Impact of Additives on Poly(acrylonitrile-butadiene-styrene) Membrane Formation Process Using Non-Solvent-Induced Phase Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. ABS Characterization
2.3. CHEM 21 Solvent Evaluation
2.4. Dope Solution Creation
2.5. Solubility of ABS
2.6. Volatility of Dope Solution
2.7. Viscosity of Dope Solution
2.8. Membrane Fabrication
2.9. SEM
2.10. Porosity
2.11. Mechanical Characterization
2.12. Water Contact Angle
2.13. Water Flux and BSA Rejection
3. Results and Discussion
3.1. Polymer Characterization
3.2. Solvent Selection
3.3. Impact of Additives on Membrane Formation
3.4. Mixture Properties Affecting Membrane Structure
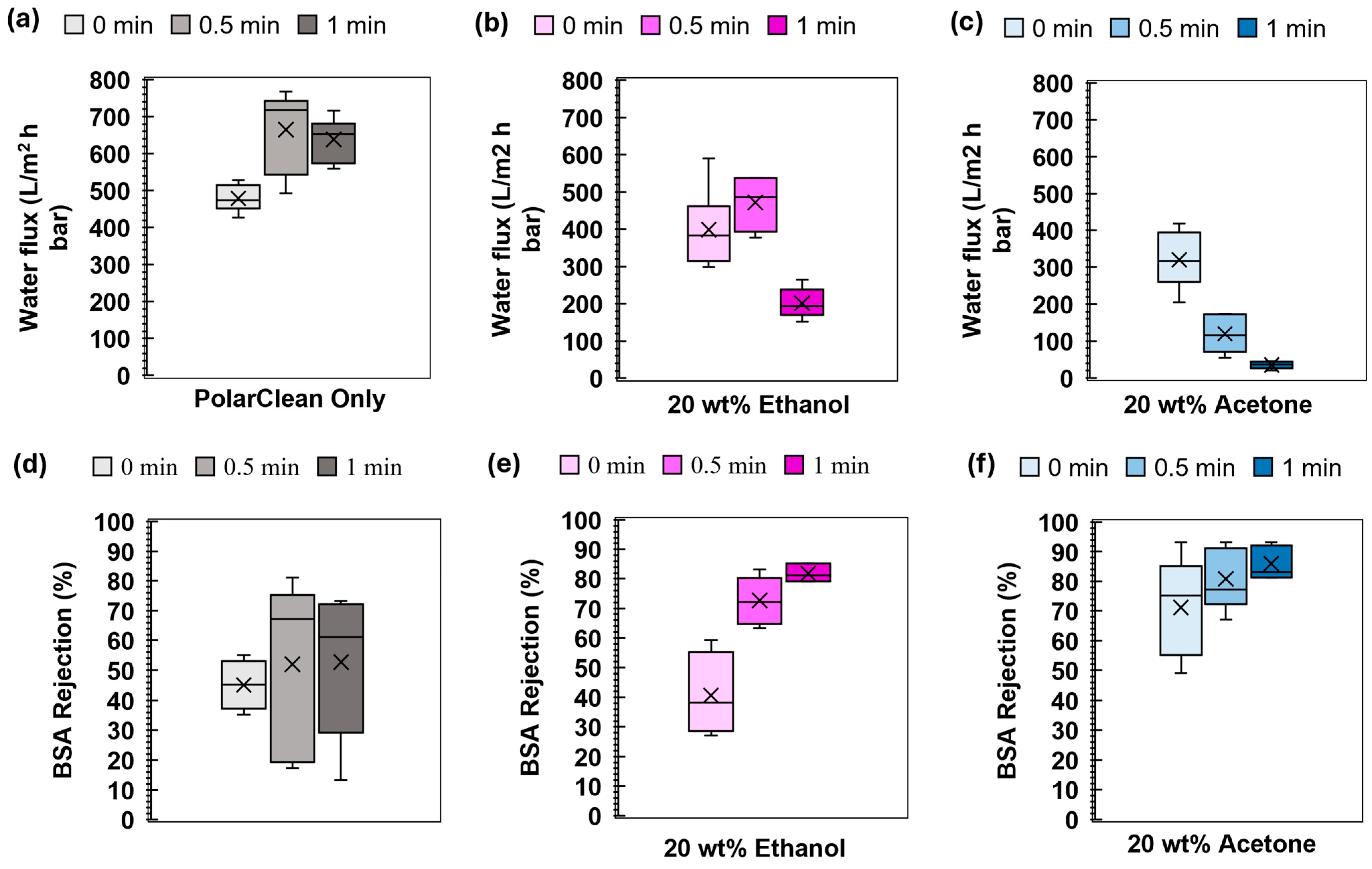
3.5. Membrane Properties and Filtration Performance
3.6. Impact of Evaporation Time on Membrane Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Poly(acrylonitrile-butadiene-styrene) |
| NIPS | Non-solvent-induced phase separation |
References
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, A.; Othman, M.H.D.; Jilani, A.; Khan, I.U.; Kamaludin, R.; Iqbal, J.; Al-Sehemi, A.G. Challenges, Opportunities and Future Directions of Membrane Technology for Natural Gas Purification: A Critical Review. Membranes 2022, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Ziabka, M.; Dziadek, M.; Pielichowska, K. Surface and Structural Properties of Medical Acrylonitrile Butadiene Styrene Modified with Silver Nanoparticles. Polymers 2020, 12, 197. [Google Scholar] [CrossRef]
- Anjos, E.G.R.d.; Marini, J.; Montagna, L.S.; Montanheiro, T.L.d.A.; Passador, F.R. Reactive processing of maleic anhydride-grafted ABS and its compatibilizing effect on PC/ABS blends. Polímeros 2020, 30. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Sanaeepur, H.; Moghadassi, A.; Kargari, A.; Ghanbari, D.; Mehrabadi, Z.S. Modification of ABS Membrane by PEG for Capturing Carbon Dioxide from CO2/N2Streams. Sep. Sci. Technol. 2010, 45, 1385–1394. [Google Scholar] [CrossRef]
- Etemadi, H.; Qazvini, H.; Alishah, N.H. Preparation of high performance polycarbonate/acrylonitrile-butadiene-styrene blend ultrafiltration membrane for water treatment. Desalination Water Treat. 2021, 218, 146–154. [Google Scholar] [CrossRef]
- Hapońska, M.; Clavero, E.; Salvadó, J.; Torras, C. Application of ABS membranes in dynamic filtration for Chlorella sorokiniana dewatering. Biomass Bioenergy 2018, 111, 224–231. [Google Scholar] [CrossRef]
- Mandegari, M.; Fashandi, H. Untapped potentials of acrylonitrile-butadiene-styrene/polyurethane (ABS/PU) blend membrane to purify dye wastewater. J. Environ. Manag. 2017, 197, 464–475. [Google Scholar] [CrossRef]
- Nur’aini, S.; Zulfi, A.; Arrosyid, B.H.; Rafryanto, A.F.; Noviyanto, A.; Hapidin, D.A.; Feriyanto, D.; Saputro, K.E.; Khairurrijal, K.; Rochman, N.T. Waste acrylonitrile butadiene styrene (ABS) incorporated with polyvinylpyrrolidone (PVP) for potential water filtration membrane. RSC Adv. 2022, 12, 33751–33760. [Google Scholar] [CrossRef]
- Kamelian, F.S.; Saljoughi, E.; Shojaee Nasirabadi, P.; Mousavi, S.M. Modifications and research potentials of acrylonitrile/butadiene/styrene (ABS) membranes: A review. Polymer Composites 2018, 39, 2835–2846. [Google Scholar] [CrossRef]
- Kamelian, F.S.; Mousavi, S.M.; Ahmadpour, A.; Ghaffarian, V. Preparation of acrylonitrile-butadiene-styrene membrane: Investigation of solvent/nonsolvent type and additive concentration. Korean J. Chem. Eng. 2014, 31, 1399–1404. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Subramaniam Ramasamy, B.S.; Narayanan, M.; Gopalakrishnan, A.V.; Kandasamy, S.; Raja, R. A review on fabrication, characterization of membrane and the influence of various parameters on contaminant separation process. Chemosphere 2022, 306, 135629. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Membrane Fabrication/Manufacturing Techniques. In Gas Separation Membranes: Polymeric and Inorganic; Ismail, A.F., Chandra Khulbe, K., Matsuura, T., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 193–220. [Google Scholar] [CrossRef]
- Sherwood, J.; Albericio, F.; de la Torre, B.G. N,N-Dimethyl Formamide European Restriction Demands Solvent Substitution in Research and Development. ChemSusChem 2024, 17, e202301639. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, C.; He, H.; Mao, X.; Wei, L.; Zhou, F.; Sun, R. Green-Solvent-Processable Composite Micro/Nanofiber Membrane with Gradient Asymmetric Structure for Efficient Microfiltration. Small 2023, 19, e2207330. [Google Scholar] [CrossRef]
- Tapia-Quiros, P.; Montenegro-Landivar, M.F.; Reig, M.; Vecino, X.; Saurina, J.; Granados, M.; Cortina, J.L. Integration of membrane processes for the recovery and separation of polyphenols from winery and olive mill wastes using green solvent-based processing. J. Environ. Manag. 2022, 307, 114555. [Google Scholar] [CrossRef]
- Chang, S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery-a review. Environ. Sci. Pollut. Res. 2020, 27, 32371–32388. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with Rhodiasolv®Polarclean as water soluble green solvent. J. Membr. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. γ-Valerolactone as Bio-Based Solvent for Nanofiltration Membrane Preparation. Membranes 2021, 11, 418. [Google Scholar] [CrossRef]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.-D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef]
- Tan, L.; Scott, H.L.; Smith, M.D.; Pingali, S.V.; O’Neill, H.M.; Morrell-Falvey, J.L.; Katsaras, J.; Smith, J.C.; Davison, B.H.; Elkins, J.G.; et al. Amphiphilic Co-Solvents Modulate the Structure of Membrane Domains. ACS Sustain. Chem. Eng. 2023, 11, 1598–1609. [Google Scholar] [CrossRef]
- Abd El-Latif, R.A.; Aziz, M.E.A.; El-Taweel, S.H.; El-Khair, M.T.A.; Saad, G.R. Effects of Co-Solvent on the Morphology, Physicochemical Properties, and Performance of PVDF Electrospun Membranes in Comparison to Flat-Sheet Membranes. J. Compos. Sci. 2022, 6, 253. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Nanda, B.; Sailaja, M.; Mohapatra, P.; Pradhan, R.K.; Nanda, B.B. Green solvents: A suitable alternative for sustainable chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Stratton, G.W. Effect of the solvent acetone on membrane integrity in the green alga Chlorella pyrenoidosa. Bull. Environ. Contam. Toxicol. 1989, 42, 754–760. [Google Scholar] [CrossRef]
- Zhu, N.; Dong, X.; Liu, Z.; Zhang, G.; Jin, W.; Xu, N. Toward highly-effective and sustainable hydrogen production: Bio-ethanol oxidative steam reforming coupled with water splitting in a thin tubular membrane reactor. Chem. Commun. 2012, 48, 7137–7139. [Google Scholar] [CrossRef]
- Rodriguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of Low-Toxicity Solvent Employment for Total Lipid and Tocopherol Compound Extraction from Patagonian Squid By-Products. Foods 2023, 12, 504. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Dong, X.; Al-Jumaily, A.; Escobar, I.C. Investigation of the Use of a Bio-Derived Solvent for Non-Solvent-Induced Phase Separation (NIPS) Fabrication of Polysulfone Membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef]
- Peng, P.; Shi, B.; Jia, L.; Li, B. Relationship Between Hansen Solubility Parameters of ABS and Its Homopolymer Components of PAN, PB, and PS. J. Macromol. Sci. Part B 2010, 49, 864–869. [Google Scholar] [CrossRef]
- Hansen, C. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 546. [Google Scholar] [CrossRef]
- Sha, J.; Zhang, R.; Zun, Y.; Zhao, R.; Wan, Y.; He, H.; Sun, R.; Jiang, G.; Li, Y.; Li, T. Equilibrium solubility, thermodynamic properties and Hansen solubility parameter of doxifluridine in (±)-2-ethyl-1-hexanol+(methanol, ethanol and acetone) at various temperatures. J. Mol. Liq. 2020, 319, 114130. [Google Scholar] [CrossRef]
- Abbott, S.; Hansen, C.M. Hansen Solubility Parameters in Practice; Hansen-Solubility. 2008. Available online: https://pirika.com/ENG/HSP/E-Book/index.html (accessed on 1 May 2025).
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Jenkins, D.; Salhadar, K.; Ashby, G.; Mishra, A.; Cheshire, J.; Beltran, F.; Grunlan, M.; Andrieux, S.; Stubenrauch, C.; Cosgriff-Hernandez, E. PoreScript: Semi-automated pore size algorithm for scaffold characterization. Bioact. Mater. 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Solvay. Rhodiasolv® Polarclean. Available online: https://www.solvay.com/sites/g/files/srpend221/files/tridion/documents/Polarclean.pdf (accessed on 8 June 2025).
- Seo, J.S.; Jeon, H.T.; Han, T.H. Rheological Investigation of Relaxation Behavior of Polycarbonate/Acrylonitrile-Butadiene-Styrene Blends. Polymers 2020, 12, 1916. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lan, C.-L. Fabrication of high-aspect-ratio ceramic microstructures by injection molding with the altered lost mold technique. J. Microelectromechanical Syst. 2001, 10, 62–68. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A novel green solvent alternative for polymeric membrane preparation via nonsolvent-induced phase separation (NIPS). J. Membr. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Yushkin, A.; Basko, A.; Balynin, A.; Efimov, M.; Lebedeva, T.; Ilyasova, A.; Pochivalov, K.; Volkov, A. Effect of Acetone as Co-Solvent on Fabrication of Polyacrylonitrile Ultrafiltration Membranes by Non-Solvent Induced Phase Separation. Polymers 2022, 14, 4603. [Google Scholar] [CrossRef]
- Yushkin, A.; Balynin, A.; Efimov, M.; Pochivalov, K.; Petrova, I.; Volkov, A. Fabrication of Polyacrylonitrile UF Membranes by VIPS Method with Acetone as Co-Solvent. Membranes 2022, 12, 523. [Google Scholar] [CrossRef]
- Hu, H.-W.; Tsao, H.-K.; Sheng, Y.-J. Impact of Nonsolvent–Solvent Affinity on Membrane Morphology and Microstructure: Unraveling the Transition from Traversing Pore to Closed Void Structures. Macromolecules 2024, 57, 7640–7653. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Dou, Z.; Xing, L.; Fang, W.; Sun, C.; Men, Z. Investigated Hydrogen-Bond Network Kinetics of Acetone-Water Solutions by Spontaneous and Stimulated Raman Spectroscopy. Opt. Express 2022, 30, 17270–17277. [Google Scholar] [CrossRef]
- Wu, N.; Li, X.; Liu, S.; Zhang, M.; Ouyang, S. Effect of Hydrogen Bonding on the Surface Tension Properties of Binary Mixture (Acetone-Water) by Raman Spectroscopy. Appl. Sci. 2019, 9, 1235. [Google Scholar] [CrossRef]
- Chen, L.; Honma, Y.; Mizutani, T.; Liaw, D.J.; Gong, J.P.; Osada, Y. Effects of polyelectrolyte complexation on the UCST of zwitterionic polymer. Polymer 2000, 41, 141–147. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Nan, Y.; Zhao, C.; Beaudoin, G.; Zhu, X.X. Synergistic Approaches in the Design and Applications of UCST Polymers. Macromol. Rapid Commun. 2023, 44, e2300261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Hailemariam, R.H.; Choi, J.-S.; Getachew, B.A.; Damtie, M.M.; Rho, H.; Park, K.-D.; Lee, J.; Woo, Y.C. Fabrication and characterization of high-performance acetone-assisted polyamide thin-film composite membranes for fluoridated saline water treatment. Desalination 2022, 538, 115922. [Google Scholar] [CrossRef]
- Tekin, F.S.; Çulfaz-Emecen, P.Z. Controlling Cellulose Membrane Performance via Solvent Choice during Precursor Membrane Formation. ACS Appl. Polym. Mater. 2023, 5, 2185–2194. [Google Scholar] [CrossRef]
- Mohamed, F.; Hasbullah, H.; Jamian, W.N.R.; Rani, A.R.A.; Saman, M.F.K.; Salleh, W.N.H.W.; Ismail, A.F. Orphological investigation of poly(Lactic acid) asymmetric membrane. J. Eng. Sci. Technol. 2015, 10, 1–8. [Google Scholar]
- Bagheripour, E.; Moghadassi, A.; Hosseini, S.M.N. Preparation of Polyvinylchloride Nanofiltration Membrane: Investigation the Effect of Thickness, Prior Evaporation Time and Addition Polyethylenglchol as Additive on Membrane Performance and Properties. Int. J. Eng. 2016, 29, 280–287. [Google Scholar]
- Eldeeb, A.; Hassaan, M.Y. Economic polymer-based waterproofing membranes by incorporation of wastes to form eco-friendly material. Egypt. J. Chem. 2020, 63, 3747–3755. [Google Scholar] [CrossRef]






| BP (°C) | FP (°C) | Viscosity (mPa·s) a | Vapor Pressure (Pa) b | Worst H3xx c | H4xx | Safety Score | Health Score | Environmental Score | Ranking | |
|---|---|---|---|---|---|---|---|---|---|---|
| PolarClean d | 280 | 146 | 9.82 | 1 | H319 | None | 2 | 2 | 1 | Recommend |
| Ethanol e | 78 | 13 | 1.1 | 8200 | H319 | None | 4 | 3 | 3 | Recommend |
| Acetone e | 56 | −18 | 0.32 | 30,800 | H319 | None | 5 | 3 | 3 | Recommend |
| ABS wt/wt% | Solvent Composition | RED a | Viscosity b (mPa·s) | Initial Volatility c (%) |
|---|---|---|---|---|
| 16 | PolarClean | 0.89 | 1013 ± 3 | 0 |
| 16 | 10 wt% Ethanol 90 wt% PolarClean | 0.90 | 551 ± 20 | 1 ± 0.8 |
| 16 | 20 wt% Ethanol 80 wt% PolarClean | 0.93 | 467 ± 33 | 2 ± 1.6 |
| 16 | 10 wt% Acetone 90 wt% PolarClean | 0.89 | 487 ± 52 | 2 ± 0.8 |
| 16 | 20 wt% Acetone 80 wt% PolarClean | 0.90 | 384 ± 27 | 5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhameri, S.; Stallings, J., Jr.; Fadhilah, E.; Ingram, E.; Leach, M.; Aronova, A.; Chwatko, M. Impact of Additives on Poly(acrylonitrile-butadiene-styrene) Membrane Formation Process Using Non-Solvent-Induced Phase Separation. Membranes 2025, 15, 181. https://doi.org/10.3390/membranes15060181
Dhameri S, Stallings J Jr., Fadhilah E, Ingram E, Leach M, Aronova A, Chwatko M. Impact of Additives on Poly(acrylonitrile-butadiene-styrene) Membrane Formation Process Using Non-Solvent-Induced Phase Separation. Membranes. 2025; 15(6):181. https://doi.org/10.3390/membranes15060181
Chicago/Turabian StyleDhameri, Sulaiman, Jason Stallings, Jr., Endras Fadhilah, Emily Ingram, Mara Leach, Anastasiia Aronova, and Malgorzata Chwatko. 2025. "Impact of Additives on Poly(acrylonitrile-butadiene-styrene) Membrane Formation Process Using Non-Solvent-Induced Phase Separation" Membranes 15, no. 6: 181. https://doi.org/10.3390/membranes15060181
APA StyleDhameri, S., Stallings, J., Jr., Fadhilah, E., Ingram, E., Leach, M., Aronova, A., & Chwatko, M. (2025). Impact of Additives on Poly(acrylonitrile-butadiene-styrene) Membrane Formation Process Using Non-Solvent-Induced Phase Separation. Membranes, 15(6), 181. https://doi.org/10.3390/membranes15060181





_Chwatko.png)

