Water Recovery from Laundry Wastewater by Integrated Purification Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater
Characterization Methods
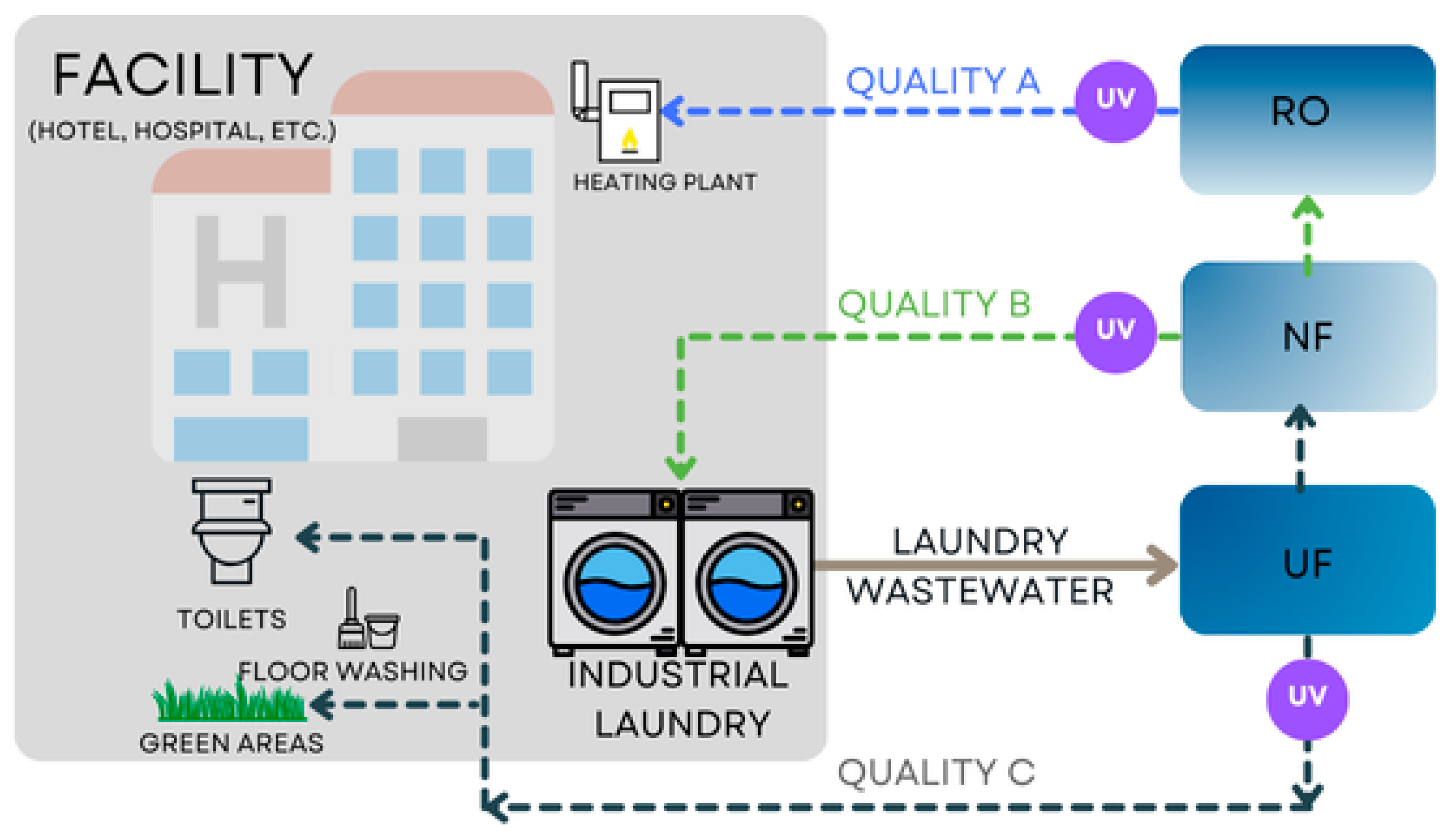
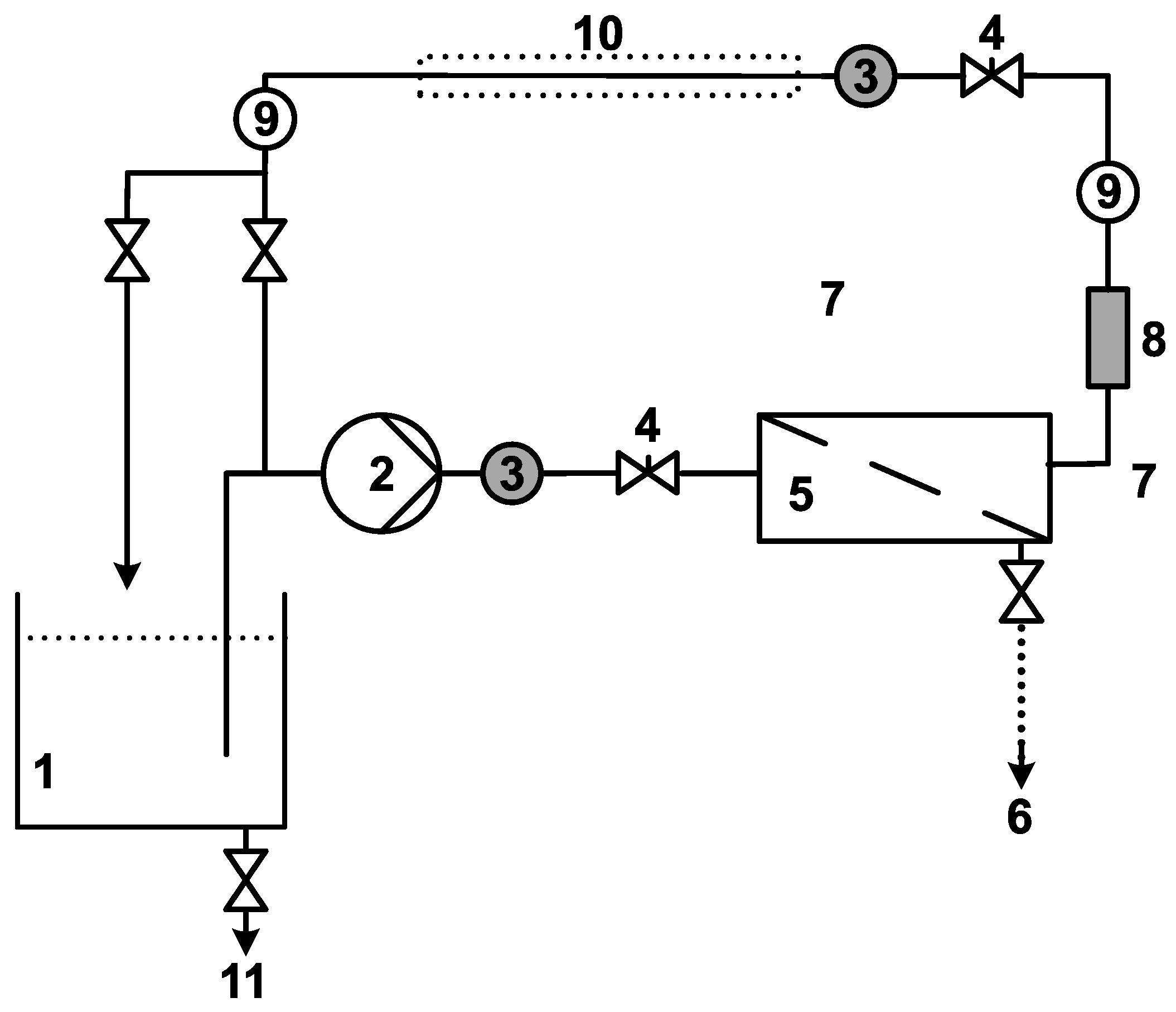
2.2. Set-Up
2.3. Membrane Modules
2.4. Purification Systems
3. Results and Discussion
3.1. Wastewater Characteristics
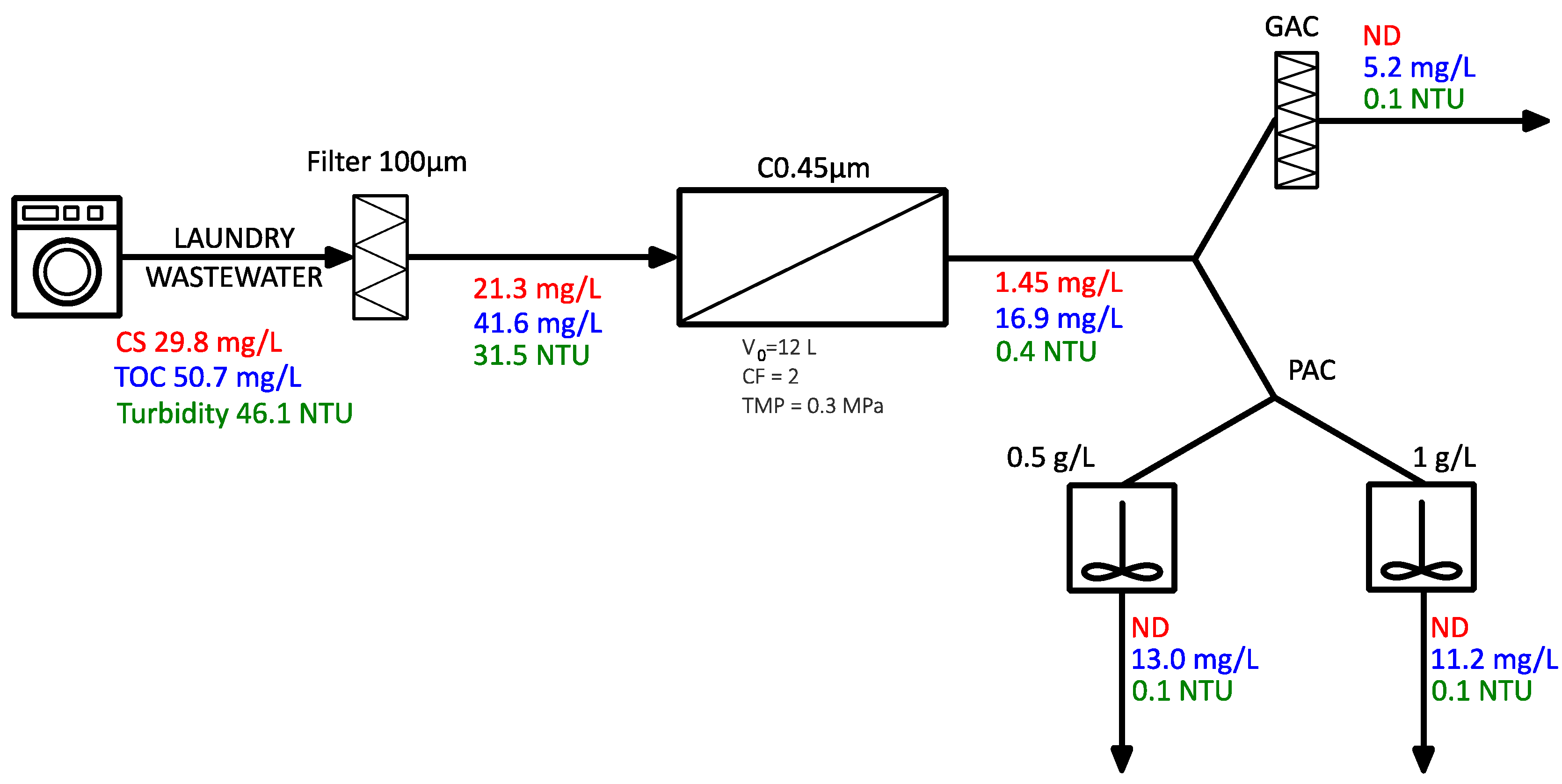
3.2. Filtration
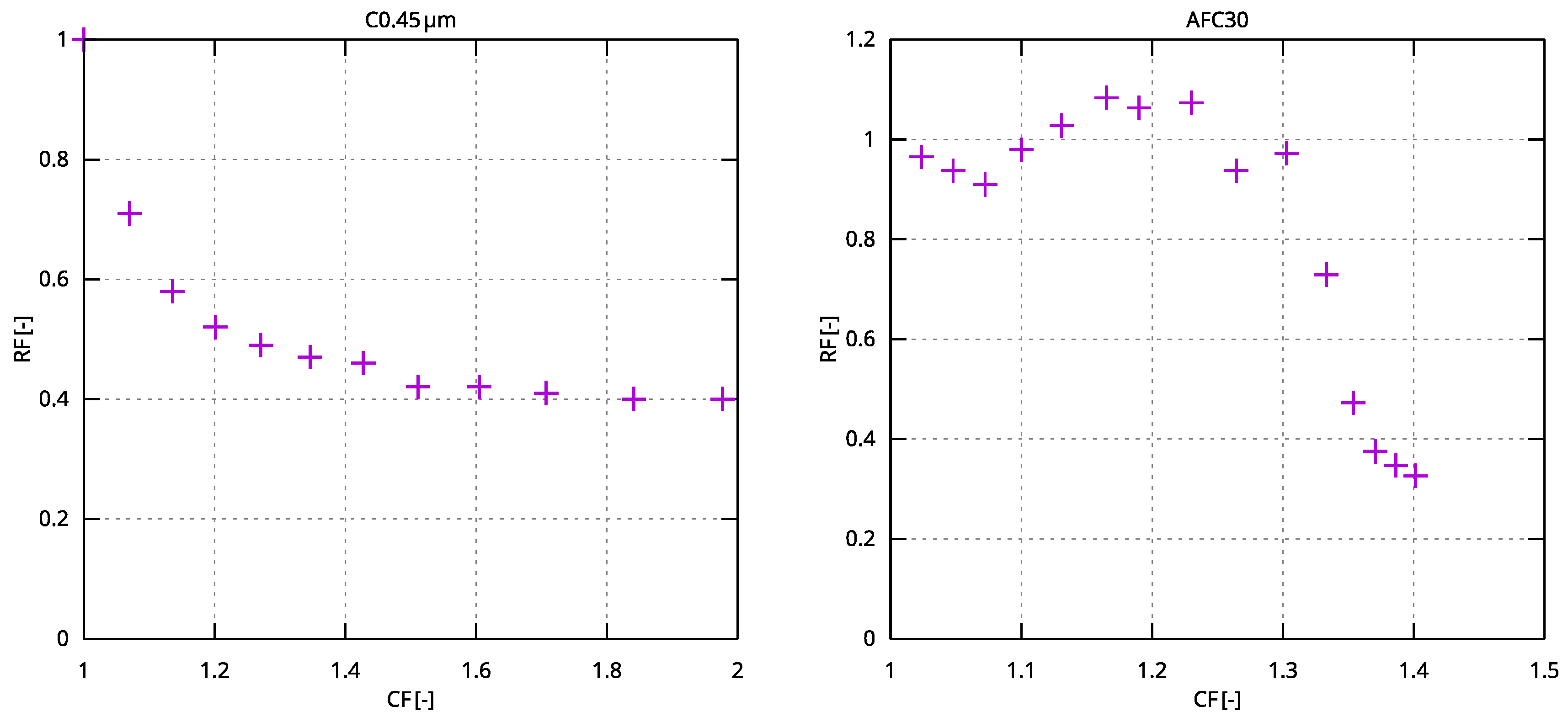
3.3. Microfiltration—0.45 µm Module
3.4. Variant I: Filtration—Microfiltration—Nanofiltration
3.5. Variant II, III: Filtration—Microfiltration—Adsorption
3.6. Variant IV: Filtration—Ultrafiltration—Nanofiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Effective Membrane Surface Area (m2) |
| BOD5 | Biochemical Oxygen Demand (5-day) |
| CF | Concentration Factor (–) |
| COD | Chemical Oxygen Demand |
| CSs | Cationic Surfactants |
| DC | Dose of Carbon (g/L) |
| F | Filtration |
| GAC | Granular Activated Carbon |
| H | Bed Height (m) |
| J | Permeate Volume Flux (L/m2·h) |
| J0 | Distilled Water Flux (L/m2·h) |
| LWW | Laundry Wastewater |
| MF | Microfiltration |
| NF | Nanofiltration |
| NTU | Nephelometric Turbidity Unit |
| PAC | Powdered Activated Carbon |
| RF | Relative Flux (J/J0) |
| t | Time (h or min, depending on context) |
| tm | Mixing Time (min) |
| ts | Sedimentation Time (min) |
| TMP | Transmembrane Pressure (MPa) |
| TOC | Total Organic Carbon |
| TS | Total Solids |
| TSS | Total Suspended Solids |
| UF | Ultrafiltration |
| v | Filtration Rate |
| VB | Volume of Carbon Bed (L) |
| V0 | Initial Feed Volume (L) |
| Vt | Volume at Time t (L) |
| ν | Flow velocity along membrane surface (m/s) |
References
- Buy Unity Now. (n.d.). Unity Commercial Washing Machines for Hotels. Available online: https://buyunitynow.com/unity-commercial-washing-machines-hotels/ (accessed on 5 February 2025).
- Nicolaidis, C.; Vyrides, I. Closing the water cycle for industrial laundries: An operational performance and techno-economic evaluation of a full-scale membrane bioreactor system. Resour. Conserv. Recycl. 2014, 92, 128–135. [Google Scholar] [CrossRef]
- Jayanto, G.D.; Widyastuti, M.; Hadi, M.P. Laundry wastewater characteristics and their relationship with river water quality as an indicator of water pollution: Case study of Code Watershed, Yogyakarta. E3S Web Conf. 2021, 325, 02011. [Google Scholar] [CrossRef]
- Ciabatti, I.; Cesaro, F.; Faralli, L.; Fatarella, E.; Tognotti, F. Demonstration of a treatment system for purification and reuse of laundry wastewater. Desalination 2009, 245, 451–459. [Google Scholar] [CrossRef]
- Vishali, S.; Poonguzhali, E.; Banerjee, I.; George, S.S.; Srinivasan, P. Purification of domestic laundry wastewater in an integrated treatment system consists of coagulation and ultrafiltration membrane process. Chemosphere 2023, 314, 137662. [Google Scholar] [CrossRef]
- Babajanzadeh, B.; Sherizadeh, S.; Ranji, H. Detergents and surfactants: A brief review. Open Access J. Sci. 2019, 3, 94–99. [Google Scholar] [CrossRef]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and performance optimisation of detergent product containing binary mixture of anionic-nonionic surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef]
- Mondal, M.G.; Pratap, A.P. Synthesis and performance properties of cationic fabric softeners derived from free fatty acid of tallow fat. J. Oleo Sci. 2016, 65, 691–700. [Google Scholar] [CrossRef]
- Yoshii, S.; Nakajima, D.; Saito, N.; Terasaki, M.; Endo, S. Triethanolamine-based esterquat in sediments: New analytical method, environmental occurrence, and homologue composition. Chemosphere 2024, 361, 142495. [Google Scholar] [CrossRef]
- Mishra, S.; Tyagi, V.K. Esterquats: The novel class of cationic fabric softeners. J. Oleo Sci. 2007, 56, 269–276. [Google Scholar] [CrossRef]
- Global Market Insights. Esterquats Market Size & Share Analysis Report, 2024–2032. 21 October 2024. Available online: https://www.gminsights.com/industry-analysis/esterquats-market (accessed on 18 January 2025).
- Šostar-Turk, S.; Petrinić, I.; Simonič, M. Laundry wastewater treatment using coagulation and membrane filtration. Resour. Conserv. Recycl. 2005, 44, 185–196. [Google Scholar] [CrossRef]
- Deressa, S.; Endale, H.; Dadi, D.; Kitte, S.A. Removal of anionic surfactant from residential laundry wastewater using jackfruit (Artocarpus heterophyllus) seeds. Adv. Environ. Technol. 2019, 1, 47–53. [Google Scholar] [CrossRef]
- Corona, R.R.B.; Sad, C.M.S.; Silva, M.; Lopes, D.L.; Leite, J.S.D.; Viegas, G.M.F.; Gonçalves, G.R.; Filgueiras, P.R.; Castro, E.V.R. Adsorption of anionic surfactant in graphite oxide: A study for treatment of laundry wastewater. J. Environ. Chem. Eng. 2021, 9, 106858. [Google Scholar] [CrossRef]
- Zotesso, J.P.; Cossich, E.S.; Janeiro, V.; Tavares, C.R.G. Treatment of hospital laundry wastewater by UV/H2O2 process. Environ. Sci. Pollut. Res. 2017, 24, 6278–6287. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.O.C.; Veit, M.T.; Palácio, S.M.; Gonçalves, G.C.; Fagundes-Klen, M.R. Combined application of coagulation/flocculation/sedimentation and membrane separation for the treatment of laundry wastewater. Int. J. Chem. Eng. 2019, 2019, 8324710. [Google Scholar] [CrossRef]
- Melián, E.P.; Santiago, D.E.; León, E.; Vaswani Reboso, J.; Herrera-Melián, J.A. Treatment of laundry wastewater by different processes: Optimization and life cycle assessment. J. Environ. Chem. Eng. 2023, 11, 109302. [Google Scholar] [CrossRef]
- Lade, O.; Gbagba, Z. Sustainable water supply: Potential of recycling laundry wastewater for domestic use. J. Civ. Eng. Environ. Sci. 2018, 4, 056–060. [Google Scholar] [CrossRef]
- Benis, K.Z.; Behnami, A.; Aghayani, E.; Farabi, S.; Pourakbar, M. Water recovery and on-site reuse of laundry wastewater by a facile and cost-effective system: Combined biological and advanced oxidation process. Sci. Total Environ. 2021, 789, 148068. [Google Scholar] [CrossRef]
- Bilad, M.R.; Mat Nawi, N.I.; Subramaniam, D.D.; Shamsuddin, N.; Khan, A.L.; Jaafar, J.; Nandiyanto, A.B.D. Low-pressure submerged membrane filtration for potential reuse of detergent and water from laundry wastewater. J. Water Process Eng. 2020, 36, 101264. [Google Scholar] [CrossRef]
- Kumar, S.; Khosravanipour Mostafazadeh, A.; Kumar, L.R.; Tyagi, R.D.; Drogui, P.; Brien, E. Advancements in laundry wastewater treatment for reuse: A review. J. Environ. Sci. Health Part A 2022, 57, 927–946. [Google Scholar] [CrossRef]
- Procházková, M.; Máša, V. Sustainable wastewater management in industrial laundries. Chem. Eng. Trans. 2022, 94, 577–582. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Sum, J.Y.; Ng, Z.J.; Mohammad, A.W. Water pathways through the ages: Integrated laundry wastewater treatment for pollution prevention. Sci. Total Environ. 2021, 760, 143966. [Google Scholar] [CrossRef]
- Khan, N.A.; Bokhari, A.; Mubashir, M.; Klemeš, J.J.; El Morabet, R.; Khan, R.A.; Alsubih, M.; Azam, M.; Saqib, S.; Mukhtar, A.; et al. Treatment of hospital wastewater with submerged aerobic fixed film reactor coupled with tube-settler. Chemosphere 2022, 286, 131838. [Google Scholar] [CrossRef] [PubMed]
- Barumbu, A.; Bilad, M.R.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Al-Gharabli, S. Detergent Recovery from Laundry Wastewater Using Self-Made Flat Sheet Membrane. Membranes 2020, 10, 260. [Google Scholar] [CrossRef]
- Sumisha, A.; Arthanareeswaran, G.; Lukka Thuyavan, Y.; Ismail, A.F.; Chakraborty, S. Treatment of laundry wastewater using polyethersulfone/polyvinylpyrollidone ultrafiltration membranes. Ecotoxicol. Environ. Saf. 2015, 121, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, J.; Massé, A.; Andrs, Y.; Combe, F.; Jaouen, P. Laundry water recycling in ship by direct nanofiltration with tubular membranes. Resour. Conserv. Recycl. 2010, 55, 148–154. [Google Scholar] [CrossRef]
- Shang, X.; Kim, H.C.; Huang, J.H.; Dempsey, B.A. Coagulation strategies to decrease fouling and increase critical flux and contaminant removal in microfiltration of laundry wastewater. Sep. Purif. Technol. 2015, 147, 44–50. [Google Scholar] [CrossRef]
- Klimonda, A.; Kowalska, I. Separation and concentration of cationic surfactant solutions with the use of ceramic modules. Environ. Prot. Eng. 2020, 46, 41–51. [Google Scholar] [CrossRef]
- Regulation of the Minister of Maritime Economy and Inland Navigation. On Substances Particularly Harmful to the Aquatic Environment and the Conditions That Must Be Met When Introducing Wastewater into Water or Soil, as Well as When Discharging Stormwater or Meltwater into Water or Water Facilities. 2019. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20190001311/O/D20191311.pdf (accessed on 11 March 2025). (In Polish)
- Kudlek, E.; Bohdziewicz, J.; Dudziak, M. Influence of water matrix on the retention of pharmaceuticals by high-pressure membrane filtration. Ecol. Chem. Eng. A 2015, 22, 469–479. [Google Scholar] [CrossRef]
- Otero-Fernández, A.; Díaz, P.; Otero, J.A.; Ibáñez, R.; Maroto-Valiente, A.; Palacio, L.; Prádanos, P.; Carmona, F.J.; Hernández, A. Morphological, chemical and electrical characterization of a family of commercial nanofiltration polyvinyl alcohol coated polypiperazineamide membranes. Eur. Polym. J. 2020, 126, 109544. [Google Scholar] [CrossRef]
- Turkay, O.; Barışçı, S.; Sillanpää, M. E-peroxone process for the treatment of laundry wastewater: A case study. J. Environ. Chem. Eng. 2017, 5, 4282–4290. [Google Scholar] [CrossRef]
- Klimonda, A.; Kowalska, I. Surfactant Fouling in Pressure-Driven Membrane Processes. Environ. Prot. Eng. 2023, 49, 65–78. [Google Scholar] [CrossRef]
- Atesci, Z.C.; Inan, H. Removal of microfiber and surfactants from household laundry washing effluents by powdered activated carbon: Kinetics and isotherm studies. Water Sci. Technol. 2023, 88, 1578. [Google Scholar] [CrossRef] [PubMed]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, J. Removal of surfactant substances from aqueous solution by adsorption. In Proceedings of the XX-th Jubilee-National, VII-th International Scientific and Technical Conference “Water Supply and Water Quality”, Poznań, Poland, 15–18 June 2008. [Google Scholar]
- Sahu, D.; Pervez, S.; Karbhal, I.; Tanrakar, A.; Mishra, A.; Verma, S.R.; Deb, M.K.; Ghosh, K.K.; Pervez, Y.F.; Shrivas, K.; et al. Applications of different adsorbent materials for the removal of organic and inorganic contaminants from water and wastewater—A review. Desalination Water Treat. 2024, 254, 100253. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Liu, T.; Li, X.; Cui, Y.; Xu, L.; Huo, S.; Zou, B.; Qian, J.; Ma, A.; et al. Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects. Catalysts 2023, 13, 1356. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, F.; He, X.; Liao, Y.; Chen, R.; Xia, R.; Shang, Y.; Wang, Q.; Yu, J. The adsorption of biogenetic odorants onto activated carbon: Adsorption characteristics and impacts of algal organic matter. Environ. Res. 2023, 238, 117072. [Google Scholar] [CrossRef]
- Paulino, R.; Tamburic, B.; Stuetz, R.M.; Zamyadi, A.; Crosbie, N.; Henderson, R.K. Critical review of adsorption and biodegradation mechanisms for removal of biogenic taste and odour compounds in granular and biological activated carbon contactors. J. Water Process Eng. 2023, 52, 103518. [Google Scholar] [CrossRef]



| Reference | Type of LWW | pH [-] | Turbidity [NTU] | TS [mg/L] | BOD5 [mg O2/L] | COD [mg O2/L] | Surfactants [mg/L] |
|---|---|---|---|---|---|---|---|
| [5] | Domestic | 7.46 | 437 | N/A | N/A | 286 | 45 |
| [13] | Domestic | 9.58 | 9.76 | N/A | 1546 | 3135 | 38.9 |
| [14] | Domestic | 9.66 ± 0.03 | 45 ± 1.42 | 2311.83 ± 11.77 | N/A | N/A | 40.82 ± 0.1 (anionic) |
| [15] | Hospital | 6.6–11.7 | 37 * ± 29 | 363 ± 146 | N/A | 411 ± 204 | N/A |
| [16] | Industrial | 10.0 ± 0.1 | 61 ± 2 | 456 ± 6 | 58 ± 0 | 587 ± 4 | 11.7 ± 0.1 |
| 10.5 ± 0 | 52 ± 2 | 530 ± 3 | 87 ± 0 | 383 ± 15 | 19.6 ± 0.1 | ||
| 10.9 ± 0 | 64 ± 1 | 532 ± 7 | 67 ± 0 | 245 ±8 | 15.9 ± 0 | ||
| [17] | Industrial | 11.08 ± 1.30 | 1290 ± 105 | 2900 ± 326 | 750 ± 80 | 1920 ± 220 | 9.42 ± 0.85 (LAS) 37.36 ± 0.17 (BiAS) |
| [4] | Industrial | 7–9 | 40–150 | 90–200 | N/A | 400–1000 | 1–10 (nonionic) 1–15 (anionic) |
| [18] | Domestic | 9.3–10 | 14–400 | 200–987 | 48–1200 | 375–4155 | N/A |
| Industrial | 9.0–11 | 40–150 | 400–1000 | 218–9810 | 80–212,000 | N/A | |
| Hospital | 11.4–11.6 | 87.9 | 66–71 | 44–50 | 477–876 | N/A |
| Landscape Irrigation | Toilet Flushing | Laundry Process | |
|---|---|---|---|
| pH [-] | 6.0–9.0 | 6.0–9.0 | 6.0–9.0 |
| Turbidity [NTU] | no limit | no limit | 2 |
| BOD5 [mg O2/L] | 30 | 30 | 10 |
| TSS [mg/L] | 30 | 30 | 10 |
| Hardness [mg CaCO3/L] | no limit | no limit | 90 |
| Symbol | Material | Cut-Off [kDa] | Pore Size [nm] | Distilled Water Flux * [L/m2h] |
|---|---|---|---|---|
| MF C0.45 µm | zirconium dioxide | - | 450 | 240 |
| UF C300 | zirconium dioxide | 300 | - | 101 |
| NF AFC40 | polyamide | 0.2 [31] | 0.51 ± 0.10 [32] | 16 |
| Variant | Stage I | Stage II | ||
|---|---|---|---|---|
| I F–MF–NF | Microfiltration | Nanofiltration | ||
| MF C0.45 µm | V0 = 10 L | NF AFC30 | V0 = 5 L | |
| TMP = 0.3 MPa | TMP = 0.3 MPa | |||
| ν = 3 m/s | ν = 0.6 m/s | |||
| CF = 2 | CF = 1.5 | |||
| II F–MF–ADS | Microfiltration | Adsorption, flow reactor | ||
| MF C0.45 µm | V0 = 10 L | GAC | V0 = 3 L | |
| TMP = 0.3 MPa | H = 0.15 m | |||
| ν = 3 m/s | VB = 0.4 L | |||
| CF = 2 | v = 5 m/h | |||
| III F–MF–ADS | Microfiltration | Adsorption, batch reactor | ||
| MF C0.45 µm | V0 = 10 L | PAC | V0 = 1 L | |
| TMP = 0.3 MPa | DC = 0.5; 1 g/L | |||
| ν = 3 m/s | tm = 30 min | |||
| CF = 2 | ts = 60 min | |||
| IV F–UF–NF | Ultrafiltration | Nanofiltration | ||
| UF C300 | V0 = 10 L | NF AFC30 | V0 = 5 L | |
| TMP = 0.3 MPa | TMP = 0.3 MPa | |||
| ν = 3 m/s | ν = 0.6 m/s | |||
| CF = 2 | CF = 1.5 | |||
| Parameter | Wastewater | Standard for Discharge into the Environment [30] |
|---|---|---|
| pH [-] | 7.16 ± 0.30 | 6.5–9 |
| Conductivity [µS/cm] | 773.5 ± 54.1 | - |
| Turbidity [NTU] | 46.1 ± 1.9 | - |
| CSs [mg/L] | 29.8 ± 7.9 | 5 * |
| COD [mg O2/L] | 86.4 ± 12.3 | 125 |
| BOD5 [mg O2/L] | 74.0 ± 5.7 | 25 |
| TOC [mg/L] | 50.7 ± 11.3 | 30 |
| Total solids [mg/L] | 343 ± 7 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimonda, A.; Kowalska, I. Water Recovery from Laundry Wastewater by Integrated Purification Systems. Membranes 2025, 15, 125. https://doi.org/10.3390/membranes15040125
Klimonda A, Kowalska I. Water Recovery from Laundry Wastewater by Integrated Purification Systems. Membranes. 2025; 15(4):125. https://doi.org/10.3390/membranes15040125
Chicago/Turabian StyleKlimonda, Aleksandra, and Izabela Kowalska. 2025. "Water Recovery from Laundry Wastewater by Integrated Purification Systems" Membranes 15, no. 4: 125. https://doi.org/10.3390/membranes15040125
APA StyleKlimonda, A., & Kowalska, I. (2025). Water Recovery from Laundry Wastewater by Integrated Purification Systems. Membranes, 15(4), 125. https://doi.org/10.3390/membranes15040125








