Mesoscopic Structure of Lipid Nanoparticles Studied by Small-Angle X-Ray Scattering: A Spherical Core-Triple Shell Model Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Model and Simulation
3.2. Empty-LNPs and mRNA-LNPs
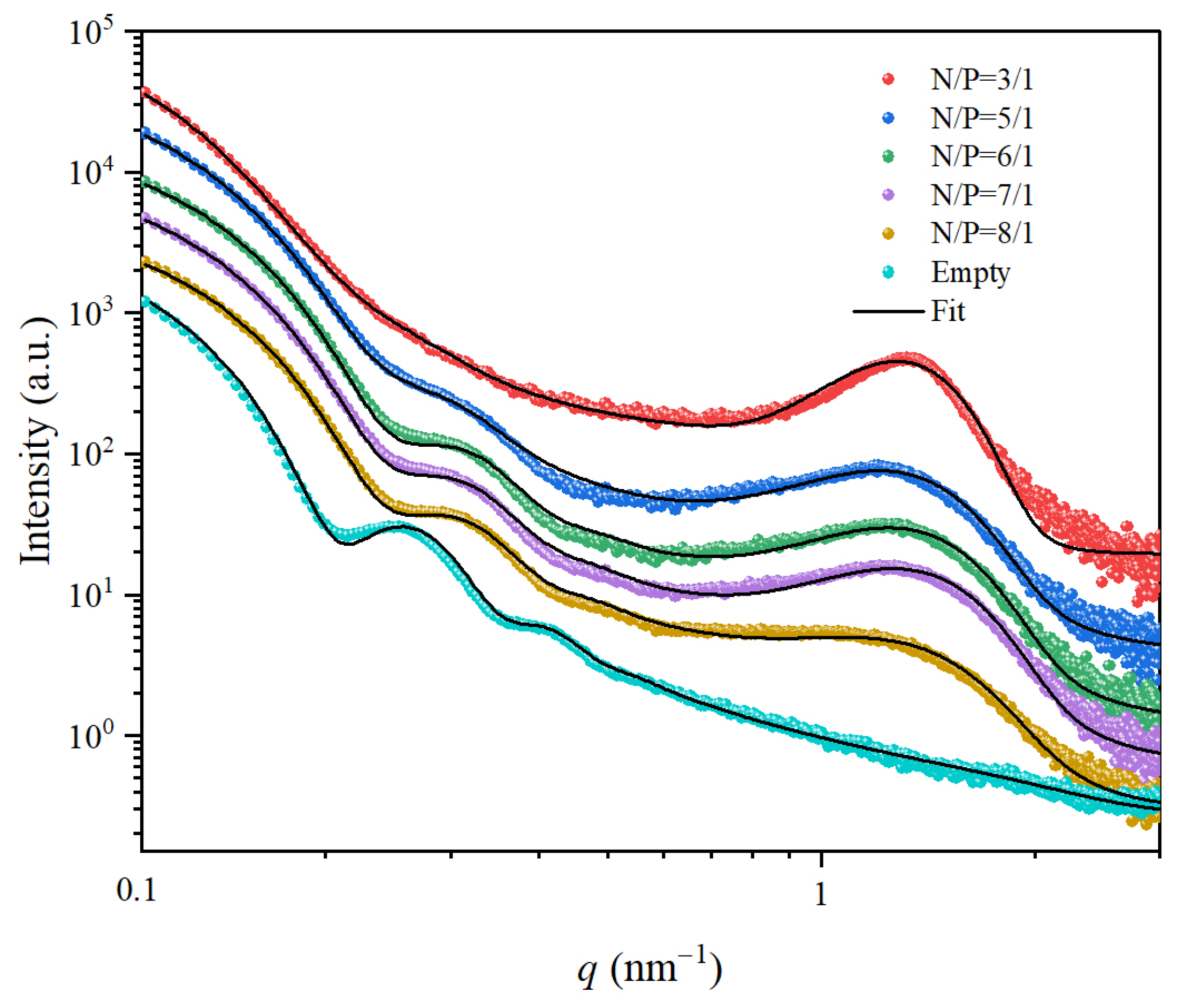
3.3. The Influence of N/P Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Felgner, P.L. The 60-Year Evolution of Lipid Nanoparticles for Nucleic Acid Delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-Assembled mRNA Vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the Role of Helper Lipids in Lipid Nanoparticle Formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.J.; Rossi, D. Oligonucleotides and the COVID-19 Pandemic: A Perspective. Nucleic Acid Ther. 2020, 30, 129–132. [Google Scholar] [CrossRef]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and Efficacy of the mRNA BNT162b2 Vaccine against SARS-CoV-2 in Five Groups of Immunocompromised Patients and Healthy Controls in a Prospective Open-Label Clinical Trial. eBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Meulewaeter, S.; Aernout, I.; Deprez, J.; Engelen, Y.; De Velder, M.; Franceschini, L.; Breckpot, K.; Van Calenbergh, S.; Asselman, C.; Boucher, K.; et al. Alpha-Galactosylceramide Improves the Potency of mRNA LNP Vaccines against Cancer and Intracellular Bacteria. J. Control. Release 2024, 370, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, M.; Zhou, L.; Wang, Y.; Jiao, Y.; Wang, C.; Gong, C.; Cen, X.; Yao, S. Glucocorticoid Pre-Administration Improves LNP-mRNA Mediated Protein Replacement and Genome Editing Therapies. Int. J. Pharm. 2025, 672, 125282. [Google Scholar] [CrossRef]
- Li, H.-Y.; Paramanandana, A.; Kim, S.Y.; Granger, L.; Raimi-Abraham, B.T.; Shattock, R.; Makatsoris, C.; Forbes, B. Targeted Nasal Delivery of LNP-mRNAs Aerosolised by Rayleigh Breakup Technology. Int. J. Pharm. 2025, 672, 125335. [Google Scholar] [CrossRef]
- Huang, T.; Che, S.; Lv, Z.; Hao, D.; Wang, R.; Yi, Q.; Mei, L.; Yuan, Y.; Zou, H.; Guo, Y.; et al. mRNA-LNP Vaccines Combined with tPA Signal Sequence Elicit Strong Protective Immunity against Klebsiella Pneumoniae. mSphere 2024, 10, e00775-24. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid Nanoparticles for siRNA Delivery in Cancer Treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-Human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Ball, R.L.; Deluty, S.B.; Singh, S.R.; Strelkova, D.; Knapp, C.M.; Whitehead, K.A. Branched-Tail Lipid Nanoparticles Potently Deliver mRNA In Vivo Due to Enhanced Ionization at Endosomal pH. Small 2019, 15, 1805097. [Google Scholar] [CrossRef]
- Patel, P.; Ibrahim, N.M.; Cheng, K. The Importance of Apparent pKa in the Development of Nanoparticles Encapsulating siRNA and mRNA. Trends Pharmacol. Sci. 2021, 42, 448–460. [Google Scholar] [CrossRef]
- Naidu, G.S.; Yong, S.; Ramishetti, S.; Rampado, R.; Sharma, P.; Ezra, A.; Goldsmith, M.; Hazan-Halevy, I.; Chatterjee, S.; Aitha, A.; et al. A Combinatorial Library of Lipid Nanoparticles for Cell Type-Specific mRNA Delivery. Adv. Sci. 2023, 10, e2301929. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, J.; Qiu, M.; Li, Y.; Glass, Z.; Xu, Q. Imidazole-Based Synthetic Lipidoids for In Vivo mRNA Delivery into Primary T Lymphocytes. Angew. Chem. Int. Ed. 2020, 59, 20083–20089. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Cheng, Q.; Siegwart, D.J. On the Mechanism of Tissue-Specific mRNA Delivery by Selective Organ Targeting Nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload Distribution and Capacity of mRNA Lipid Nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef]
- Ripoll, M.; Martin, E.; Enot, M.; Robbe, O.; Rapisarda, C.; Nicolai, M.-C.; Deliot, A.; Tabeling, P.; Authelin, J.-R.; Nakach, M.; et al. Optimal Self-Assembly of Lipid Nanoparticles (LNP) in a Ring Micromixer. Sci. Rep. 2022, 12, 9483. [Google Scholar] [CrossRef]
- Spinozzi, F.; Moretti, P.; Perinelli, D.R.; Corucci, G.; Piergiovanni, P.; Amenitsch, H.; Sancini, G.A.; Franzese, G.; Blasi, P. Small-Angle X-Ray Scattering Unveils the Internal Structure of Lipid Nanoparticles. J. Colloid Interface Sci. 2024, 662, 446–459. [Google Scholar] [CrossRef]
- Caselli, L.; Conti, L.; De Santis, I.; Berti, D. Small-Angle X-Ray and Neutron Scattering Applied to Lipid-Based Nanoparticles: Recent Advancements across Different Length Scales. Adv. Colloid Interface Sci. 2024, 327, 103156. [Google Scholar] [CrossRef]
- Fan, Y.; Rigas, D.; Kim, L.J.; Chang, F.-P.; Zang, N.; McKee, K.; Kemball, C.C.; Yu, Z.; Winkler, P.; Su, W.-C.; et al. Physicochemical and Structural Insights into Lyophilized mRNA-LNP from Lyoprotectant and Buffer Screenings. J. Control. Release 2024, 373, 727–737. [Google Scholar] [CrossRef]
- Svergun, D.I.; Koch, M.H.J.; Timmins, P.A.; May, R.P. Small Angle X-Ray and Neutron Scattering from Solutions of Biological Macromolecules; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-963953-3. [Google Scholar]
- Harvey, R.D.; Bello, G.; Kikhney, A.G.; Torres, J.; Surya, W.; Wölk, C.; Shen, C. Absolute Scattering Length Density Profile of Liposome Bilayers Obtained by SAXS Combined with GIXOS: A Tool to Determine Model Biomembrane Structure. J. Appl. Crystallogr. 2023, 56, 1639–1649. [Google Scholar] [CrossRef]
- Varga, Z.; Wacha, A.; Vainio, U.; Gummel, J.; Bóta, A. Characterization of the PEG Layer of Sterically Stabilized Liposomes: A SAXS Study. Chem. Phys. Lipids 2012, 165, 387–392. [Google Scholar] [CrossRef]
- Sakai, K.; Tomizawa, H.; Tsuchiya, K.; Ishida, N.; Sakai, H.; Abe, M. Characterizing the Structural Transition of Cationic DPPC Liposomes from the Approach of TEM, SAXS and AFM Measurements. Colloids Surf. B Biointerfaces 2008, 67, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Ogura, T.; Tsuchiya, K.; Ohishi, K.; Masubuchi, Y.; Akamatsu, M.; Sakai, K.; Abe, M.; Sakai, H. Effect of Polyols on Membrane Structures of Liposomes: A Study Using Small-Angle X-Ray Scattering Data and Generalized Indirect Fourier Transformation. Chem. Phys. Lipids 2022, 249, 105253. [Google Scholar] [CrossRef]
- Dos Santos, N.; Allen, C.; Doppen, A.-M.; Anantha, M.; Cox, K.A.K.; Gallagher, R.C.; Karlsson, G.; Edwards, K.; Kenner, G.; Samuels, L.; et al. Influence of Poly (Ethylene Glycol) Grafting Density and Polymer Length on Liposomes: Relating Plasma Circulation Lifetimes to Protein Binding. Biochim. Biophys. Acta 2007, 1768, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nagle, J.F.; Sachs, J.N.; Feller, S.E.; Pencer, J.; Jackson, A.; Katsaras, J. Lipid Bilayer Structure Determined by the Simultaneous Analysis of Neutron and X-Ray Scattering Data. Biophys. J. 2008, 95, 2356–2367. [Google Scholar] [CrossRef]
- Di Cola, E.; Grillo, I.; Ristori, S. Small Angle X-Ray and Neutron Scattering: Powerful Tools for Studying the Structure of Drug-Loaded Liposomes. Pharmaceutics 2016, 8, 10. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural Information from Multilamellar Liposomes at Full Hydration: Full q-Range Fitting with High Quality X-Ray Data. Phys. Rev. E 2000, 62, 4000–4009. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; He, G.; Guo, C.; Dong, J.; Wu, L. Development of mRNA Lipid Nanoparticles: Targeting and Therapeutic Aspects. Int. J. Mol. Sci. 2024, 25, 10166. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid Nanoparticles for Delivery of RNA Therapeutics: Current Status and the Role of in vivo Imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, Y.; Park, J.; Jung, H.; Lee, H. Lipid Nanoparticles (LNPs) for in Vivo RNA Delivery and Their Breakthrough Technology for Future Applications. Adv. Drug Deliv. Rev. 2023, 200, 114990. [Google Scholar] [CrossRef] [PubMed]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through mRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Gilbert, J.; Sebastiani, F.; Arteta, M.Y.; Terry, A.; Fornell, A.; Russell, R.; Mahmoudi, N.; Nylander, T. Evolution of the Structure of Lipid Nanoparticles for Nucleic Acid Delivery: From in Situ Studies of Formulation to Colloidal Stability. J. Colloid Interface Sci. 2024, 660, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Unruh, T.; Götz, K.; Vogel, C.; Fröhlich, E.; Scheurer, A.; Porcar, L.; Steiniger, F. Mesoscopic Structure of Lipid Nanoparticle Formulations for mRNA Drug Delivery: Comirnaty and Drug-Free Dispersions. ACS Nano 2024, 18, 9746–9764. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. An Ionizable Lipid Toolbox for RNA Delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhou, Z. Lipid Nanoparticle-Based Delivery System─A Competing Place for mRNA Vaccines. ACS Omega 2024, 9, 6219–6234. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Toft, K.N.; Snakenborg, D.; Jeppesen, M.G.; Jacobsen, J.K.; Vestergaard, B.; Kutter, J.P.; Arleth, L. BioXTAS RAW, a Software Program for High-Throughput Automated Small-Angle X-Ray Scattering Data Reduction and Preliminary Analysis. J. Appl. Crystallogr. 2009, 42, 959–964. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A Tool for Small-Angle Scattering Data Analysis Using a Library of Analytical Expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef]
- Jeffries, C.M.; Ilavsky, J.; Martel, A.; Hinrichs, S.; Meyer, A.; Pedersen, J.S.; Sokolova, A.V.; Svergun, D.I. Small-Angle X-Ray and Neutron Scattering. Nat. Rev. Methods Primers 2021, 1, 70. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool Suite for Modeling and Analysis of Small-Angle Scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Von Gundlach, A.R.; Garamus, V.M.; Willey, T.M.; Ilavsky, J.; Hilpert, K.; Rosenhahn, A. Use of Small-Angle X-Ray Scattering to Resolve Intracellular Structure Changes of Escherichia Coli Cells Induced by Antibiotic Treatment. J. Appl. Crystallogr. 2016, 49, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, P.; Humbert, S.; Sorbier, L.; Moreaud, M. Small-Angle X-Ray Scattering Intensity of Multiscale Models of Spheroids. J. Appl. Crystallogr. 2023, 56, 237–246. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; van der Meel, R.; Zaifman, J.; Darjuan, M.M.; Grisch-Chan, H.M.; Thöny, B.; Tam, Y.Y.C.; Cullis, P.R. Fusion-Dependent Formation of Lipid Nanoparticles Containing Macromolecular Payloads. Nanoscale 2019, 11, 9023–9031. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating Lipid Nanoparticles for Organ-Targeted mRNA Accumulation and Translation. Nat. Commun. 2024, 15, 5659. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid Nanoparticle-Mediated Lymph Node–Targeting Delivery of mRNA Cancer Vaccine Elicits Robust CD8+ T Cell Response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-Destabilizing Ionizable Phospholipids for Organ-Selective mRNA Delivery and CRISPR–Cas Gene Editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef]
- Cárdenas, M.; Campbell, R.A.; Yanez Arteta, M.; Lawrence, M.J.; Sebastiani, F. Review of Structural Design Guiding the Development of Lipid Nanoparticles for Nucleic Acid Delivery. Curr. Opin. Colloid Interface Sci. 2023, 66, 101705. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Song, P.; Miao, X.; Liu, G.; Yang, C.; Wei, X.; Li, N.; Bian, F. Small-Angle X-Ray Scattering for PEGylated Liposomal Doxorubicin Drugs: An Analytical Model Comparison Study. Mol. Pharm. 2023, 20, 4654–4663. [Google Scholar] [CrossRef]
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Bawab, A.A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent Advances in Using Liposomes for Delivery of Nucleic Acid-Based Therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Wu, K.; Xu, F.; Dai, Y.; Jin, S.; Zheng, A.; Zhang, N.; Xu, Y. Characterization of mRNA-LNP Structural Features and Mechanisms for Enhanced mRNA Vaccine Immunogenicity. J. Control. Release 2024, 376, 1288–1299. [Google Scholar] [CrossRef] [PubMed]



| Sample | σ (nm) | R0 (nm) | t1 (nm) | ρ1 (×10−4) | ρ2 (×10−4) | ρ3 (×10−5) | CGaussian (nm−1) | WPeak (nm−1) |
|---|---|---|---|---|---|---|---|---|
| empty-LNPs | 2.50 ± 0.01 | 22.94 ± 0.01 | 2.04 ± 0.02 | −1.16 ± 0.02 | 2.77 ± 0.05 | 0.22 ± 0.01 | - | - |
| mRNA-LNPs | 2.65 ± 0.01 | 22.01 ± 0.01 | 1.85 ± 0.04 | −1.14 ± 0.01 | 2.19 ± 0.05 | 0.55 ± 0.03 | 1.27 ± 0.01 | 0.28 ± 0.01 |
| Sample | σ (nm) | R0 (nm) | t1 (nm) | ρ1 (×10−4) | ρ2 (×10−4) | ρ3 (×10−5) | CGaussian (nm−1) | WPeak (nm−1) |
|---|---|---|---|---|---|---|---|---|
| 3/1 | 5.18 ± 0.01 | 17.74 ± 0.03 | 2.57 ± 0.02 | −1.07 ± 0.02 | 2.87 ± 0.01 | 1.67 ± 0.01 | 1.28 ± 0.01 | 0.26 ± 0.01 |
| 5/1 | 3.70 ± 0.01 | 17.92 ± 0.01 | 1.82 ± 0.02 | −1.08 ± 0.01 | 2.96 ± 0.05 | 0.80 ± 0.01 | 1.24 ± 0.01 | 0.32 ± 0.01 |
| 6/1 | 3.12 ± 0.01 | 17.97 ± 0.01 | 1.92 ± 0.01 | −1.13 ± 0.01 | 2.89 ± 0.04 | 0.63 ± 0.01 | 1.27 ± 0.01 | 0.33 ± 0.01 |
| 7/1 | 3.14 ± 0.01 | 18.23 ± 0.01 | 1.93 ± 0.02 | −1.15 ± 0.01 | 2.86 ± 0.03 | 0.59 ± 0.01 | 1.29 ± 0.01 | 0.34 ± 0.01 |
| 8/1 | 3.05 ± 0.01 | 18.53 ± 0.01 | 1.87 ± 0.01 | −1.18 ± 0.01 | 2.23 ± 0.03 | 0.58 ± 0.01 | 1.21 ± 0.01 | 0.38 ± 0.01 |
| empty | 2.85 ± 0.01 | 22.13 ± 0.01 | 1.91 ± 0.01 | −1.23 ± 0.01 | 3.12 ± 0.02 | 0.18 ± 0.01 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Song, P.; Li, Y.; Tu, S.; Mehmood, M.; Chen, L.; Li, N.; Tian, Q. Mesoscopic Structure of Lipid Nanoparticles Studied by Small-Angle X-Ray Scattering: A Spherical Core-Triple Shell Model Analysis. Membranes 2025, 15, 153. https://doi.org/10.3390/membranes15050153
Li H, Song P, Li Y, Tu S, Mehmood M, Chen L, Li N, Tian Q. Mesoscopic Structure of Lipid Nanoparticles Studied by Small-Angle X-Ray Scattering: A Spherical Core-Triple Shell Model Analysis. Membranes. 2025; 15(5):153. https://doi.org/10.3390/membranes15050153
Chicago/Turabian StyleLi, Hao, Panqi Song, Yiwen Li, Shuyang Tu, Mehwish Mehmood, Liang Chen, Na Li, and Qiang Tian. 2025. "Mesoscopic Structure of Lipid Nanoparticles Studied by Small-Angle X-Ray Scattering: A Spherical Core-Triple Shell Model Analysis" Membranes 15, no. 5: 153. https://doi.org/10.3390/membranes15050153
APA StyleLi, H., Song, P., Li, Y., Tu, S., Mehmood, M., Chen, L., Li, N., & Tian, Q. (2025). Mesoscopic Structure of Lipid Nanoparticles Studied by Small-Angle X-Ray Scattering: A Spherical Core-Triple Shell Model Analysis. Membranes, 15(5), 153. https://doi.org/10.3390/membranes15050153







