1. Introduction
Anaerobic digestion (AD) is a widely used wastewater treatment technology with significant advantages, such as the efficient processing of various feedstocks with high organic load and the production of biogas, which is considered a renewable energy source [
1,
2]. In 2021, the total number of AD/biogas plants in Europe amounted to 18,843, mainly processing feedstocks of agricultural origin, i.e., agricultural residues, sequential crops, energy crops etc. (
Figure S1-1 in Supplementary Materials) [
3]. However, there is also a significant potential for biogas production through AD treatment of effluents/wastes from the food processing industry, which is currently at a lower level. This study deals with the valorization of food industry digestates, which proves to be quite complicated, mainly due to their variable composition depending on AD feed conditions [
4]. Therefore, the adaptation/development of appropriate processing steps is required to achieve project sustainability when such AD feedstocks are used.
The main slurry type effluent of the AD plant (i.e., the
digestate) contains minerals, volatile fatty acids (VFAs), and nutrients (i.e., nitrogen, N, phosphorus, P, and potassium, K compounds) [
5]. Primary liquid–solid separation of digestate results in two fractions: a solid (10–20% mass fraction) and a liquid (80–90%) containing the water-soluble nutrients [
6]. The relatively high nutrient content of these streams has drawn attention to their valorization, especially as fertilizers, which is also in line with the new European Union (EU) Regulation 2019/1009 on Fertilizing Products [
7]. The
solid fraction is used for agricultural purposes, the production of energy, and other value-added products [
8]. The
liquid digestate (LD) can also be used for direct fertilization under controlled conditions [
9,
10,
11]. However, in general, parameters like high salinity and other organic/inorganic micropollutants [
12,
13] can render LD harmful to plants and vegetation, thus hindering its direct application [
14,
15]. Additionally, variations in AD feedstock composition and operating parameters result in varying nutrient content [
6,
15,
16], which should remain relatively constant when fertilization of specific plant(s) is considered. Several processes have been investigated for the treatment of LD to recover nutrients and use them efficiently in fertilizers, including ion exchange and adsorption [
15,
17,
18], ammonia stripping [
19,
20], chemical precipitation [
11,
15], and membrane separation [
10,
21]. However, the development of a sustainable LD treatment scheme is still being pursued, which is challenging and requires the integration of different processes to obtain suitable fertilizer products and water for reuse or safe disposal [
22,
23,
24].
This paper is focused on the valorization of
dairy processing effluents, characterized by modest concentration of organic matter and seasonal variability [
25,
26]. For this important type of effluent, the estimated volume produced by EU-27 industries is at the level of 200 × 10
6 m
3/yr [
27]. Furthermore, the EU has established/listed [
28] best available techniques (BAT) for treating such effluents, ensuring maximum environmental protection and sustainability. In a recent review [
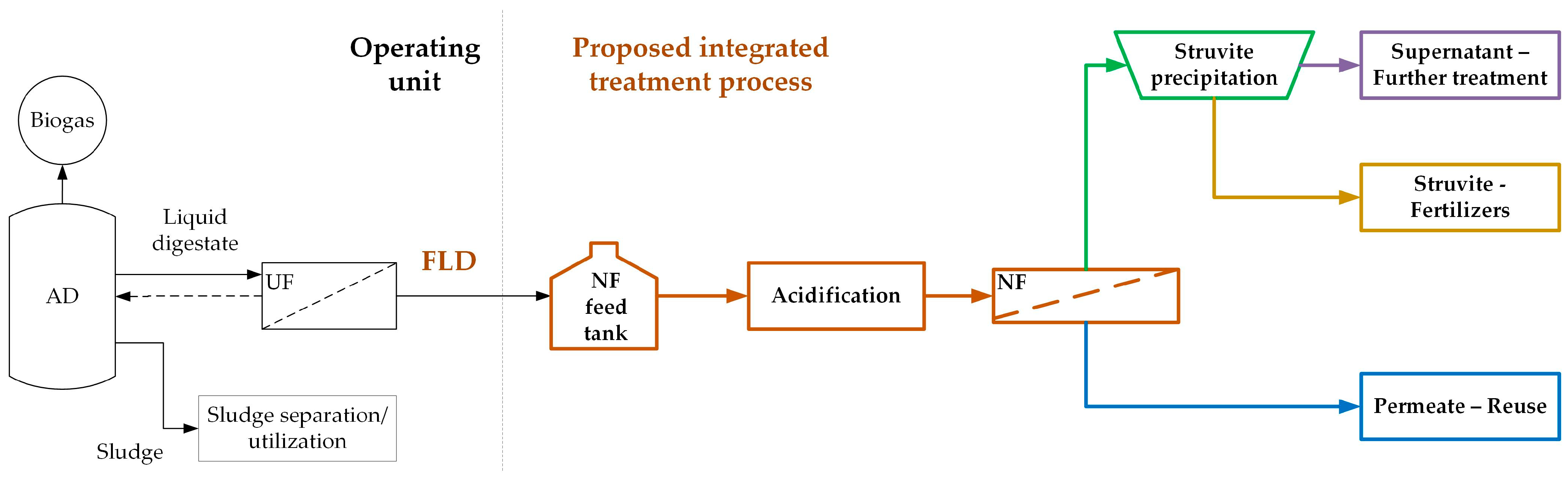
27] of listed BAT and other appropriate applied techniques, the need is stressed for additional R&D work to improve dairy effluent valorization. Towards this goal, an integrated system was investigated [
29] aiming at the valorization of LD from an industrial AD facility (
Figure 1) treating effluents from a dairy plant (“BIZIOS S.A.”) located in Thessaly, Greece. The dairy processing effluents fed to the AD unit consist mainly of cheese whey, lactose, “flushings”, and CIP (Cleaning in Place) water from the production lines. The liquid digestate from the AD unit is treated with ultrafiltration (UF) membranes (Memthane
® technology from Veolia Water Technologies, Delft, The Netherlands [
30]) to remove suspended solids (SS), whereas the excess sludge is separated through centrifugation. In the investigated scheme [
29] (
Figure 1), the produced filtered liquid digestate (FLD, i.e., the UF permeate), free from most of its original organic load, is further treated by nanofiltration (NF) membranes to effectively retain the dissolved inorganic species in the concentrate. However, due to the significant FLD alkalinity, a pretreatment step prior to NF is necessary [
29], i.e., controlled acidification to reduce alkalinity to mitigate NF membrane scaling. The NF permeate stream is suitable for industrial reuse or restricted irrigation [
31], while the concentrate can be utilized for nutrient recovery. Other relevant studies [
32,
33] have shown that NF treatment can play a critical role by concentrating phosphate-containing effluents, leading to sufficient saturation of phosphate salts and a reduction in the chemicals needed for their crystallization/recovery. This study focuses on the recovery of nutrients in the form of struvite from such NF concentrates.
The precipitation of magnesium ammonium phosphate (MgNH
4PO
4·6H
2O, MAP), an effective fertilizer commonly known as struvite [
34,
35,
36,
37,
38], has been efficiently used for the simultaneous recovery of nutrients (i.e., ammonium and phosphate ions). In fact, industrial systems are already in operation, with annual production of MAP reaching ~78 kt in the EU-28 [
39,
40,
41]. Nevertheless, there are significant knowledge gaps, as process performance is influenced by several parameters, including pH [
42,
43,
44], duration of precipitation [
45], the molar ratio of struvite components in the feed solution (NH
4:Mg:PO
4) [
44,
46,
47], magnesium (Mg) source [
45,
46,
48], and the composition of the effluent [
49,
50,
51]. The effects of these parameters on struvite precipitation have been extensively studied using mostly synthetic solutions [
48,
52,
53,
54]. However, the efficiency of the process should be carefully evaluated using real LD samples in order to obtain realistic design data for such plants. Moreover, investigations have been reported on dairy industry waste-streams with
high nutrient content [
55,
56]. Yet, the recovery of nutrients from wastewaters with rather
low nutrient concentration (of interest to this study) remains a challenge that should be addressed [
22,
23,
24].
Literature studies along the lines indicated above are briefly outlined. Lopez et al. [
57] evaluated the feasibility of struvite precipitation from a NF retentate stream using a synthetic solution that simulated the secondary effluent of a wastewater treatment plant. Through experiments and simulations, they concluded that 70% permeate removal resulted in adequate concentration for struvite precipitation without Mg addition. Nir et al. [
32] investigated the recovery of phosphorous from low P concentration effluents through continuous NF and calcium phosphate crystallization. Arola et al. [
33] applied a two-stage NF treatment to the effluent of a membrane bioreactor treating municipal wastewater. The final concentrate stream was fed into a settling tank to precipitate phosphates in the form of hydroxyapatite. Ramaswami et al. [
34] investigated the recovery of ammonia from raw methanogenic landfill leachates and different streams from membrane processes through struvite precipitation.
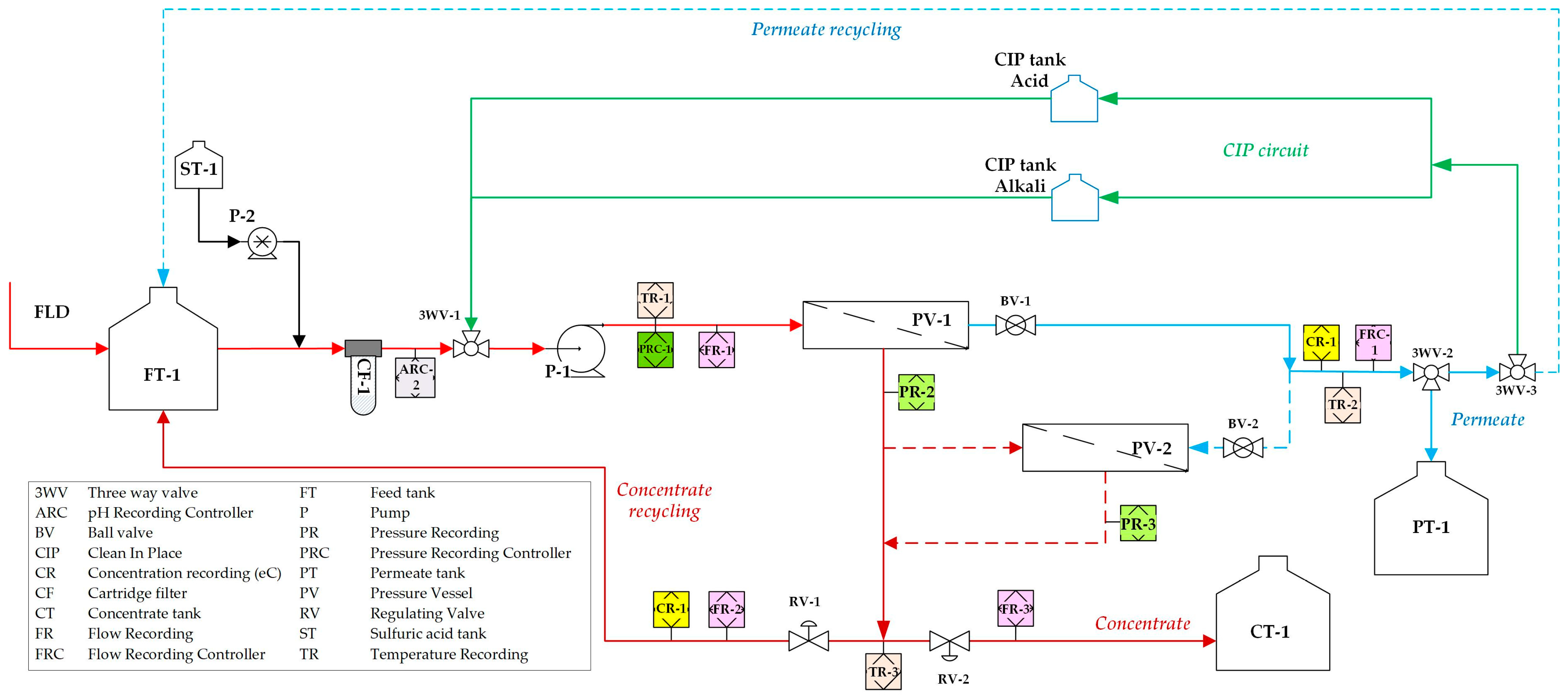
In the aforementioned study by Tsaridou et al. [
29], a comprehensive methodology was applied that included bench and cross-flow tests with synthetic and real FLD, as well as reliable simulations to determine the key design parameters of an NF pilot plant. Such a pilot unit was initially tested with synthetic solutions. In the present study, the performance of an appropriate NF pilot unit is tested in an industrial environment to optimize the concentration of FLD, also taking into account FLD variability. Furthermore, the valorization of the NF concentrate through struvite precipitation is systematically investigated. The effects of key parameters, including pH, duration of struvite precipitation, molar ratio of NH
4:Mg:PO
4 in the feed solution, and Mg source, on process performance are investigated. Such a systematic investigation of the key process parameters for struvite recovery from
real effluents is rarely found in the literature, although it is essential to draw clear conclusions regarding process sustainability.
2. Struvite Formation Reaction and Process Performance Indicators
Struvite is formed through the following chemical reaction:
In principle, the stoichiometric ratio of Mg
2+, NH
4+, and PO
43− (1:1:1) is required to form struvite, and (depending on the FLD composition) phosphorous and/or magnesium must be added to the AD effluent to achieve the desired supersaturation conditions [
48]. In addition, pH is a key factor in the formation of struvite [
58]. Therefore, pH adjustment of the feed solution was investigated in the alkaline range, as it has been repeatedly reported that optimal pH values are in the range of pH 8.0–10.0 [
23,
59,
60].
To evaluate struvite precipitation from a given feed solution, the removal ratio (R
i) [
61,
62] of each ionic component (i.e., Mg
2+, NH
4+ and PO
43−) is determined as follows:
where
Cbi and
Cfi are the concentrations of component i in the initial solution
after the addition of the Mg2+ and PO43− ions and in the final solution (after precipitation), respectively. This parameter, which is expressed as the percentage removal efficiency (i.e., %
Ri), is a useful indicator for an effective comparison between feed solutions with different compositions of Mg
2+ and PO
43− ions.
To assess the quality of the formed precipitate, the struvite purity (%) is determined according to Cui et al. [
63] using the following expression:
where
CN (mg/L) is the concentration of NH
4-N in the HCl solution after dissolution of the precipitate,
V (L) is the volume of the HCl solution,
MN and
MMAP in g/mol are the molar masses of nitrogen and struvite, respectively, and
mMAP is the mass of the recovered precipitate.
5. Discussion
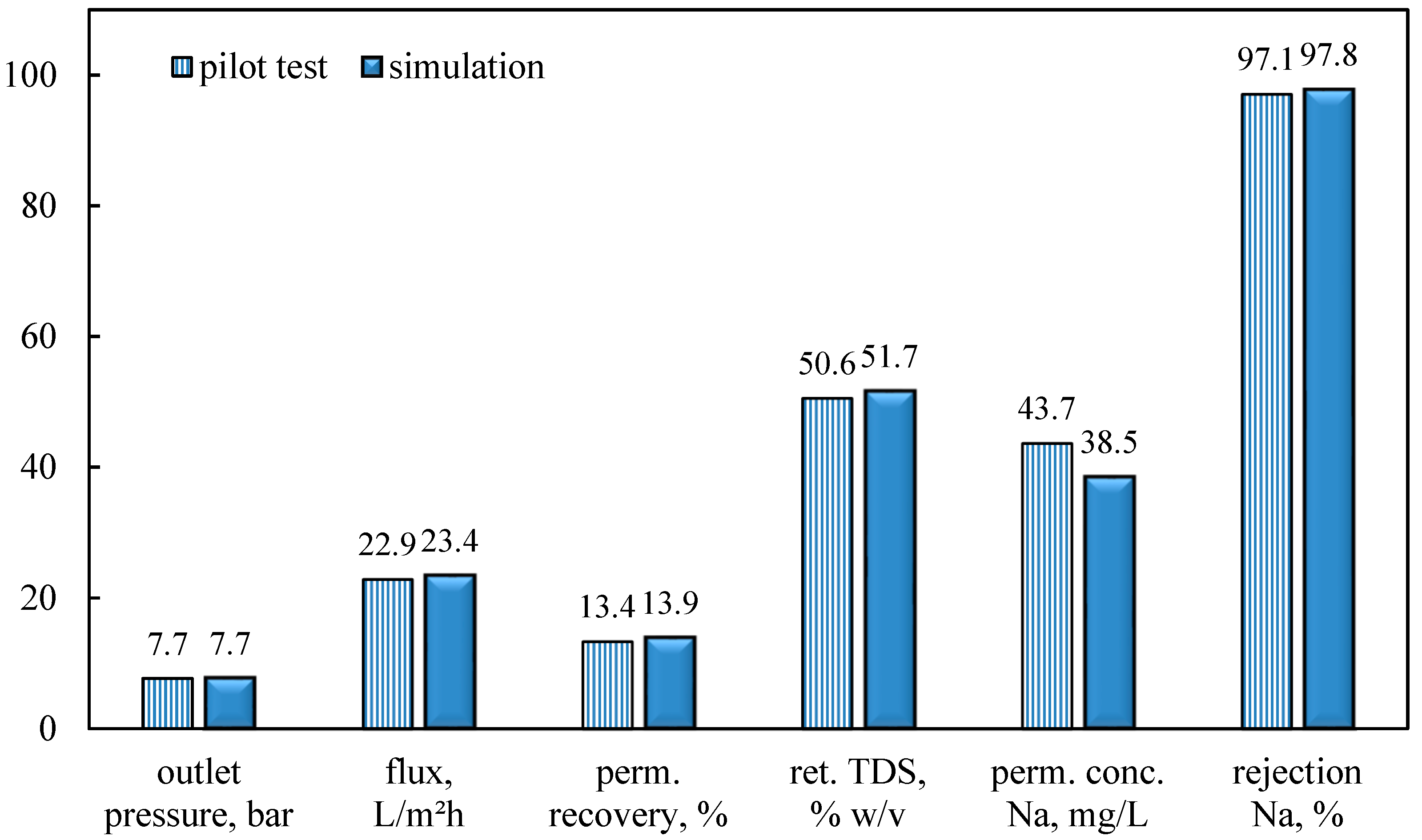
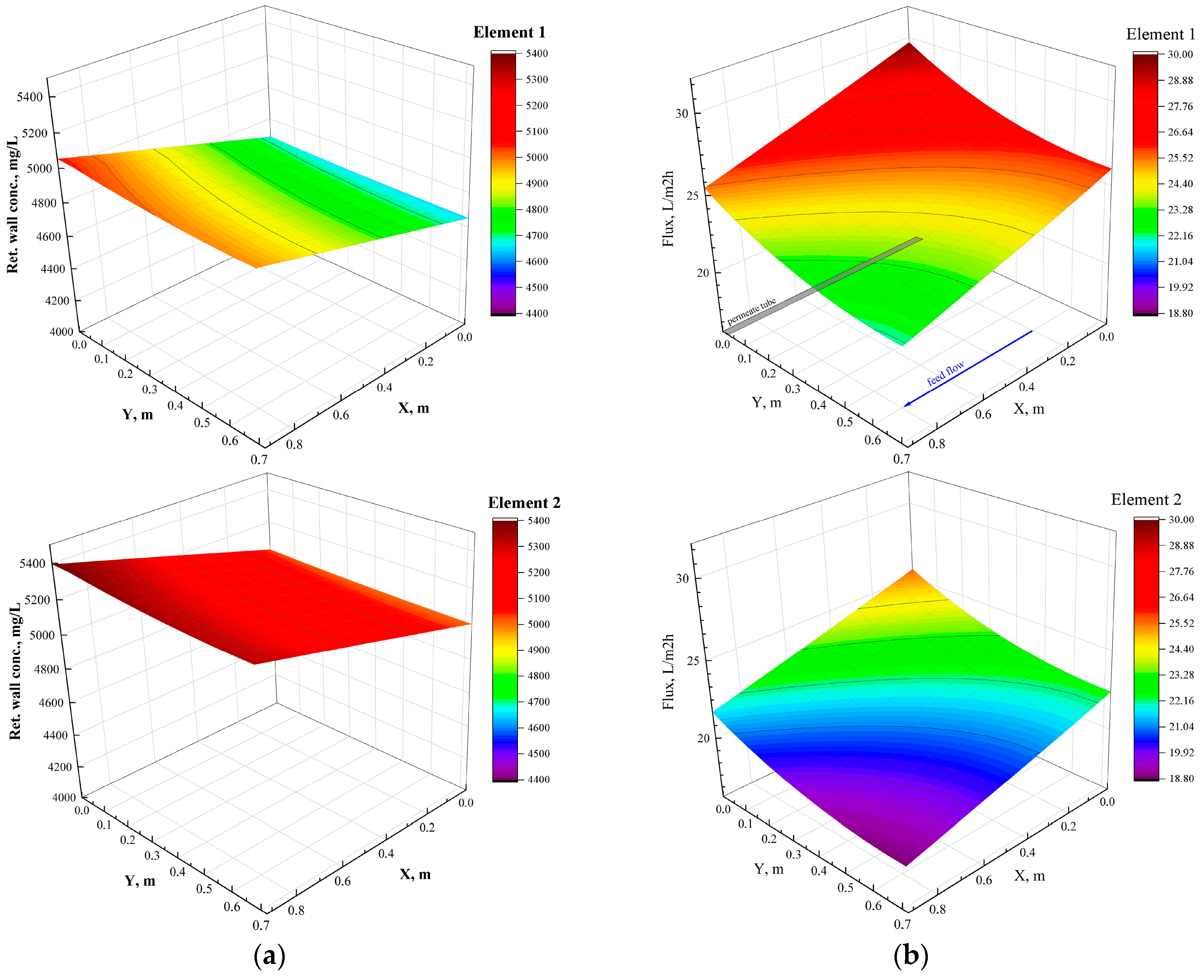
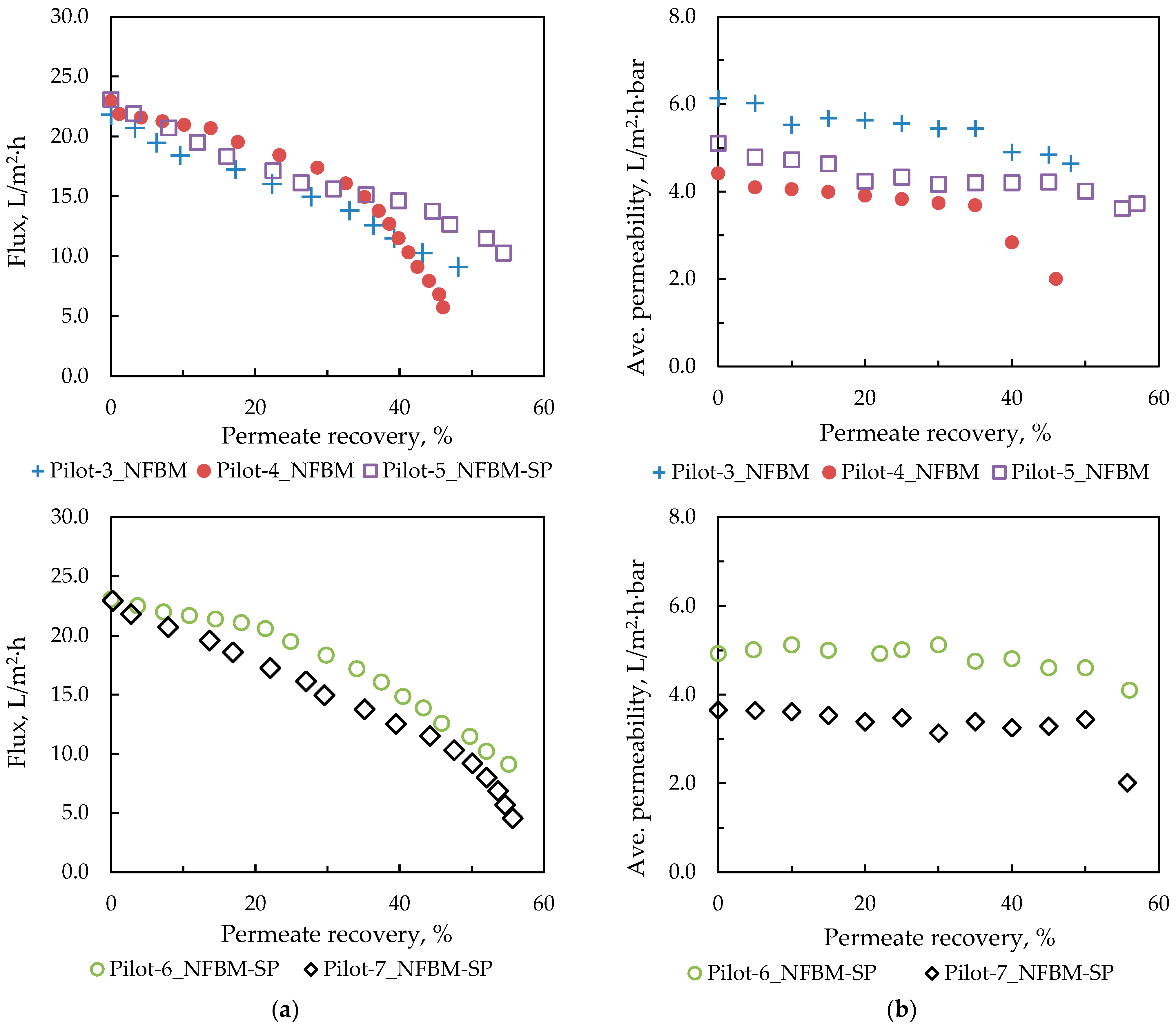
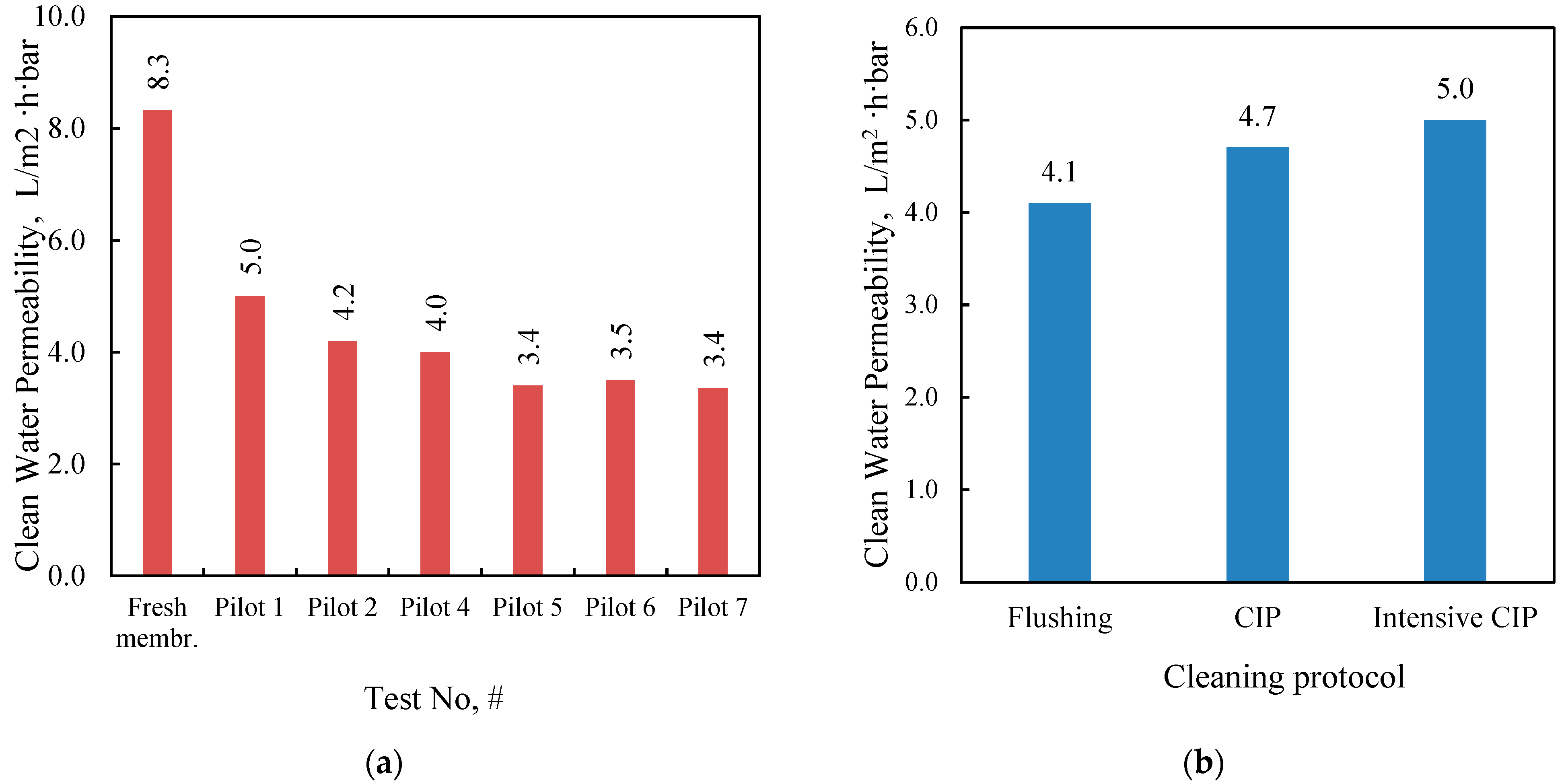
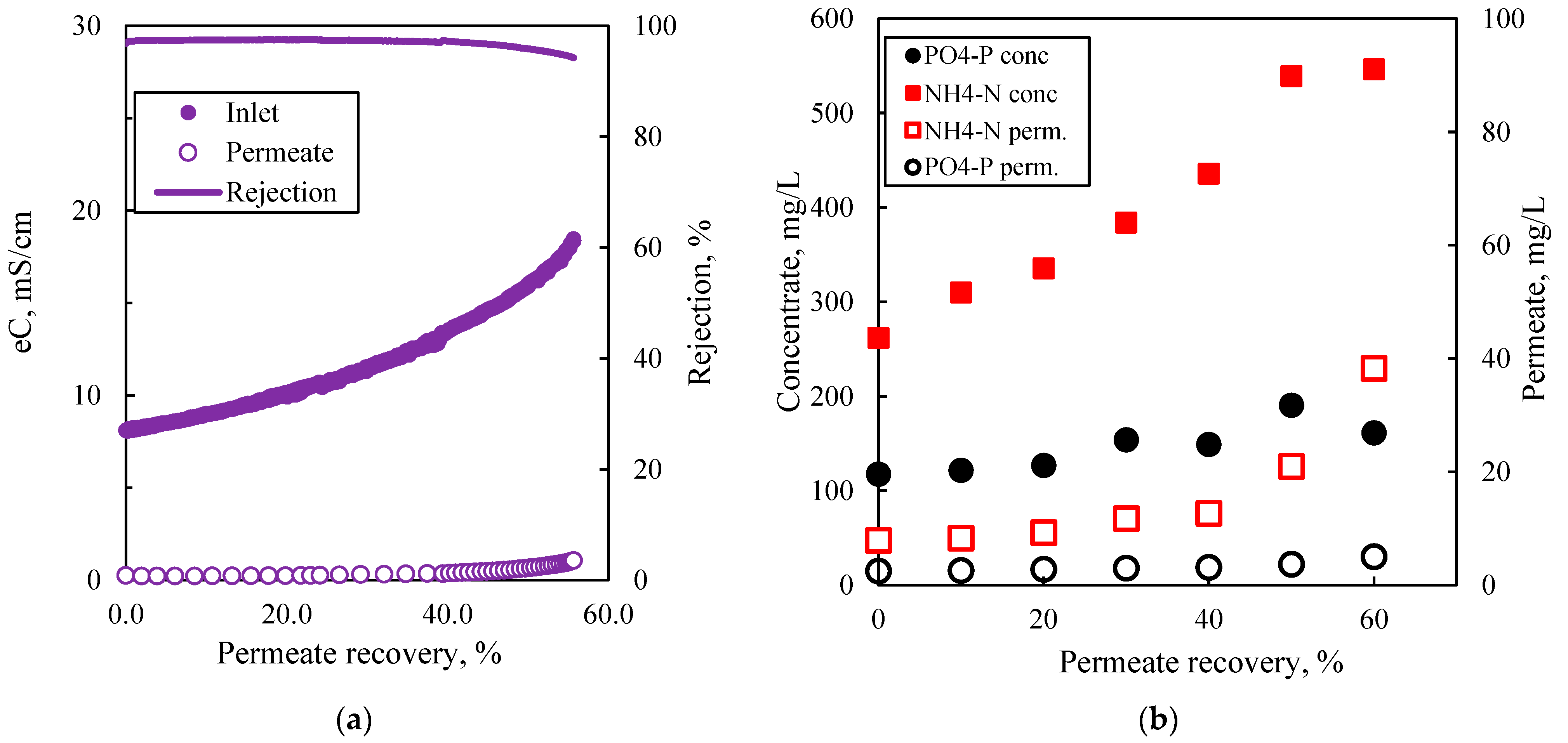
In this study, an integrated method for the valorization of the liquid digestate of an AD plant is investigated. The methodology includes first the concentration of industrial FLD with an NF pilot unit and the subsequent recovery of nutrients from the concentrate stream through struvite precipitation. The results of the nanofiltration pilot tests have shown that for this particular FLD, permeate recovery of 50 ± 10% and a concentration factor of nutrients ~2 is feasible, i.e., under controlled membrane scaling. An acidification processing step (before NF) was necessary to control the increased and variable FLD alkalinity, which would lead to undesirable salt precipitation and membrane scaling. Conditions for maintaining/restoring the NF membrane’s permeability through periodic cleaning (flushing, CIP) have been investigated at pilot scale. An advanced NF process simulator, whose satisfactory performance was confirmed through comparison with the realistic pilot data, can be used to assist process scale-up and control. The NF permeate cannot be disposed of in sensitive receiving water bodies as the NH
4-N disposal limits (≤2 mg/L) are not met. However, it can be safely used for industrial purposes and/or restricted irrigation [
31]. Unrestricted irrigation is also an option after appropriate dilution.
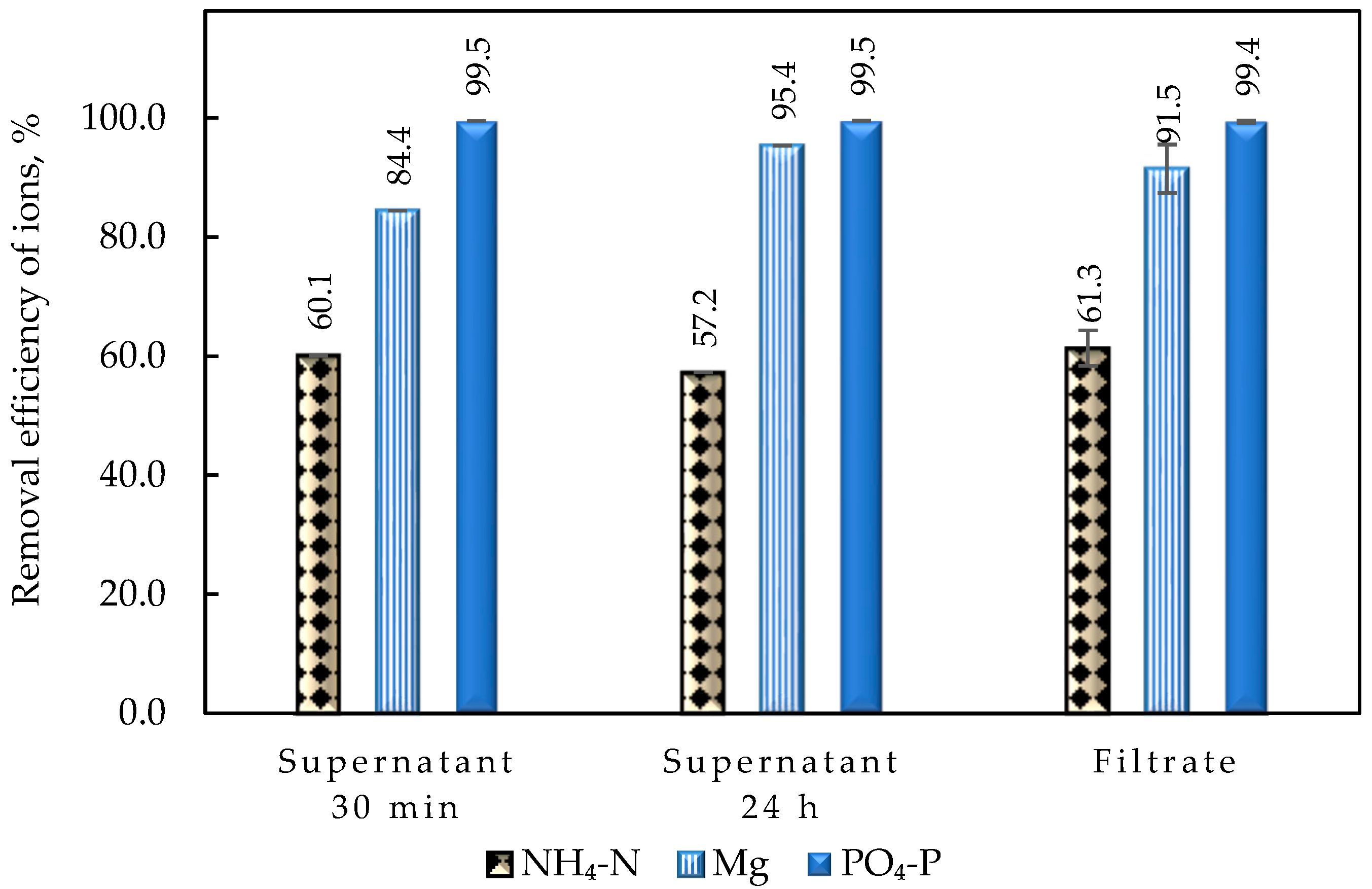
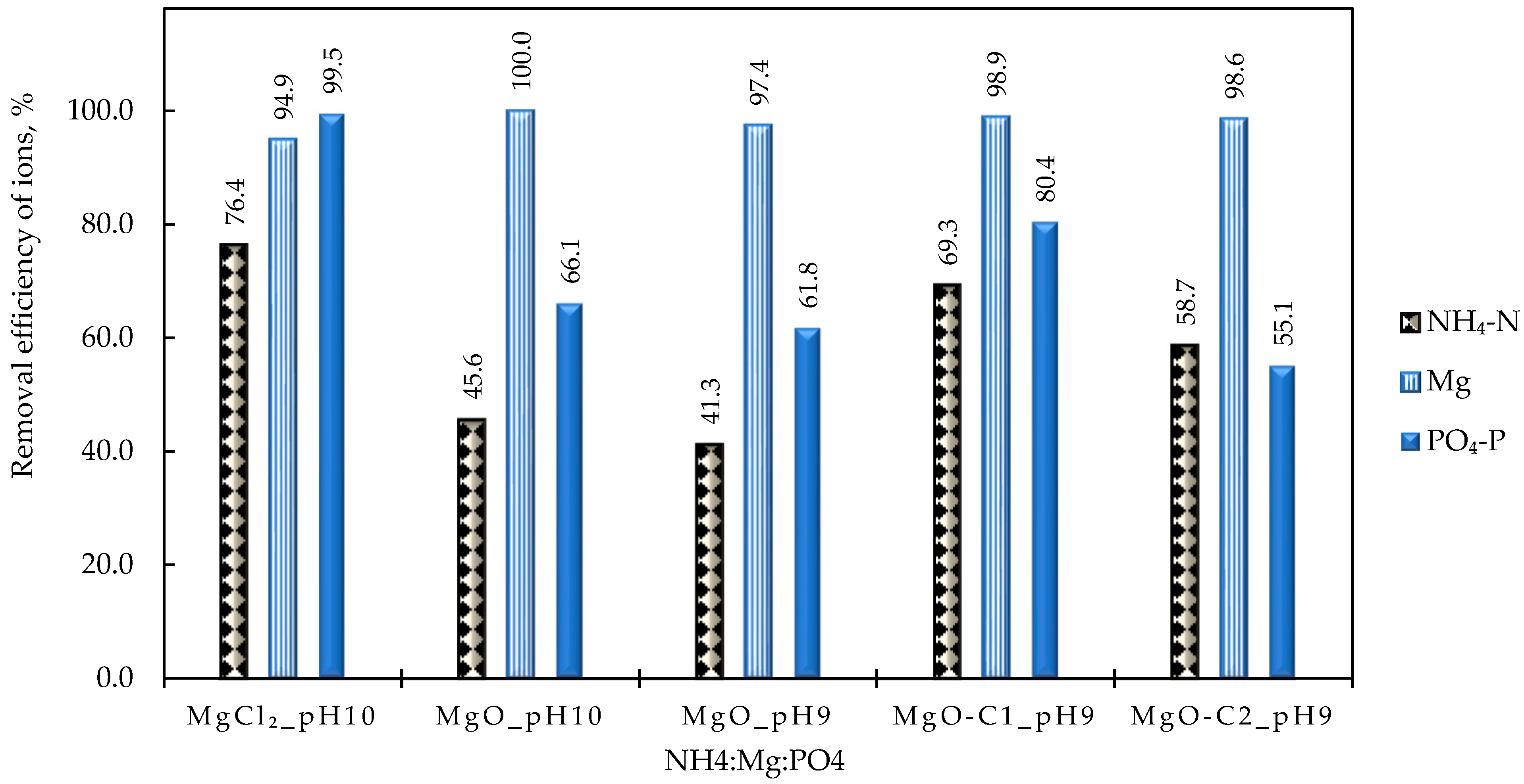
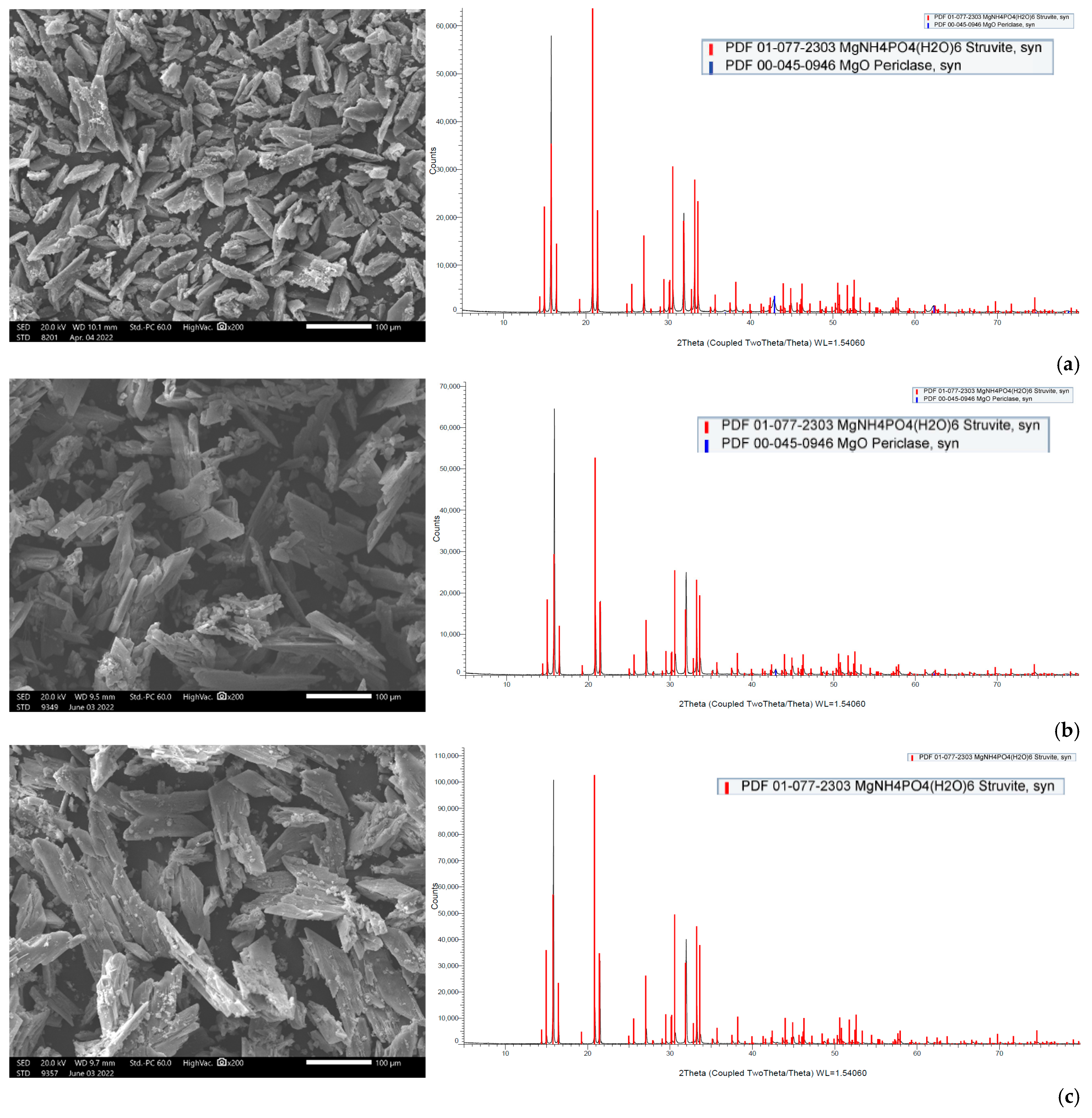
Particular attention was paid to the parametric study of struvite precipitation of the NF concentrate stream, as it contains valuable nutrients, which are the main target of this work. Therefore, a systematic investigation of the key operating parameters for higher precipitation efficiency was carried out. In all cases, the addition of a PO43− and Mg2+ source was necessary due to the excess of NH4+ in the liquid digestate. Magnesium chloride proved to be more effective, even though the addition of NaOH was necessary to adjust the pH. The increased pH values favor the formation of struvite compared to other phosphate precipitates and contribute to increased Mg2+ binding. Thus, pH 10 was selected for treatment of the specific NF concentrate, taking into account the operating costs. The concentration of the three building blocks of struvite (NH4+, Mg2+, PO43−) in the supernatant remains almost constant 30 min after the start of struvite precipitation and over a period of approximately 24 h. Therefore, it can be concluded that the ion complexation for struvite formation occurs quite quickly, and chemical precipitation is practically completed within 30 min. Drying the precipitate at high temperature (105 °C) leads to the dissociation of struvite, as confirmed through XRD analysis. In contrast, at lower drying temperatures (25 °C and 40 °C), the crystalline form of struvite was clearly identified. Therefore, it is suggested that the precipitate should be dried at ambient temperature. To improve ammonium removal through struvite formation, the addition of Mg2+ and PO43− salts in excess is necessary. A near-optimal ΝH4+:Mg2+:PO43− molar ratio of 1:1.5:1.5 was determined experimentally.
Under the aforementioned near-optimal operating conditions, the following removal efficiencies were achieved: 76.4% for NH
4+, 94.9% for Mg
2+, and 99.5% for PO
43−. The mass of dry struvite produced was ~11.7 g/L of the NF concentrate’s volume, consisting of ~3%
w/
w ΝH
4-N, ~11% Mg
2+, and 12%
w/
w PO
4−P. Although the concentration of nutrients in the struvite precipitate does not meet the minimum content specified in the EU regulation for commercial use [
7], it could be utilized in the production of fertilizers or directly on selected crops, depending on the nutrient content and ratio. The reproducibility of the results for struvite recovery obtained in the laboratory and on a larger scale is considered satisfactory for future applications on a pilot/industrial scale. Furthermore, because the recovered struvite originates from controlled dairy industry effluents, the potential fertilizer produced would be free of heavy metals, emerging contaminants, etc. Finally, the inorganic load of the supernatant solution/effluent resulting from the precipitation process is still quite high (despite the lower nutrient content) for direct disposal. Therefore, this effluent should be treated further before reuse or disposal.
Finally, it is emphasized that the compositional variability, the relatively high conductivity, and the alkalinity of the liquid digestate are important issues in the management of agro-industrial effluents by AD. Therefore, combined appropriate operations (such as those presented in this study) are necessary to overcome such issues and achieve a sustainable process for FLD valorization.