Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water–Energy Nexus
Abstract
1. Introduction
| Desalination Technology | Plant Capacity (m3/Day) | Energy Consumption (kWh/m3) | Ref. |
|---|---|---|---|
| RO | 100,000–305,000 | 2.5–4.0 | [36] |
| NA | 2.58–8.5 | [37] | |
| 128,000 | 4–6 | [38] | |
| MSF | 50,000–70,000 | 19.58–27.25 | [38] |
| MED | 5000–15,000 | 14.45–21.35 | [38] |
| Mechanical vapor compression | 100–3000 | 7–12 | [38] |
| Thermal vapor compression | 10,000–30,000 | 16.26 | [38] |
| NF | NA | 2.54–4.2 | [39] |
| FO (standalone) | NA | 0.084–0.275 | [40] |
| NA | 0.11 | [22] | |
| MD | 1–15 | 1.58–2.63 | [41] |
2. Forward Osmosis (FO)
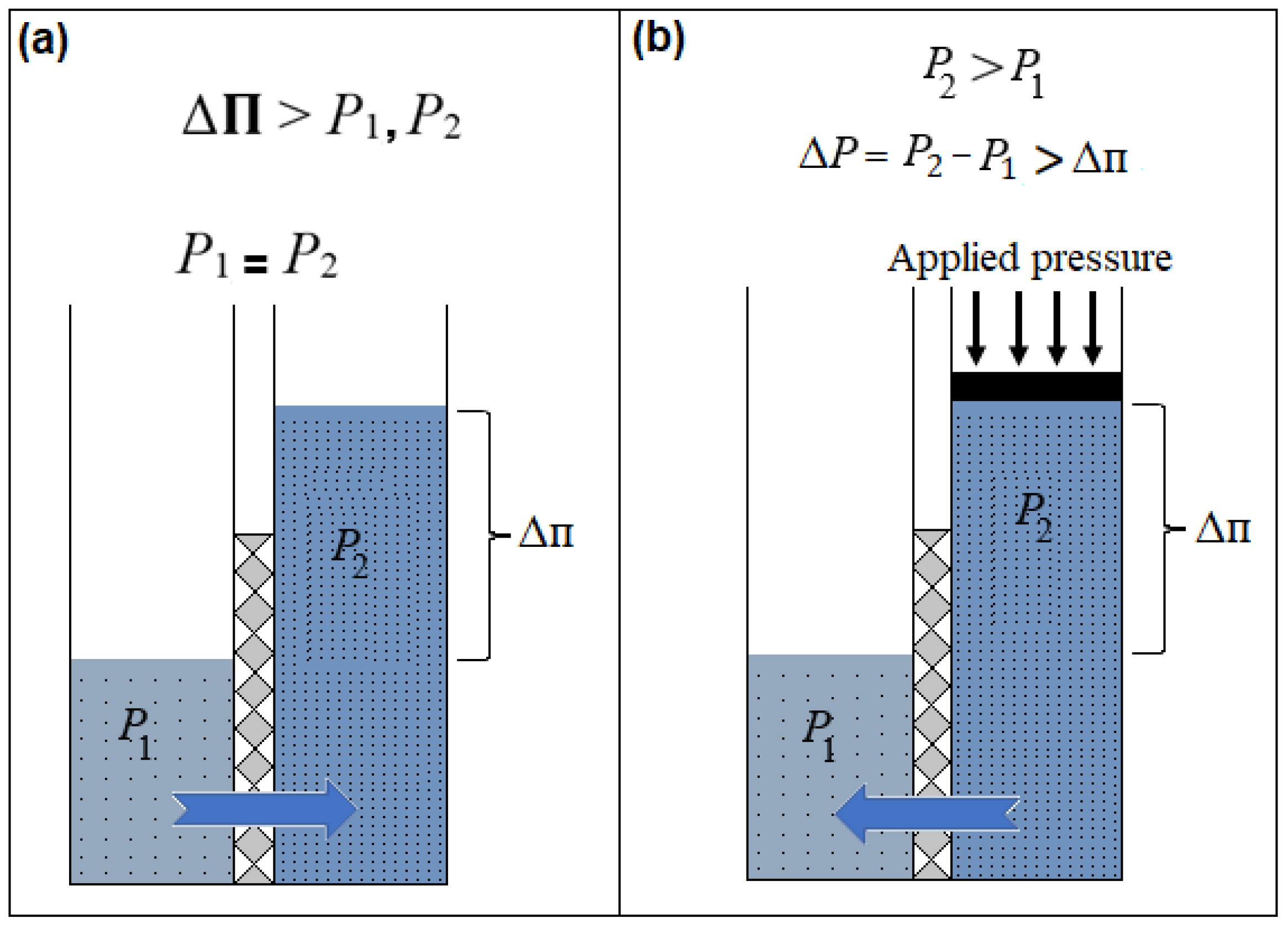
2.1. Overview
2.2. FO Opportunities and Challenges
2.3. Draw Solution
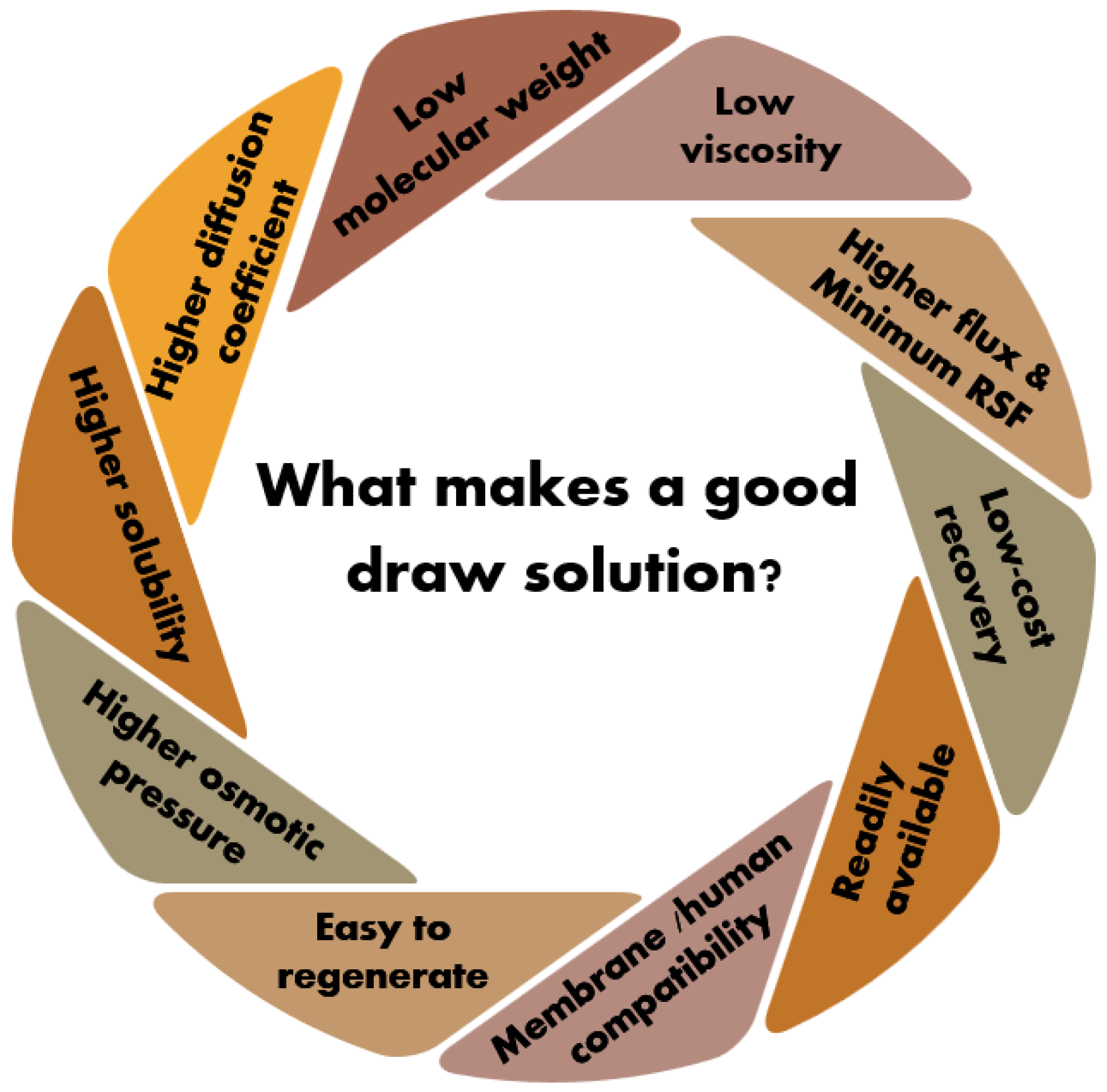
2.3.1. Characteristics of an Ideal Draw Solution
2.3.2. Selection of an Appropriate DS
2.3.3. Classification of Draw Solutes
Inorganic Salts
| DS | DS Conc. | FS | FO Membrane | Flux (LMH) | Recovery | Ref. |
|---|---|---|---|---|---|---|
| NaCl | 1 M | DI water | Flat-sheet CTA membrane from FTSH20©, Albany, OR, USA | NA | NA | [90] |
| MgCl2 | 1 M | DI water | CTA flat-sheet membrane from FTSH20©, USA | NA | NA | [90] |
| NaCl | 0.5 M | DI water, wastewater | CTA membrane from HTI, Albany, OR, USA | 6 | MD | [93] |
| NaCl | 0.8 M | Wastewater | CTA based FO membrane from HTI, USA | 9–52 | NF | [94] |
| NaCl | 1.5 M | Raw sewage | Flat-sheet cellulose-based membrane from HTI, USA | 8 | MD | [95] |
| MgCl2 | 1.5 M | Digested sludge | Flat-sheet CTA membrane from HTI, USA | 9 | MD | [91] |
| NaCl | 4.82 M | Waste landfill leachate | TFC membrane from HTI, USA | NA | MD | [96] |
| NaCl | 0.5–2 M | BSA solution | Hydrophobic PBI NF hollow fiber membrane | NA | MD | [72] |
| KCl | 2 M | DI water | CA membrane from HTI, USA | 22.6 | NA | [97] |
| NaNO3 | 20.35 | |||||
| KNO3 | 15.8 | |||||
| NH4NO3 | 14.9 | |||||
| NH4Cl | 19.07 | |||||
| (NH4)2SO4 | 19.23 | |||||
| NH4H2PO4 | 15.51 | |||||
| Ca(NO3)2 | 17.91 | |||||
| (NH4)2HPO4 | 13.88 | |||||
| CaCl2 | 1.6 M | DI water | PA based flat-sheet TFC membrane from HTI, USA | NA | MD | [92] |
| HCOONa | 4.1 M | |||||
| KBr | 3.2 M | |||||
| LiBr | 2.2 M | |||||
| LiCl | 2.6 M | |||||
| MgCl2 | 1.5 M | |||||
| Na(C2H5COO) | 4.1 M | |||||
| NaCl | 3.0 M |
Organic Compounds
Thermo-Responsive Polymers
Magnetic Nanoparticles (MNPs)
| DS Category | DS | FO Membrane | Flux (LMH) | Recovery | Ref. |
|---|---|---|---|---|---|
| Organic compounds | PAA-Na | Flat-sheet CTA membrane from HTI (USA) | 5 | UF | [100] |
| Na salt of Poly (aspartic acid) | TFC FO membrane from HTI (USA) | 31.8 | NF, MD | [101] | |
| PEI | TFC membrane from HTI, USA | NA | NF | [102] | |
| Dimethyl ether | NA | NA | Thermal heating | [104] | |
| Sodium Polystyrene sulfonate | Commercial TFC FO membrane from HTI (USA) | 13 | UF | [105] | |
| Thermo-responsive polymer | PAGB | Commercial flat-sheet CTA membrane (HTI, USA) | NA | NA | [114] |
| EO-PO copolymer | Hollow fiber FO membrane (TOYOBO, Osaka, Japan) | NA | Coalescer and NF | [115] | |
| Pluronic® L-35 | TFC hollow fiber FO membrane | 1.22 | Phase separation | [116] | |
| MNPs | Citrate coated Fe3O4 | Commercial CTA membrane (HTI, USA) | 17.3 | Magnetic separation | [117] |
| PEG diacid coated MNPs | Flat-sheet membrane (HTI, USA) | >10 | NA | [118] | |
| Dextran coated Fe3O4 MNPs | HTI FO membrane | NA | Magnetic separation | [109] | |
| High charged compound | EDTA sodium salt | CTA FO membrane (HTI, USA) | 8.45 | NF | [87] |
| EDTA-2Na | NA | NA | MD | [119] | |
| Hydrogels | Hydrolyzed polyacrylamide | Polyamide-based TFC FO membrane | NA | NA | [103] |
| Polyacrylamide | NA | 0.36 | Pressure stimuli | [88] |
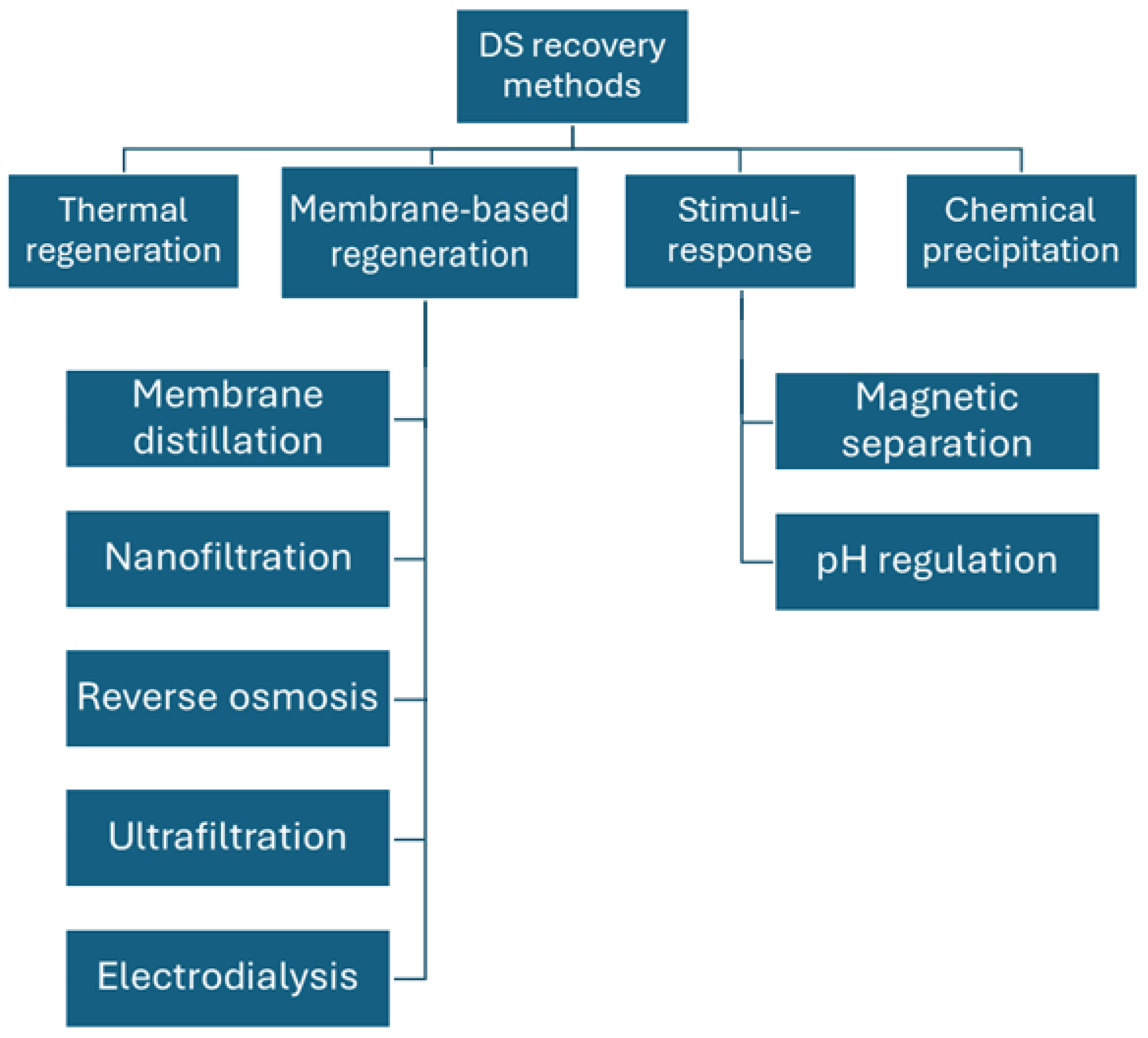
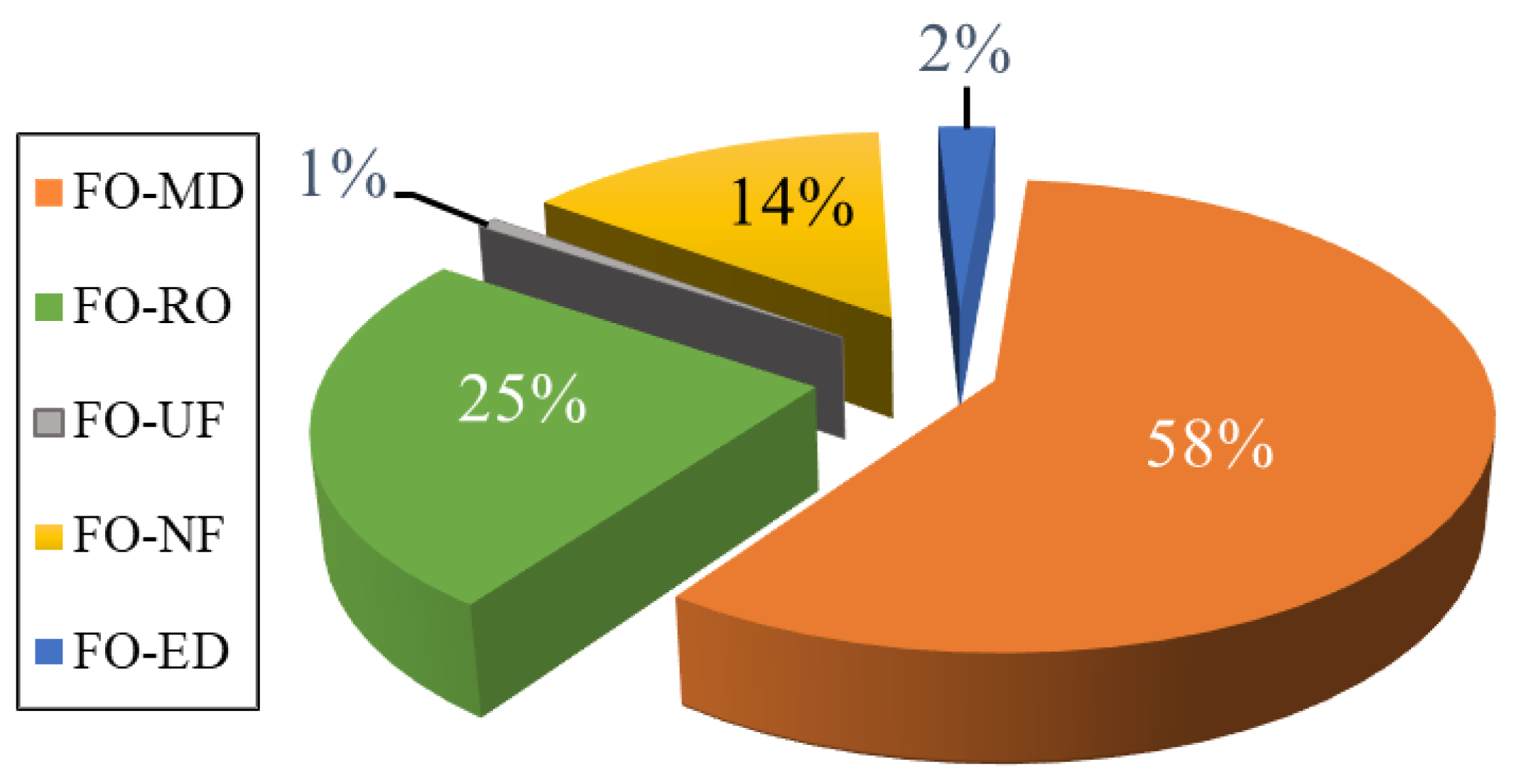
2.4. DS Regeneration Techniques
| Category | Recovery Method | DS Type | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|
| Thermal regeneration | Heating at 60 °C | Switchable polarity solvent | 1. Thermal regeneration is energy intensive. 2. Low-grade or renewable heat sources can be used. 3. Product quality is poor. | [120] |
| Heating at 60 °C | NH3/CO2 | [122] | ||
| Heating above 60 °C | Thermo-responsive polymer | [86] | ||
| Membrane-based regeneration | MD | NaCl | 1. Besides MD, other processes are less energy intensive. 2. High water quality and recovery rate. | [123] |
| NF | MgCl2 | [124] | ||
| UF | Polyelectrolyte | [100] | ||
| RO | Real seawater | [125] | ||
| ED | NaCl | [74] | ||
| Stimuli response | Magnetic separation | Functionalized MNPs | 1. Only suitable for materials whose solubility changes with pH. 2. Low water quality. 3. Cost-effective. | [108] |
| Magnetic separation | Fe3O4 MNPs | [109] | ||
| pH regulation | EDTA-2Na | [126] | ||
| Chemical precipitation | Metathesis precipitation | Copper sulfate | 1. The product could be toxic. 2. Chemicals are costly. | [121] |
3. Membrane Distillation
3.1. Overview
3.2. Factors Limiting Commercialization and Industrial Application of MD
3.2.1. Fouling
3.2.2. Membrane Pore Wetting
3.2.3. High Thermal Energy Consumption
4. Integrated FO-MD Process
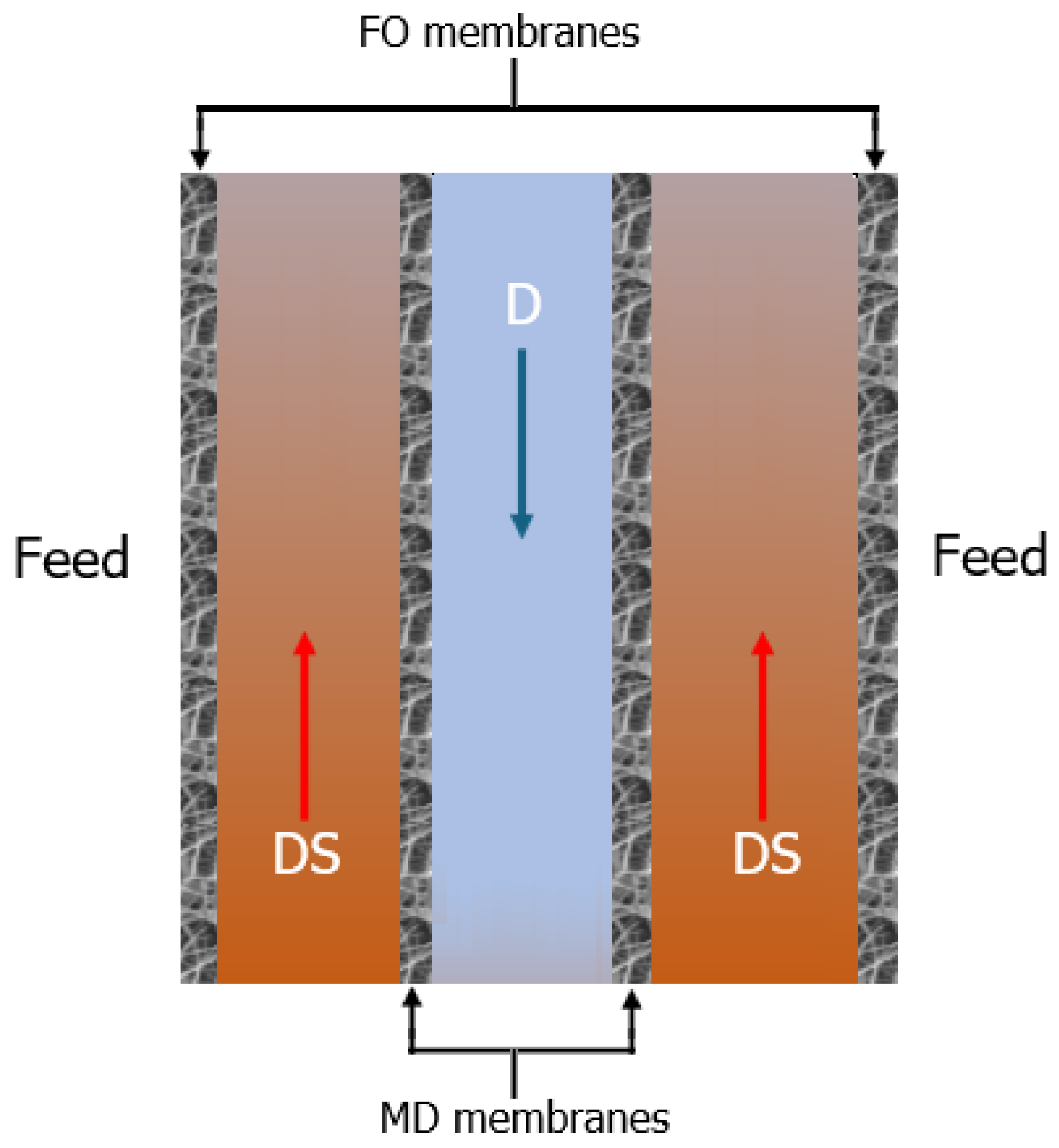
4.1. Fundamentals of the Hybrid Process
4.2. Opportunities to Integrate FO with MD in a Hybrid Process
4.3. Fouling and Its Mitigation Through Advanced Membrane Material
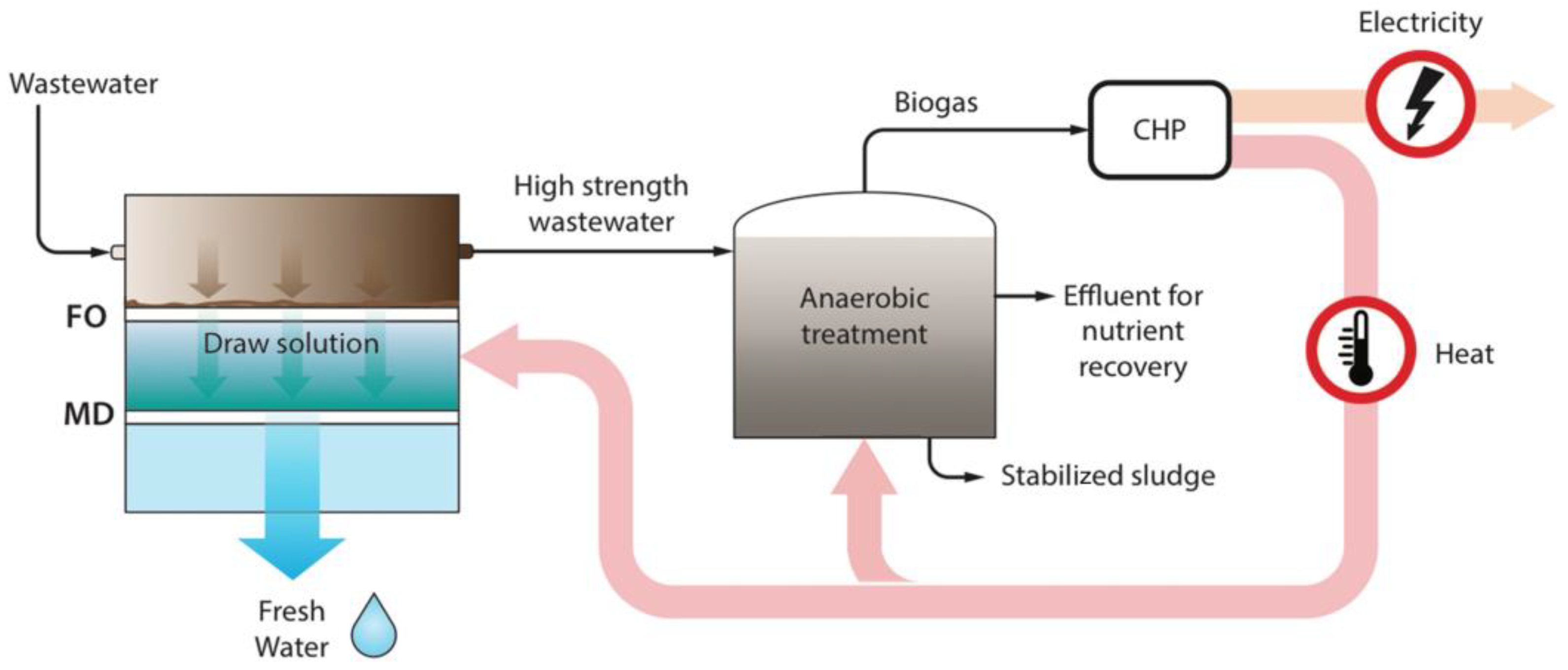
4.4. Applications of FO-MD Hybrid Processes
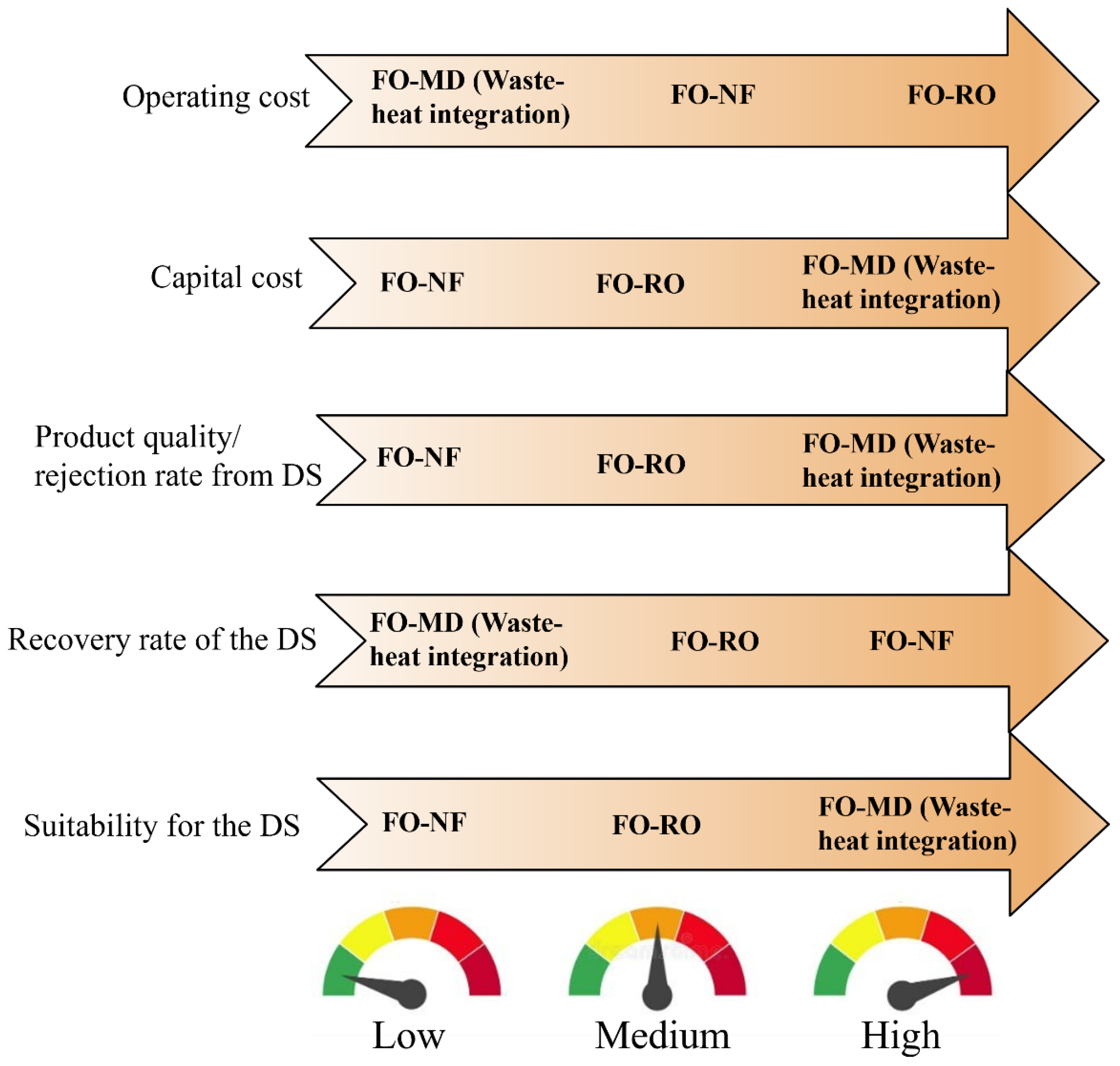
4.5. Economics of FO-MD Hybrid Processes
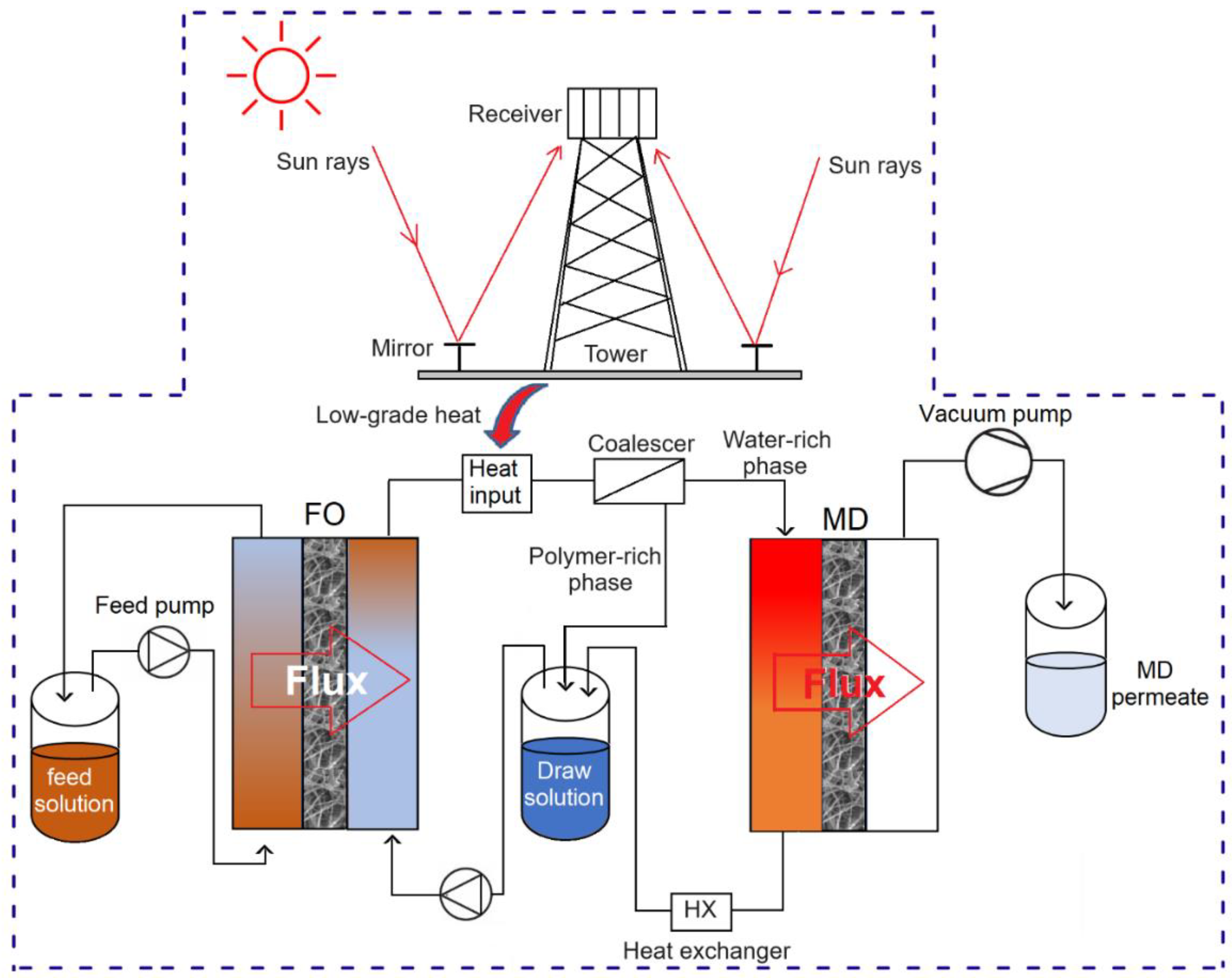
4.6. Energy Consumption in FO-MD Hybrid Processes
4.7. Scale-Up of the FO-MD Hybrid Processes
5. Conclusions and Outlook
- Despite progress in membrane technology, further research is essential in areas such as energy-efficient module design and scalable configurations to bring the technology closer to commercialization.
- An optimal DS must offer high osmotic pressure, a high diffusion coefficient, increased solubility, low molecular weight and viscosity, minimal RSF, cost-effective recovery, and compatibility with membranes and human consumption-related applications, such as food and drinking water. However, existing DSs are economically not feasible, due to either high costs or expensive regeneration processes.
- Although FO-MD hybrid processes demonstrate significant promise for industrial applications, particularly in treating complex wastewater, they remain so far predominantly at the laboratory scale due to the challenges associated with DS selection and its regeneration costs.
- The FO-MD hybrid process has the potential to achieve significantly lower OPEX compared to other membrane-based hybrid systems, such as FO-RO, particularly when utilizing waste or renewable energy sources for MD. For example, an FO-MD system powered by waste heat can produce water at a cost of USD 1.28/m3, making it 61.9% cheaper than using a conventional heat source for FO-MD, which costs USD 3.36/m3. Additionally, it is 12.93% more cost-effective than the FO-RO process, which produces water at USD 1.47/m3.
- Although there have been few studies examining FO-MD hybrid processes on an industrial scale, such as wastewater treatment utilizing waste heat, there is a pressing need for further techno-economic analysis to assess its feasibility in various industries.
Author Contributions
Funding
Conflicts of Interest
References
- Karagiannis, I.C.; Soldatos, P.G. Water desalination cost literature: Review and assessment. Desalination 2008, 223, 448–456. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh water resource, scarcity, water salinity challenges and possible remedies: A review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Chiam, C.-K.; Sarbatly, R. Vacuum membrane distillation processes for aqueous solution treatment—A review. Chem. Eng. Process. Process Intensif. 2013, 74, 27–54. [Google Scholar] [CrossRef]
- Ahmed, M. A study on the influence of feed and draw solution concentrations on the performance of the pilot-scale forward osmosis-membrane distillation system. Desalination Water Treat. 2024, 317, 100225. [Google Scholar] [CrossRef]
- El-Bourawi, M.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Sangeetha, D.; Chang, H.-M.; Thanh, C.N.D.; Le, Q.H.; Ku, H.-M. Developments in forward osmosis and membrane distillation for desalination of waters. Environ. Chem. Lett. 2018, 16, 1247–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Dow, N.; Duke, M.; Ostarcevic, E.; Gray, S. Identification of material and physical features of membrane distillation membranes for high performance desalination. J. Membr. Sci. 2010, 349, 295–303. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Membr. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Lin, Y.-H.; Chen, J. Enhanced air gap membrane desalination by novel finned tubular membrane modules. J. Membr. Sci. 2011, 378, 398–406. [Google Scholar] [CrossRef]
- Maab, H.; Francis, L.; Al-Saadi, A.; Aubry, C.; Ghaffour, N.; Amy, G.; Nunes, S.P. Synthesis and fabrication of nanostructured hydrophobic polyazole membranes for low-energy water recovery. J. Membr. Sci. 2012, 423, 11–19. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Li, X.; Wang, X.; Drioli, E.; Wang, Z.; Zhao, S. Optimization of novel composite membranes for water and mineral recovery by vacuum membrane distillation. Desalination 2018, 440, 39–47. [Google Scholar] [CrossRef]
- Bagger-Jørgensen, R.; Meyer, A.S.; Pinelo, M.; Varming, C.; Jonsson, G. Recovery of volatile fruit juice aroma compounds by membrane technology: Sweeping gas versus vacuum membrane distillation. Innov. Food Sci. Emerg. Technol. 2011, 12, 388–397. [Google Scholar] [CrossRef]
- Criscuoli, A.; Drioli, E. Vacuum membrane distillation for the treatment of coffee products. Sep. Purif. Technol. 2019, 209, 990–996. [Google Scholar] [CrossRef]
- Bandini, S.; Sarti, G.C. Concentration of must through vacuum membrane distillation. Desalination 2002, 149, 253–259. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, G.; Xu, Z.; Zhao, X.; Xie, W.; Wang, L.; Zhai, Z.; Liu, J.; Zhao, S.; Zhao, Y. Multi-hierarchical structured PTFE membrane for liquid desiccant dewatering via membrane distillation. J. Membr. Sci. 2025, 715, 123449. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Hanot, B.; Molina-Fernández, C.; Luis, P. Experimental mass transfer comparison between vacuum and direct contact membrane distillation for the concentration of carbonate solutions. Sep. Purif. Technol. 2021, 275, 119193. [Google Scholar] [CrossRef]
- Bagger-Jørgensen, R.; Meyer, A.S.; Varming, C.; Jonsson, G. Recovery of volatile aroma compounds from black currant juice by vacuum membrane distillation. J. Food Eng. 2004, 64, 23–31. [Google Scholar] [CrossRef]
- Fatima, S.; Govardhan, B.; Kalyani, S.; Sridhar, S. Extraction of volatile organic compounds from water and wastewater by vacuum-driven membrane process: A comprehensive review. Chem. Eng. J. 2022, 434, 134664. [Google Scholar] [CrossRef]
- Mazlan, N.M.; Peshev, D.; Livingston, A.G. Energy consumption for desalination—A comparison of forward osmosis with reverse osmosis, and the potential for perfect membranes. Desalination 2016, 377, 138–151. [Google Scholar] [CrossRef]
- Xie, M.; Lee, J.; Nghiem, L.D.; Elimelech, M. Role of pressure in organic fouling in forward osmosis and reverse osmosis. J. Membr. Sci. 2015, 493, 748–754. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.-S. Draw solutions for forward osmosis processes: Developments, challenges, and prospects for the future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Altaee, A.; Zaragoza, G.; van Tonningen, H.R. Comparison between Forward Osmosis-Reverse Osmosis and Reverse Osmosis processes for seawater desalination. Desalination 2014, 336, 50–57. [Google Scholar] [CrossRef]
- Altaee, A.; Millar, G.J.; Zaragoza, G.; Sharif, A. Energy efficiency of RO and FO–RO system for high-salinity seawater treatment. Clean Technol. Environ. Policy 2017, 19, 77–91. [Google Scholar] [CrossRef]
- Choi, B.G.; Zhan, M.; Shin, K.; Lee, S.; Hong, S. Pilot-scale evaluation of FO-RO osmotic dilution process for treating wastewater from coal-fired power plant integrated with seawater desalination. J. Membr. Sci. 2017, 540, 78–87. [Google Scholar] [CrossRef]
- He, F.; Gilron, J.; Lee, H.; Song, L.; Sirkar, K.K. Potential for scaling by sparingly soluble salts in crossflow DCMD. J. Membr. Sci. 2008, 311, 68–80. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Grzechulska, J.; Morawski, A.W. Membrane distillation of NaCl solution containing natural organic matter. J. Membr. Sci. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently Combining Water Reuse and Desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef]
- Patel, D.; Mudgal, A.; Patel, V.; Patel, J. Water desalination and wastewater reuse using integrated reverse osmosis and forward osmosis system. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1146, 012029. [Google Scholar] [CrossRef]
- Voutchkov, N. Energy use for membrane seawater desalination—Current status and trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of-the-art review. Desalination 2020, 491, 114569. [Google Scholar] [CrossRef]
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, L.; Fu, Y.; Zhu, M.; Xue, L. Development of lower cost seawater desalination processes using nanofiltration technologies—A review. Desalination 2015, 376, 109–116. [Google Scholar] [CrossRef]
- Iskander, S.M.; Zou, S.; Brazil, B.; Novak, J.T.; He, Z. Energy consumption by forward osmosis treatment of landfill leachate for water recovery. Waste Manag. 2017, 63, 284–291. [Google Scholar] [CrossRef]
- Bahar, R.; Ng, K.C. Fresh water production by membrane distillation (MD) using marine engine’s waste heat. Sustain. Energy Technol. Assess. 2020, 42, 100860. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R.; Elimelech, M. Performance evaluation of sucrose concentration using forward osmosis. J. Membr. Sci. 2009, 338, 61–66. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, J.J. Mass transfer limitations in forward osmosis: Are some potential applications overhyped? Desalination 2013, 318, 118–124. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Kamel, A.H.; Al-Juboori, R.A.; al-shaeli, M.; Ladewig, B.; Ibrahim, S.S.; Alsalhy, Q.F. Potential application of hybrid forward osmosis—Membrane distillation (FO-MD) system for various water treatment processes. Process Saf. Environ. Prot. 2023, 180, 1023–1052. [Google Scholar] [CrossRef]
- Hu, B.; Huang, L.; Chang, H.; Ji, Z.; Yan, Z.; Qu, D.; Wang, J.; Qu, F.; Liang, H. A review of key parameters affecting inorganic scaling in thermal, pressure, and osmosis-driven membranes for produced water desalination. Sep. Purif. Technol. 2025, 354, 129023. [Google Scholar] [CrossRef]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Hilal, N. Recent trends in membranes and membrane processes for desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Zou, S.; Yuan, H.; Childress, A.; He, Z. Energy Consumption by Recirculation: A Missing Parameter When Evaluating Forward Osmosis. Environ. Sci. Technol. 2016, 50, 6827–6829. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Anh-Vu, N.; Nomura, Y.; Hidaka, T.; Fujiwara, T. Forward osmosis membrane process: A review of temperature effects on system performance and membrane parameters from experimental studies. J. Environ. Chem. Eng. 2024, 12, 113429. [Google Scholar] [CrossRef]
- Hancock, N.T.; Cath, T.Y. Solute Coupled Diffusion in Osmotically Driven Membrane Processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Wang, H.; Xie, Z. Smart utilisation of reverse solute diffusion in forward osmosis for water treatment: A mini review. Sci. Total Environ. 2023, 873, 162430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Wu, S. A comprehensive review of responsive draw solutes in forward osmosis: Categories, characteristics, mechanisms and modifications. Desalination 2024, 583, 117676. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Widjojo, N.; Chung, T.-S. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Bui, N.-N.; McCutcheon, J.R. Hydrophilic Nanofibers as New Supports for Thin Film Composite Membranes for Engineered Osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. [Google Scholar] [CrossRef]
- Chekli, L.; Kim, Y.; Phuntsho, S.; Li, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Evaluation of fertilizer-drawn forward osmosis for sustainable agriculture and water reuse in arid regions. J. Environ. Manag. 2017, 187, 137–145. [Google Scholar] [CrossRef]
- Raval, H.D.; Koradiya, P. Direct fertigation with brackish water by a forward osmosis system converting domestic reverse osmosis module into forward osmosis membrane element. Desalination Water Treat. 2016, 57, 15740–15747. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Effects of draw solutions and membrane conditions on electricity generation and water flux in osmotic microbial fuel cells. Bioresour. Technol. 2012, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
- Al-aibi, S.; Mahood, H.B.; Sharif, A.O.; Alpay, E.; Simcoe-Read, H. Evaluation of draw solution effectiveness in a forward osmosis process. Desalination Water Treat. 2016, 57, 13425–13432. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; He, Z. Tackle reverse solute flux in forward osmosis towards sustainable water recovery: Reduction and perspectives. Water Res. 2019, 149, 362–374. [Google Scholar] [CrossRef]
- Chung, T.-S.; Li, X.; Ong, R.C.; Ge, Q.; Wang, H.; Han, G. Emerging forward osmosis (FO) technologies and challenges ahead for clean water and clean energy applications. Curr. Opin. Chem. Eng. 2012, 1, 246–257. [Google Scholar] [CrossRef]
- Gray, G.T.; McCutcheon, J.R.; Elimelech, M. Internal concentration polarization in forward osmosis: Role of membrane orientation. Desalination 2006, 197, 1–8. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, S.; Elimelech, M.; Lee, S.; Hong, S. Effect of hydraulic pressure and membrane orientation on water flux and reverse solute flux in pressure assisted osmosis. J. Membr. Sci. 2014, 465, 159–166. [Google Scholar] [CrossRef]
- Su, J.; Chung, T.-S. Sublayer structure and reflection coefficient and their effects on concentration polarization and membrane performance in FO processes. J. Membr. Sci. 2011, 376, 214–224. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.; Hong, S. A novel analysis of reverse draw and feed solute fluxes in forward osmosis membrane process. Desalination 2014, 352, 128–135. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.; Hasan, S. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Bamaga, O.A.; Yokochi, A.; Zabara, B.; Babaqi, A.S. Hybrid FO/RO desalination system: Preliminary assessment of osmotic energy recovery and designs of new FO membrane module configurations. Desalination 2011, 268, 163–169. [Google Scholar] [CrossRef]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.-S. Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Tan, C.H.; Ng, H.Y. A novel hybrid forward osmosis—Nanofiltration (FO-NF) process for seawater desalination: Draw solution selection and system configuration. Desalination Water Treat. 2010, 13, 356–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. A Natural Driven Membrane Process for Brackish and Wastewater Treatment: Photovoltaic Powered ED and FO Hybrid System. Environ. Sci. Technol. 2013, 47, 10548–10555. [Google Scholar] [CrossRef]
- Altaee, A.; Zaragoza, G. A conceptual design of low fouling and high recovery FO–MSF desalination plant. Desalination 2014, 343, 2–7. [Google Scholar] [CrossRef]
- Altaee, A.; Mabrouk, A.; Bourouni, K. A novel Forward osmosis membrane pretreatment of seawater for thermal desalination processes. Desalination 2013, 326, 19–29. [Google Scholar] [CrossRef]
- Altaee, A.; Mabrouk, A.; Bourouni, K.; Palenzuela, P. Forward osmosis pretreatment of seawater to thermal desalination: High temperature FO-MSF/MED hybrid system. Desalination 2014, 339, 18–25. [Google Scholar] [CrossRef]
- Ling, M.M.; Chung, T.-S. Surface-Dissociated Nanoparticle Draw Solutions in Forward Osmosis and the Regeneration in an Integrated Electric Field and Nanofiltration System. Ind. Eng. Chem. Res. 2012, 51, 15463–15471. [Google Scholar] [CrossRef]
- Yen, S.K.; Su, M.; Wang, K.Y.; Chung, T.-S. Study of draw solutes using 2-methylimidazole-based compounds in forward osmosis. J. Membr. Sci. 2010, 364, 242–252. [Google Scholar] [CrossRef]
- Gwak, G.; Hong, S. Chapter 3—Draw Solute Selection. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation; Sarp, S., Hilal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 87–122. [Google Scholar]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Yu, B. Recent Advance on Draw Solutes Development in Forward Osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef]
- McGovern, R.K.; Lienhard V, J.H. On the potential of forward osmosis to energetically outperform reverse osmosis desalination. J. Membr. Sci. 2014, 469, 245–250. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Ghaffour, N.; Amy, G.; Leiknes, T.; Vrouwenvelder, J.S. Life cycle cost of a hybrid forward osmosis—Low pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 2016, 88, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Dey, K.; Dsilva Winfred Rufuss, D.; Arulvel, S.; Akinaga, T. Forward osmosis desalination: A critical review focussing on recent advancements in draw solution recovery techniques for enhanced efficiency and regeneration. J. Environ. Chem. Eng. 2024, 12, 113968. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Petrinic, I.; Jancic, N.; Jansen van Vuuren, R.D.; Buksek, H. Commercial thermo-responsive polyalkylene glycols as draw agents in forward osmosis. Desalination 2024, 582, 117576. [Google Scholar] [CrossRef]
- Hau, N.T.; Chen, S.-S.; Nguyen, N.C.; Huang, K.Z.; Ngo, H.H.; Guo, W. Exploration of EDTA sodium salt as novel draw solution in forward osmosis process for dewatering of high nutrient sludge. J. Membr. Sci. 2014, 455, 305–311. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Yao, J.; Simon, G.P.; Wang, H. Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem. Commun. 2011, 47, 1710–1712. [Google Scholar] [CrossRef]
- Alejo, T.; Arruebo, M.; Carcelen, V.; Monsalvo, V.M.; Sebastian, V. Advances in draw solutes for forward osmosis: Hybrid organic-inorganic nanoparticles and conventional solutes. Chem. Eng. J. 2017, 309, 738–752. [Google Scholar] [CrossRef]
- Eddouibi, J.; Abderafi, S.; Vaudreuil, S.; Bounahmidi, T. Water desalination by forward osmosis: Dynamic performance assessment and experimental validation using MgCl2 and NaCl as draw solutes. Comput. Chem. Eng. 2021, 149, 107313. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Toward Resource Recovery from Wastewater: Extraction of Phosphorus from Digested Sludge Using a Hybrid Forward Osmosis–Membrane Distillation Process. Environ. Sci. Technol. Lett. 2014, 1, 191–195. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Vanneste, J.; Cath, T.Y. Assessment of alternative draw solutions for optimized performance of a closed-loop osmotic heat engine. J. Membr. Sci. 2016, 504, 162–175. [Google Scholar] [CrossRef]
- Parveen, F.; Hankins, N. Integration of Forward Osmosis Membrane Bioreactor (FO-MBR) and Membrane Distillation (MD) units for water reclamation and regeneration of draw solutions. J. Water Process Eng. 2021, 41, 102045. [Google Scholar] [CrossRef]
- Pal, P.; Chakrabortty, S.; Nayak, J.; Senapati, S. A flux-enhancing forward osmosis–nanofiltration integrated treatment system for the tannery wastewater reclamation. Environ. Sci. Pollut. Res. 2017, 24, 15768–15780. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. A Forward Osmosis–Membrane Distillation Hybrid Process for Direct Sewer Mining: System Performance and Limitations. Environ. Sci. Technol. 2013, 47, 13486–13493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: Evaluating the performance of fertilizer draw solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.M. A critical review on draw solutes development for forward osmosis. Desalination 2016, 391, 16–29. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.-S. Polyelectrolyte-Promoted Forward Osmosis–Membrane Distillation (FO–MD) Hybrid Process for Dye Wastewater Treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Su, J.; Amy, G.L.; Chung, T.-S. Exploration of polyelectrolytes as draw solutes in forward osmosis processes. Water Res. 2012, 46, 1318–1326. [Google Scholar] [CrossRef]
- Gwak, G.; Jung, B.; Han, S.; Hong, S. Evaluation of poly (aspartic acid sodium salt) as a draw solute for forward osmosis. Water Res. 2015, 80, 294–305. [Google Scholar] [CrossRef]
- Jun, B.-M.; Nguyen, T.P.N.; Ahn, S.-H.; Kim, I.-C.; Kwon, Y.-N. The application of polyethyleneimine draw solution in a combined forward osmosis/nanofiltration system. J. Appl. Polym. Sci. 2015, 132, 42198–42206. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, B.; Yue, Q.; Kong, J.; Shon, H.K.; Liu, P.; Gao, Y. Explore the forward osmosis performance using hydrolyzed polyacrylamide as draw solute for dye wastewater reclamation in the long-term process. Chem. Eng. J. 2015, 273, 316–324. [Google Scholar] [CrossRef]
- Monjezi, A.A.; Mahood, H.B.; Campbell, A.N. Regeneration of dimethyl ether as a draw solute in forward osmosis by utilising thermal energy from a solar pond. Desalination 2017, 415, 104–114. [Google Scholar] [CrossRef]
- Tian, E.; Hu, C.; Qin, Y.; Ren, Y.; Wang, X.; Wang, X.; Xiao, P.; Yang, X. A study of poly (sodium 4-styrenesulfonate) as draw solute in forward osmosis. Desalination 2015, 360, 130–137. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, Q.; Chen, N.; Lu, X. Thermoresponsive copolymer-based draw solution for seawater desalination in a combined process of forward osmosis and membrane distillation. Desalination 2014, 348, 26–32. [Google Scholar] [CrossRef]
- Kim, J.-j.; Chung, J.-S.; Kang, H.; Yu, Y.A.; Choi, W.J.; Kim, H.J.; Lee, J.-C. Thermo-responsive copolymers with ionic group as novel draw solutes for forward osmosis processes. Macromol. Res. 2014, 22, 963–970. [Google Scholar] [CrossRef]
- Ling, M.M.; Wang, K.Y.; Chung, T.-S. Highly Water-Soluble Magnetic Nanoparticles as Novel Draw Solutes in Forward Osmosis for Water Reuse. Ind. Eng. Chem. Res. 2010, 49, 5869–5876. [Google Scholar] [CrossRef]
- Joafshan, M.; Shakeri, A.; Razavi, S.R.; Salehi, H. Gas responsive magnetic nanoparticle as novel draw agent for removal of Rhodamine B via forward osmosis: High water flux and easy regeneration. Sep. Purif. Technol. 2022, 282, 119998. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D. Highly water soluble and recovered dextran coated Fe3O4 magnetic nanoparticles for brackish water desalination. Sep. Purif. Technol. 2011, 81, 392–399. [Google Scholar] [CrossRef]
- Dey, P.; Izake, E.L. Magnetic nanoparticles boosting the osmotic efficiency of a polymeric FO draw agent: Effect of polymer conformation. Desalination 2015, 373, 79–85. [Google Scholar] [CrossRef]
- Ling, M.M.; Chung, T.-S. Desalination process using super hydrophilic nanoparticles via forward osmosis integrated with ultrafiltration regeneration. Desalination 2011, 278, 194–202. [Google Scholar] [CrossRef]
- Tayel, A.; Nasr, P.; Sewilam, H. Enhanced water flux using uncoated magnetic nanoparticles as a draw solution in forward osmosis desalination. Desalination Water Treat. 2020, 193, 169–176. [Google Scholar] [CrossRef]
- Yasukawa, M.; Tanaka, Y.; Takahashi, T.; Shibuya, M.; Mishima, S.; Matsuyama, H. Effect of molecular weight of draw solute on water permeation in forward osmosis process. Ind. Eng. Chem. Res. 2015, 54, 8239–8246. [Google Scholar] [CrossRef]
- Ahmed, M.; Kumar, R.; Garudachari, B.; Thomas, J.P. Performance evaluation of a thermoresponsive polyelectrolyte draw solution in a pilot scale forward osmosis seawater desalination system. Desalination 2019, 452, 132–140. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.-N.; Chong, J.Y.; Wang, R. Thermo-responsive nonionic amphiphilic copolymers as draw solutes in forward osmosis process for high-salinity water reclamation. Water Res. 2022, 221, 118768. [Google Scholar] [CrossRef]
- Na, Y.; Yang, S.; Lee, S. Evaluation of citrate-coated magnetic nanoparticles as draw solute for forward osmosis. Desalination 2014, 347, 34–42. [Google Scholar] [CrossRef]
- Ge, Q.; Su, J.; Chung, T.-S.; Amy, G. Hydrophilic Superparamagnetic Nanoparticles: Synthesis, Characterization, and Performance in Forward Osmosis Processes. Ind. Eng. Chem. Res. 2011, 50, 382–388. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Ho, S.-T.; Nguyen, H.T.; Ray, S.S.; Nguyen, N.T.; Hsu, H.-T.; Le, N.C.; Tran, T.T. Optimising the recovery of EDTA-2Na draw solution in forward osmosis through direct contact membrane distillation. Sep. Purif. Technol. 2018, 198, 108–112. [Google Scholar] [CrossRef]
- Stone, M.L.; Rae, C.; Stewart, F.F.; Wilson, A.D. Switchable polarity solvents as draw solutes for forward osmosis. Desalination 2013, 312, 124–129. [Google Scholar] [CrossRef]
- Alnaizy, R.; Aidan, A.; Qasim, M. Draw solute recovery by metathesis precipitation in forward osmosis desalination. Desalination Water Treat. 2013, 51, 5516–5525. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot demonstration of the NH3/CO2 forward osmosis desalination process on high salinity brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Fu, X.; Chung, T.-S. Sustainable water recovery from oily wastewater via forward osmosis-membrane distillation (FO-MD). Water Res. 2014, 52, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Dave, P.; Nath, K. Performance of low pressure nanofiltration membrane in forward osmosis using magnesium chloride as draw solute. J. Water Process Eng. 2020, 33, 101092. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Li, Z.; Valladares, R.; Li, Q.; Amy, G. Indirect desalination of Red Sea water with forward osmosis and low pressure reverse osmosis for water reuse. Desalination 2011, 280, 160–166. [Google Scholar] [CrossRef]
- Halakarni, M.A.; Samage, A.; Mahto, A.; Polisetti, V.; Nataraj, S.K. Forward osmosis process for energy materials recovery from industrial wastewater with simultaneous recovery of reusable water: A sustainable approach. Mater. Today Sustain. 2023, 22, 100361. [Google Scholar] [CrossRef]
- Calabro, V.; Jiao, B.L.; Drioli, E. Theoretical and experimental study on membrane distillation in the concentration of orange juice. Ind. Eng. Chem. Res. 1994, 33, 1803–1808. [Google Scholar] [CrossRef]
- Cath, T.Y.; Adams, V.D.; Childress, A.E. Experimental study of desalination using direct contact membrane distillation: A new approach to flux enhancement. J. Membr. Sci. 2004, 228, 5–16. [Google Scholar] [CrossRef]
- Cojocaru, C.; Khayet, M. Sweeping gas membrane distillation of sucrose aqueous solutions: Response surface modeling and optimization. Sep. Purif. Technol. 2011, 81, 12–24. [Google Scholar] [CrossRef]
- Basini, L.; D’Angelo, G.; Gobbi, M.; Sarti, G.; Gostoli, C. A desalination process through sweeping gas membrane distillation. Desalination 1987, 64, 245–257. [Google Scholar] [CrossRef]
- Sarti, G.; Gostoli, C.; Bandini, S. Extraction of organic components from aqueous streams by vacuum membrane distillation. J. Membr. Sci. 1993, 80, 21–33. [Google Scholar] [CrossRef]
- Mohammadi, T.; Bakhteyari, O. Concentration of l-lysine monohydrochloride (l-lysine- HCl) syrup using vacuum membrane distillation. Desalination 2006, 200, 591–594. [Google Scholar] [CrossRef]
- Mohammadi, T.; Akbarabadi, M. Separation of ethylene glycol solution by vacuum membrane distillation (VMD). Desalination 2005, 181, 35–41. [Google Scholar] [CrossRef]
- Komesli, O.T.; Teschner, K.; Hegemann, W.; Gokcay, C.F. Vacuum membrane applications in domestic wastewater reuse. Desalination 2007, 215, 22–28. [Google Scholar] [CrossRef]
- Bandini, S.; Saavedra, A.; Sarti, G.C. Vacuum membrane distillation: Experiments and modeling. AIChE J. 1997, 43, 398–408. [Google Scholar] [CrossRef]
- Garcıa-Payo, M.; Izquierdo-Gil, M.A.; Fernández-Pineda, C. Air gap membrane distillation of aqueous alcohol solutions. J. Membr. Sci. 2000, 169, 61–80. [Google Scholar] [CrossRef]
- Lee, J.; Alsaadi, A.S.; Ghaffour, N. Multi-stage air gap membrane distillation reversal for hot impaired quality water treatment: Concept and simulation study. Desalination 2019, 450, 1–11. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Ghaffour, N.; Li, J.-D.; Gray, S.; Francis, L.; Maab, H.; Amy, G.L. Modeling of air-gap membrane distillation process: A theoretical and experimental study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef]
- Khayet, M.; Cojocaru, C. Air gap membrane distillation: Desalination, modeling and optimization. Desalination 2012, 287, 138–145. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation operated at high seawater concentration factors: Role of the membrane on CaCO3 scaling in presence of humic acid. J. Membr. Sci. 2010, 346, 263–269. [Google Scholar] [CrossRef]
- Gryta, M. Effect of iron oxides scaling on the MD process performance. Desalination 2007, 216, 88–102. [Google Scholar] [CrossRef]
- Krivorot, M.; Kushmaro, A.; Oren, Y.; Gilron, J. Factors affecting biofilm formation and biofouling in membrane distillation of seawater. J. Membr. Sci. 2011, 376, 15–24. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Ruiz-Aguirre, A.; Zaragoza, G.; Arafat, H.A. Membrane fouling and cleaning in long term plant-scale membrane distillation operations. J. Membr. Sci. 2014, 468, 360–372. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Karakulski, K. Wastewater treatment by membrane distillation. Desalination 2006, 198, 67–73. [Google Scholar] [CrossRef]
- Drioli, E.; Wu, Y. Membrane distillation: An experimental study. Desalination 1985, 53, 339–346. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S.; Farooque, A.M. Performance restoration and autopsy of NF membranes used in seawater pretreatment. Desalination 2005, 178, 261–271. [Google Scholar] [CrossRef]
- Gryta, M. The assessment of microorganism growth in the membrane distillation system. Desalination 2002, 142, 79–88. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Guijt, C.M.; de Haan, A.B. Desalination and water recycling by air gap membrane distillation. Desalination 2006, 187, 291–301. [Google Scholar] [CrossRef]
- Khayet, M.; Velázquez, A.; Mengual, J.I. Direct contact membrane distillation of humic acid solutions. J. Membr. Sci. 2004, 240, 123–128. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.-J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Susanto, H. Towards practical implementations of membrane distillation. Chem. Eng. Process. Process Intensif. 2011, 50, 139–150. [Google Scholar] [CrossRef]
- Gryta, M. Calcium sulphate scaling in membrane distillation process. Chem. Pap. 2009, 63, 146–151. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- He, F.; Sirkar, K.K.; Gilron, J. Effects of antiscalants to mitigate membrane scaling by direct contact membrane distillation. J. Membr. Sci. 2009, 345, 53–58. [Google Scholar] [CrossRef]
- Mat Nawi, N.I.; Bilad, M.R.; Anath, G.; Nordin, N.A.H.; Kurnia, J.C.; Wibisono, Y.; Arahman, N. The Water Flux Dynamic in a Hybrid Forward Osmosis-Membrane Distillation for Produced Water Treatment. Membranes 2020, 10, 225. [Google Scholar] [CrossRef]
- Chamani, H.; Woloszyn, J.; Matsuura, T.; Rana, D.; Lan, C.Q. Pore wetting in membrane distillation: A comprehensive review. Prog. Mater. Sci. 2021, 122, 100843. [Google Scholar] [CrossRef]
- Gryta, M. Long-term performance of membrane distillation process. J. Membr. Sci. 2005, 265, 153–159. [Google Scholar] [CrossRef]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Gryta, M. Mitigation of Membrane Wetting by Applying a Low Temperature Membrane Distillation. Membranes 2020, 10, 158. [Google Scholar] [CrossRef]
- Barbe, A.M.; Hogan, P.A.; Johnson, R.A. Surface morphology changes during initial usage of hydrophobic, microporous polypropylene membranes. J. Membr. Sci. 2000, 172, 149–156. [Google Scholar] [CrossRef]
- Karakulski, K.; Gryta, M. Water demineralisation by NF/MD integrated processes. Desalination 2005, 177, 109–119. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Franken, A.C.M.; Nolten, J.A.M.; Mulder, M.H.V.; Bargeman, D.; Smolders, C.A. Wetting criteria for the applicability of membrane distillation. J. Membr. Sci. 1987, 33, 315–328. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Mavukkandy, M.O.; Bilad, M.R.; Arafat, H.A. Understanding wetting phenomena in membrane distillation and how operational parameters can affect it. J. Membr. Sci. 2016, 515, 163–174. [Google Scholar] [CrossRef]
- Christie, K.S.S.; Horseman, T.; Lin, S. Energy efficiency of membrane distillation: Simplified analysis, heat recovery, and the use of waste-heat. Environ. Int. 2020, 138, 105588. [Google Scholar] [CrossRef]
- Criscuoli, A.; Carnevale, M.C.; Drioli, E. Evaluation of energy requirements in membrane distillation. Chem. Eng. Process. Process Intensif. 2008, 47, 1098–1105. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Khanna, V.; Vidic, R.D. Importance of feed recirculation for the overall energy consumption in membrane distillation systems. Desalination 2018, 428, 250–254. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating energy consumption of air gap membrane distillation for seawater desalination at pilot scale level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, W.-S. Numerical modeling of the vacuum membrane distillation process. Desalination 2013, 331, 46–55. [Google Scholar] [CrossRef]
- Elmarghany, M.R.; El-Shazly, A.H.; Salem, M.S.; Sabry, M.N.; Nady, N. Thermal analysis evaluation of direct contact membrane distillation system. Case Stud. Therm. Eng. 2019, 13, 100377. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Alternative heating techniques in membrane distillation: A review. Desalination 2020, 496, 114713. [Google Scholar] [CrossRef]
- Guan, G.; Wang, R.; Wicaksana, F.; Yang, X.; Fane, A.G. Analysis of Membrane Distillation Crystallization System for High Salinity Brine Treatment with Zero Discharge Using Aspen Flowsheet Simulation. Ind. Eng. Chem. Res. 2012, 51, 13405–13413. [Google Scholar] [CrossRef]
- Suleman, M.; Asif, M.; Jamal, S.; Dong, P.; Xi, X. A numerical study on effects of operational parameters and membrane characteristics on performance of vacuum membrane distillation (VMD). Desalination Water Treat. 2020, 183, 182–193. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R.; Field, R.; Fane, A.G. Energy efficiency evaluation and economic analyses of direct contact membrane distillation system using Aspen Plus. Desalination 2011, 283, 237–244. [Google Scholar] [CrossRef]
- Gilron, J.; Song, L.; Sirkar, K.K. Design for Cascade of Crossflow Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2007, 46, 2324–2334. [Google Scholar] [CrossRef]
- Tlili, I.; Mohammad Sajadi, S.; Baleanu, D.; Ghaemi, F. Flat sheet direct contact membrane distillation study to decrease the energy demand for solar desalination purposes. Sustain. Energy Technol. Assess. 2022, 52, 102100. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Dutta, N.; Singh, B.; Subbiah, S.; Muthukumar, P. Performance analysis of a single and multi-staged direct contact membrane distillation module integrated with heat recovery units. Chem. Eng. J. Adv. 2020, 4, 100055. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Thu, K.; Ghaffour, N.; Ng, K.C. Performance investigation of a solar-assisted direct contact membrane distillation system. J. Membr. Sci. 2013, 427, 345–364. [Google Scholar] [CrossRef]
- Lee, H.; He, F.; Song, L.; Gilron, J.; Sirkar, K.K. Desalination with a cascade of cross-flow hollow fiber membrane distillation devices integrated with a heat exchanger. AIChE J. 2011, 57, 1780–1795. [Google Scholar] [CrossRef]
- Shim, S.M.; Lee, J.G.; Kim, W.S. Performance simulation of a multi-VMD desalination process including the recycle flow. Desalination 2014, 338, 39–48. [Google Scholar] [CrossRef]
- Razaqpur, A.G.; Wang, Y.; Liao, X.; Liao, Y.; Wang, R. Progress of photothermal membrane distillation for decentralized desalination: A review. Water Res. 2021, 201, 117299. [Google Scholar] [CrossRef]
- Wu, J.; Zodrow, K.R.; Szemraj, P.B.; Li, Q. Photothermal nanocomposite membranes for direct solar membrane distillation. J. Mater. Chem. A 2017, 5, 23712–23719. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Enhanced performance of direct contact membrane distillation via selected electrothermal heating of membrane surface. J. Membr. Sci. 2020, 610, 118224. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; Le-Clech, P. Opportunities to reach economic sustainability in forward osmosis–reverse osmosis hybrids for seawater desalination. Desalination 2015, 363, 26–36. [Google Scholar] [CrossRef]
- Ortega-Delgado, B.; Palenzuela, P.; Altaee, A.; Alarcón-Padilla, D.C.; Hawari, A.H.; Zaragoza, G. Thermo-economic assessment of forward osmosis as pretreatment to boost the performance and sustainability of multi-effect distillation for seawater desalination. Desalination 2022, 541, 115989. [Google Scholar] [CrossRef]
- Sbardella, L.; Blandin, G.; Fàbregas, A.; Carlos Real Real, J.; Serra Clusellas, A.; Ferrari, F.; Bosch, C.; Martinez-Lladó, X. Optimization of pilot scale forward osmosis process integrated with electrodialysis to concentrate landfill leachate. Chem. Eng. J. 2022, 434, 134448. [Google Scholar] [CrossRef]
- Park, H.W.; Baek, J.; Kim, W.-J. Forward osmosis and direct contact membrane distillation: Emerging membrane technologies in food and beverage processing. Innov. Food Sci. Emerg. Technol. 2024, 93, 103626. [Google Scholar] [CrossRef]
- Islam, M.S.; Touati, K.; Rahaman, M.S. Feasibility of a hybrid membrane-based process (MF-FO-MD) for fracking wastewater treatment. Sep. Purif. Technol. 2019, 229, 115802. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Naji, O.; Alanezi, A.A.; Ghaffour, N.; Déon, S.; Subbiah, S.; Altaee, A. Development in forward Osmosis-Membrane distillation hybrid system for wastewater treatment. Sep. Purif. Technol. 2022, 286, 120498. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- García-Rodríguez, L. Seawater desalination driven by renewable energies: A review. Desalination 2002, 143, 103–113. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Spahis, N.; Goosen, M.F.; Ghaffour, N.; Drouiche, N.; Ouagued, A. Application of geothermal energy for heating and fresh water production in a brackish water greenhouse desalination unit: A case study from Algeria. Renew. Sustain. Energy Rev. 2010, 14, 512–517. [Google Scholar] [CrossRef]
- Lin, S.; Yip, N.Y.; Cath, T.Y.; Osuji, C.O.; Elimelech, M. Hybrid Pressure Retarded Osmosis–Membrane Distillation System for Power Generation from Low-Grade Heat: Thermodynamic Analysis and Energy Efficiency. Environ. Sci. Technol. 2014, 48, 5306–5313. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Li, J.-d.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.-k.; Xu, Y.-y. Pilot test of vacuum membrane distillation for seawater desalination on a ship. Desalination 2006, 189, 165–169. [Google Scholar] [CrossRef]
- Koschikowski, J.; Wieghaus, M.; Rommel, M.; Ortin, V.S.; Suarez, B.P.; Betancort Rodríguez, J.R. Experimental investigations on solar driven stand-alone membrane distillation systems for remote areas. Desalination 2009, 248, 125–131. [Google Scholar] [CrossRef]
- Sarbatly, R.; Chiam, C.-K. Evaluation of geothermal energy in desalination by vacuum membrane distillation. Appl. Energy 2013, 112, 737–746. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Zaragoza, G. Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems. Membranes 2019, 9, 69. [Google Scholar] [CrossRef]
- Husnain, T.; Liu, Y.; Riffat, R.; Mi, B. Integration of forward osmosis and membrane distillation for sustainable wastewater reuse. Sep. Purif. Technol. 2015, 156, 424–431. [Google Scholar] [CrossRef]
- Son, H.S.; Kim, Y.; Nawaz, M.S.; Al-Hajji, M.A.; Abu-Ghdaib, M.; Soukane, S.; Ghaffour, N. Impact of osmotic and thermal isolation barrier on concentration and temperature polarization and energy efficiency in a novel FO-MD integrated module. J. Membr. Sci. 2021, 620, 118811. [Google Scholar] [CrossRef]
- Sushvanth Reddy, A.; Kalla, S.; Murthy, Z.V.P. Textile wastewater treatment via membrane distillation. Environ. Eng. Res. 2022, 27, 210228. [Google Scholar] [CrossRef]
- Dow, N.; Villalobos García, J.; Niadoo, L.; Milne, N.; Zhang, J.; Gray, S.; Duke, M. Demonstration of membrane distillation on textile waste water: Assessment of long term performance, membrane cleaning and waste heat integration. Environ. Sci. Water Res. Technol. 2017, 3, 433–449. [Google Scholar] [CrossRef]
- Wu, X.; Ma, S.; Ng, D.; Acharya, D.; Fan, L.; Xie, Z. Enhancing water recovery through integrated graphene oxide-modified forward osmosis and membrane distillation for real textile wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112512. [Google Scholar] [CrossRef]
- Kim, Y.; Li, S.; Francis, L.; Li, Z.; Linares, R.V.; Alsaadi, A.S.; Abu-Ghdaib, M.; Son, H.S.; Amy, G.; Ghaffour, N. Osmotically and Thermally Isolated Forward Osmosis–Membrane Distillation (FO–MD) Integrated Module. Environ. Sci. Technol. 2019, 53, 3488–3498. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Martinetti, C.R. Combined Membrane-Distillation-Forward-Osmosis Systems and Methods of Use. U.S. Patent US8029671B2, 4 October 2011. [Google Scholar]
- Ghaffour, N.; Francis, L.; Li, Z.; Valladares, R.; Alsaadi, A.S.; Ghdaib, M.A.; Amy, G.L. Osmotically and thermally Isolated Forward Osmosis-Membrane Distillation (FO-MD) Integrated Module for Water Treatment Applications. U.S. Patent US10688439B2, 23 June 2020. [Google Scholar]
- Soukane, S.; Naceur, M.W.; Francis, L.; Alsaadi, A.; Ghaffour, N. Effect of feed flow pattern on the distribution of permeate fluxes in desalination by direct contact membrane distillation. Desalination 2017, 418, 43–59. [Google Scholar] [CrossRef]
- Ricci, B.C.; Skibinski, B.; Koch, K.; Mancel, C.; Celestino, C.Q.; Cunha, I.L.C.; Silva, M.R.; Alvim, C.B.; Faria, C.V.; Andrade, L.H.; et al. Critical performance assessment of a submerged hybrid forward osmosis—Membrane distillation system. Desalination 2019, 468, 114082. [Google Scholar] [CrossRef]
- Arcanjo, G.S.; Costa, F.C.R.; Ricci, B.C.; Mounteer, A.H.; de Melo, E.N.M.L.; Cavalcante, B.F.; Araújo, A.V.; Faria, C.V.; Amaral, M.C.S. Draw solution solute selection for a hybrid forward osmosis-membrane distillation module: Effects on trace organic compound rejection, water flux and polarization. Chem. Eng. J. 2020, 400, 125857. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Union, E. Demonstration of Concentrated Solar Power Coupled with Advanced Desalination System in the Gulf Region. Available online: https://desolination.eu/ (accessed on 21 May 2025).
- Edwie, F.; Chung, T.-S. Development of hollow fiber membranes for water and salt recovery from highly concentrated brine via direct contact membrane distillation and crystallization. J. Membr. Sci. 2012, 421–422, 111–123. [Google Scholar] [CrossRef]
- Lu, D.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Treatment and energy utilization of oily water via integrated ultrafiltration-forward osmosis–membrane distillation (UF-FO-MD) system. J. Membr. Sci. 2018, 548, 275–287. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Ullah, R.; Khraisheh, M.; Esteves, R.J.; McLeskey, J.T.; AlGhouti, M.; Gad-el-Hak, M.; Vahedi Tafreshi, H. Energy efficiency of direct contact membrane distillation. Desalination 2018, 433, 56–67. [Google Scholar] [CrossRef]
- Otávio Rosa e Silva, G.; Rodrigues dos Santos, C.; Souza Casella, G.; Pinheiro Drumond, G.; Cristina Santos Amaral, M. Membrane fouling in integrated forward osmosis and membrane distillation systems—A review. Sep. Purif. Technol. 2025, 356, 129955. [Google Scholar] [CrossRef]
- Tan, B.; He, Z.; Fang, Y.; Zhu, L. Removal of organic pollutants in shale gas fracturing flowback and produced water: A review. Sci. Total Environ. 2023, 883, 163478. [Google Scholar] [CrossRef]
- Han, F.; Zhao, J.; Bian, Y.; Guo, J.; Chen, L. Electro mitigation of calcium carbonate and calcium sulfate scaling in an optimized thermal conductive membrane distillation process. Sep. Purif. Technol. 2023, 316, 123796. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Silica scaling and scaling reversibility in forward osmosis. Desalination 2013, 312, 75–81. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.C. Calcium carbonate scaling by reverse draw solute diffusion in a forward osmosis membrane for shale gas wastewater treatment. J. Membr. Sci. 2017, 522, 257–266. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, J.; Tang, C.Y. Gypsum scaling during forward osmosis process—A direct microscopic observation study. Desalination Water Treat. 2016, 57, 3317–3327. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, S.-H.; Jeong, S.; Hwang, T.-M. Application of ultrasound to mitigate calcium sulfate scaling and colloidal fouling. Desalination 2014, 336, 153–159. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Chen, Y.; Gao, B.; Wang, Z. Scaling control of forward osmosis-membrane distillation (FO-MD) integrated process for pre-treated landfill leachate treatment. Desalination 2021, 520, 115342. [Google Scholar] [CrossRef]
- Mokarizadeh, H.; Moayedfard, S.; Maleh, M.S.; Mohamed, S.I.G.P.; Nejati, S.; Esfahani, M.R. The role of support layer properties on the fabrication and performance of thin-film composite membranes: The significance of selective layer-support layer connectivity. Sep. Purif. Technol. 2021, 278, 119451. [Google Scholar] [CrossRef]
- Ibraheem, B.M.; Aani, S.A.; Alsarayreh, A.A.; Alsalhy, Q.F.; Salih, I.K. Forward Osmosis Membrane: Review of Fabrication, Modification, Challenges and Potential. Membranes 2023, 13, 379. [Google Scholar] [CrossRef]
- Bhinder, A.; Shabani, S.; Sadrzadeh, M. Effect of Internal and External Concentration Polarizations on the Performance of Forward Osmosis Process. In Osmotically Driven Membrane Processes: Approach, Development and Current Status; IntechOpen: London, UK, 2018. [Google Scholar]
- Akther, N.; Yuan, Z.; Chen, Y.; Lim, S.; Phuntsho, S.; Ghaffour, N.; Matsuyama, H.; Shon, H. Influence of graphene oxide lateral size on the properties and performances of forward osmosis membrane. Desalination 2020, 484, 114421. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.; Tang, C.Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 2012, 405–406, 149–157. [Google Scholar] [CrossRef]
- Amini, M.; Rahimpour, A.; Jahanshahi, M. Forward osmosis application of modified TiO2-polyamide thin film nanocomposite membranes. Desalination Water Treat. 2016, 57, 14013–14023. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Highly Hydrophilic Thin-Film Composite Forward Osmosis Membranes Functionalized with Surface-Tailored Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 5044–5053. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, C.; Hou, L.-a. Comparison of performance and biofouling resistance of thin-film composite forward osmosis membranes with substrate/active layer modified by graphene oxide. RSC Adv. 2019, 9, 6502–6509. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Ulbricht, M. Nano-sized metal organic framework to improve the structural properties and desalination performance of thin film composite forward osmosis membrane. J. Membr. Sci. 2017, 531, 59–67. [Google Scholar] [CrossRef]
- Su, J.; Ong, R.C.; Wang, P.; Chung, T.-S.; Helmer, B.J.; de Wit, J.S. Advanced FO membranes from newly synthesized CAP polymer for wastewater reclamation through an integrated FO-MD hybrid system. AIChE J. 2013, 59, 1245–1254. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation. J. Membr. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Haija, M.A. Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Khumalo, N.; Derese, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Superhydrophobic PVDF nanofibre membranes coated with an organic fouling resistant hydrophilic active layer for direct-contact membrane distillation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 363–372. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Li, M.; Li, K.; Wang, L.; Zhang, X. Feasibility of concentrating textile wastewater using a hybrid forward osmosis-membrane distillation (FO-MD) process: Performance and economic evaluation. Water Res. 2020, 172, 115488. [Google Scholar] [CrossRef]
- Kwon, D.; Bae, W.; Kim, J. Hybrid forward osmosis/membrane distillation integrated with anaerobic fluidized bed bioreactor for advanced wastewater treatment. J. Hazard. Mater. 2021, 404, 124160. [Google Scholar] [CrossRef]
- Husnain, T.; Mi, B.; Riffat, R. A Combined Forward Osmosis and Membrane Distillation System for Sidestream Treatment. J. Water Resour. Prot. 2015, 7, 1111–1120. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Impact of humic acid fouling on membrane performance and transport of pharmaceutically active compounds in forward osmosis. Water Res. 2013, 47, 4567–4575. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater—A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Nguyen, H.T.; Ho, S.-T.; Chen, S.-S.; Ngo, H.H.; Guo, W.; Ray, S.S.; Hsu, H.-T. Exploring high charge of phosphate as new draw solute in a forward osmosis–membrane distillation hybrid system for concentrating high-nutrient sludge. Sci. Total Environ. 2016, 557–558, 44–50. [Google Scholar] [CrossRef]
- Saqib Nawaz, M.; Alqulayti, A.; Torres Serrano, V.M.; Soukane, S.; Gudideni, V.; Al-Qahtani, A.; Yan, I.C.; Ghaffour, N. Optimizing electrocoagulation pre-treatment efficiency during simultaneous treatment of different produced water streams in a FO-MD hybrid system. Sep. Purif. Technol. 2024, 336, 126290. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Deng, J.; Zhang, P.; Feng, S.; Chen, Y. Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution. Membranes 2024, 14, 106. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, X.; Yang, L.; Wang, W.; Wang, H.; Li, Z. A green approach for non-thermal concentration of skim milk by forward osmosis combined with membrane distillation for draw solution regeneration. Chem. Eng. Res. Des. 2024, 210, 469–480. [Google Scholar] [CrossRef]
- An, X.; Hu, Y.; Wang, N.; Zhou, Z.; Liu, Z. Continuous juice concentration by integrating forward osmosis with membrane distillation using potassium sorbate preservative as a draw solute. J. Membr. Sci. 2019, 573, 192–199. [Google Scholar] [CrossRef]
- Song, H.; Xie, F.; Chen, W.; Liu, J. FO/MD hybrid system for real dairy wastewater recycling. Environ. Technol. 2018, 39, 2411–2421. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Zhao, L.; Ma, W.; Liu, H.; Ma, J. Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 2016, 91, 45–54. [Google Scholar] [CrossRef]
- Cath, T.Y.; Adams, D.; Childress, A.E. Membrane contactor processes for wastewater reclamation in space: II. Combined direct osmosis, osmotic distillation, and membrane distillation for treatment of metabolic wastewater. J. Membr. Sci. 2005, 257, 111–119. [Google Scholar] [CrossRef]
- Cath, T.Y.; Cartinella, J.L.; Adams, V.D.; Childress, A.; Gormly, S.; Flynn, M. New Concepts and Performance of the Direct Osmotic Concentration Process for Wastewater Recovery in Advanced Life Support Systems; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2006. [Google Scholar]
- Ray, H.; Perreault, F.; Boyer, T.H. Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD). Environ. Sci. Water Res. Technol. 2019, 5, 1993–2003. [Google Scholar] [CrossRef]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Ghaffour, N.; Vrouwenvelder, J.S.; Shon, H.K. Optimisation of a forward osmosis and membrane distillation hybrid system for the treatment of source-separated urine. Sep. Purif. Technol. 2019, 212, 368–375. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, K.; Zhang, Y.; Wang, J.; Zhang, X. Domestic wastewater treatment by forward osmosis-membrane distillation (FO-MD) integrated system. Water Sci. Technol. 2018, 77, 1514–1523. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Hong, S. Treatment of industrial wastewater produced by desulfurization process in a coal-fired power plant via FO-MD hybrid process. Chemosphere 2018, 210, 44–51. [Google Scholar] [CrossRef]
- Aydiner, C.; Sen, U.; Topcu, S.; Ekinci, D.; Altinay, A.D.; Koseoglu-Imer, D.Y.; Keskinler, B. Techno-economic viability of innovative membrane systems in water and mass recovery from dairy wastewater. J. Membr. Sci. 2014, 458, 66–75. [Google Scholar] [CrossRef]
- Pan, S.-F.; Dong, Y.; Zheng, Y.-M.; Zhong, L.-B.; Yuan, Z.-H. Self-sustained hydrophilic nanofiber thin film composite forward osmosis membranes: Preparation, characterization and application for simulated antibiotic wastewater treatment. J. Membr. Sci. 2017, 523, 205–215. [Google Scholar] [CrossRef]
- Cabrera-Castillo, E.H.; Castillo, I.; Ciudad, G.; Jeison, D.; Ortega-Bravo, J.C. FO-MD setup analysis for acid mine drainage treatment in Chile: An experimental-theoretical economic assessment compared with FO-RO and single RO. Desalination 2021, 514, 115164. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Benes, N.; Nijmeijer, A.; McCutcheon, J.R. Use of a Forward Osmosis–Membrane Distillation Integrated Process in the Treatment of High-Salinity Oily Wastewater. Ind. Eng. Chem. Res. 2019, 58, 956–962. [Google Scholar] [CrossRef]
- Li, X.M.; Zhao, B.; Wang, Z.; Xie, M.; Song, J.; Nghiem, L.D.; He, T.; Yang, C.; Li, C.; Chen, G. Water reclamation from shale gas drilling flow-back fluid using a novel forward osmosis-vacuum membrane distillation hybrid system. Water Sci. Technol. 2014, 69, 1036–1044. [Google Scholar] [CrossRef]
- Ahmed, M.; Alambi, R.K.; Bhadrachari, G.; Al-Muqahwi, S.; Thomas, J.P. Design and optimization of a unique pilot scale forward osmosis integrated membrane distillation system for seawater desalination. J. Environ. Chem. Eng. 2023, 11, 109949. [Google Scholar] [CrossRef]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. A techno-economic assessment of membrane distillation for treatment of Marcellus shale produced water. Desalination 2017, 416, 24–34. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Elimelech, M.; Amy, G.; Vrouwenvelder, H. (Eds.) Recent Developments in Forward Osmosis Processes; IWA Publishing: London, UK, 2017. [Google Scholar]
- dos Santos, C.R.; Rosa e Silva, G.O.; Dias Araújo, A.A.; Serafim, T.G.; Drumond, G.P.; dos Santos, V.L.; Fernandes, L.d.A.; de Araújo, J.C.; Arcanjo, G.S.; de Souza Santos, L.V.; et al. Granular anaerobic membrane bioreactor coupled hybrid forward osmosis—Membrane distillation module for organic matter, nutrient and bisphenol A removal: Integrated assessment of performance, cost, toxicity, and risks. Chem. Eng. J. 2025, 504, 158022. [Google Scholar] [CrossRef]
- Lee, D.-J.; Hsieh, M.-H. Forward osmosis membrane processes for wastewater bioremediation: Research needs. Bioresour. Technol. 2019, 290, 121795. [Google Scholar] [CrossRef]
- Tavakkoli, S. A Systems-Level Approach for Integrated Shale Gas Wastewater Management. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2018; 171p. [Google Scholar]
- Lee, J.-G.; Kim, W.-S. Numerical study on multi-stage vacuum membrane distillation with economic evaluation. Desalination 2014, 339, 54–67. [Google Scholar] [CrossRef]
- Tan, N.P.B.; Ucab, P.M.L.; Dadol, G.C.; Jabile, L.M.; Talili, I.N.; Cabaraban, M.T.I. A review of desalination technologies and its impact in the Philippines. Desalination 2022, 534, 115805. [Google Scholar] [CrossRef]
- Elsaid, K.; Taha Sayed, E.; Yousef, B.A.A.; Kamal Hussien Rabaia, M.; Ali Abdelkareem, M.; Olabi, A.G. Recent progress on the utilization of waste heat for desalination: A review. Energy Convers. Manag. 2020, 221, 113105. [Google Scholar] [CrossRef]
- Zaragoza, G.; Andrés-Mañas, J.A.; Ruiz-Aguirre, A. Commercial scale membrane distillation for solar desalination. NPJ Clean Water 2018, 1, 20. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N. Economic evaluation of desalination by small-scale autonomous solar-powered membrane distillation units. Desalination 2008, 220, 566–573. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Deshmukh, S.K.; Guddad, M.V. Economic assessment of multi-effect membrane distillation (MEMD) for water treatment. Int. J. Eng. Res. Technol. 2017, 10, 253–257. [Google Scholar]
- Karagiannis, I.C.; Soldatos, P.G. Current status of water desalination in the Aegean Islands. Desalination 2007, 203, 56–61. [Google Scholar] [CrossRef]
- Kesieme, U.K.; Milne, N.; Aral, H.; Cheng, C.Y.; Duke, M. Economic analysis of desalination technologies in the context of carbon pricing, and opportunities for membrane distillation. Desalination 2013, 323, 66–74. [Google Scholar] [CrossRef]
- Bitaw, T.N.; Park, K.; Kim, J.; Chang, J.W.; Yang, D.R. Low-recovery, -energy-consumption, -emission hybrid systems of seawater desalination: Energy optimization and cost analysis. Desalination 2019, 468, 114085. [Google Scholar] [CrossRef]
- Wittholz, M.K.; O’Neill, B.K.; Colby, C.B.; Lewis, D. Estimating the cost of desalination plants using a cost database. Desalination 2008, 229, 10–20. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Papadakis, G.; Mathioulakis, E.; Belessiotis, V. The effect of hydraulic energy recovery in a small sea water reverse osmosis desalination system; experimental and economical evaluation. Desalination 2005, 184, 241–246. [Google Scholar] [CrossRef]
- Zarebska-Mølgaard, A.; Li, K.; Niedzielska, A.; Schneider, C.; Yangali-Quintanilla, V.; Tsapekos, P.; Angelidaki, I.; Wang, J.; Helix-Nielsen, C. Techno-economic assessment of a hybrid forward osmosis and membrane distillation system for agricultural water recovery. Sep. Purif. Technol. 2022, 283, 120196. [Google Scholar] [CrossRef]
- Judd, S.J. Membrane technology costs and me. Water Res. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Duong, H.C.; Ansari, A.J.; Hailemariam, R.H.; Woo, Y.C.; Pham, T.M.; Ngo, L.T.; Dao, D.T.; Nghiem, L.D. Membrane Distillation for Strategic Water Treatment Applications: Opportunities, Challenges, and Current Status. Curr. Pollut. Rep. 2020, 6, 173–187. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Olesen, L.; Lorenzen, J.; Rasmussen, C.; Laursen, H.; Vestergaard, E.; Keiding, K. Lowering desalination costs by alternative desalination and water reuse scenarios. Desalination Water Treat. 2015, 55, 2437–2445. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing potential of nanofiltration, reverse osmosis and membrane distillation drinking water treatment for pharmaceutically active compounds (PhACs) removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Ghaffour, N.; Lattemann, S.; Missimer, T.; Ng, K.C.; Sinha, S.; Amy, G. Renewable energy-driven innovative energy-efficient desalination technologies. Appl. Energy 2014, 136, 1155–1165. [Google Scholar] [CrossRef]
- Cipollina, A.; Di Sparti, M.G.; Tamburini, A.; Micale, G. Development of a Membrane Distillation module for solar energy seawater desalination. Chem. Eng. Res. Des. 2012, 90, 2101–2121. [Google Scholar] [CrossRef]
- Charcosset, C. A review of membrane processes and renewable energies for desalination. Desalination 2009, 245, 214–231. [Google Scholar] [CrossRef]
- Hanemaaijer, J.; Medevoort, J.; Jansen, A.; Dotremont, C.; van Sonsbeek, E.; Yuan, T.; Ryck, L. Memstill membrane distillation—A future desalination technology. Desalination 2006, 199, 175–176. [Google Scholar] [CrossRef]
- Jouhara, H.; Olabi, A.G. Industrial waste heat recovery. Energy 2018, 160, 1–2. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.Y.; Jang, Y.H.; Kim, M.-g.; Yang, D.R.; Hong, S. Comprehensive analysis of a hybrid FO/crystallization/RO process for improving its economic feasibility to seawater desalination. Water Res. 2020, 171, 115426. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; Lienhard, J.H. Energy efficiency of membrane distillation up to high salinity: Evaluating critical system size and optimal membrane thickness. Appl. Energy 2018, 211, 715–734. [Google Scholar] [CrossRef]
- Suleman, M.; Asif, M.; Jamal, S.A. Temperature and concentration polarization in membrane distillation: A technical review. Desalin. Water Treat 2021, 229, 52–68. [Google Scholar] [CrossRef]
- Giagnorio, M.; Morciano, M.; Zhang, W.; Hélix-Nielsen, C.; Fasano, M.; Tiraferri, A. Coupling of forward osmosis with desalination technologies: System-scale analysis at the water-energy nexus. Desalination 2022, 543, 116083. [Google Scholar] [CrossRef]
- Tarnacki, K.; Meneses, M.; Melin, T.; van Medevoort, J.; Jansen, A. Environmental assessment of desalination processes: Reverse osmosis and Memstill®. Desalination 2012, 296, 69–80. [Google Scholar] [CrossRef]
- BCS, Incorporated. Waste Heat Recovery: Technology and Opportunities in US Industry; Department of Energy (US): Washington, DC, USA, 2008. [Google Scholar]
- Mahmoudi, A.; Fazli, M.; Morad, M. A recent review of waste heat recovery by Organic Rankine Cycle. Appl. Therm. Eng. 2018, 143, 660–675. [Google Scholar] [CrossRef]
- Elson, A.; Tidball, R.K.; Hampson, A. Waste Heat to Power Market Assessment; Building Technologies Research and Integration Center: Oak Ridge, TN, USA, 2015. [Google Scholar]
- Gingerich, D.B.; Mauter, M.S. Quantity, Quality, and Availability of Waste Heat from United States Thermal Power Generation. Environ. Sci. Technol. 2015, 49, 8297–8306. [Google Scholar] [CrossRef]
- Anderson, W.V.; Cheng, C.-M.; Butalia, T.S.; Weavers, L.K. Forward Osmosis–Membrane Distillation Process for Zero Liquid Discharge of Flue Gas Desulfurization Wastewater. Energy Fuels 2021, 35, 5130–5140. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Chung, T.-S.; Zhang, S.; Wang, K.Y.; Su, J.; Ling, M.M. Forward osmosis processes: Yesterday, today and tomorrow. Desalination 2012, 287, 78–81. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation using low-grade energy for desalination: A review. J. Environ. Chem. Eng. 2021, 9, 105818. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Son, H.S.; Alpatova, A.; Nawaz, M.S.; Soukane, S.; Medina, S.C.; Gudideni, V.; Al-Qahtani, A.; Anjum, D.H.; Ghaffour, N. Pre-pilot forward osmosis—Membrane distillation hybrid system for sustainable produced water treatment and reducing volume of hazardous waste in oil and gas industry. J. Water Process Eng. 2025, 69, 106628. [Google Scholar] [CrossRef]
- Ali, A.S.; Bounahmidi, T. Coupling of photovoltaic thermal with hybrid forward osmosis-membrane distillation: Energy and water production dynamic analysis. J. Water Process Eng. 2024, 64, 105710. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Unlocking the application potential of forward osmosis through integrated/hybrid process. Sci. Total Environ. 2020, 706, 136047. [Google Scholar] [CrossRef] [PubMed]



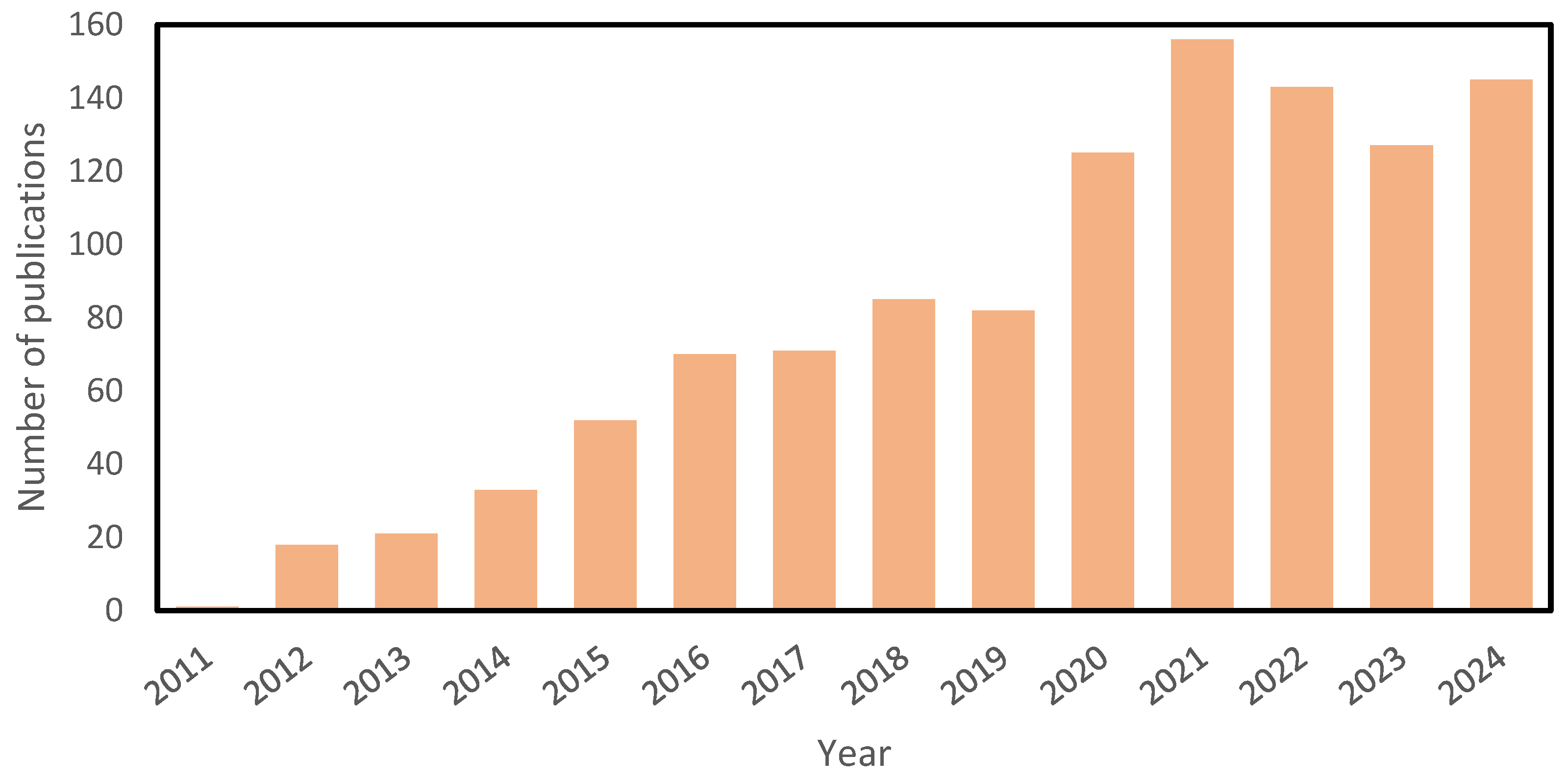
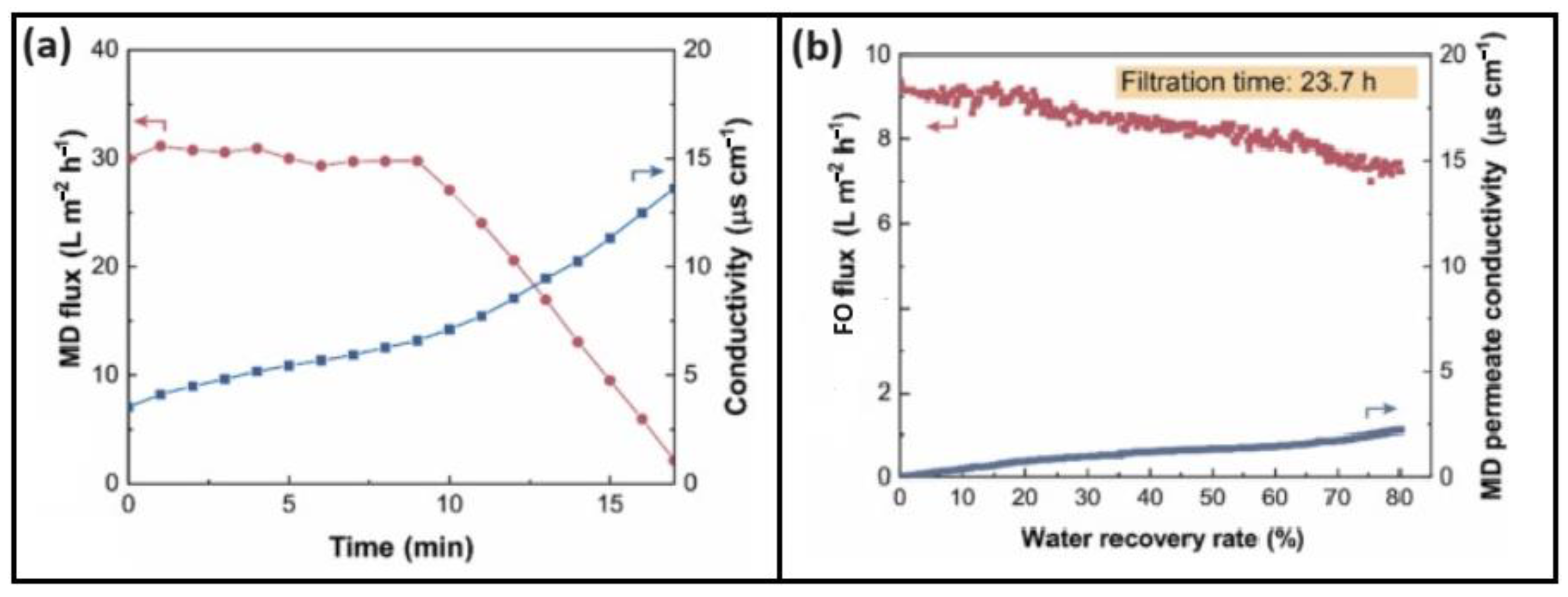


| FS, DS, and Membranes | Conditions and Results |
|---|---|
| FS: raw sewage from a sewage treatment plant, DS: NaCl solution. FO membrane: commercial CTA flat-sheet membrane HTI (USA). MD membrane: PTFE membrane from porous membrane technology (Ningbo, China). | FO feed and draw CFV: 9 cm/s, FS temperature: 20 °C, DS temperature: 40 °C, DS concentration: 1.5 M NaCl, FO flux: 8 LMH. MD feed and distillate flow rates: 1 L/min, feed temperature: 40 °C, distillate temperature: 20 °C, MD flux: 8 LMH. Achieved 80% recovery of the raw sewage by the FO-MD hybrid system. A cumulative permeate volume of 8.25 L was attained after 78 h [95]. |
| FS: DI water, municipal wastewater. DS: NaCl solution, TEAB, and PDAC. CTA membrane from HTI (USA). | FO CFV: 0.12 m/s, DS concentration: 0.5 M NaCl, 0.44 M PDAC, and 0.5 M TEAB. MD with three different feeds: 0.5 M NaCl, 0.44 M PDAC, and 0.5 M TEAB. Feed temperature: 45 °C, permeate temperature: 20 °C, CFV: 0.12 m/s. FO-MD flux: 6–8 LMH. More than 99% rejection of all DSs through MD [93]. |
| FS: DI water, 0.3–0.495 g/L of NH4Cl solution, non-fat dry milk with concentration of 985–1800 mg/L COD, synthetic wastewater, real wastewater, and FS containing 0.1–0.2 g/L NaAsO2. DS: 1 M NaCl. FO membrane: CTA membrane HTI (USA). | FO feed temperature: 20 °C, DS temperature: 50 °C. MD feed temperature: 50 °C, permeate temperature: 20 °C, CFV: 0.4 L/min. Feed as DI water: 14.4 LMH of flux for both FO and MD. Feed with 300–495 mg/L of ammonium concentration, ammonium detected in the permeate was 0.18 mg/L, indicating 100% rejection. COD removal from non-fat dry milk was 90% by FO alone, preventing fouling and wetting in MD. More than 99.9% removal of the chemical solutes from synthetic and real wastewater [202]. |
| FS: wastewater. DS: NaCl solution. FO membrane: commercial flat-sheet CTA membrane from HTI (USA). MD membrane: PVDF hollow fiber membrane. | FO DS concentration: 35 g/L, feed and DS CFV: 0.28 m/s. DS temperature: 53 °C, FS temperature: 20 °C. MD flux: 17.6 LMH, product water conductivity: 7.5 µS·cm−1. More than 90% removal efficiency of the contaminants (Cl−, NO3−, SO42−, PO43−, TOC, NH3-N, TN, and total phosphorus) was obtained [263]. |
| FS: raw flue gas desulfurization wastewater. DS: 3 M NaCl. FO membrane: TFC polyamide membrane from Porifera, Inc. (San Leandro, CA, USA). MD membrane: PTFE supported by PP from Pall Gelman Sciences (Port Washington, NY, USA). | FO process CFV: 8.5 cm/s, temperature: 25 °C. FO process was carried out under the AL-FS mode. MD feed and permeate temperatures were 55 and 20 °C, respectively. At a 50% recovery rate, flux decreased from 18.5 to 13.1 LMH. Afterward, a sharp decrease in flux (13.1 to 2.8 LMH) was observed at 55% recovery. An organic removal rate of 98.61% was achieved [264]. |
| FS: dairy wastewater. DS: 2 M NaCl. FO membrane: commercial flat-sheet CTA membrane from HTI (USA). MD membrane: PP and PVDF membranes from GE Osmonics (Minnetonka, MN, USA). | FO feed and DS flow rates: 300 L/h, temperature: 25 °C. Feed and DS CFV: 0.5 m/s. MD feed temperature: 48 °C, distillate temperature: 20 °C. Feed and permeate crossflow rates: 180 L/h, MD (PVDF) flux: 17.1 LMH, MD (PP) flux: 27.7 LMH [265]. |
| FS: wastewater from the pharmaceutical industry. DS: 0.6 M NaCl. FO membrane: polyamide membrane. MD membrane: flat-sheet PVDF membrane. | FO feed as 500 mg/L tetracycline solution, feed and DS CFV: 12.5 cm/s, FS temperature: 25 °C, DS temperature: 70 °C, FO initial flux: 40 LMH. MD transmembrane temperature: 43 °C, feed CFV: 1.67 cm/s, MD flux: 18 LMH. Rejection of tetracycline by FO-MD: 99.9%. After 7 h run, tetracycline content in distillate water was less than 0.1 mg/L [266]. |
| FS: acid mine drainage. DS: 1 M NaCl. FO membrane: flat-sheet TFC membrane from Porifera, Inc. (USA). MD membrane: PTFE/PP flat sheet from Membrane Solutions (Shanghai, China). | FO FS temperature: 20–22 °C, DS temperature: 60 °C, FO initial flux: 23 LMH. After 12 h, FO flux was 64% of the initial value. MD feed temperature: 60 °C, distillate temperature: 20 °C, flux: 10.5 LMH with only a small decrease during 3.5 h of operation. 50% recovery was achieved [267]. |
| FS: oily wastewater. DS: 5 M NaCl, 4 M KCl, 4.8 and 10 M LiCl, 4.8 M MgCl2. FO membrane: commercial TFC membrane from HTI (USA). MD membrane: PP flat-sheet membrane obtained from 3 M®. | FO FS temperature: 20 °C, DS temperature: 50 °C, CFV: 0.25 m/s. MD feed temperature: 50 °C, permeate temp: 20 °C, CFV: 0.25 m/s. LiCl showed higher flux in FO since the osmotic pressure is more at higher concentrations. 10 M LiCl exhibited lower MD flux due to lower vapor pressure [268]. |
| FS: oily wastewater. DS: 2 M NaCl. FO membrane: CTA-TFC membrane. MD membrane: PVDF hollow fiber. | FO FS temperature: 23 °C, DS temperature: 60 °C. FO flux: 20–32.5 LMH with oily wastewater (4000 ppm petroleum) as feed. MD feed temperature: 60 °C, MD flux: 5.8 LMH [123]. |
| FS: synthetic oily water, sewage. DS: oily water (5–50 mg/L of oil concentration). FO membrane: CTA membrane HTI (USA). | FO DS temperature: 50 °C, DS concentration: 5–50 mg/L of oil concentration. FO flux: 0.65 LMH. FO-MD: at 50 mg/L of oil concentration in DS, flux declined from 5 to 2.6 LMH [216]. |
| FS: shale gas drilling fluid from a drilling site. DS: KCl, NaCl, and MgCl2. FO membrane: commercial CTA membrane obtained from HTI (USA). MD membrane: CF4-plasma-modified PVDF membrane. | FO feed and DS flow rates: 0.6 L/min, DS concentration: 3 M KCl, 3.26 M NaCl, and 1.75 M MgCl2. Order of FO water flux at the same osmotic pressure: KCl > NaCl > MgCl2. MD process: VMD configuration. Applied vacuum: −40 kPa. Sweeping air flow rate: 6 L/min. 23 LMH of flux were achieved at 3 M KCl as DS at a temperature of 25 °C [269]. |
| FS: wastewater. DS: 1.5 M Na2SO4. FO membrane: CTA membrane from HTI (USA). MD membrane: PTFE membrane from Sterlitech Corporation (Auburn, WA, USA). | FO feed and DS cross flow rates: 0.2 L/min, FS temperature: 25 °C. MD feed flow rate: 0.2 L/min, distillate flow rate: 0.1 L/min, feed temperature: 55 °C, distillate temperatures: 5, 15, and 25 °C. Optimal MD performance at a temperature difference of 50 °C. Flux: 18.6 LMH, RSF: 5.1–8 gMH [246]. |
| FS: seawater. DS: NaCl 70,000 ppm. FO membrane: Spiral-wound TFC membrane from Toray (USA). MD membranes: Spiral-wound AGMD module from Aquastill (Sittard, The Netherlands). | FO feed and DS flow rates: 600 L/h and 400 L/h, respectively. FS temperature: 25 °C, flux: 6.3 to 7.3 LMH. MD process: AGMD configuration. Feed temperature: 85 °C, permeate temperature: 10 °C, flux: 3.75 to 4 LMH. Average water recovery: 33% [270]. |
| Membrane Process | Capacity (m3/Day) | Conditions | Heat Source for MD | OPEX (USD/m3) | Ref. |
|---|---|---|---|---|---|
| MD | 0.1–0.5 | Brackish water and untreated seawater as feed, spiral air gap MD modules. | Solar energy | 15–18 | [280] |
| MD | 0.51 | Four stage multi-effect air gap MD module. Membrane: commercial flat-sheet PTFE membrane (Madhu Chemicals, Mumbai, India). | Conventional heat source with heat recovery | 8.91 | [281] |
| MD | 0.51 | Four stage multi-effect air gap MD module. Membrane: commercial flat-sheet PTFE membrane (Madhu Chemicals, India). | Waste-heat source | 3.82 | [281] |
| MD | 24,000 | DCMD configuration. | Conventional heat source without heat recovery | 1.23 | [11] |
| MD | 24,000 | DCMD configuration. Feed inlet temperature: 55 °C. | Industrial waste heat without heat recovery | 0.64 | [11] |
| RO | 1000 | - | - | 0.82 | [1,282] |
| MD | 30,000 | DCMD configuration, flat-sheet PTFE membrane from Ningbo, China. Feed temperature: 60 °C. | Driven with high-temperature waste heat and electricity | 0.66 | [283] |
| ED | 100 | - | - | 1.87 | [284] |
| MD | 30,000 | DCMD configuration, flat-sheet PTFE membrane with PP support from Ningbo, China. | Driven with low-temperature waste heat and electricity | 0.57 | [283] |
| RO | 275,000 | - | - | 0.5 | [285] |
| MD | 105,000 | Memstill® technology with hollow fiber membranes, feed inlet temperature: 65 °C. | Industrial waste heat source at USD 0.10/GJ | 0.26 | [148] |
| RO | 2.6 | - | - | 3.61 | [286] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleman, M.; Al-Rudainy, B.; Lipnizki, F. Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water–Energy Nexus. Membranes 2025, 15, 162. https://doi.org/10.3390/membranes15060162
Suleman M, Al-Rudainy B, Lipnizki F. Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water–Energy Nexus. Membranes. 2025; 15(6):162. https://doi.org/10.3390/membranes15060162
Chicago/Turabian StyleSuleman, Muhammad, Basel Al-Rudainy, and Frank Lipnizki. 2025. "Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water–Energy Nexus" Membranes 15, no. 6: 162. https://doi.org/10.3390/membranes15060162
APA StyleSuleman, M., Al-Rudainy, B., & Lipnizki, F. (2025). Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water–Energy Nexus. Membranes, 15(6), 162. https://doi.org/10.3390/membranes15060162







