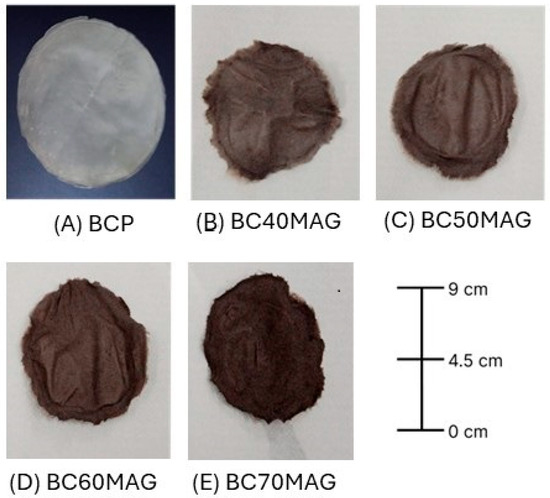
Figure 1.
Bacterial cellulose biomembranes. (A) BCP = processed and dried BC in film form; (B) BC40MAG; (C) BC50MAG; (D) BC60MAG; (E) BC70MAG.
3.1. X-Ray Diffraction (XRD)
According to the XRD spectra shown in Figure 2, the samples exhibited the crystalline planes (−1 1 0) and (2 0 0), characteristic of cellulose [37,47], with the planes (2 2 0), (1 0 4), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) corresponding to magnetite [25,48,49,50]. The magnetite produced through the optimized methodology exhibited crystallites with an average diameter (6.96 nm), significantly smaller than those prepared by Souza et al. [29], using ex situ coprecipitation and processing (20, 20 nm). Table 1 provides a comparative overview of the crystallite sizes and crystallinity of the Fe3O4 particles formed in this study and those reported by Souza et al. [29], who performed ex situ synthesis without optimizations.
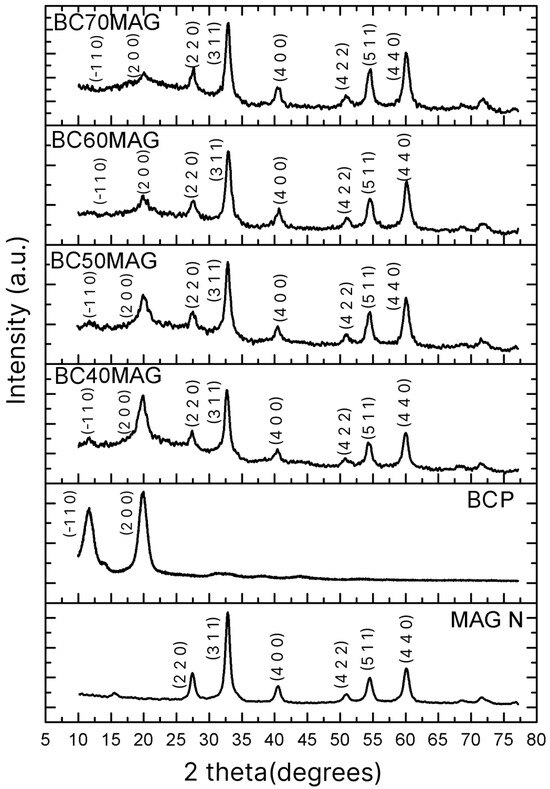
Figure 2.
XRD spectra of the produced materials: BC70MAG, BC60MAG, BC50MAG, BC40MAG, BCP, and MAG N; MAG N = magnetite nanoparticles.

Table 1.
Comparative values of magnetite made by the coprecipitation method obtained in this work and in the study by Souza et al. [18].
Such a reduction in diameter can be attributed to the enhanced control of parameters involved in the production of Fe3O4, as discussed previously. Namely, the adjusted ratio of iron ions, increased stirring, and sample purification may have significantly impacted the final nanoparticle size [30,31,32,33].
With this average diameter, the new magnetite nanoparticles can be classified as superparamagnetic, since, according to Giradet et al. [51], the critical diameter for Fe3O4 particles exhibiting such behavior is 20 ± 5 nm. The crystallinity of the nanoparticles also differed from that of the previous study. Souza et al. [29] reported nanoparticles with a crystallinity of 43%, whereas in the present study, a crystallinity of 60.5% was achieved, thanks to the optimization of the synthesis process. This increase in crystallinity indicates that the particles obtained under optimized conditions have a higher degree of molecular organization, with more aligned and regular structural patterns, resulting in higher stability, lower water absorption, and consequently a reduced risk of conversion to maghemite [52].
3.2. Fourier-Transform Infrared (FTIR) Spectrometry
As shown in Figure 3, the samples exhibited vibrational peaks characteristic of the chemical bonds present in cellulose at wavenumbers of 2914, 1415, 1080, 839, and 3122 cm−1, corresponding to the asymmetric stretching of C-H, CH2, C-O, C-C, and O-H [17,49], respectively. Additionally, vibrational peaks related to Fe-O and Fe-O-C bonds were identified between 586 and 598 cm−1, confirming the presence of magnetite and its association with BC. Finally, the clear increase in the transmittance of iron-related peaks observed in the BC60MAG and BC70MAG samples can be ascribed to the increased amount of incorporated Fe3O4.
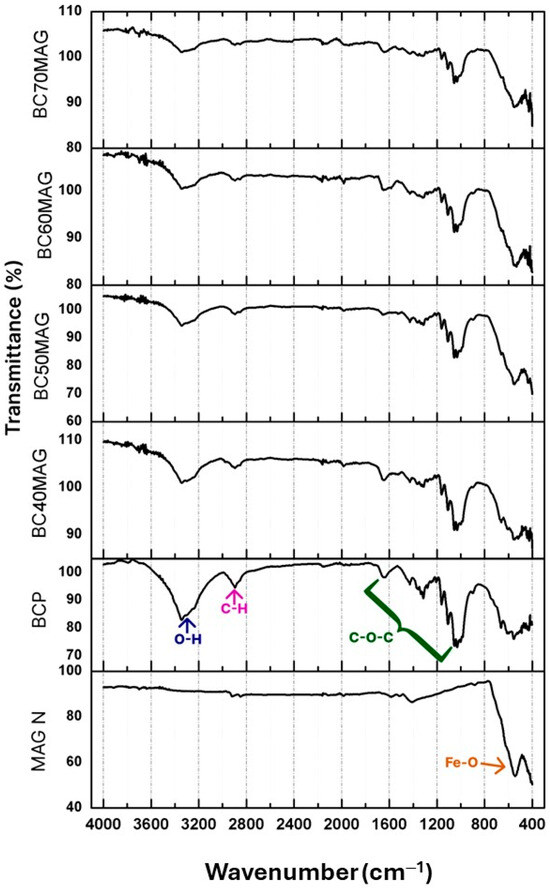
Figure 3.
FTIR spectra of the produced materials, with the demarcation of its main peaks: BCP, BC70MAG, BC60MAG, BC50MAG, BC40MAG, and MAG N.
3.3. Scanning Electron Microscopy (SEM)
According to the SEM images (Figure 4), the samples had surfaces on which BC fibers and magnetite nanoparticles were visible. The arrangement of compounds in the samples was similar to that observed by Chaabane et al. [17] and Chanthiwong et al. [53] in cellulose nanofibers, whose coating with nanoparticles made them thicker.
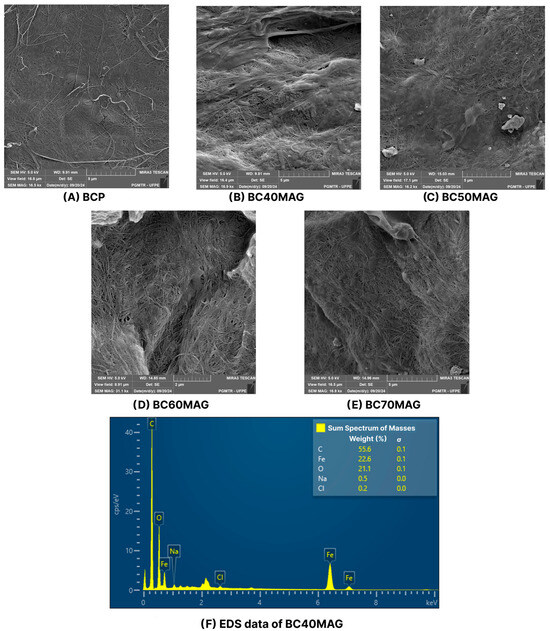
Figure 4.
SEM images of the produced materials, with the magnifications of: BCP = 16.5 kx (A); BC40MAG = 16.9 kx (B); BC50MAG = 16.2 kx (C); BC60MAG = 31.1 kx (D); and BC70MAG = 16.8 kx (E). EDS of the CB40MAG sample, with the percentage of iron (F).
The fibers obtained from crushed BC membranes appeared to be reorganized in conjunction with the added nanoparticles, forming a more homogeneous surface with few agglomeration points (Figure 4). Figure 4A shows the BC fibers in the BCP sample, without the nanoparticles, that coated the fibers in the other samples, as shown in Figure 4B–E. This characteristic is similar to that recently observed by Souza et al. [29] for a magnetic BC produced using the ex situ coprecipitation method, which, however, exhibited a surface richer in Fe3O4 particles, likely due to the higher incorporation of nanoparticles into the BC (82.92%) and the larger average size of its crystallites (31.03 nm).
The EDS graph presented in Figure 4F indicates a strong presence of iron in the composition of the samples, as well as residual reagents in smaller quantities, such as sodium and chlorine, likely derived from the reagents used during the production process.
3.4. Vibrating Sample Magnetometry (VSM)
The hysteresis loops of specimens measured by VSM are illustrated in Figure 5, and their respective saturation magnetization (Ms) values, obtained through the Langevin curve, are listed in Table 2. For comparison purposes, Table 2 also presents the magnetic properties corresponding to the pure magnetite samples and the BC-based materials with magnetite, as reported in the study by Souza et al. [29], which were also synthesized using the ex situ coprecipitation method with BC processing, albeit without the optimizations.
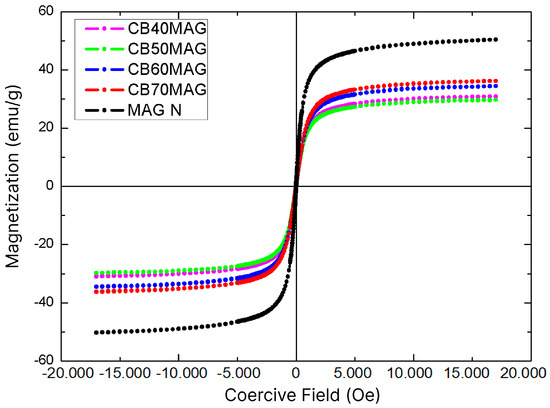
Figure 5.
VSM graphs of the produced materials: BC70MAG, BC60MAG, BC50MAG, and MAG N.

Table 2.
Values of saturation magnetization (Ms) for each produced sample and the others found in the study by Souza et al. [29].
The crystallinity of the particles is an important factor for the saturation magnetization (Ms), as crystalline structures with more irregularities can disrupt the alignment of magnetic moments, while a greater structural organization allows more magnetic moments to effectively contribute to an increase in Ms. Low crystallinity may also indicate defects in the crystal structure, altering the magnetic interaction between Fe2+ and Fe3+ ions in the inverse spinel structure of magnetite [42,54,55]. Thus, due to the crystallite size and the high purity of the obtained magnetite, the samples achieved higher Ms values than those recently obtained by Souza et al. [29], even with lower percentages of magnetite in their compositions.
Particularly, the Ms of magnetite synthesized in this study under optimized conditions (36.2 emu/g) and non-optimized conditions (29.7 emu/g) were about 113% and 75% higher than the highest value reported by those authors for magnetic BC prepared by in situ coprecipitation and processing (17 emu/g). Moreover, the obtained samples did not exhibit a significant coercive field, demonstrating superparamagnetic behavior [53,56] with a faster response to external magnetic fields, an ideal characteristic for sensors and biosensors [3,57,58], as these devices rely on quick responses to magnetic oscillations, as well as for electromagnetic resonance contrast agents [59] and drug delivery systems [25,60], which require materials that do not exhibit magnetic remanence, a feature that could hinder these applications [22]. This contrasts with the particles obtained through the same synthetic route but without optimizations, as reported in the previous study [29], where the material exhibited a significant coercive field.
Based on the relationship between the graphs shown in Figure 5 and the Langevin curve, it was also possible to estimate the radius of the Fe3O4 nanoparticles in each sample. All values were close to 5.3 nm, which was relatively consistent with the crystallite size obtained from XRD analysis using the Scherrer equation, a typical size for a superparamagnetic magnetite nanoparticle. Similar results have been reported in literature; in Khanam et al. [61], the authors synthesized magnetite nanoparticles using the coprecipitation method and observed an average particle size of 6.8 nm, along with a saturation magnetization of 49.88 emu/g—values comparable to those found in the present study. The particle size values obtained were smaller than those reported by Osipov et al. [62], who synthesized the nanoparticles using a laser-based method. However, they were similar to the superparamagnetic particles produced by Darbandi et al. [63], who employed microemulsion techniques. Chanthiwong et al. [50] and Sriplai et al. [56] also produced superparamagnetic magnetite particles with sizes and saturation magnetization values similar to those obtained in the present study.
4. Conclusions
Magnetic materials based on BC were produced with different concentrations of magnetite. The results confirmed that, through modified parameters such as the molar ratio of reagents, stirring, and purification, it was possible to optimize the methodology to obtain an innovative biomaterial containing superparamagnetic magnetite particles. The obtained magnetite nanoparticles exhibited crystallites with reduced average diameter, higher crystallinity, and higher saturation magnetization than those obtained in previous studies conducted in our laboratories.
The samples with a higher percentage of incorporated magnetite (BCMAG60 and BCMAG70) showed higher saturation magnetization, indicating greater sensitivity to external magnetic fields. The produced biomaterials can be applied in areas such as electronics, sensors, EMI shielding, and enzyme immobilization, among others.
Author Contributions
Conceptualization, T.C.d.S. and H.M.M.; methodology, T.C.d.S. and H.M.M.; software, T.C.d.S. and E.P.H.; validation, T.C.d.S. formal analysis, T.C.d.S.; investigation, T.C.d.S. and H.M.M.; resources, G.M.V. and L.A.S.; data curation, T.C.d.S. and A.C.; writing—original draft preparation, T.C.d.S.; writing—review and editing, I.J.B.D., A.F.d.S.C., E.P.H., A.C., G.M.V. and L.A.S.; supervision, A.F.d.S.C., G.M.V. and L.A.S.; project administration, G.M.V. and L.A.S.; funding acquisition, L.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Brazilian development agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Finance Code 001).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Instituto Avançado de Tecnologia e Inovação (IATI), Brazil; Department of Physics, Federal University of Pernambuco (UFPE), Brazil; and Department of Fundamental Chemistry, Federal University of Pernambuco (UFPE), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Betlej, I.; Zakaria, S.; Krajewski, K.J.; Boruszewski, P. Bacterial cellulose–properties and its potential application. Sains Malays. 2021, 50, 493–505. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Sting, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Xu, W.; Xiong, R.; Huang, C. Cellulose-based fibrous materials for self-powered wearable pressure sensor: A mini review. Cellulose 2023, 30, 1981–1998. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2019, 76, 333–335. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Dhar, P.; Etula, J.; Bankar, S.B. In Situ Bioprocessing of Bacterial Cellulose with Graphene: Percolation Network Formation, Kinetic Analysis with Physicochemical and Structural Properties Assessment. ACS Appl. Bio. Mater. 2019, 2, 4052–4066. [Google Scholar] [CrossRef]
- Saleh, A.K.; Alessa, A.H.; Omran, A.M.E. Characterization of ex situ developed bacterial cellulose/ZnO-NPs nanocomposite for antimicrobial evaluation. Biomass Convers. Biorefin. 2024, 15, 4597–4605. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Potential of magnetic nano cellulose in biomedical applications: Recent advances. Biomater. Polym. Horiz. 2022, 1, 32–47. [Google Scholar] [CrossRef]
- Jin, K.; Jin, C.; Wu, Y. Synthetic biology-powered microbial co-culture strategy and application of bacterial cellulose-based composite materials. Carbohydr. Polym. 2022, 283, 119171. [Google Scholar] [CrossRef]
- Ulla, M.W.; Alabbosh, K.F.S.; Fatima, A.; Ul Islam, S.; Manan, S.; Ul-Islam, M.; Yang, G. Advanced biotechnological applications of bacterial nanocellulose-based nanohybrids: A review. Adv. Ind. Eng. Polym. Res. 2024, 7, 100–121. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.E.; Ionescu, D.; Dinu-Pirvu, C.E. Bacterial cellulose—A remarkable polymer as a source for biomaterials tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Muiruri, J.K.; Yeo, J.C.C.; Zhu, Q.; Ye, E.; Loh, X.J.; Li, Z. Bacterial cellulose: Recent advances in biosynthesis, functionalization strategies and emerging applications. Eur. Polym. J. 2023, 199, 112446. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z. A self-healing magneto-responsive nanocellulose ferrogel and flexible soft strain sensor. J. Biol. Macromol. 2023, 234, 15. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Cleveland, D.; Tran, G.; Joseph, F. Potential of bacterial cellulose for sustainable fashion and textile applications: A review. J. Mater. Sci. 2024, 59, 6685–6710. [Google Scholar] [CrossRef]
- Katyal, M.; Mahajan, R.; Sharma, A.; Gupta, R.K.; Aggarwal, N.; Yadav, A. Bacterial cellulose: Nature's greener tool for industries. Biotechnol. Appl. Biochem. 2023, 70, 1629–1640. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Riquelme, D.; Rajivgandhi, G.; Muneeswaran, T.; Cho, W.S.; Anand, M.; Manoharan, N.; Quero, F. Dual-role of graphene/bacterial cellulose/magnetite nanocomposites as highly effective antibacterial agent and visible-light-driven photocatalyst. J. Environ. Chem. Eng. 2021, 9, 5. [Google Scholar] [CrossRef]
- Chaabane, L.; Chahdoura, H.; Mehdaoui, R.; Snoussi, M.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr. Polym. 2020, 247, 116707. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional Magnetic Hydrogels Based on Millettia Speciosa Champ Residue Cellulose and Chitosan: Highly Efficient and Reusable Adsorbent for Congo Red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Du, Z.; Hu, S.; Huang, J.; Shi, Z.; Su, B.; Yang, G. CoFe2O4 Embedded Bacterial Cellulose for Flexible, Biodegradable, and Self-Powered Electromagnetic Sensor. Nano Energy 2022, 102, 107740. [Google Scholar] [CrossRef]
- Fan, M.; Xia, X.; Li, S.; Zhang, R.; Wu, L.; Qu, M.; Tang, P.; Bin, Y. Sustainable bacterial cellulose reinforced carbon nanotube buckypaper and its multifunctionality for electromagnetic interference shielding, Joule heating and humidity sensing. Chem. Eng. J. 2022, 441, 136103. [Google Scholar] [CrossRef]
- Petrov, K.D.; Chubarov, A.S. Magnetite nanoparticles for biomedical applications. Encyclopedia 2022, 2, 1811–1828. [Google Scholar] [CrossRef]
- Souza, T.C.; Costa, A.F.d.S.; Vinhas, G.M.; Sarubbo, L.A. Synthesis of iron oxides and influence on final sizes and distribution in bacterial cellulose applications. Polymers 2023, 15, 3284. [Google Scholar] [CrossRef]
- Saipul Bahri, N.S.N.; Nguyen, T.; Matsumoto, K.; Watanabe, M.; Morita, Y.; Septiani, E.L.; Le, K.A.C.; Hirano, T.; Ogi, T. Controlling the magnetic responsiveness of cellulose nanofiber particles embedded with iron oxide nanoparticles. ACS Appl. Bio Mater. 2024, 7, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Aphesteguy, J.C.; Jacobo, S.E.; Schegoleva, N.N.; Kurlyandskaya, G.V. Characterization of nanosized spinel ferrite powders synthesized by coprecipitation and autocombustion method. J. Alloys Compd. 2010, 495, 509–512. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Jun, Y.; Seo, J.; Cheon, J. Nanoscaling Laws of Magnetic Nanoparticles and Their Applicabilities in Biomedical Sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef]
- Ze, X.L.; Sun, Y.J.; Ge, S.W.; Zhu, F.; Yin, F.; Gu, L.; Yang, F.; Hu, P.; Chen, G.P.; Wang, K.; et al. An overview of synthesis and structural regulation of magnetic nanomaterials prepared by chemical coprecipitation. Superalloys 2023, 13, 152. [Google Scholar] [CrossRef]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Souza, T.C.; dos Santos, A.R.; Chacon, J.L.d.S.P.; Durval, Í.J.B.; Costa, A.F.d.S.; Padrón Hernández, E.; Converti, A.; Vinhas, G.M.; Sarubbo, L.A. Innovation in methods for incorporating magnetite into biocellulose for electromagnetic interference shielding effectiveness applications. Energies 2024, 17, 3202. [Google Scholar] [CrossRef]
- Goss, C. Saturation magnetisation, coercivity and lattice parameter changes in the system Fe₃O₄-γFe₂O₃, and their relationship to structure. Phys. Chem. Miner. 1988, 16, 164–171. [Google Scholar] [CrossRef]
- Jiang, W.; Lai, K.; Hu, H.; Zeng, X.; Lan, F.; Liu, K.; Wu, Y.; Gu, Z. The effect of [Fe³⁺]/[Fe²⁺] molar ratio and iron salts concentration on the properties of superparamagnetic iron oxide nanoparticles in the water/ethanol/toluene system. J. Nanopart. Res. 2011, 13, 5135–5145. [Google Scholar] [CrossRef]
- Eom, T.; Kim, S.; An, S.; Oh, K.; Suhr, D. Effect on the formation of Fe₃O₄ with ferrous sulfate/ferric sulfate molar ratio and precipitants. J. Korean Med. Sci. 2011, 21, 157–162. [Google Scholar] [CrossRef]
- Shahid, M.; Choi, Y. Characterization and application of magnetite particles, synthesized by reverse coprecipitation method in open air from mill scale. J. Magn. Magn. Mater. 2020, 495, 165823. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum: II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Hungund, B.; Gupta, S.G. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol. 2010, 2, 127–133. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient source. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Galdino, C.J.S., Jr.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Valenzuela, R.; Bastidas, D.M.; Rojas, A.; Urreta, S.E.; Chiacchiarini, P.; Sapag, K. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe₃O₄) by the co-precipitation method. J. Alloys Compd. 2009, 488, 227–231. [Google Scholar] [CrossRef]
- Rahmawati, R.; Harjanto, S.; Soepriyanto, S.; Susanto, H.; Suharso, D. Optimization of frequency and stirring rate for synthesis of magnetite (Fe₃O₄) nanoparticles by using coprecipitation-ultrasonic irradiation methods. Procedia Eng. 2017, 170, 55–59. [Google Scholar] [CrossRef]
- Syahida, A.; Sutanto, H.; Manawan, M.; Setiadi, E.; Wibowo, A.; Irianti, F.; Alkian, I.; Hidayanto, E.; Priyono, P.; Marhaendrajaya, I.; et al. Diffraction and magnetization properties of Fe₃O₄ nanoparticle from natural iron sand in various stirring rate for potential biomedical applications. Rasayan J. Chem. 2022, 15, 316–325. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room temperature coprecipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Cheah, Y.S.; Masaharu, K. Comparison of crystallinity index computational methods based on lignocellulose X-ray diffractogram. Mater. Res. Found. 2023, 29, 128–134. [Google Scholar] [CrossRef]
- Saragi, T.; Widyaiswari, U.; Manalu, D.J.; Adiperdana, B.; Risdiana, R.; Adachi, T. Particle Size and Its Distribution Deduced from Magnetic Properties of Magnetite Nanoparticles by the Modified Langevin Equation. AIP Adv. 2023, 13, 095103. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, D.; Yokouchi, Y.; Tanaka, H.; Iwata, I. Crystalline investigations of cellulose films and fibers prepared using ionic liquid mixed solvent. J. Jpn. Soc. Test. Mater. 2022, 71, 579–584. [Google Scholar] [CrossRef]
- Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High yield synthesis and application of magnetite nanoparticles (Fe₃O₄). Magnetochemistry 2020, 6, 22. [Google Scholar] [CrossRef]
- Zheng, H.; Daghagheleh, O.; Ma, Y.; Taferner, B.; Schenk, J.; Kapelyushin, Y. Phase transition of magnetite ore fines during oxidation probed by in situ high-temperature X-ray diffraction. Metall. Mater. Trans. B 2023, 54BI, 1195. [Google Scholar] [CrossRef]
- Sabur, A.; Gafur, A. Crystallographic, morphological, magnetic, and thermal characterization of superparamagnetic magnetite nanoparticles (Fe₃O₄) synthesized by chemical coprecipitation method and calcined at 250 °C for 4 hr. J. Nanomater. 2024, 2024, 577778. [Google Scholar] [CrossRef]
- Girardet, T.; Venturini, P.; Martinez, H.; Dupin, J.-C.; Cleymand, F.; Fleutot, S. Spinel. Magnetic iron oxide nanoparticles: Properties, synthesis and washing methods. Appl. Sci. 2022, 12, 8127. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of nanocomposite film comprising of polyvinyl alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Chanthiwong, M.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Controlling the processing of co-precipitated magnetic bacterial cellulose/iron oxide nanocomposites. Mater. Des. 2020, 196, 109148. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Xu, N.; Guo, K.; Xu, Z.; Chen, C.; Liu, H.; Gao, J. Regulation of saturation magnetization of magnetite by doping with group III elements. Phys. Chem. Chem. Phys. 2023, 25, 33152–33158. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, Q.; He, Y.; Liu, R. Current sensor based on an integrated micro-ring resonator and superparamagnetic nanoparticles. Opt. Express 2020, 28, 5684–5691. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Litvinova, L.S.; Safronov, A.P.; Schupletsova, V.V.; Tyukova, I.S.; Khaziakhmatova, O.G.; Slepchenko, G.B.; Yurova, K.A.; Cherempey, E.G.; Kulesh, N.A.; et al. Water-Based Suspensions of Iron Oxide Nanoparticles with Electrostatic or Steric Stabilization by Chitosan: Fabrication, Characterization and Biocompatibility. Sensors 2017, 17, 2605. [Google Scholar] [CrossRef]
- Mira-Cuenca, C.; Meslier, T.; Roig-Sanchez, S.; Laromaine, A.; Roig, A. Patterning bacterial cellulose films with iron oxide nanoparticles and magnetic resonance imaging monitoring. ACS Appl. Polym. Mater. 2021, 3, 4959–4965. [Google Scholar] [CrossRef]
- Yingkamhaeng, N.; Intapan, I.; Sukyai, P. Fabrication and characterisation of functionalised superparamagnetic bacterial nanocellulose using ultrasonic-assisted in situ synthesis. Fibers Polym. 2018, 19, 489–497. [Google Scholar] [CrossRef]
- Khanam, J.; Ahmed, M.F.; Zaman, S.M.; Sharmin, N. Synthesis of nano-sized magnetic iron oxide by a simple and facile co-precipitation method. Bangladesh J. Sci. Ind. Res. 2022, 57, 67–76. [Google Scholar] [CrossRef]
- Osipov, V.V.; Platonov, V.V.; Uimin, M.A.; Podkin, A.V. Laser synthesis of magnetic iron oxide nanopowders. Tech. Phys. 2012, 57, 543–549. [Google Scholar] [CrossRef]
- Darbandi, M.; Stromberg, F.; Landers, J.; Reckers, N.; Sanyal, B.; Keune, W.; Wende, H. Nanoscale size effect on surface spin canting in iron oxide nanoparticles synthesized by the microemulsion method. J. Phys. D Appl. Phys. 2012, 45, 195001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).