Porphyrin-Modified Polyethersulfone Ultrafiltration Membranes for Enhanced Bacterial Inactivation and Filtration Performance
Abstract
1. Introduction
2. Materials and Methods
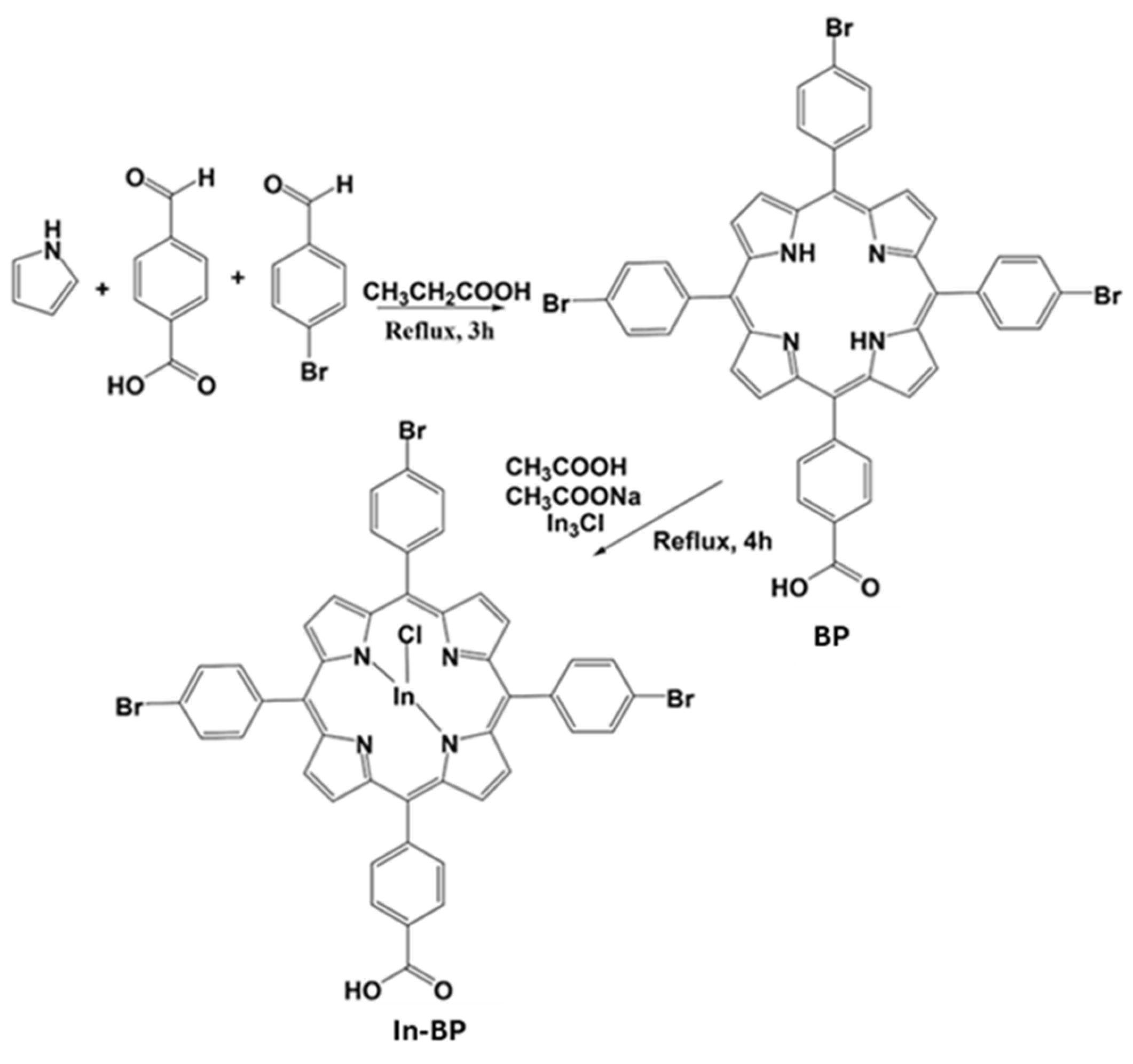
2.1. Synthesis of 5,10,15-Tris(5-bromophenyl)porphyrin (BP) as Well as Its Corresponding Indium Chloride Derivative ClIn(III), 5,10,15,Tris-(4-bromophenyl)-20-(4-carboxyphenyl)porphyrin (In-BP)
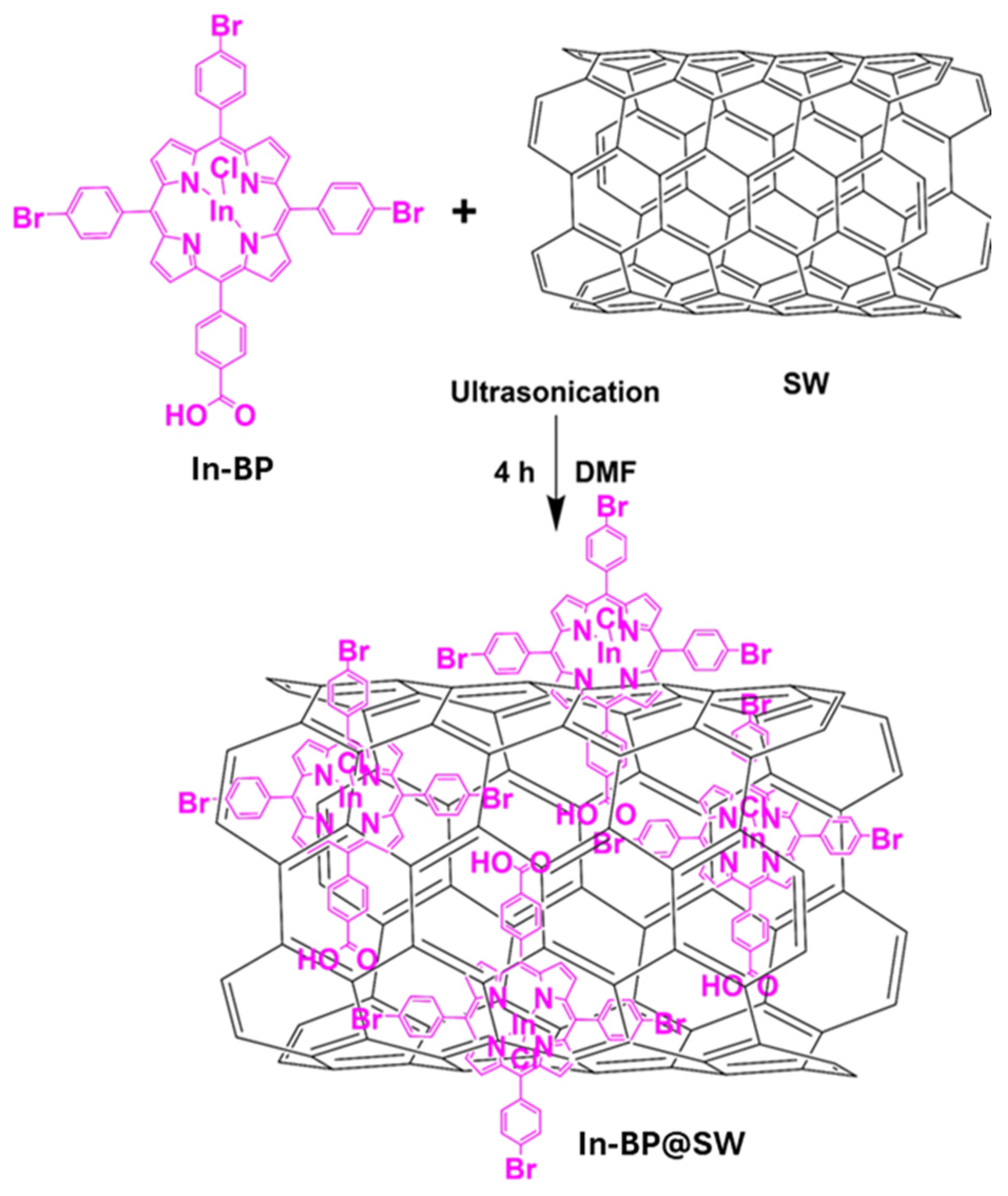
2.2. Conjugation 5,10,15-Tris(5-bromophenyl)porphyrin (BP) as Well as Its Corresponding Indium Chloride Derivative ClIn(III), 5,10,15,Tris-(4-bromophenyl)-20-(4-carboxyphenyl)porphyrin (In-BP) with Single-Walled Carbon Nanotubes (SWCNTs)
2.3. Fabrication of Membranes
2.4. Characterization Methods
2.5. Membrane Performance Assessment
2.6. Municipal Wastewater Sampling
2.7. Antimicrobial Photodynamic Inactivation (aPDI)
3. Results and Discussions
3.1. Nanofiller Characteristics
3.1.1. FTIR and Mass Spectrometry
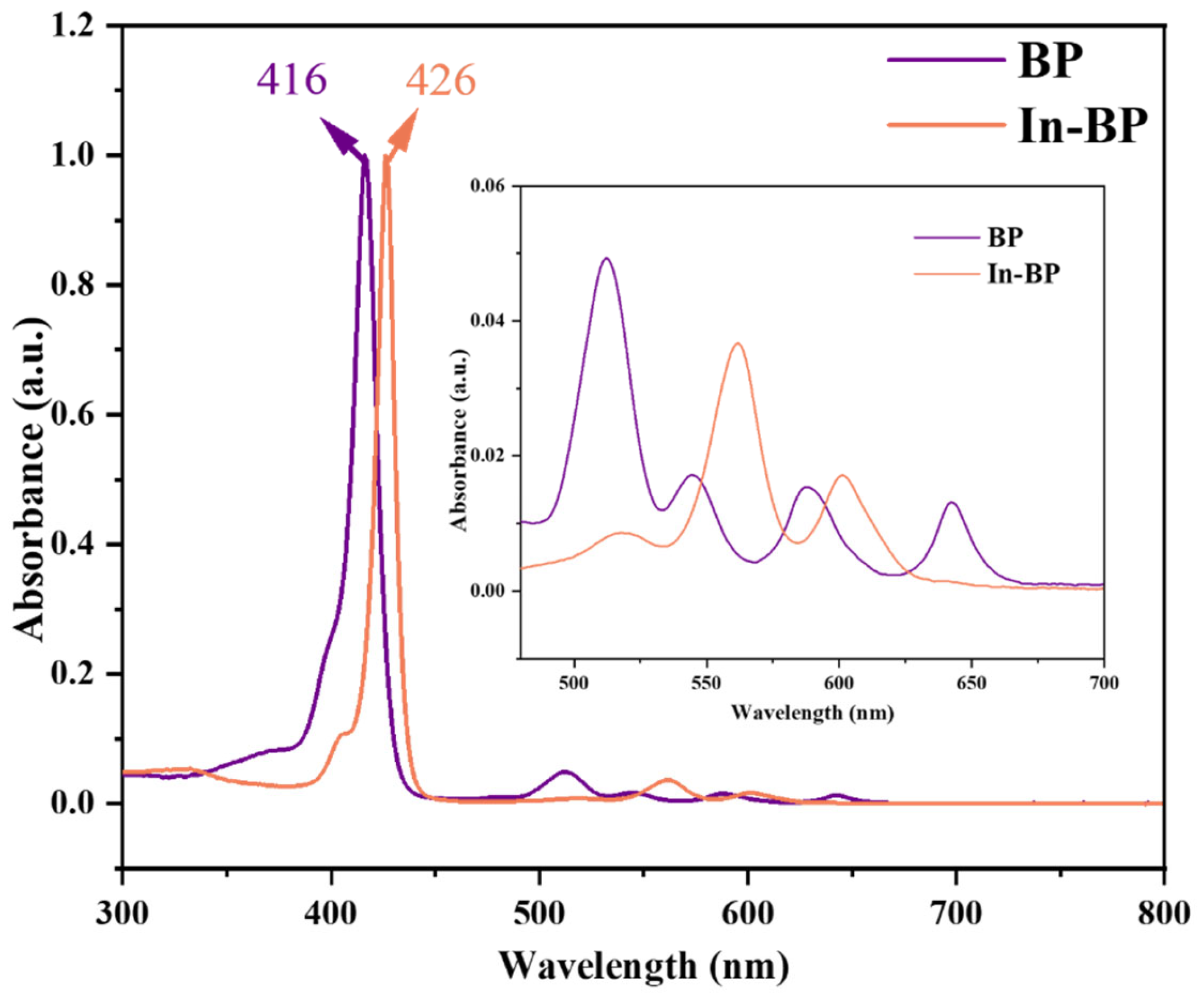
3.1.2. UV-Vis Spectrophotometry
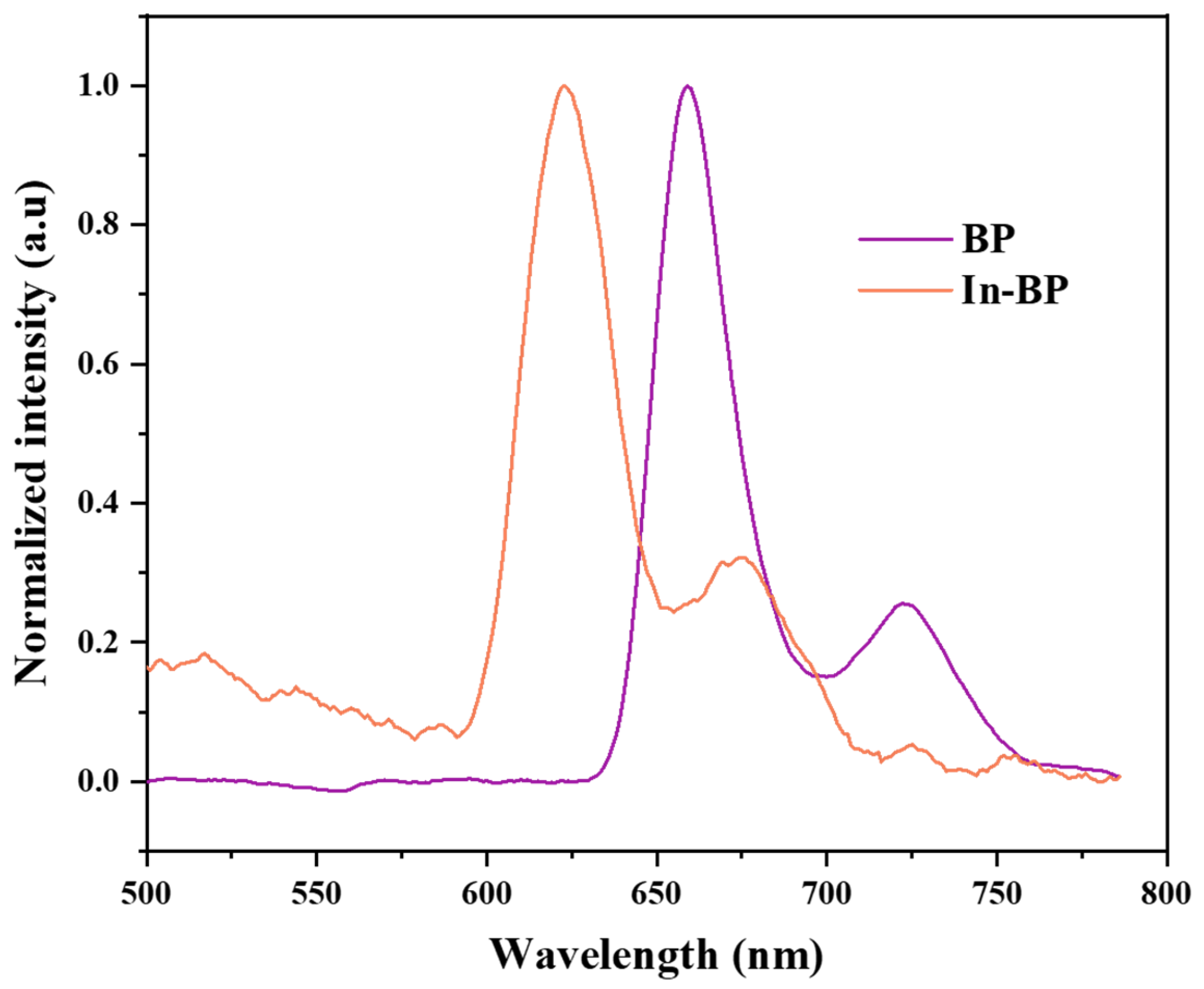
3.1.3. Fluorescence Emission
3.2. Membrane Characteristics
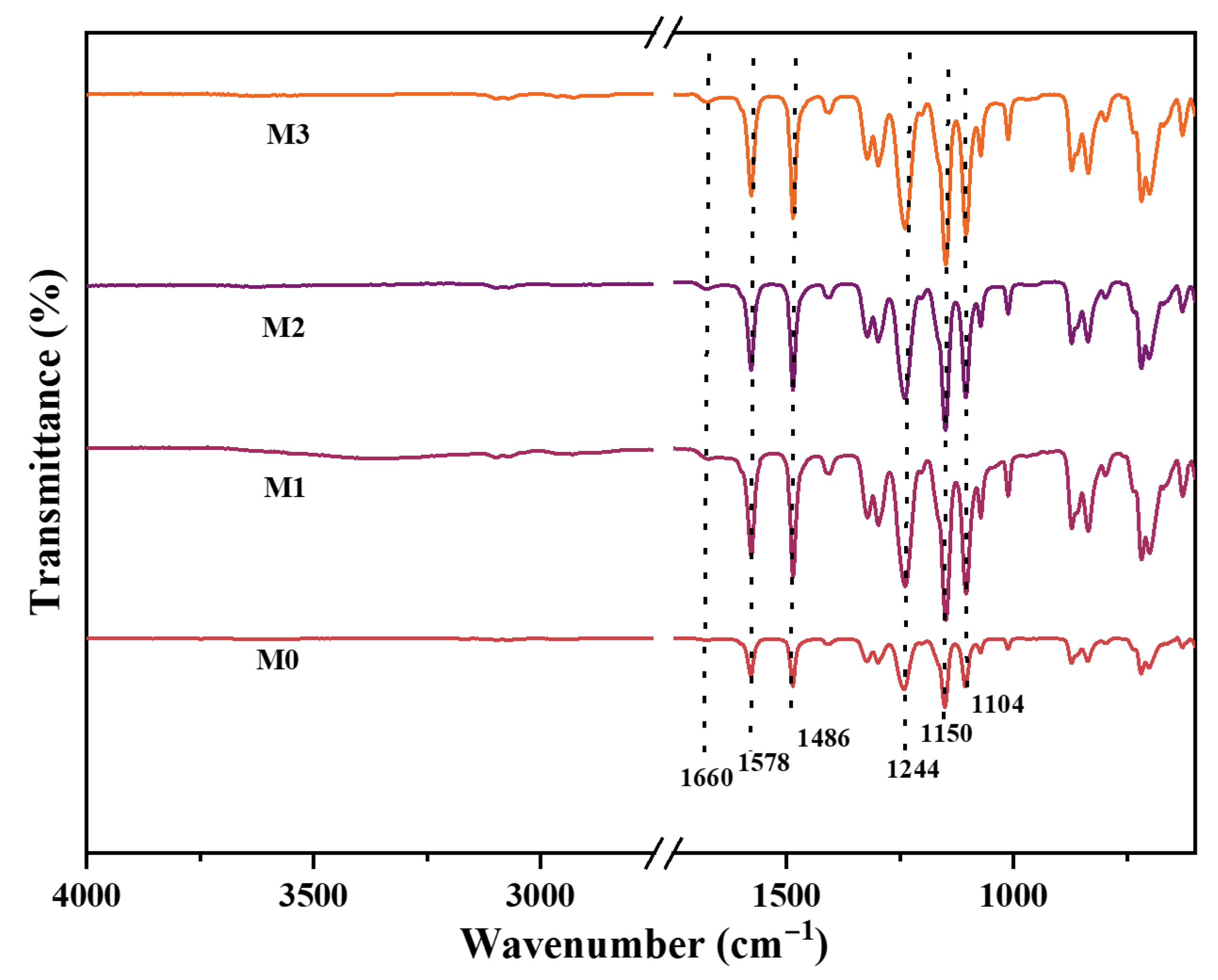
3.2.1. Fourier Transform Infrared Spectroscopy
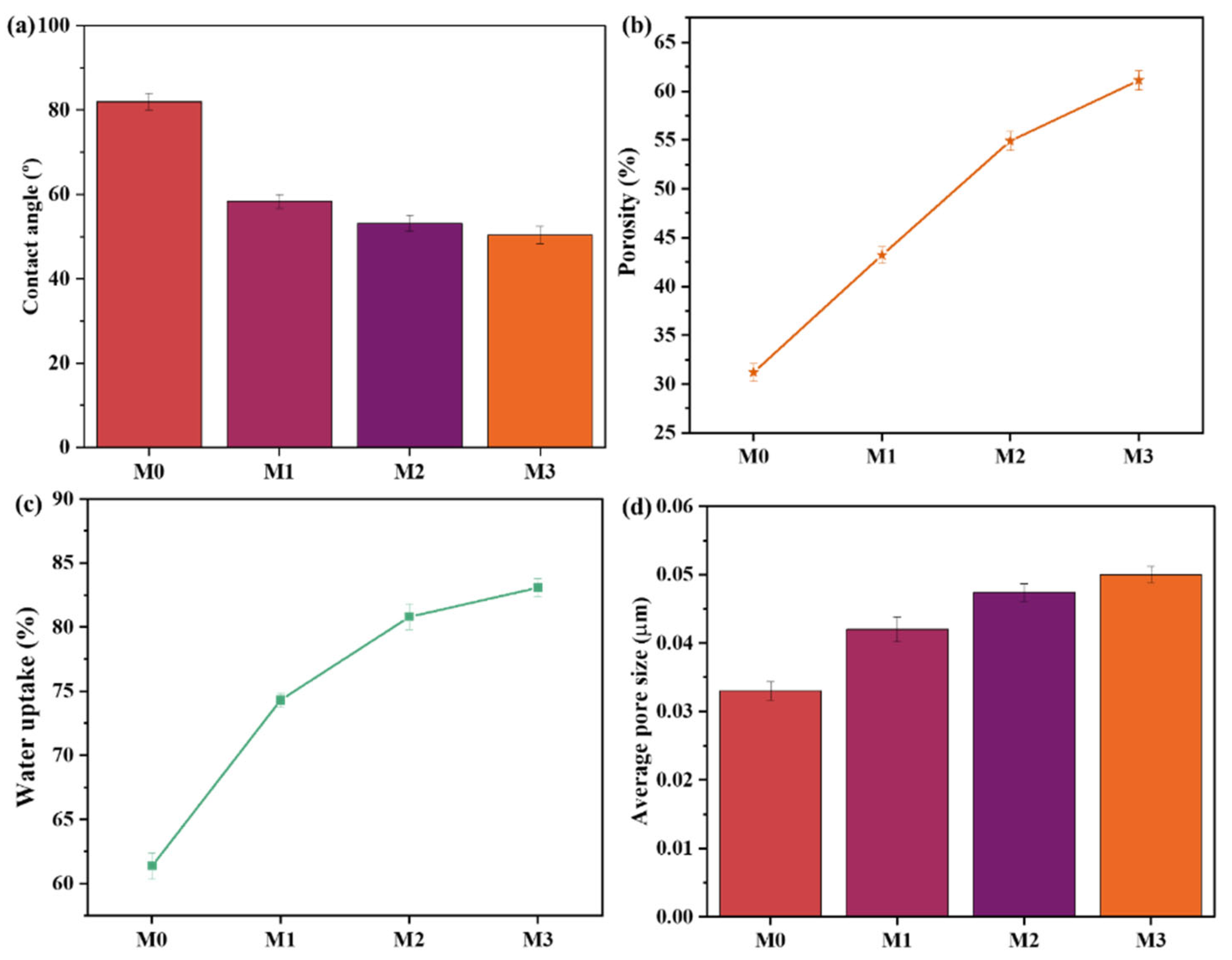
3.2.2. Water Contact Angle, Porosity, and Pore Size Measurements
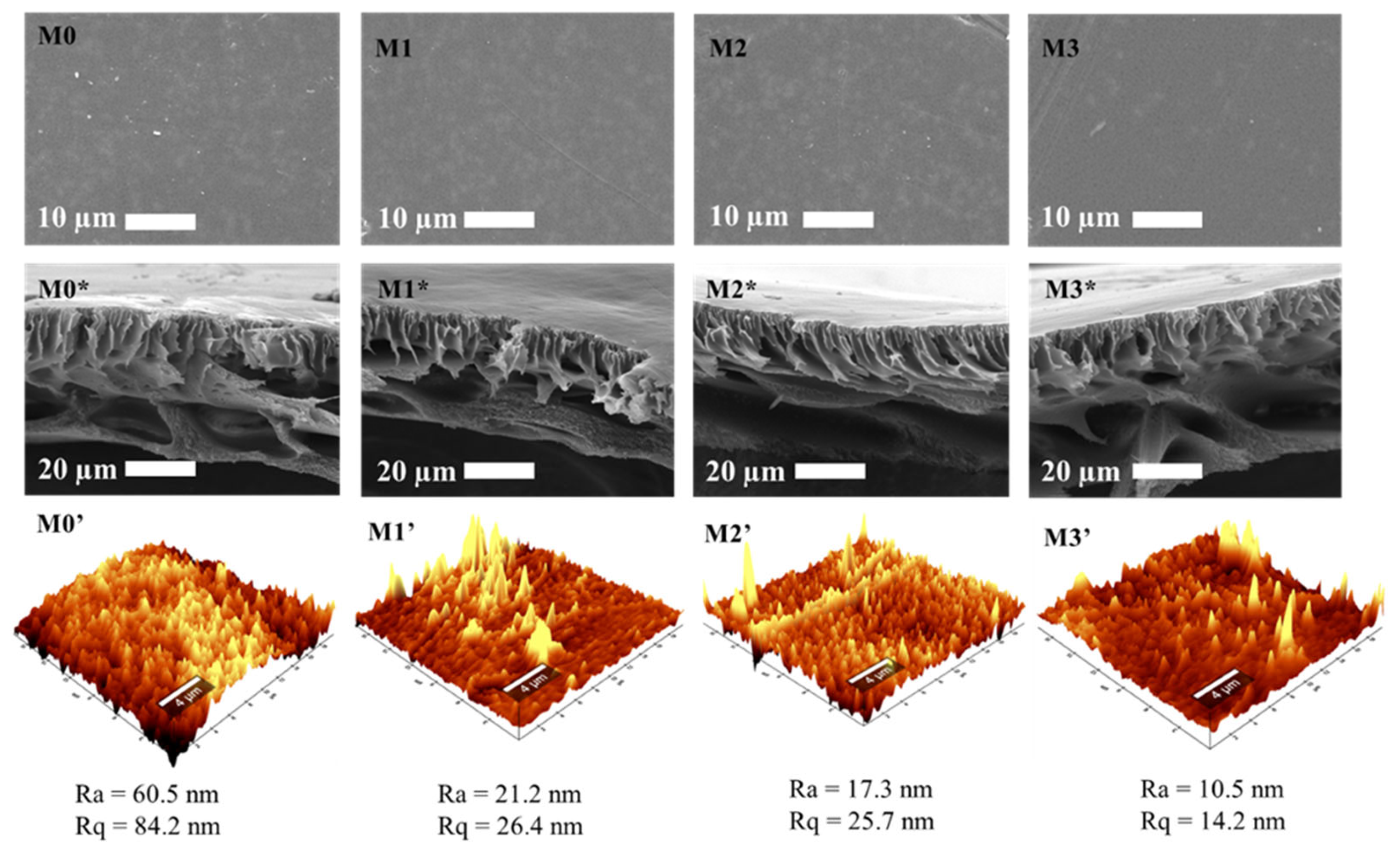
3.2.3. Scanning Electron Microscopy and Atomic Force Microscope (AFM)
3.3. Membrane Application
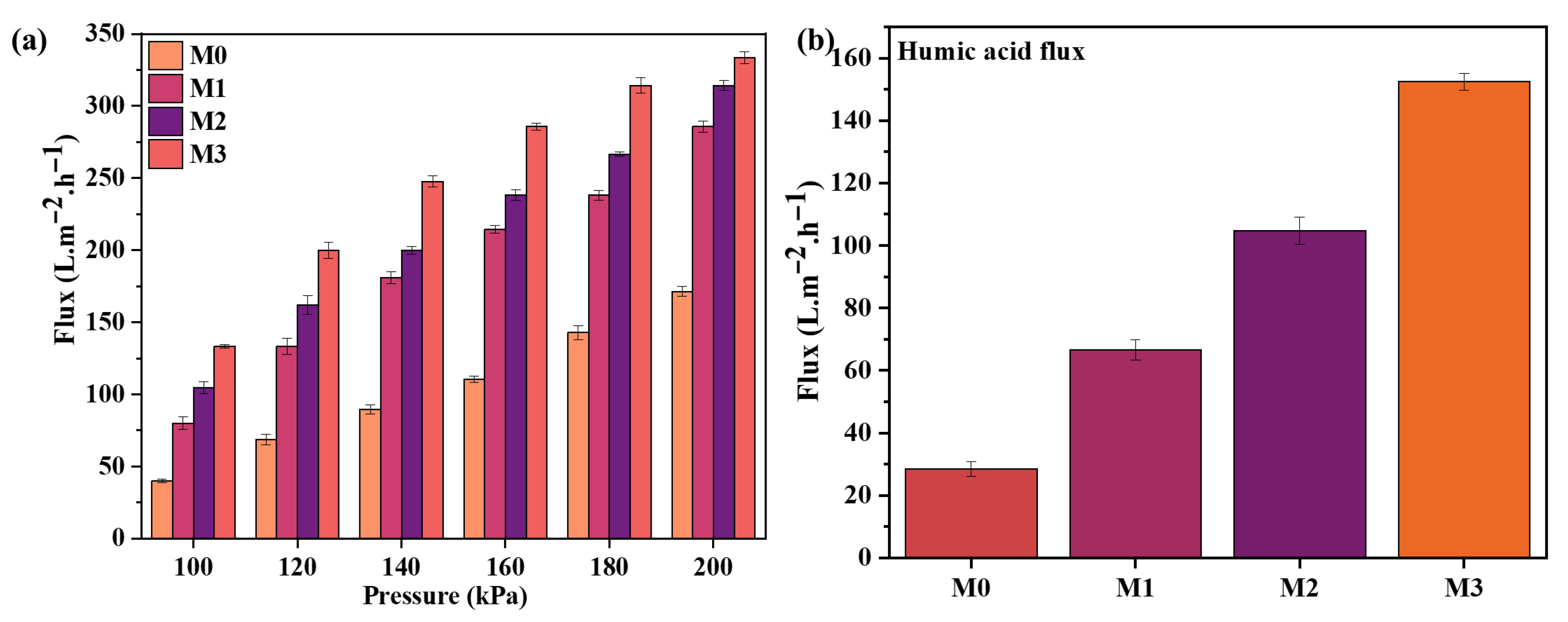
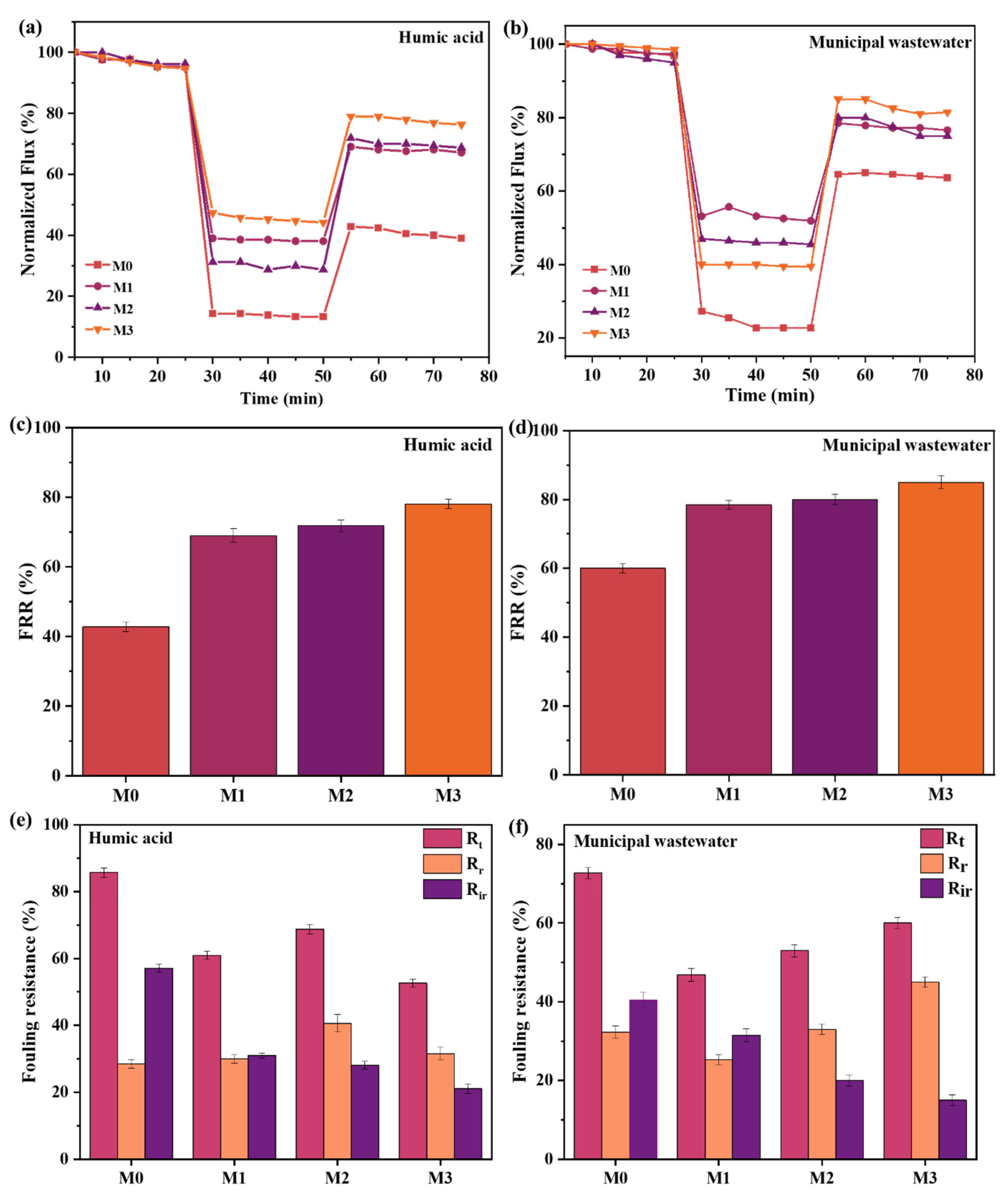
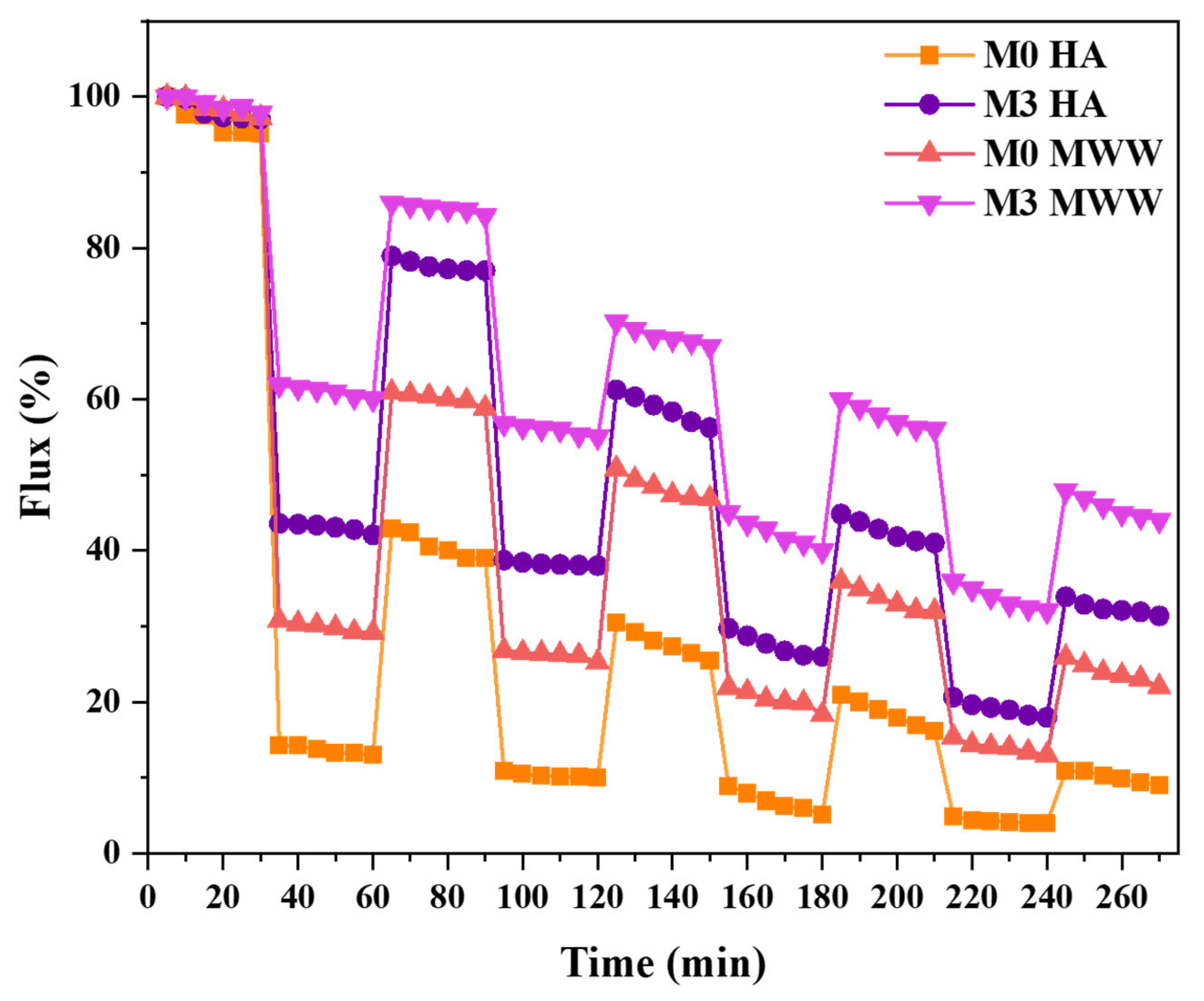
3.3.1. Flux
3.3.2. Fouling Resistance Reusability Potential of Membranes
3.3.3. Municipal Wastewater Characteristics and Quality
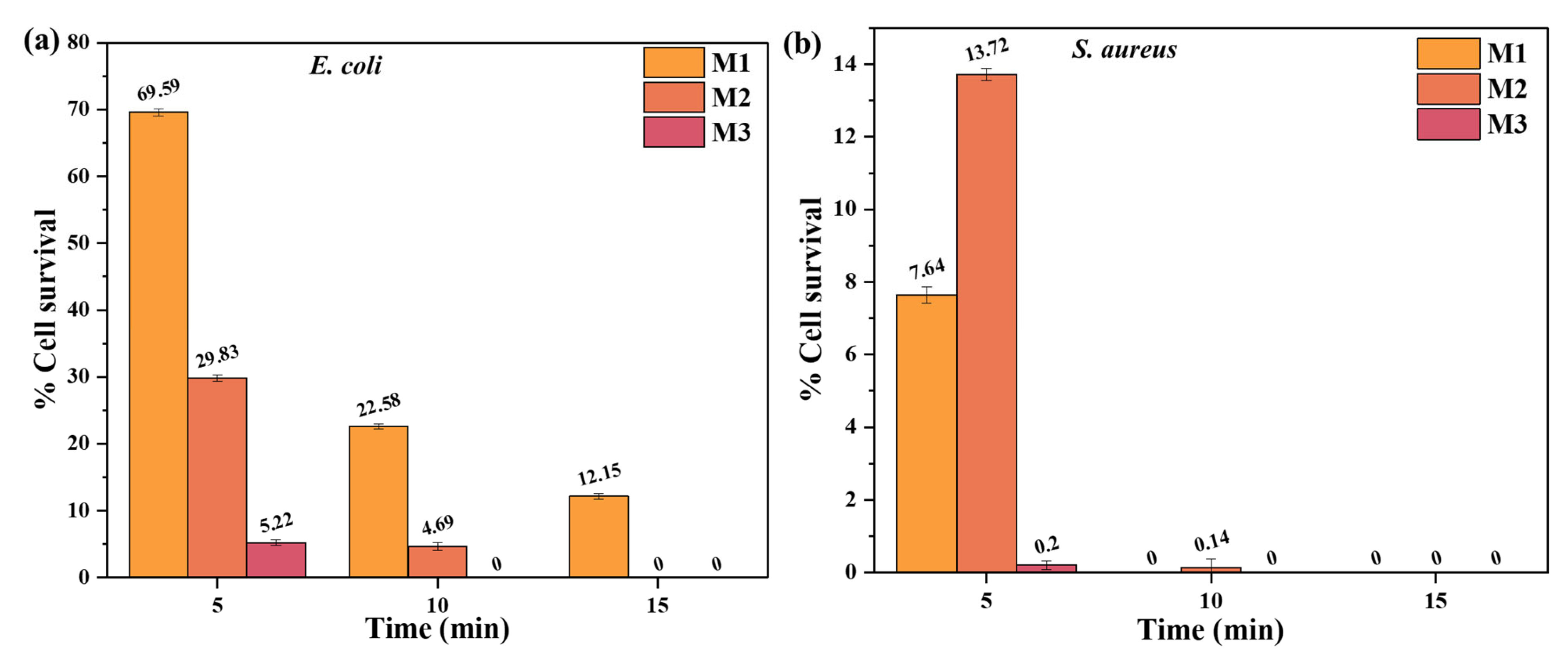
3.3.4. Bacterial Inactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PES | Porphyrin-modified polyethersulfone |
| UF | Ultrafiltration |
| CA | Contact angle |
| MWW | Municipal wastewater |
| FRRs | Recovery ratios |
| HA | Humic acid |
| ROS | Porphyrins generate reactive oxygen species |
| SWCNTs | Single-walled carbon nanotubes |
| ACS | Propionic acid |
| PB | 5,10,15-tris(5-bromophenyl)porphyrin |
| In-PB | 5,10,15,tris-(4-bromophenyl)-20-(4-carboxyphenyl)porphyrin Indium-5,10,15,20-tetracarboxy porphyrin (In-PB) |
| PB@SW | 5,10,15,20-tetracarboxy porphyrin@swcnts |
| In-PB@SW | Indium-5,10,15,20-tetracarboxy porphyrin@swcnts |
| FTIR | Fourier transform infrared |
| UV-Vis | Ultraviolet-visible |
| SEM | Scanning electron microscope |
References
- Sedlak, D. Water for All: Global Solutions for a Changing Climate; Yale University Press: New Haven, CT, USA, 2023; pp. 1–426. [Google Scholar]
- Chand, K.; Mehrotra, R.; Arya, U.; Bahuguna, D.; Tiwari, R. Water Security in Africa. In Water Scarcity Management: Enabling Technologies; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2025; pp. 39–52. [Google Scholar] [CrossRef]
- Amparo-Salcedo, M.; Pérez-Gimeno, A.; Navarro-Pedreño, J. Water Security Under Climate Change: Challenges and Solutions Across 43 Countries. Water 2025, 17, 633. [Google Scholar] [CrossRef]
- Shayo, G.M.; Elimbinzi, E.; Shao, G.N.; Fabian, C. Severity of Waterborne Diseases in Developing Countries and the Effectiveness of Ceramic Filters for Improving Water Quality. Bull. Natl. Res. Cent. Bull. 2023, 47, 113. [Google Scholar] [CrossRef]
- Amicizia, D.; Micale, R.T.; Pennati, B.M.; Zangrillo, F.; Iovine, M.; Lecini, E.; Marchini, F.; Lai, P.L.; Panatto, D. Burden of Typhoid Fever and Cholera: Similarities and Differences. Prevention Strategies for European Travelers to Endemic/Epidemic Areas. J. Prev. Med. Hyg. 2019, 60, E271–E285. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Ji, X.; Huang, J.; Teng, L.; Li, S.; Li, X.; Cai, W.; Chen, Z.; Lai, Y. Advances in Particulate Matter Filtration: Materials, Performance, and Application. Green Energy Environ. 2023, 8, 673–697. [Google Scholar] [CrossRef]
- Awad, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering 2021, 5, 34. [Google Scholar] [CrossRef]
- Alenazi, R.A.; Alsohaimi, I.H.; El-Aassar, M.R.; El-Ossaily, Y.A.; Alenezy, E.K.; Alanazi, S.J.F.; Alshahrani, A.A.; Alanazi, A.H.; Aldawsari, A.M.; Hassan, H.M.A. Comparative Analysis of High-Performance UF Membranes with Sulfonated Polyaniline: Improving Hydrophilicity and Antifouling Capabilities for Water Purification. Sep. Purif. Technol. 2025, 353, 128409. [Google Scholar] [CrossRef]
- Güneş-Durak, S.; Acarer-Arat, S.; Tüfekci, M.; Pir, İ.; Üstkaya, Z.; Öz, N.; Tüfekci, N. Mechanical Enhancement and Water Treatment Efficiency of Nanocomposite PES Membranes: A Study on Akçay Dam Water Filtration Application. ACS Omega 2024, 9, 31556–31568. [Google Scholar] [CrossRef]
- Nasir, A.M.; Adam, M.R.; Mohamad Kamal, S.N.E.A.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Aziz, F.; Yusof, N.; Bilad, M.R.; Mohamud, R.; et al. A Review of the Potential of Conventional and Advanced Membrane Technology in the Removal of Pathogens from Wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar] [CrossRef]
- Su, X.; Feng, X.; Wang, M.; Song, Z.; Dong, W.; Li, X.; Ren, N.; Sun, F. Temporal Dynamic of Biofouling on the Ultrafiltration Membrane for Wastewater Reclamation and Strategy for Biofouling Pertinence Mitigation. J. Memb. Sci. 2023, 687, 122053. [Google Scholar] [CrossRef]
- Tong, C.Y.; Chang, Y.S.; Ooi, B.S.; Chan, D.J.C. Physico-Chemistry and Adhesion Kinetics of Algal Biofilm on Polyethersulfone (PES) Membrane with Different Surface Wettability. J. Environ. Chem. Eng. 2021, 9, 106531. [Google Scholar] [CrossRef]
- Chauke, N.M.; Munonde, T.S.; Mketo, N. A Critical Review of the Anti-Biofouling Properties of Biogenic-Based Silver Nanoparticles (AgNPs) Embedded on Polymer Membranes for Wastewater Treatment. J. Ind. Eng. Chem. 2025, 149, 209–232. [Google Scholar] [CrossRef]
- Ahmed, N.; Suhaimi, A.; Masood, A.; Mahmoudi, E.; Siow, K.S.; Mohd Razip Wee, M.F. Antimicrobial Property of Polyethersulfone (PES) Membrane by Plasma Copolymerization of TEOS and Oxazoline for Organic Dyes Filtration. Results Eng. 2023, 19, 101339. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient Removal of Water Bacteria and Viruses Using Electrospun Nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef]
- Monteiro, C.J.P.; Faustino, M.A.F.; Serpa, C. Porphyrin-Based Compounds: Synthesis and Application. Molecules 2023, 28, 7108. [Google Scholar] [CrossRef]
- Balu, K.; Kaliyamoorthy, S.; Durai, M.; Aguiar, A.; Sobral, M.C.M.; Muthuvel, I.; Kumaravel, S.; Avula, B.; Sobral, A.J.F.N.; Ahn, Y.H. Porphyrins and ZnO Hybrid Semiconductor Materials: A Review. Inorg. Chem. Commun. 2023, 154, 110973. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Wang, Z.; Chen, F.; Liao, X.; Hu, X.; Dong, L. Sodium Copper Chlorophyll Mediated Photodynamic Treatment Inactivates Escherichia Coli via Oxidative Damage. Food Res. Int. 2022, 157, 111472. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Soy, R.; Mgidlana, S.; Mack, J.; Nyokong, T. Light-Driven Antimicrobial Therapy of Palladium Porphyrins and Their Chitosan Immobilization Derivatives and Their Photophysical-Chemical Properties. Dye. Pigment. 2022, 203, 110313. [Google Scholar] [CrossRef]
- Collen Makola, L.; Nyokong, T.; Amuhaya, E.K. Impact of Axial Ligation on Photophysical and Photodynamic Antimicrobial Properties of Indium (III) Methylsulfanylphenyl Porphyrin Complexes Linked to Silver-Capped Copper Ferrite Magnetic Nanoparticles. Polyhedron 2021, 193, 114882. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Ren, X.; Yao, S.; Fan, W.; Nafady, A.; Al-Enizi, A.M.; Ma, S. A Continuous Porous Porphyrinic Polymer Thin-Film Composite Membrane for Anti-Biofouling and Molecular Sieving. J. Mater. Chem. A 2024, 12, 26170–26177. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial Photodynamic Therapy: Single-Walled Carbon Nanotube (SWCNT)-Porphyrin Conjugate for Visible Light Mediated Inactivation of Staphylococcus Aureus. Colloids Surfaces B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef]
- Magaela, N.B.; Ledwaba, M.M.; Malomane, N.; Mack, J.; Nyokong, T.; Managa, M. Photodynamic Inactivation of Staphylococcus Aureus and Escherichia Coli with Free-Base and Indium(III) 5,10,15,20-Tetrakis(4-Pyridyl) Porphyrin Adsorbed onto Single-Walled Carbon Nanotubes. J. Porphyr. Phthalocyanines 2024, 28, 260–271. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the Preparation of Metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Mafukidze, D.; Britton, J.; Nyokong, T. Photophysical Studies of Meso-Tetrakis(4-Nitrophenyl) and Meso-Tetrakis(4-Sulfophenyl) Gallium Porphyrins Loaded into Pluronic F127 Polymeric Micelles. J. Photochem. Photobiol. A Chem. 2017, 348, 179–187. [Google Scholar] [CrossRef]
- Murakami, H.; Nomura, T.; Nakashima, N. Noncovalent Porphyrin-Functionalized Single-Walled Carbon Nanotubes in Solution and the Formation of Porphyrin–Nanotube Nanocomposites. Chem. Phys. Lett. 2003, 378, 481–485. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Sønderskov, S.M.; Xia, D.; Wu, X.; Liang, C.; Dong, M. Tannic Acid-Functionalized 3D Porous Nanofiber Sponge for Antibiotic-Free Wound Healing with Enhanced Hemostasis, Antibacterial, and Antioxidant Properties. J. Nanobiotechnol. 2023, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Characterization of Surface-Modified Porous PVDF Hollow Fibers for Refinery Wastewater Treatment Using Microscopic Observation. Desalination 2011, 283, 206–213. [Google Scholar] [CrossRef]
- Shafiei, M.; Hajian, M. Preparation and Characterization of Polyvinyl Butyral/Zeolitic Imidazolate Framework-8 Nanocomposite Ultrafiltration Membranes to Improve Water Flux. Adv. Polym. Technol. 2018, 37, 3607–3618. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High Flux and Fouling Resistant Flat Sheet Polyethersulfone Membranes Incorporated with Graphene Oxide for Ultrafiltration Applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A.; Aljundi, I.H.; AbuMousa, R.A. Facile Fabrication of Graphitic Carbon Nitride Nanosheets and Its Integrated Polyamide Hyper-Cross-Linked TFC Nanofiltration Membrane with Intrinsic Molecular Porosity for Salts and Organic Pollutant Rejection from Water. J. Mater. Res. Technol. 2021, 15, 6319–6328. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Albayati, T.M.; Rashid, K.T.; Saady, N.M.C.; Zendehboudi, S. Green Nanoparticles Blending with Polyacrylonitrile Ultrafiltration Membrane for Antifouling Oily Wastewater Treatment. Sep. Purif. Technol. 2025, 353, 128256. [Google Scholar] [CrossRef]
- Esmaili, Z.; Sadeghian, Z.; Ashrafizadeh, S.N. Tailoring of BiVO4 Morphology for Efficient Antifouling of Visible-Light-Driven Photocatalytic Ceramic Membranes for Oily Wastewater Treatment. J. Water Process Eng. 2024, 67, 106145. [Google Scholar] [CrossRef]
- Chakansin, C.; Yostaworakul, J.; Warin, C.; Kulthong, K.; Boonrungsiman, S. Resazurin Rapid Screening for Antibacterial Activities of Organic and Inorganic Nanoparticles: Potential, Limitations and Precautions. Anal. Biochem. 2022, 637, 114449. [Google Scholar] [CrossRef]
- Nobatana, V.; Oyim, J.; Nwahara, N.; Sindelo, A.; Nyokong, T. The Photodynamic Anti-Cancer and Anti-Bacterial Behaviour of Meso-Substituted Trans-A2B2 Porphyrin Conjugated Silica-gold Nanoparticles. Inorganica Chim. Acta 2025, 579, 122584. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Daza, M.C.; Páez-Mozo, E.A.; Martínez O., F.; Guedes, C.L.B.; Di Mauro, E. Visible Light Singlet Oxygen Production with Tetra(4-Carboxyphenyl)Porphyrin/SiO2. J. Photochem. Photobiol. A Chem. 2013, 259, 47–52. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Daza, M.C.; Martínez, F.; Páez-Mozo, E.A.; Guedes, C.L.B.; Di Mauro, E. Visible Light Superoxide Radical Anion Generation by Tetra(4-Carboxyphenyl)Porphyrin/TiO2: EPR Characterization. J. Photochem. Photobiol. A Chem. 2010, 215, 172–178. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, D.; Wu, Z.; Wan, J.; Zheng, X.; Yu, L.; Phillips, D.L. The Visible Light Degradation Activity and the Photocatalytic Mechanism of Tetra(4-Carboxyphenyl) Porphyrin Sensitized TiO2. Mater. Res. Bull. 2014, 57, 311–319. [Google Scholar] [CrossRef]
- Choi, W.; Ingole, P.G.; Li, H.; Kim, J.H.; Lee, H.K.; Baek, I.H. Preparation of Facilitated Transport Hollow Fiber Membrane for Gas Separation Using Cobalt Tetraphenylporphyrin Complex as a Coating Material. J. Clean. Prod. 2016, 133, 1008–1016. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Pan, B. Potential of Carbon Nanotubes in Water Treatment. Recent Prog. Carbon Nanotub. Res. 2012, 201110, 51332. [Google Scholar]
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes. Molecules 2020, 25, 691. [Google Scholar] [CrossRef]
- Alsohaimi, I.H. Comparative Study of PS and PES and Their Sulfonated Forms in Antifouling Behavior and Rejection Efficiency. J. King Saud Univ. Sci. 2024, 36, 103576. [Google Scholar] [CrossRef]
- Bai, L.; Ding, J.; Wang, H.; Ren, N.; Li, G.; Liang, H. High-Performance Nanofiltration Membranes with a Sandwiched Layer and a Surface Layer for Desalination and Environmental Pollutant Removal. Sci. Total Environ. 2020, 743, 140766. [Google Scholar] [CrossRef] [PubMed]
- Sisay, E.J.; Al-Tayawi, A.N.; László, Z.; Kertész, S. Recent Advances in Organic Fouling Control and Mitigation Strategies in Membrane Separation Processes: A Review. Sustainability 2023, 15, 13389. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Fouling in Reverse Osmosis Membranes: Monitoring, Characterization, Mitigation Strategies and Future Directions. Heliyon 2023, 9, e14908. [Google Scholar] [CrossRef]
- Mahmud, N.A.C.; Saufi, S.M.; Abu Seman, M.N.; Takriff, M.S.; Ang, W.L. Effect of Cellulose Nanocrystals and Carboxylated Multiwalled Carbon Nanotubes on Performance of Polyethersulfone Membrane for Humic Acid Removal. Chem. Eng. Res. Des. 2024, 201, 185–193. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Li, S.; Wang, Z.; Wen, F.; Li, C.; Matsuyama, H.; Hu, S. Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method. Membranes 2020, 10, 66. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Moutloali, R.M. Antifouling Properties of Cu(Tpa)@GO/PES Composite Membranes and Selective Dye Rejection. J. Memb. Sci. 2018, 554, 195–210. [Google Scholar] [CrossRef]
- oulad, F.; Zinadini, S.; Zinatizadeh, A.A.; Derakhshan, A.A. Novel (4,4-Diaminodiphenyl Sulfone Coupling Modified PES/PES) Mixed Matrix Nanofiltration Membranes with High Permeability and Anti-Fouling Property. Sep. Purif. Technol. 2020, 236, 116292. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Pang, W.Y.; Mohd Shafie, Z.M.H.; Zaulkiflee, N.D. PES/PVP/TiO2 Mixed Matrix Hollow Fiber Membrane with Antifouling Properties for Humic Acid Removal. J. Water Process Eng. 2019, 31, 100827. [Google Scholar] [CrossRef]
- Ouda, M.; Hai, A.; Krishnamoorthy, R.; Govindan, B.; Othman, I.; Kui, C.C.; Choi, M.Y.; Hasan, S.W.; Banat, F. Surface Tuned Polyethersulfone Membrane Using an Iron Oxide Functionalized Halloysite Nanocomposite for Enhanced Humic Acid Removal. Environ. Res. 2022, 204, 112113. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Pan, Y.; Lu, X.; Li, Y.Y.; Zhang, Z.; Niu, C.; Kumar, G.; Kobayashi, T.; Zhao, Y.; Xu, K. Anaerobic Membrane Bioreactor towards Biowaste Biorefinery and Chemical Energy Harvest: Recent Progress, Membrane Fouling and Future Perspectives. Renew. Sustain. Energy Rev. 2019, 115, 109392. [Google Scholar] [CrossRef]
- Bahamonde Soria, R.; Luis, P. Antifouling Membranes for Polluted Solvents Treatment. In Current Trends and Future Developments on (Bio-) Membranes: Membrane Technologies in Environmental Protection and Public Health: Challenges and Opportunities; Elsevier: Amsterdam, The Netherlands, 2023; pp. 295–334. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing Antifouling Capability of PES Membrane via Mixing with Various Types of Polymer Modified Multi-Walled Carbon Nanotube. J. Memb. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Chen, Y.; Zhang, H. A Comparison Study: The Different Impacts of Sodium Hypochlorite on PVDF and PSF Ultrafiltration (UF) Membranes. Water Res. 2017, 109, 227–236. [Google Scholar] [CrossRef]
- Fathanah, U.; Rosnelly, C.M.; Zuhra, Z.; Muchtar, S.; Razi, F.; Rinaldi, W.; Syamsuddin, Y. Integrated Approach to Elevating PES Membrane Performance with a Dynamic Silica and Chitosan Additive Duo. S. Afr. J. Chem. Eng. 2025, 53, 1–11. [Google Scholar] [CrossRef]
- Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation. Processes 2018, 6, 28. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.M.C.; Cornelissen, E.R.; Vrouwenvelder, J.S.; Verliefde, A.; van Loosdrecht, M.C.M.; Picioreanu, C. Cost of Fouling in Full-Scale Reverse Osmosis and Nanofiltration Installations in the Netherlands. Desalination 2021, 500, 114865. [Google Scholar] [CrossRef]
- Jashrapuria, K.; Singh, S.P. Zwitterionic Polymer Brush Functionalized Graphene Oxide Blended Polyethersulfone Membrane with Enhanced Performance and Anti-Biofouling Properties. J. Memb. Sci. 2023, 687, 122032. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Synergetic Effects of Dodecylamine-Functionalized Graphene Oxide Nanoparticles on Antifouling and Antibacterial Properties of Polysulfone Ultrafiltration Membranes. J. Water Process Eng. 2021, 42, 102120. [Google Scholar] [CrossRef]
- Jahani, Z.; Mosaffa, E.; Oroujzadeh, M.; Ghafuri, H. Performance Evaluation of Polyethersulfone Membranes Modified with Poly (Acrylic Acid-Co-N-Vinyl Pyrrolidone) Grafted Mesoporous Carbon Nitride for Effective Removal of Cadmium(II) from Wastewater. Polym. Adv. Technol. 2023, 34, 3803–3817. [Google Scholar] [CrossRef]
- Kamińska, G.; Marszałek, A. Advanced Treatment of Real Grey Water by SBR Followed by Ultrafiltration-Performance and Fouling Behavior. Water 2020, 12, 154. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Rafey, A.; Khalid, U.; Zafar, A.; Ameen, M.; Lima, E.C. Removal of Micropollutants from Municipal Wastewater Using Different Types of Activated Carbons. J. Environ. Manag. 2021, 278, 111302. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, F.S.; Koutchma, T.; Ferreira, E.H.R.; Walter, E.H.M.; Rosenthal, A. Resistance of Escherichia Coli, Salmonella Spp., and Listeria Monocytogenes in High and Low-Acidity Juices Processed by High Hydrostatic Pressure. Int. J. Food Microbiol. 2023, 395, 110189. [Google Scholar] [CrossRef] [PubMed]
- Sáfar, G.D.A.M.; Martins, D.C.D.S.; Defreitas-Silva, G.; Rebouças, J.S.; Idemori, Y.M.; Righi, A. Interactions of Porphyrins and Single Walled Carbon Nanotubes: A Fine Duet. Synth. Met. 2014, 193, 64–70. [Google Scholar] [CrossRef]
- Ehli, C.; Campidelli, S.; Brunetti, F.G.; Prato, M.; Guldi, D.M. Single-Wall Carbon Nanotube Porphyrin Nanoconjugates. J. Porphyr. Phthalocyanines 2007, 11, 442–447. [Google Scholar] [CrossRef]
| Membrane ID | PES (wt.%) | NMP (wt.%) | Nanofillers | |
|---|---|---|---|---|
| Nanofiller ID | Nanofiller (wt.%) | |||
| M0 | 18 | 82 | - | - |
| M1 | 18 | 81.75 | BP | 0.25 |
| M2 | 18 | 81.75 | In-BP | 0.25 |
| M3 | 18 | 81.75 | In-BP@SW | 0.25 |
| % Bacterial Viability | ||||||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | |||||
| 5 | 10 | 15 | 5 | 10 | 15 | |
| M1 | 69.59 | 29.83 | 5.22 | 7.64 | 0 | 0 |
| M2 | 22.58 | 4.69 | 0 | 13.72 | 0.14 | 0 |
| M3 | 12.15 | 0 | 0 | 0.20 | 0 | 0 |
| Log Reduction | ||
|---|---|---|
| S. aureus | E. coli | |
| Light | Light | |
| M1 | 6.88 | 5.74 |
| M2 | 7.38 | 6.76 |
| M3 | 9.79 | 8.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matebese, F.; Malomane, N.; Motloutsi, M.L.; Moutloali, R.M.; Managa, M. Porphyrin-Modified Polyethersulfone Ultrafiltration Membranes for Enhanced Bacterial Inactivation and Filtration Performance. Membranes 2025, 15, 239. https://doi.org/10.3390/membranes15080239
Matebese F, Malomane N, Motloutsi ML, Moutloali RM, Managa M. Porphyrin-Modified Polyethersulfone Ultrafiltration Membranes for Enhanced Bacterial Inactivation and Filtration Performance. Membranes. 2025; 15(8):239. https://doi.org/10.3390/membranes15080239
Chicago/Turabian StyleMatebese, Funeka, Nonkululeko Malomane, Meladi L. Motloutsi, Richard M. Moutloali, and Muthumuni Managa. 2025. "Porphyrin-Modified Polyethersulfone Ultrafiltration Membranes for Enhanced Bacterial Inactivation and Filtration Performance" Membranes 15, no. 8: 239. https://doi.org/10.3390/membranes15080239
APA StyleMatebese, F., Malomane, N., Motloutsi, M. L., Moutloali, R. M., & Managa, M. (2025). Porphyrin-Modified Polyethersulfone Ultrafiltration Membranes for Enhanced Bacterial Inactivation and Filtration Performance. Membranes, 15(8), 239. https://doi.org/10.3390/membranes15080239







