Membrane-Based CO2 Capture Across Industrial Sectors: Process Conditions, Case Studies, and Implementation Insights
Abstract
1. Introduction
2. Membrane Process for CO2 Capture
3. The Membrane-Based CO2 Capture Process in Various Industrial Plants
3.1. Pre-Combustion CO2 Capture
3.2. Post-Combustion CO2 Capture
3.2.1. Natural Gas Power Plant
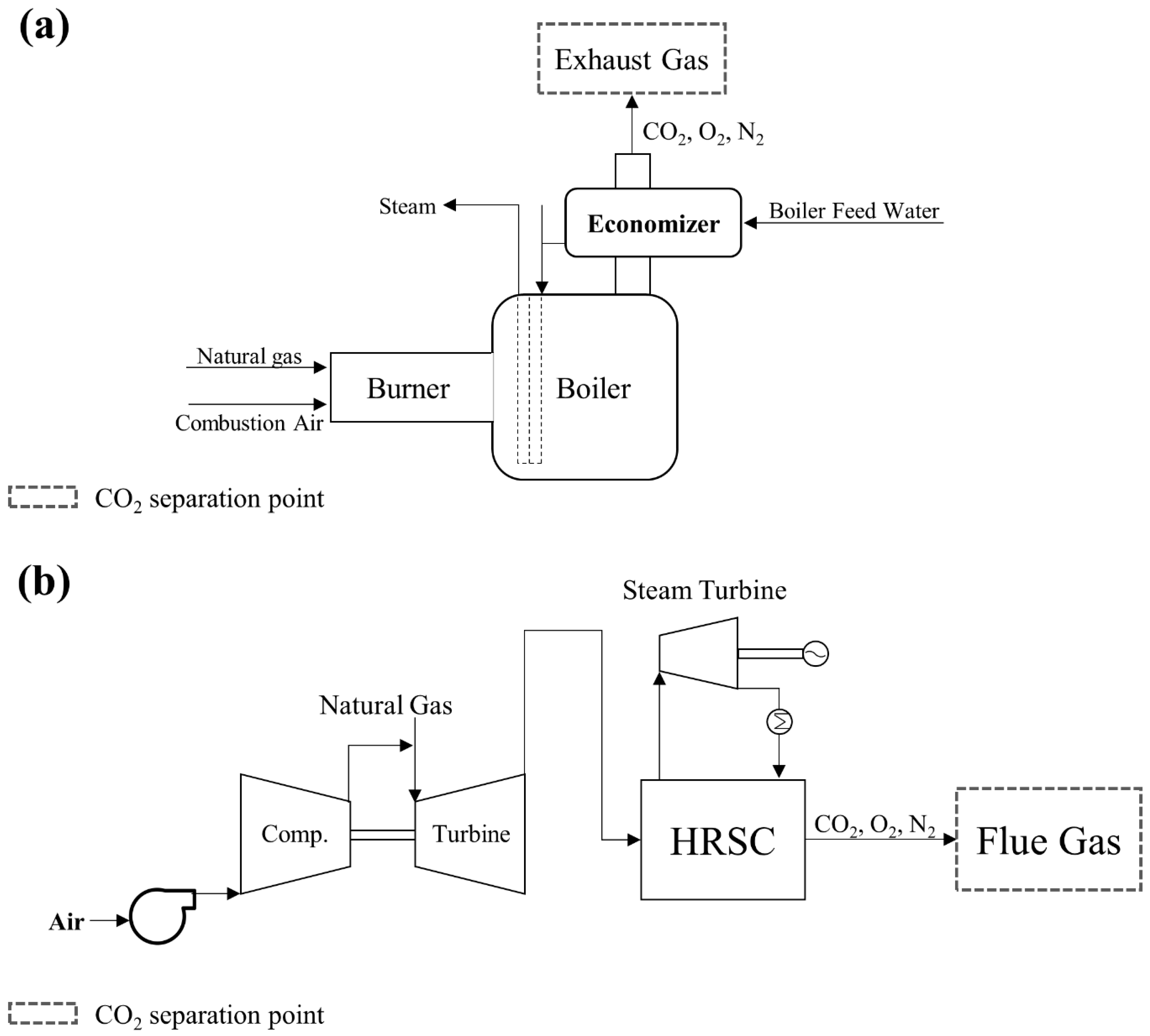
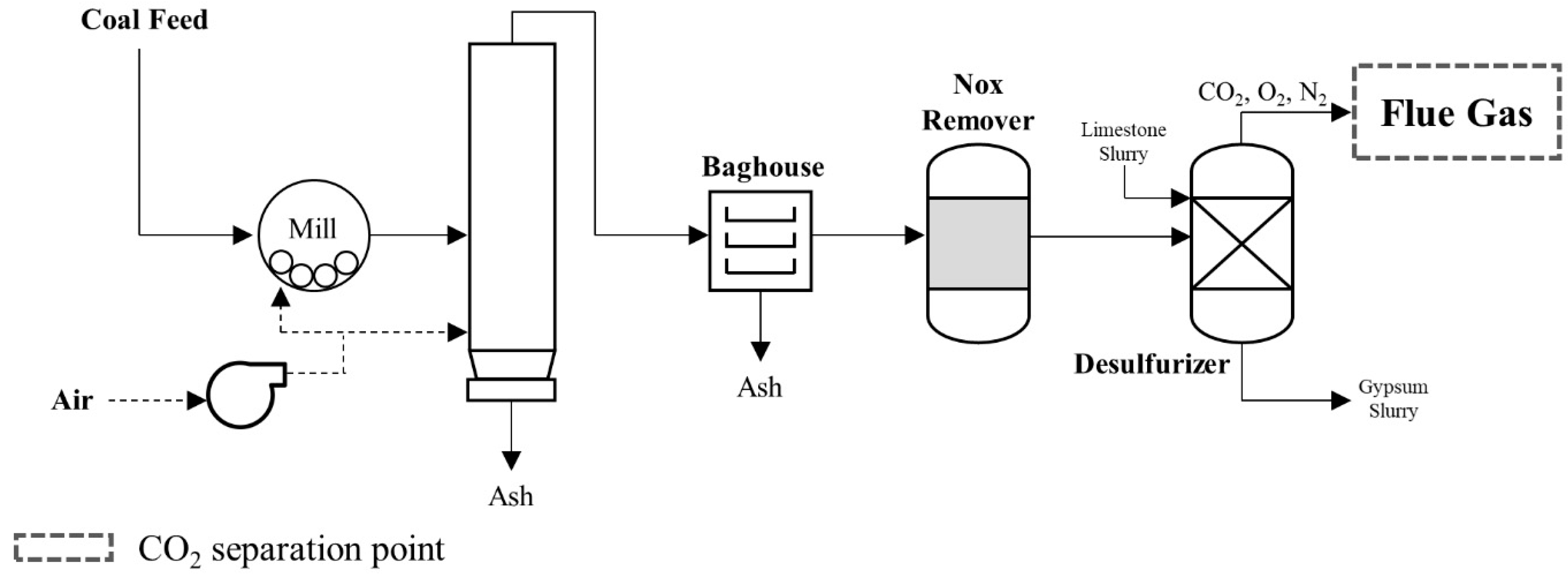
3.2.2. Coal-Fired Power Plant
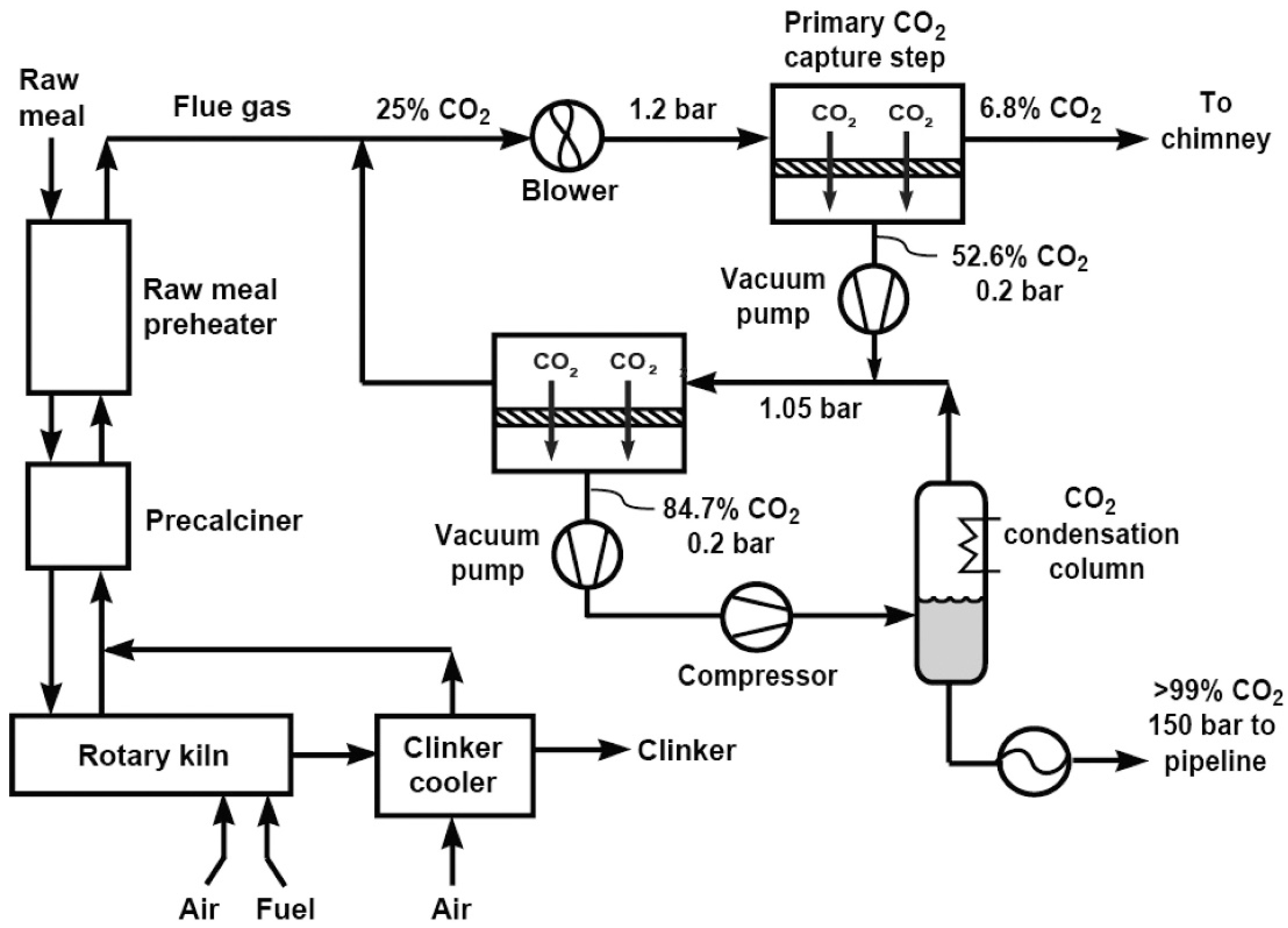
3.2.3. Cement Industry
3.3. Iron and Steel Industry
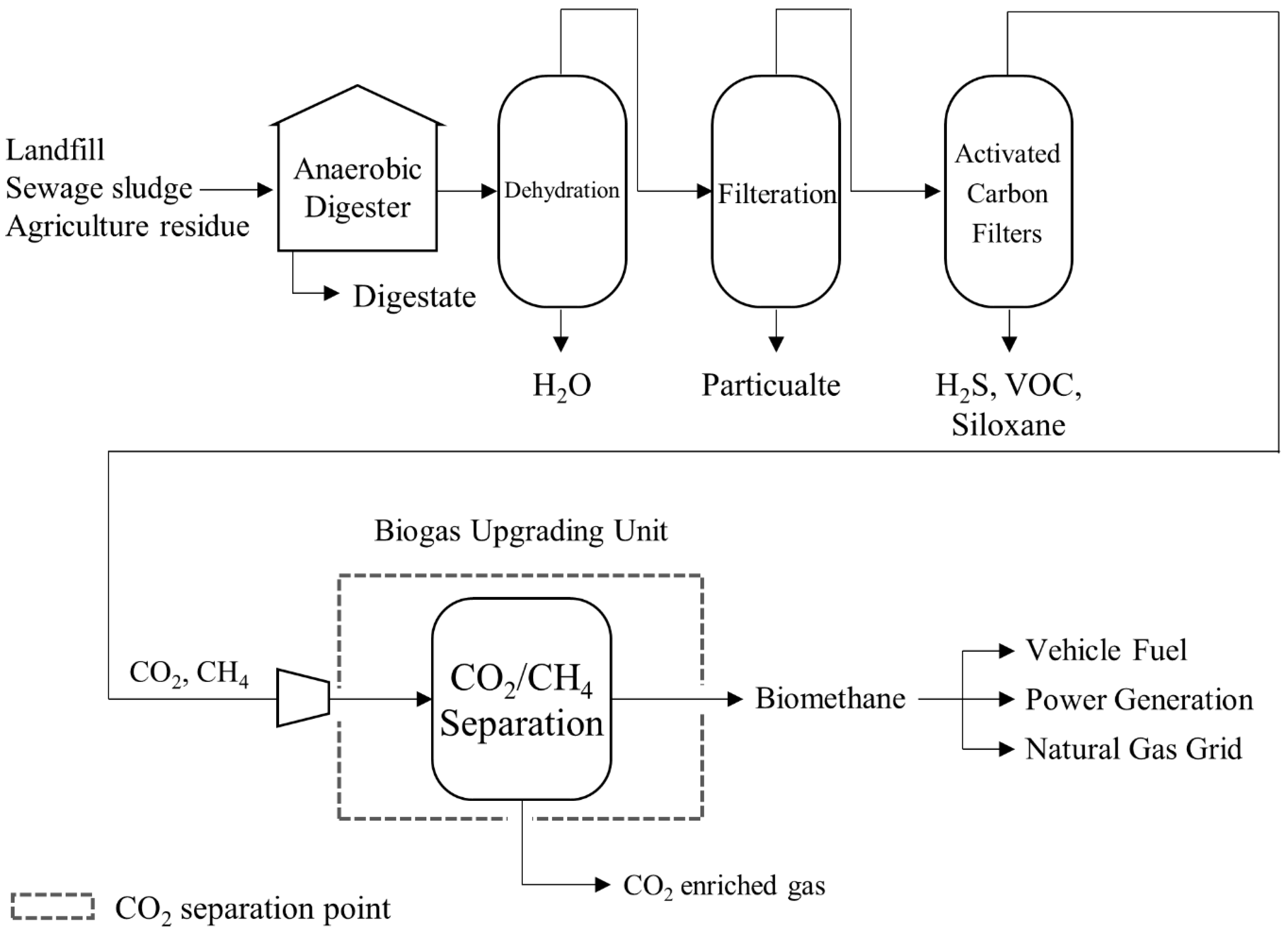
3.4. Biogas Uprading
4. Summary
| Emission Source | CO2 Conc. (%) | Composition | Pressure (Bar) | Temperature (°C) | References | |
|---|---|---|---|---|---|---|
| Pre-combustion | Shifted syngas | 15–40 | CO2, H2, H2O, N2, CH4, H2S | 20–50 | 250–400 | [75,76,77,78,79,80,140] |
| Post-combustion | Natural gas power plant | 4–10 | CO2, N2, H2O, O2, NOx, SOx | Ambient | 100–200 | [88,89,92,141] |
| Coal-fired power plant | 10–15 | CO2, N2, O2, H2O, CO, NOx, SOx, dust | Ambient | 90–180 | [96,97,98,142,143] | |
| Cement manufacturing | 5–15 | CO2, N2, O2, H2O, NOx, SOx, dust | Ambient | 100–200 | [144,145,146] | |
| Etc. | Steel manufacturing | 16–42 | CO2, N2, CO, H2 | 1–3 | 150–300 | [119,147,148] |
| Biogas upgrading | 30–45 | CO2, CH4, H2O, NH3, H2, O2, N2, H2S, siloxane | 3–7 | 40–60 | [124,125,149] | |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| BF | Blast Furnace |
| Bio-CNG | Bio-Compressed Natural Gas |
| BOF | Basic Oxygen Furnace |
| COS | Carbonyl Sulfide |
| FEED | Front-End Engineering Design |
| FGD | Flue Gas Desulfurization |
| FTM | Facilitated Transport Membrane |
| HRSG | Heat Recovery Steam Generator |
| IGCC | Integrated Gasification Combined Cycle |
| LCOE | Levelized Cost of Electricity |
| MMM | Mixed-Matrix Membrane |
| MTR | Membrane Technology Research |
| NCCC | National Carbon Capture Center |
| NG | Natural Gas |
| NGCC | Natural Gas Combined Cycle |
| PBI | Polybenzimidazole |
| PDMS | Polydimethylsiloxane |
| PES | Polyether Sulfone |
| PVAm | Polyvinylamine |
| SCR | Selective Catalytic Reduction |
| S-EGR | Selective Exhaust Gas Recirculation |
| TEA | Techno-Economic Analysis |
| TFC | Thin-Film Composite |
| TGR | Top Gas Recycling |
| WGS | Water–Gas Shift |
References
- Hekmatmehr, H.; Esmaeili, A.; Pourmahdi, M.; Atashrouz, S.; Abedi, A.; Ali Abuswer, M.; Nedeljkovic, D.; Latifi, M.; Farag, S.; Mohaddespour, A. Carbon capture technologies: A review on technology readiness level. Fuel 2024, 363, 130898. [Google Scholar] [CrossRef]
- National, A.; Space, A. Atmospheric CO2 Concentrations from Mauna Loa Observatory. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 21 April 2025).
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Liu, D.; Guo, X.; Xiao, B. What causes growth of global greenhouse gas emissions? Evidence from 40 countries. Sci. Total Environ. 2019, 661, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Gołasa, P.; Wysokiński, M.; Bieńkowska-Gołasa, W.; Gradziuk, P.; Golonko, M.; Gradziuk, B.; Siedlecka, A.; Gromada, A. Sources of Greenhouse Gas Emissions in Agriculture, with Particular Emphasis on Emissions from Energy Used. Energies 2021, 14, 3784. [Google Scholar] [CrossRef]
- Azhar Khan, M.; Zahir Khan, M.; Zaman, K.; Naz, L. Global estimates of energy consumption and greenhouse gas emissions. Renew. Sustain. Energy Rev. 2014, 29, 336–344. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- Darwin, R. Effects of Greenhouse Gas Emissions on World Agriculture, Food Consumption, and Economic Welfare. Clim. Change 2004, 66, 191–238. [Google Scholar] [CrossRef]
- Tovar-Ortiz, S.A.; Rodriguez-Gonzalez, P.T.; Tovar-Gómez, R. Modeling the Impact of Global Warming on Ecosystem Dynamics: A Compartmental Approach to Sustainability. World 2024, 5, 1077–1100. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, L. Membranes for CO2 capture and separation: Progress in research and development for industrial applications. Sep. Purif. Technol. 2024, 335, 126022. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Baliban, R.C.; Elia, J.A.; Floudas, C.A. Modeling, Simulation, and Optimization of Postcombustion CO2 Capture for Variable Feed Concentration and Flow Rate. 1. Chemical Absorption and Membrane Processes. Ind. Eng. Chem. Res. 2012, 51, 15642–15664. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Guo, B.; Sheng, M.; Wang, D.; Mao, S.; Ye, N.; Qiao, Z.; Kang, G.; Cao, Y.; et al. Industrial-scale spiral-wound facilitated transport membrane modules for post-combustion CO2 capture: Development, investigation and optimization. J. Membr. Sci. 2023, 670, 121368. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical analysis of CO2 capture pathways and technologies. J. Environ. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- Bhown, A.S. Status and Analysis of Next Generation Post-combustion CO2 Capture Technologies. Energy Procedia 2014, 63, 542–549. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. Technology readiness level assessment of carbon capture and storage technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Xing, L.; Chen, Z.; Zhan, G.; Huang, Z.; Wang, L.; Li, J. Efficient CO2 Desorption Catalysts: From Material Design to Kinetics Analysis and Application Evaluation. Engineering 2025, 49, 251–259. [Google Scholar] [CrossRef]
- Vorokhta, M.; Kusdhany, M.I.M.; Švábová, M.; Nishihara, M.; Sasaki, K.; Lyth, S.M. Hierarchically porous carbon foams coated with carbon nitride: Insights into adsorbents for pre-combustion and post-combustion CO2 separation. Sep. Purif. Technol. 2025, 354, 129054. [Google Scholar] [CrossRef]
- Kuppireddy, S.; Varghese, A.M.; Araj, H.A.; Hart, P.; Ramantani, T.; Bampos, G.; Karanikolos, G.N. A combined experimental and simulations assessment of CO2 capture and CO2/H2 separation performance of aminosilane-grafted MCM-41 and pore-expanded MCM-41. Microporous Mesoporous Mater. 2024, 377, 113220. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, Z.; Wen, Y.; Dai, Z.; Song, S.; Lin, H.; Feng, X.; Lu, X.; Ji, T.; Zhu, J. Networking SiO2-Al2O3 and amines into robust and long-lasting molded sorbents for continuous CO2 capture. Chem. Eng. J. 2024, 499, 156446. [Google Scholar] [CrossRef]
- He, T.; Si, B.; Gundersen, T.; Lin, W.; Chen, L.; Zhang, K. High ethane content enables efficient CO2 capture from natural gas by cryogenic distillation. Sep. Purif. Technol. 2025, 352, 128153. [Google Scholar] [CrossRef]
- Qiao, S.; Xu, M.; Lv, X.; Zhao, H. Analysis and Optimization of Cryogenic Distillation Systems: For Reducing Distillation Energy Consumption. Chem. Eng. Technol. 2025, 48, e202400296. [Google Scholar] [CrossRef]
- Li, H.; Zhang, R.; Wang, T.; Sun, X.; Hou, C.; Xu, R.; Wu, Y.; Tang, Z. Simulation of H2S and CO2 removal from IGCC syngas by cryogenic distillation. Carbon Capture Sci. Technol. 2022, 3, 100012. [Google Scholar] [CrossRef]
- Kumar, S.; Priyadarshini, M.; Ahmad, A.; Ghangrekar, M.M. Advanced biological and non-biological technologies for carbon sequestration, wastewater treatment, and concurrent valuable recovery: A review. J. CO2 Util. 2023, 68, 102372. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Castro, M.; Gómez-Díaz, D.; Navaza, J.M.; Rumbo, A. Carbon Dioxide Capture by Chemical Solvents Based on Amino Acids: Absorption and Regeneration. Chem. Eng. Technol. 2021, 44, 248–257. [Google Scholar] [CrossRef]
- Liu, R.-S.; Shi, X.-D.; Wang, C.-T.; Gao, Y.-Z.; Xu, S.; Hao, G.-P.; Chen, S.; Lu, A.-H. Advances in Post-Combustion CO2 Capture by Physical Adsorption: From Materials Innovation to Separation Practice. ChemSusChem 2021, 14, 1428–1471. [Google Scholar] [CrossRef]
- Sun, S.; Billings, A.; Zhang, K.; Huang, K. Direct, efficient and selective capture of low concentration of CO2 from natural gas flue gas using a high temperature tubular carbon capture membrane. J. Membr. Sci. 2022, 661, 120929. [Google Scholar] [CrossRef]
- Shah, C.; Raut, S.; Kacha, H.; Patel, H.; Shah, M. Carbon capture using membrane-based materials and its utilization pathways. Chem. Pap. 2021, 75, 4413–4429. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Yuan, Z.; Lin, Z.; Zhang, M.; Li, Y.; Tang, J.; Liang, Z.; Li, Y.; Chen, L.; et al. Boosting membranes for CO2 capture toward industrial decarbonization. Carbon Capture Sci. Technol. 2023, 7, 100117. [Google Scholar] [CrossRef]
- Welton, C.; Chen, F.; Zhou, H.-C.; Yi, S. Mixed matrix membrane formation with porous metal–organic nanomaterials for CO2 capture and separation: A critical review. Carbon Capture Sci. Technol. 2025, 14, 100347. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 415–416, 424–434. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Luo, W.; Wang, Y.; Mu, P.; Li, J. A review on the recent advances in composite membranes for CO2 capture processes. Sep. Purif. Technol. 2023, 307, 122752. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Recent advances on the membrane processes for CO2 separation. Chin. J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R. Cost-optimal CO2 capture ratio for membrane-based capture from different CO2 sources. Chem. Eng. J. 2017, 327, 618–628. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Qiao, Z.; Wu, H.; Dong, S.; Zhao, S.; Wang, J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. [Google Scholar] [CrossRef]
- Asadi, J.; Kazempoor, P. Techno-economic analysis of membrane-based processes for flexible CO2 capturing from power plants. Energy Convers. Manag. 2021, 246, 114633. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Techno-Economic Assessment of Polymer Membrane Systems for Postcombustion Carbon Capture at Coal-Fired Power Plants. Environ. Sci. Technol. 2013, 47, 3006–3014. [Google Scholar] [CrossRef]
- Li, Q.; Wu, H.; Wang, Z.; Wang, J. Analysis and optimal design of membrane processes for flue gas CO2 capture. Sep. Purif. Technol. 2022, 298, 121584. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-selective membranes for hydrogen production and CO2 capture—Part II: Techno-economic analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J.; Lee, J.S. New facile process evaluation for membrane-based CO2 capture: Apparent selectivity model. Chem. Eng. J. 2023, 460, 141624. [Google Scholar] [CrossRef]
- Huang, Y.; Merkel, T.C.; Baker, R.W. Pressure ratio and its impact on membrane gas separation processes. J. Membr. Sci. 2014, 463, 33–40. [Google Scholar] [CrossRef]
- Russo, G.; Prpich, G.; Anthony, E.J.; Montagnaro, F.; Jurado, N.; Di Lorenzo, G.; Darabkhani, H.G. Selective-exhaust gas recirculation for CO2 capture using membrane technology. J. Membr. Sci. 2018, 549, 649–659. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Brunetti, A.; Drioli, E.; Lee, Y.M.; Barbieri, G. Engineering evaluation of CO2 separation by membrane gas separation systems. J. Membr. Sci. 2014, 454, 305–315. [Google Scholar] [CrossRef]
- Park, J.W.; Song, Y.; Lee, J.; Heo, S.; Yeo, J.-G.; Lee, C.; Han, S.; Cho, C.-H.; Kim, J.-K.; Lee, J.H. High-performance carbon dioxide capture: 99% purity and 90% recovery using a low-temperature, two-stage membrane process. J. Ind. Eng. Chem. 2024, 148, 433–444. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Murshid, G. Process simulation and optimal design of membrane separation system for CO2 capture from natural gas. Comput. Chem. Eng. 2012, 36, 119–128. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Wang, J.; Wang, S. Parametric Study of the Membrane Process for Carbon Dioxide Removal from Natural Gas. Ind. Eng. Chem. Res. 2009, 48, 9013–9022. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Recent advances in polymeric membranes for CO2 capture. Chin. J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Qi, R.; Li, Z.; Zhang, H.; Fu, H.; Zhang, H.; Gao, D.; Chen, H. CO2 capture performance of ceramic membrane with superhydrophobic modification based on deposited SiO2 particles. Energy 2023, 283, 129202. [Google Scholar] [CrossRef]
- Fu, H.; Shen, Y.; Li, Z.; Zhang, H.; Chen, H.; Gao, D. CO2 capture using superhydrophobic ceramic membrane: Preparation and performance analysis. Energy 2023, 282, 128873. [Google Scholar] [CrossRef]
- Hu, L.; Clark, K.; Alebrahim, T.; Lin, H. Mixed matrix membranes for post-combustion carbon capture: From materials design to membrane engineering. J. Membr. Sci. 2022, 644, 120140. [Google Scholar] [CrossRef]
- Kamio, E.; Tomohisa, Y.; Matsuyama, H. Recent Advances in Carbon Dioxide Separation Membranes: A Review. J. Chem. Eng. Jpn. 2023, 56, 2222000. [Google Scholar] [CrossRef]
- Habib, N.; Tarhanlı, I.; Senses, E.; Keskin, S.; Uzun, A. IL-modified MOF-177 filler boosts the CO2/N2 selectivity of Pebax membrane. J. Membr. Sci. 2024, 710, 123143. [Google Scholar] [CrossRef]
- Abdul Samat, N.A.S.; Goh, P.S.; Lau, W.J.; Guo, Q.; Arthanareeswaran, G.; Ismail, A.F. Tailoring the properties and hydrogen separation performance of ultrathin polymethyl methacrylate composite membrane. Int. J. Hydrogen Energy 2025, 113, 749–760. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Arabi Shamsabadi, A.; Moftakhari Sharifzadeh, M.M.; Salehi, A.; Sanaeepur, H.; Dadashi Firouzjaei, M.; Rad, V.; Jafarian, H.; Elliott, M.A.; Garcia, H. Tailoring MXene composition and structure for High-Performance mixed matrix membranes in CO2 separation applications. Sep. Purif. Technol. 2025, 371, 133360. [Google Scholar] [CrossRef]
- Woo, K.T.; Dong, G.; Lee, J.; Kim, J.S.; Do, Y.S.; Lee, W.H.; Lee, H.S.; Lee, Y.M. Ternary mixed-gas separation for flue gas CO2 capture using high performance thermally rearranged (TR) hollow fiber membranes. J. Membr. Sci. 2016, 510, 472–480. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Nam, S.Y.; Koh, H.C.; Ha, S.Y.; Barbieri, G.; Drioli, E. The effect of operating conditions on the performance of hollow fiber membrane modules for CO2/N2 separation. J. Ind. Eng. Chem. 2012, 18, 205–211. [Google Scholar] [CrossRef]
- Low, B.T.; Zhao, L.; Merkel, T.C.; Weber, M.; Stolten, D. A parametric study of the impact of membrane materials and process operating conditions on carbon capture from humidified flue gas. J. Membr. Sci. 2013, 431, 139–155. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-K. Process-integrated design of a sub-ambient membrane process for CO2 removal from natural gas power plants. Appl. Energy 2020, 260, 114255. [Google Scholar] [CrossRef]
- Wilkes, M.D.; Ejeh, J.; Roberts, D.; Brown, S. Cost of small-scale dispatchable CO2 capture: Techno-economic comparison and case study evaluation. Int. J. Greenh. Gas Control 2023, 127, 103931. [Google Scholar] [CrossRef]
- Zanco, S.E.; Pérez-Calvo, J.-F.; Gasós, A.; Cordiano, B.; Becattini, V.; Mazzotti, M. Postcombustion CO2 Capture: A Comparative Techno-Economic Assessment of Three Technologies Using a Solvent, an Adsorbent, and a Membrane. ACS Eng. Au 2021, 1, 50–72. [Google Scholar] [CrossRef]
- Chance, R.; Chen, G.; Dai, Y.; Fan, Y.; Jones, C.; Kalyanaraman, J.; Kawajiri, Y.; Koros, W.; Lively, R.; McCool, B.; et al. Rapid Temperature Swing Adsorption using Polymeric/Supported Amine Hollow Fibers; Georgia Institute of Technology: Atlanta, GA, USA, 2015; p. 537. [Google Scholar]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Ni, Z.; Li, S.; Zhang, X.; Bao, J.; Zhang, N. Analysis and comparison of the membrane-cryogenic hybrid process and multistage membrane process for pre-combustion carbon capture based on the superstructure method. Sep. Purif. Technol. 2025, 353, 128636. [Google Scholar] [CrossRef]
- Hoeger, C.; Burt, S.; Baxter, L. Cryogenic Carbon Capture™ Technoeconomic Analysis; Sustainable Energy Solutions: Orem, UT, USA, 2021. [Google Scholar]
- Pratiwi, V.D.; Renanto, R.; Juwari, J.; Altway, A.; Anugraha, R.P. Cost Analysis of the Performance of CO2 Separation with Various CO2 Concentrations from Gas Wells. J. Chem. Technol. Metall. 2024, 59, 935–944. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Ramezani, R.; Tanaka, D.A.P.; Gallucci, F. Carbon Molecular Sieve Membranes for Selective CO2/CH4 and CO2/N2 Separation: Experimental Study, Optimal Process Design, and Economic Analysis. Ind. Eng. Chem. Res. 2023, 62, 19116–19132. [Google Scholar] [CrossRef]
- Yu, M.-C.; Bai, L.-J.; Moioli, S.; Tontiwachwuthikul, P.; Plisko, T.V.; Bildyukevich, A.V.; Feng, Y.-N.; Liu, H. Hybrid CO2 capture processes consisting of membranes: A technical and techno-economic review. Adv. Membr. 2023, 3, 100071. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Ghasem, N. A Review of the CFD Modeling of Hydrogen Production in Catalytic Steam Reforming Reactors. Int. J. Mol. Sci. 2022, 23, 16064. [Google Scholar] [CrossRef]
- Wu, F.; Argyle, M.D.; Dellenback, P.A.; Fan, M. Progress in O2 separation for oxy-fuel combustion–A promising way for cost-effective CO2 capture: A review. Prog. Energy Combust. Sci. 2018, 67, 188–205. [Google Scholar] [CrossRef]
- Ryzhkov, A.; Bogatova, T.; Gordeev, S. Technological solutions for an advanced IGCC plant. Fuel 2018, 214, 63–72. [Google Scholar] [CrossRef]
- Descamps, C.; Bouallou, C.; Kanniche, M. Efficiency of an Integrated Gasification Combined Cycle (IGCC) power plant including CO2 removal. Energy 2008, 33, 874–881. [Google Scholar] [CrossRef]
- Field, R.P.; Brasington, R. Baseline Flowsheet Model for IGCC with Carbon Capture. Ind. Eng. Chem. Res. 2011, 50, 11306–11312. [Google Scholar] [CrossRef]
- Ovalle-Encinia, O.; Raupp, G.B.; Lin, J.Y.S. CO2-selective membrane reactor process for water-gas-shift reaction with CO2 capture in a coal-based IGCC power plant. Chem. Eng. Res. Des. 2024, 212, 71–80. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, S.; Luo, Y.; Wang, K.; Liu, Y.; Ji, X. Recent Progress on Hydrogen-Rich Syngas Production from Coal Gasification. Processes 2023, 11, 1765. [Google Scholar] [CrossRef]
- Lin, H.; Swihart, M.; Kniep, J.; Liu, K. Sorption Enhanced Mixed Matrix Membranes for Hydrogen (H2) Purification and Carbon Dioxide (CO2) Capture; University at Buffalo: Buffalo, NY, USA, 2020; 32p. [Google Scholar]
- Emerson, S.; Magdefrau, N.; She, Y.; Thibaud-Erkey, C. Advanced Palladium Membrane Scale-up for Hydrogen Separation; United Technologies Corporation: Farmington, CT, USA, 2012. [Google Scholar]
- Krishnan, G.; Jayaweera, I.; Sanjrujo, A.; O’Brien, K.; Callahan, R.; Berchtold, K.; Roberts, D.-L.; Johnson, W. Fabrication and Scale-up of Polybenzimidazole (PBI) Membrane Based System for Precombustion-Based Capture of Carbon Dioxide; U.S. Department of Energy, National Energy Technology Laboratory: Pittsburgh, PA, USA, 2012.
- Kniep, J.; Salim, W.; Merkel, T.; Casillas, C.; Amo, K.; He, J.; Huang, I.; Nguyen, V.; Batoon, V. Bench-Scale Development Of A Transformative Membrane Process For Pre-Combustion CO2 Capture; Membrane Technology and Research, Inc.: Newark, CA, USA, 2022. [Google Scholar]
- Ovalle-Encinia, O.; Lin, J.Y.S. High-pressure CO2 permeation properties and stability of ceramic-carbonate dual-phase membranes. J. Membr. Sci. 2022, 646, 120249. [Google Scholar] [CrossRef]
- Zhang, K. 5—Recent progress on sulfur-resistant palladium membranes. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Gallucci, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–137. [Google Scholar]
- Siegelman, R.L.; Milner, P.J.; Forse, A.C.; Lee, J.-H.; Colwell, K.A.; Neaton, J.B.; Reimer, J.A.; Weston, S.C.; Long, J.R. Water Enables Efficient CO2 Capture from Natural Gas Flue Emissions in an Oxidation-Resistant Diamine-Appended Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 13171–13186. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Ho, W.S.W. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef]
- Baker, R.W.; Freeman, B.; Kniep, J.; Wei, X.; Merkel, T. CO2 capture from natural gas power plants using selective exhaust gas recycle membrane designs. Int. J. Greenh. Gas Control 2017, 66, 35–47. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Han, Y.; Lin, L.-C. Novel Transformational Membranes and Process for CO2 Capture from Flue Gas (FE0031731); The Ohio State University: Columbus, OH, USA, 2023. [Google Scholar]
- Choi, S.-H.; Kim, J.-H.; Lee, Y. Pilot-scale multistage membrane process for the separation of CO2 from LNG-fired flue gas. Sep. Purif. Technol. 2013, 110, 170–180. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Y.; Prasad, B.; Pang, R.; Zou, C.; Ho, W.S.W. Carbon capture from flue gases using an integrated membrane skid. J. Membr. Sci. 2025, 718, 123674. [Google Scholar] [CrossRef]
- Campanari, S.; Manzolini, G.; Chiesa, P. Using MCFC for high efficiency CO2 capture from natural gas combined cycles: Comparison of internal and external reforming. Appl. Energy 2013, 112, 772–783. [Google Scholar] [CrossRef]
- Zamarripa, M.A.; Eslick, J.C.; Matuszewski, M.S.; Miller, D.C. Multi-Objective Optimization of Membrane-Based CO2 Capture; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2017.
- National Energy Technology Laboratory, Post-Combustion Membranes Technology Update; U.S. Department of Energy (DOE): Washington, DC, USA, 2013.
- Lee, J.-Y.; Keener, T.C.; Yang, Y.J. Potential Flue Gas Impurities in Carbon Dioxide Streams Separated from Coal-Fired Power Plants. J. Air Waste Manag. Assoc. 2009, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energy 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Song, C.; Pan, W.; Srimat, S.T.; Zheng, J.; Li, Y.; Wang, Y.-H.; Xu, B.-Q.; Zhu, Q.-M. Tri-reforming of Methane over Ni Catalysts for CO2 Conversion to Syngas With Desired H2/CO Ratios Using Flue Gas of Power Plants Without CO2 Separation. In Studies in Surface Science and Catalysis; Park, S.-E., Chang, J.-S., Lee, K.-W., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 153, pp. 315–322. [Google Scholar]
- Huang, S.; Li, C.; Tan, T.; Fu, P.; Xu, G.; Yang, Y. An Improved System for Utilizing Low-Temperature Waste Heat of Flue Gas from Coal-Fired Power Plants. Entropy 2017, 19, 423. [Google Scholar] [CrossRef]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, W.; Wu, A.; Shu, W.; Zhang, Y. Recent progress on CO2 separation membranes. RSC Adv. 2024, 14, 20714–20734. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Song, H.; Yang, L.; Ba, L. Colloidal Force Study of Particle Fouling on Gas Capture Membrane. Sci. Rep. 2017, 7, 12939. [Google Scholar] [CrossRef]
- Adams II, T.A.; Hoseinzade, L.; Madabhushi, P.B.; Okeke, I.J. Comparison of CO2 Capture Approaches for Fossil-Based Power Generation: Review and Meta-Study. Processes 2017, 5, 44. [Google Scholar] [CrossRef]
- Pohlmann, J.; Bram, M.; Wilkner, K.; Brinkmann, T. Pilot scale separation of CO2 from power plant flue gases by membrane technology. Int. J. Greenh. Gas Control 2016, 53, 56–64. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Sheng, M.; Wang, Z.; Zhao, S.; Wang, J.; Mao, S.; Wang, D.; Guo, B.; Ye, N.; et al. Membrane technology for CO2 capture: From pilot-scale investigation of two-stage plant to actual system design. J. Membr. Sci. 2021, 624, 119137. [Google Scholar] [CrossRef]
- United States Department of Education; National Energy Technology Laboratory. Carbon Capture Technology Compendium 2022; U.S. Department of Energy, National Energy Technology Laboratory: Pittsburgh, PA, USA, 2022; pp. 264–271.
- Merkel, T.C.; Wei, X.; White, L.S.; Pande, S.; Wijmans, H.J.; Baker, R.W. Pilot Testing of a Membrane System for Post-Combustion CO2 Capture; U.S. Department of Energy, National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2016.
- Freeman, B. Large Pilot Testing of the MTR Membrane Post-Combution CO2 Capture Process; Membrane Technology Research Inc.: Newark, CA, USA, 2024. [Google Scholar]
- News, M. MTR Carbon Capture Awarded DOE Funding for Integrated CCS Demonstration Project at Dry Fork Station. Available online: https://mtrccs.com/mtr-carbon-capture-awarded-doe-funding-for-integrated-ccs-demonstration-project-at-dry-fork-station (accessed on 6 March 2024).
- MTR Carbon Capture Selected to Provied a Capture Plant to TSMC. Available online: https://mtrccs.com/mtr-carbon-capture-selected-to-provide-a-capture-plant-to-tsmc/ (accessed on 27 January 2025).
- Lindehoff, E.; Mattsson, L.; Olofsson, M.; Svensson, F.; Farnelid, H.; Legrand, C. Biomass performance and stability of 5-year outdoor microalgal cultivation for CO2 removal from cement flue gas. Bioresour. Technol. Rep. 2024, 25, 101730. [Google Scholar] [CrossRef]
- Zanobetti, F.; Dal Pozzo, A.; Cozzani, V. Sustainability assessment of CO2 capture across different scales of hard-to-abate emission sources. Chem. Eng. J. 2025, 505, 159466. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ho, M.T.; Aguiar, A.A.; Wiley, D.E.; Stevens, G.W.; Kentish, S.E. Membrane gas separation processes for CO2 capture from cement kiln flue gas. Int. J. Greenh. Gas Control 2014, 24, 78–86. [Google Scholar] [CrossRef]
- Hassan, S.M.N.; Douglas, P.L.; Croiset, E. Techno-Economic Study of CO2 Capture from an Existing Cement Plant Using MEA Scrubbing. Int. J. Green Energy 2007, 4, 197–220. [Google Scholar] [CrossRef]
- Baker, R.W.; Freeman, B.; Kniep, J.; Huang, Y.I.; Merkel, T.C. CO2 Capture from Cement Plants and Steel Mills Using Membranes. Ind. Eng. Chem. Res. 2018, 57, 15963–15970. [Google Scholar] [CrossRef]
- Merkel, T.; Freeman, B.; Baker, R.; Hao, P.; St-Martin, P.; Pierik, D. Engineering Design of a Polaris Membrane CO2 Capture System at a Cement Plant; Membrane Technology and Research, Inc.: Newark, CA, USA, 2024. [Google Scholar]
- Gu, C.; Xie, J.; Li, X.; Gao, X. Levelized Cost Analysis for Blast Furnace CO2 Capture, Utilization, and Storage Retrofit in China’s Blast Furnace–Basic Oxygen Furnace Steel Plants. Energies 2023, 16, 7817. [Google Scholar] [CrossRef]
- Perpiñán, J.; Peña, B.; Bailera, M.; Eveloy, V.; Kannan, P.; Raj, A.; Lisbona, P.; Romeo, L.M. Integration of carbon capture technologies in blast furnace based steel making: A comprehensive and systematic review. Fuel 2023, 336, 127074. [Google Scholar] [CrossRef]
- Yun, S.; Jang, M.-G.; Kim, J.-K. Techno-economic assessment and comparison of absorption and membrane CO2 capture processes for iron and steel industry. Energy 2021, 229, 120778. [Google Scholar] [CrossRef]
- IEA Greenhouse Gas R&D Programme (IEAGHG). Challenges and Opportunities of CO2 Capture and Storage for the Iron and Steel Industry; IEA Greenhouse Gas R&D Programme (IEAGHG): Cheltenham, UK, 2012. [Google Scholar]
- Wang, K.; Hopkinson, D.; He, Q.; Nulwala, H.B.; Luebke, D.R. Membrane Development for CO2 Capture from Steel Manufacturing; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2024.
- Al-Mamoori, M.A.; Lin, J.Y.S. Water-gas shift reaction in ceramic-carbonate dual-phase membrane reactor at high temperatures and pressures. Chem. Eng. J. 2022, 448, 137652. [Google Scholar] [CrossRef]
- Vrbová, V.; Ciahotný, K. Upgrading Biogas to Biomethane Using Membrane Separation. Energy Fuels 2017, 31, 9393–9401. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; le Saché, E.; Pastor-Pérez, L.; Reina, T.R. Membrane-based technologies for biogas upgrading: A review. Environ. Chem. Lett. 2020, 18, 1649–1658. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Shin, M.S.; Jung, K.-H.; Kwag, J.-H.; Jeon, Y.-W. Biogas separation using a membrane gas separator: Focus on CO2 upgrading without CH4 loss. Process Saf. Environ. Prot. 2019, 129, 348–358. [Google Scholar] [CrossRef]
- Wojnarova, P.; Rusin, J.; Basinas, P.; Kostejn, M.; Nemec, J.; Stanovský, P.; Kim, A.S.; Izak, P. Unveiling the potential of composite water-swollen spiral wound membrane for design of low-cost raw biogas purification. Sep. Purif. Technol. 2023, 326, 124783. [Google Scholar] [CrossRef]
- Simcik, M.; Ruzicka, M.C.; Karaszova, M.; Sedlakova, Z.; Vejrazka, J.; Vesely, M.; Capek, P.; Friess, K.; Izak, P. Polyamide thin-film composite membranes for potential raw biogas purification: Experiments and modeling. Sep. Purif. Technol. 2016, 167, 163–173. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Plasticization and Swelling in Polymeric Membranes in CO2 Removal from Natural Gas. Chem. Eng. Technol. 2016, 39, 1604–1616. [Google Scholar] [CrossRef]
- Poloncarzova, M.; Vejrazka, J.; Vesely, V.; Izak, P. Effective Purification of Biogas by a Condensing-Liquid Membrane. Angew. Chem. Int. Ed. 2011, 50, 669–671. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J.S.; Yoon, H.; Kim, T.; Kim, Y.H. Water membrane for carbon dioxide separation. Sep. Purif. Technol. 2018, 203, 268–273. [Google Scholar] [CrossRef]
- Jameel, M.K.; Mustafa, M.A.; Ahmed, H.S.; Mohammed, A.j.; Ghazy, H.; Shakir, M.N.; Lawas, A.M.; Mohammed, S.k.; Idan, A.H.; Mahmoud, Z.H.; et al. Biogas: Production, properties, applications, economic and challenges: A review. Results Chem. 2024, 7, 101549. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Overview of Biogas Reforming Technologies for Hydrogen Production: Advantages and Challenges. In Proceedings of the First International Conference on Recent Advances in Bioenergy Research; Springer: New Delhi, India, 2016; pp. 227–243. [Google Scholar]
- Chuda, A.; Ziemiński, K. Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process. Energies 2021, 14, 7321. [Google Scholar] [CrossRef]
- He, X.; Chen, D.; Liang, Z.; Yang, F. Insight and Comparison of Energy-efficient Membrane Processes for CO2 Capture from Flue Gases in Power Plant and Energy-intensive Industry. Carbon Capture Sci. Technol. 2022, 2, 100020. [Google Scholar] [CrossRef]
- Chen, K.K.; Salim, W.; Han, Y.; Wu, D.; Ho, W.S.W. Fabrication and scale-up of multi-leaf spiral-wound membrane modules for CO2 capture from flue gas. J. Membr. Sci. 2020, 595, 117504. [Google Scholar] [CrossRef]
- He, X. A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain. Soc. 2018, 8, 34. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Nikkhah Dafchahi, M.; Khoshhal Salestan, S.; Chew, J.W.; Mozafari, M.; Soroush, M.; Hrapovic, S.; Hemraz, U.D.; Giro, R.; Steiner, M.B.; et al. Polymeric membranes in carbon capture, utilization, and storage: Current trends and future directions in decarbonization of industrial flue gas and climate change mitigation. Energy Environ. Sci. 2025, 18, 5025–5092. [Google Scholar] [CrossRef]
- Jordal, K.; Anantharaman, R.; Peters, T.A.; Berstad, D.; Morud, J.; Nekså, P.; Bredesen, R. High-purity H2 production with CO2 capture based on coal gasification. Energy 2015, 88, 9–17. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Y.; Lv, H.; Xu, G.; Dong, S. A New Integration System for Natural Gas Combined Cycle Power Plants with CO2 Capture and Heat Supply. Energies 2018, 11, 3055. [Google Scholar] [CrossRef]
- Merkel, T.; Kniep, J.; Fulton, D.; Watson, R.; Sakib, N.; Westling, E.; Prakash, A.; Hofmann, T.; Huang, I.; Nguyen, V.; et al. Integrated Testing of a Membrane CO2 Capture Process with a Coal-Fired Boiler; Membrane Technology and Research, Inc.: Newark, CA, USA, 2018. [Google Scholar]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Yu, H.; Guo, M.-Z.; Ling, T.-C. Carbon sequestration via CO2 curing of cement compacts: Optimizing flue gas inherent heat and water vapor. J. Ind. Eng. Chem. 2024, 137, 252–259. [Google Scholar] [CrossRef]
- Schakel, W.; Hung, C.R.; Tokheim, L.-A.; Strømman, A.H.; Worrell, E.; Ramírez, A. Impact of fuel selection on the environmental performance of post-combustion calcium looping applied to a cement plant. Appl. Energy 2018, 210, 75–87. [Google Scholar] [CrossRef]
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 Capture in the Cement Industry. Energy Procedia 2009, 1, 87–94. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kim, Y.T.; Kwak, G.; Kim, J. Techno-economic evaluation of the integrated polygeneration system of methanol, power and heat production from coke oven gas. Energy Convers. Manag. 2019, 182, 240–250. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, W.; Zhang, Y.; Lu, X.; Zhang, Y.; Shen, P. Catalytic partial oxidation of coke oven gas to syngas in an oxygen permeation membrane reactor combined with NiO/MgO catalyst. Int. J. Hydrog. Energy 2010, 35, 6239–6247. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Muñoz, R.; González-Sánchez, A.; Ruiz, H.A.; Quijano, G. Membrane materials for biogas purification and upgrading: Fundamentals, recent advances and challenges. J. Environ. Chem. Eng. 2024, 12, 114106. [Google Scholar] [CrossRef]

| Sources | Processes | CO2 Content (%) | Traces |
|---|---|---|---|
| Power plant: pre-combustion | IGCC | 30–45 | CH4, H2O, H2S, NH3 |
| Power plant: post-combustion | Petroleum power plant | 6–10 | N2 |
| Natural gas power plant | 4–8 | NOx, SOx, O2 | |
| Coal-fired power plant | 10–15 | NOx, SOx, dust | |
| Iron and steel industry | Blast furnace | 20–27 | CO, H2, H2S |
| Basic oxygen furnace | 16–42 | ||
| Cement industry | Precalciner | 1–30 | NOx, SO2, CO, dust |
| Calcination | 14–33 | ||
| Biogas | Biogas upgrading | 30–45 | H2O, H2, O2, N2, NH3, H2S, siloxane |
| Method | Concept | Advantage | Disadvantage |
|---|---|---|---|
| Absorption | Dissolves CO2 using chemical solvent (e.g., amines) | Effective for large-scale emission sources | Solvent corrosion and regeneration costs |
| Adsorption | Captures CO2 using porous material (e.g., zeolites, activated carbon) | Regenerable adsorbents | Requires high thermal energy |
| Cryogenic Separation | Liquefies CO2 at cryogenic temperature for separation | Produces high-purity CO2 | High energy consumption |
| Biological Capture | Uses microorganisms, algae, or enzymes to absorb CO2 | Environmentally friendly | Slow absorption rate, requires large space |
| Membrane | Separates CO2 using selective permeation through membranes | Flexible operation, low energy consumption, easy to modularize | Impurity sensitivity, limited performance at low CO2 concentrations |
| Method | Cost Range (USD/Ton CO2) | Key Factors Affecting Cost | Primary Applications | References |
|---|---|---|---|---|
| Absorption | USD 44–71 | Solvent properties, energy requirements, plant scale | Power plants, industrial flue gas treatment | [63,64] |
| Adsorption | USD 37–57 | Adsorbent characteristics, regeneration method, energy penalty | Industrial point sources, moderate CO2 concentrations | [65,66] |
| Cryogenic Separation | USD 12–150 | Process integration, energy efficiency, scale | High-purity CO2 streams, industrial gas processing | [67,68,69] |
| Biological Capture | USD 88–116 | Biomass availability, co-product value, scale | Power generation with negative emissions | [63,70] |
| Membrane | USD 42–50 | Compression and vacuum energy, membrane fabrication, module costs | Post-combustion capture, industrial flue gas | [71,72] |
| Component | Agricultural Residue | Sewage Sludge | Landfill | Wastewater |
|---|---|---|---|---|
| CH4 (%) | 50–70 | 60–70 | 35–65 | 55–58 |
| CO2 (%) | 25–45 | 34–38 | 30–45 | 32–50 |
| H2O (%) | 1–6 | 1–7 | 1–5 | 1–5 |
| H2 (%) | Traces | Traces | 0–5 | Traces |
| O2 (%) | 0–1 | Traces | 0–1 | Traces |
| N2 (%) | 0–5 | 0–2 | 5–15 | Traces |
| NH3 (ppm) | 0–100 | 50–100 | 0–5 | 0–100 |
| H2S (ppm) | 0–1000 | 0–400 | 0–100 | 0–4000 |
| Siloxane (%) | 0–0.2 | 0–0.2 | 0–0.2 | 0–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Heo, S.; Yeo, J.-G.; Lee, S.; Kim, J.-K.; Lee, J.H. Membrane-Based CO2 Capture Across Industrial Sectors: Process Conditions, Case Studies, and Implementation Insights. Membranes 2025, 15, 200. https://doi.org/10.3390/membranes15070200
Park JW, Heo S, Yeo J-G, Lee S, Kim J-K, Lee JH. Membrane-Based CO2 Capture Across Industrial Sectors: Process Conditions, Case Studies, and Implementation Insights. Membranes. 2025; 15(7):200. https://doi.org/10.3390/membranes15070200
Chicago/Turabian StylePark, Jin Woo, Soyeon Heo, Jeong-Gu Yeo, Sunghoon Lee, Jin-Kuk Kim, and Jung Hyun Lee. 2025. "Membrane-Based CO2 Capture Across Industrial Sectors: Process Conditions, Case Studies, and Implementation Insights" Membranes 15, no. 7: 200. https://doi.org/10.3390/membranes15070200
APA StylePark, J. W., Heo, S., Yeo, J.-G., Lee, S., Kim, J.-K., & Lee, J. H. (2025). Membrane-Based CO2 Capture Across Industrial Sectors: Process Conditions, Case Studies, and Implementation Insights. Membranes, 15(7), 200. https://doi.org/10.3390/membranes15070200







