Inhibition of ISAV Membrane Fusion by a Peptide Derived from Its Fusion Protein
Abstract
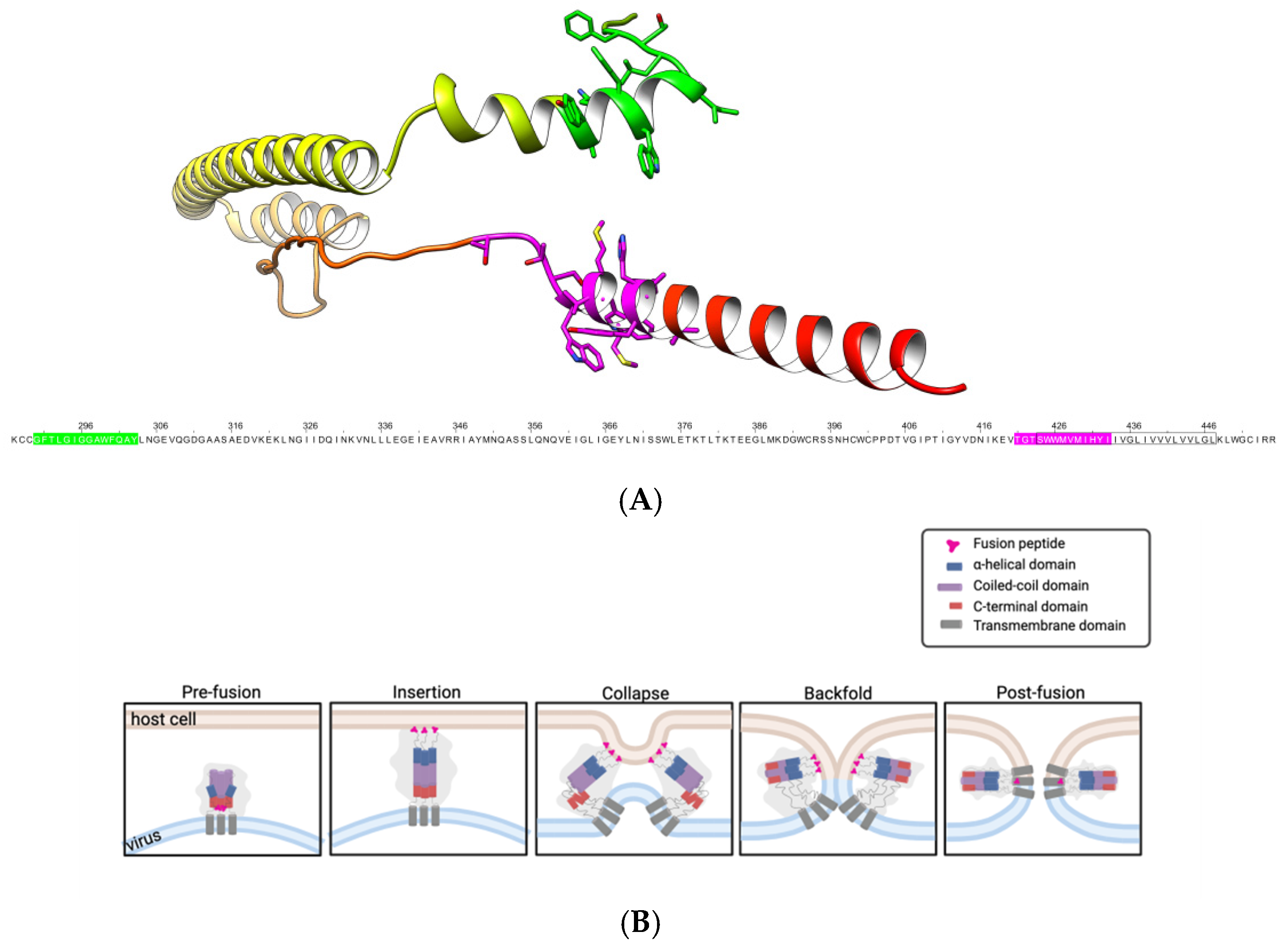
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Peptide Synthesis and Characterization
2.3. Preparation of Vesicles
2.4. Fluorescence Spectroscopy Analysis
2.4.1. Lipid Mixing Assay
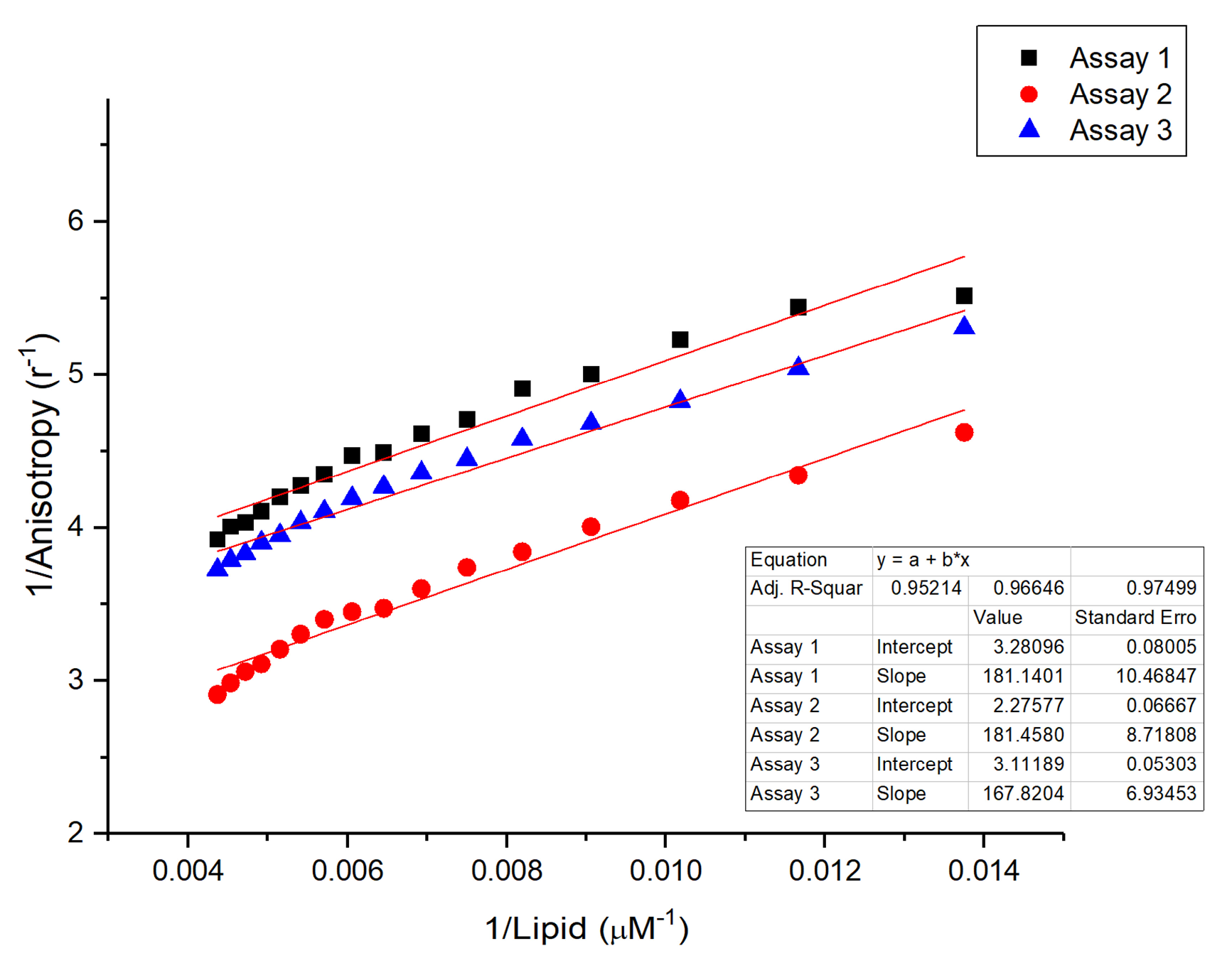
2.4.2. Determination of Binding Affinity Using Fluorescence Anisotropy
2.4.3. Generalized Polarization of Laurdan (GP)
2.4.4. Steady-State Fluorescence Anisotropy of DPH Probes
2.4.5. Fluorescence Resonance Energy Transfer (FRET)
3. Results
3.1. Fluorescence Spectroscopy
3.1.1. Lipid Mixing Assay
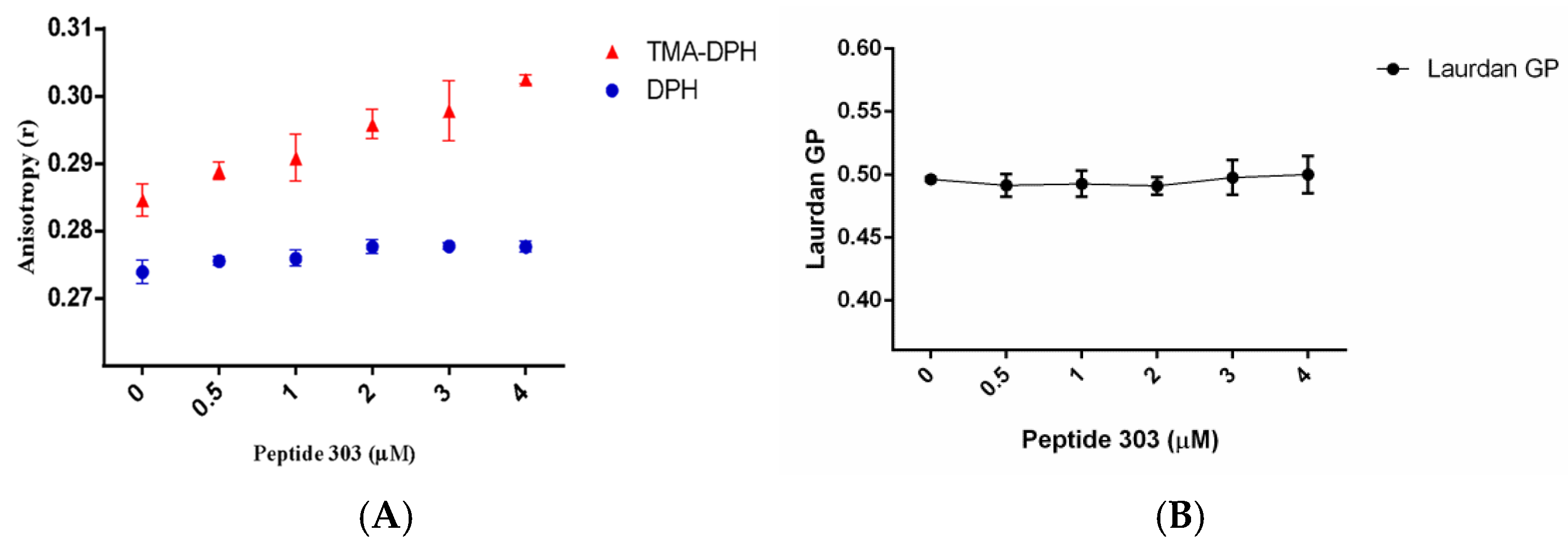
3.1.2. Steady-State Fluorescence Analysis of Peptide 303 Interaction with LUVs and Fusion Peptides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISAV | Infectious Salmon Anemia Virus |
| ISAV-FP1 | ISAV fusion peptide |
| ISA | Infectious Salmon Anemia |
| LUVs | large unilamellar vesicles |
| DPPC | dipalmitoylphosphatidylcholine |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| HE | hemagglutinin esterase |
| DPH | 1,6-diphenyl-1,3,5-hexatriene |
| TMA-DPH | 1-(4-trimethylammonium)-6-phenyl-1,3,5-hexatriene |
| Laurdan | 6-lauroyl-2-dimethylaminonaphthalene |
| R18 | octadecyl rhodamine B chloride |
| TX-100 | triton X-100 |
| SPPS | solid-phase peptide synthesis |
| Fmoc | 9-fluorenylmethoxycarbonyl |
| Kd | dissociation constant |
| GP | generalized polarization |
| FRET | Förster Resonance Energy Transfer |
References
- Vike, S.; Duesund, H.; Andersen, L.; Nylund, A. Release and survival of infectious salmon anaemia (ISA) virus during decomposition of Atlantic salmon (Salmo salar L.). Aquaculture 2014, 420–421, 119–125. [Google Scholar] [CrossRef]
- Krossøy, B.; Hordvik, I.; Nilsen, F.; Nylund, A.R.E.; Endresen, C. The putative polymerase sequence of infectious salmon anemia virus suggests a new genus within the orthomyxoviridae. Am. Soc. Microbiol. 1999, 73, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.G.; Aedo, A.; Kibenge, M.J.T.; Groman, D.B.; Yason, C.V.; Grothusen, H.; Lisperguer, A.; Calbucura, M.; Avendaño, F.; Imilán, M.; et al. First detection, isolation and molecular characterization of infectious salmon anaemia virus associated with clinical disease in farmed Atlantic salmon (Salmo salar) in Chile. BMC Vet. Res. 2008, 4, 28. [Google Scholar] [CrossRef]
- Mullins, J.E.; Groman, D.; Wadowska, D. Infectious salmon anaemia in salt water Atlantic salmon (Salmo salar L.) in new Brunswick, Canada. Fish Pathol. 1998, 18, 110–114. [Google Scholar]
- Thorud, K.; Djupvik, H.O. Experimental transmission of Goussia carpelli (Leger & Stankovitch, 1921; protista apicomplexa) to common carp, Cyprinus carpio L. Fish Pathol. 1988, 8, 109–112. [Google Scholar]
- Ibieta, P.; Tapia, V.; Venegas, C. Chilean Salmon farming on the horizon of sustainability: Review of the development of a highly intensive production, the ISA crisis and implemented actions to reconstruct a more sustainable aquaculture. In Aquaculture and the Environment—A Shared Destiny; InTechOpen: London, UK, 2011; pp. 215–246. [Google Scholar] [CrossRef]
- Oelckers, K.; Vike, S.; Duesund, H.; Gonzalez, J.; Wadsworth, S.; Nylund, A. Caligus rogercresseyi as a potential vector for transmission of infectious salmon anaemia (ISA) virus in Chile. Aquaculture 2014, 420–421, 126–132. [Google Scholar] [CrossRef]
- Hammell, K.L.; Dohoo, I.R. Risk factors associated with mortalities attributed to infectious salmon anaemia virus in New Brunswick, Canada. J. Fish Dis. 2005, 28, 651–661. [Google Scholar] [CrossRef]
- Aspehaug, V.; Mikalsen, B.A.B.; Snow, M.; Biering, E.; Villoing, S. Characterization of the infectious salmon anemia virus fusion protein. J. Virol. 2005, 79, 12544. [Google Scholar] [CrossRef]
- Hellebø, A.; Vilas, U.; Falk, K.; Vlasak, R. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J. Virol. 2004, 78, 3055–3062. [Google Scholar] [CrossRef]
- Fourrier, M.; Lester, K.; Markussen, T.; Falk, K.; Secombes, C.J.; McBeath, A.; Collet, B. Dual mutation events in the haemagglutinin-esterase and fusion protein from an infectious salmon anaemia virus HPR0 genotype promote viral fusion and activation by an ubiquitous host protease. PLoS ONE 2015, 10, e0142020. [Google Scholar] [CrossRef]
- Cook, J.D.; Soto-Montoya, H.; Korpela, M.K.; Lee, J.E. Electrostatic architecture of the infectious salmon anemia virus (ISAV) core fusion protein illustrates a carboxyl-carboxylate pH sensor. J. Biol. Chem. 2015, 290, 18495–18504. [Google Scholar] [CrossRef] [PubMed]
- Tarnok, M.E.; Guzmán, F.; Aguilar, L.F. Effects of cholesterol on lipid vesicle fusion mediated by infectious salmon anaemia virus fusion peptides. Colloids Surf. B Biointerfaces 2022, 217, 112684. [Google Scholar] [CrossRef] [PubMed]
- Rajik, M.; Jahanshiri, F.; Omar, A.R.; Ideris, A.; Hassan, S.S.; Yusoff, K. Identification and characterisation of a novel anti-viral peptide against avian influenza virus H9N2. Virol. J. 2009, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.J.C. Protein-lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 487–499. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef]
- Cross, K.J.; Langley, W.A.; Russell, R.J.; Skehel, J.J.; Steinhauer, D.A. Composition and functions of the influenza fusion peptide. Protein Pept. Lett. 2009, 16, 766–778. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gúzman, F.; Aróstica, M.; Román, T.; Beltrán, D.; Gauna, A.; Albericio, F.; Cárdenas, C. Peptides, solid- phase synthesis and characterization: Tailor-made methodologies. Electron. J. Biotechnol. 2023, 64, 27–33. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. BBA-Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Mondal, S.; Bansode, A.S.; Sarkar, M. Effect of increase in orientational order of lipid chains and head group spacing on non steroidal anti-inflammatory drug induced membrane fusion. Langmuir 2010, 26, 18967–18975. [Google Scholar] [CrossRef]
- Pallicer, J.M.; Krämer, S.D. Evaluation of fluorescence anisotropy to assess drug-lipid membrane partitioning. J. Pharm. Biomed. Anal. 2012, 71, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Poojari, C.; Wilkosz, N.; Lira, R.B.; Dimova, R.; Jurkiewicz, P. Behavior of the DPH fluorescence probe in membranes perturbed by drugs. Chem. Phys. Lipids 2019, 223, 104784. [Google Scholar] [CrossRef] [PubMed]
- Düzgüneş, N.; Fernandez-Fuentes, N.; Konopka, K. Inhibition of viral membrane fusion by peptides and approaches to peptide design. Pathogens 2021, 10, 1599. [Google Scholar] [CrossRef]
- Pattnaik, G.P.; Chakraborty, H. Coronin 1 derived tryptophan-aspartic acid containing peptides inhibit membrane fusion. Chem. Phys. Lipids. 2018, 217, 35–42. [Google Scholar] [CrossRef]
- Pandia, S.; Mahapatra, A.; Chakraborty, H. A Coronin 1-derived peptide inhibits membrane fusion by modulating membrane organization and dynamics. J. Phys. Chem. B 2024, 128, 4986–4995. [Google Scholar] [CrossRef]
- Kirschner, A.N.; Lowrey, A.S.; Longnecker, R.; Jardetzky, T.S. Binding-site interactions between Epstein-Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion. J. Virol. 2007, 81, 9216–9229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnok, M.E.; Caravia-Merlo, L.; Cárdenas, C.; Guzmán, F.; Aguilar, L.F. Inhibition of ISAV Membrane Fusion by a Peptide Derived from Its Fusion Protein. Membranes 2025, 15, 180. https://doi.org/10.3390/membranes15060180
Tarnok ME, Caravia-Merlo L, Cárdenas C, Guzmán F, Aguilar LF. Inhibition of ISAV Membrane Fusion by a Peptide Derived from Its Fusion Protein. Membranes. 2025; 15(6):180. https://doi.org/10.3390/membranes15060180
Chicago/Turabian StyleTarnok, María Elena, Lucía Caravia-Merlo, Constanza Cárdenas, Fanny Guzmán, and Luis F. Aguilar. 2025. "Inhibition of ISAV Membrane Fusion by a Peptide Derived from Its Fusion Protein" Membranes 15, no. 6: 180. https://doi.org/10.3390/membranes15060180
APA StyleTarnok, M. E., Caravia-Merlo, L., Cárdenas, C., Guzmán, F., & Aguilar, L. F. (2025). Inhibition of ISAV Membrane Fusion by a Peptide Derived from Its Fusion Protein. Membranes, 15(6), 180. https://doi.org/10.3390/membranes15060180





