In-Situ Measurement of Gas Permeability for Membranes in Water Electrolysis
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Material
2.2. Methods of Measurement
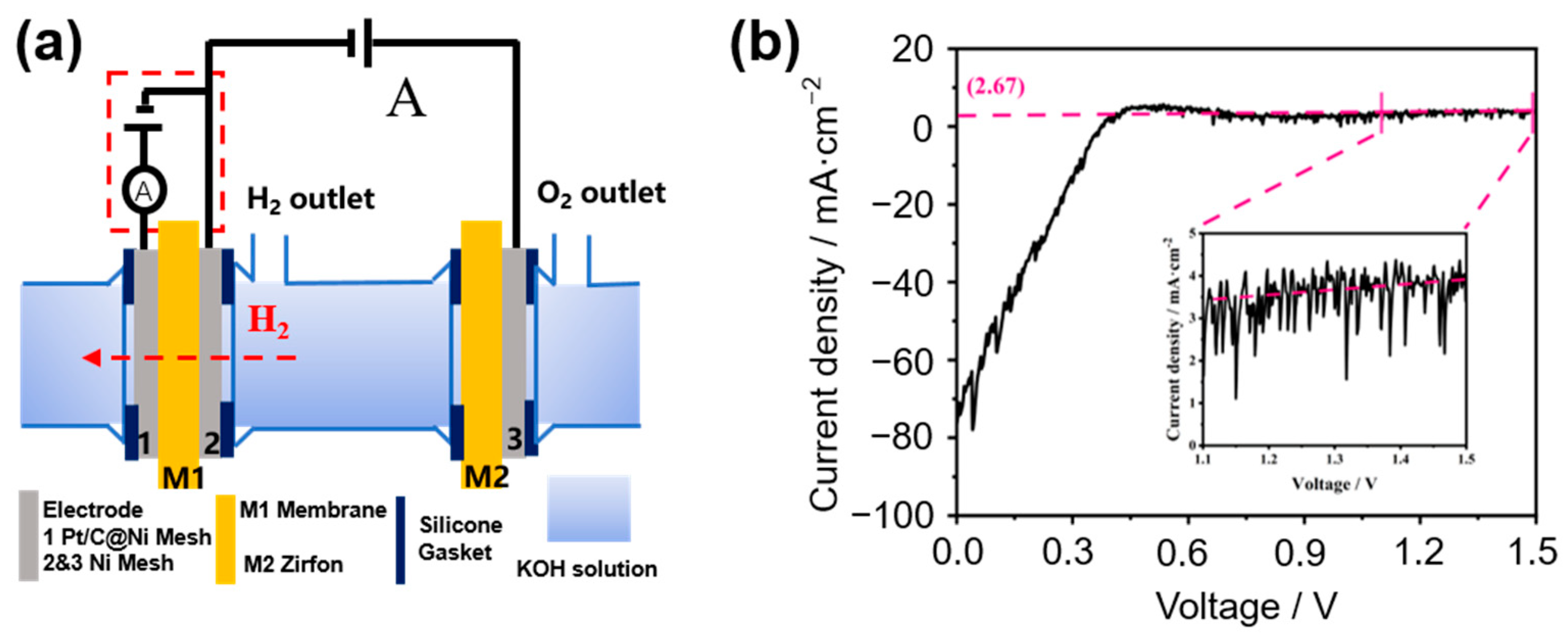
2.2.1. Principle of Measurement
2.2.2. Preparation of Electrodes
2.2.3. Electrochemical Detection
2.2.4. Calculation of the H2 Permeability PH
3. Results and Discussion
3.1. Measured Results
3.2. Comparison of In-Situ and Ex-Situ Electrochemical Measurement
3.3. Effect of Electrolytic Current Density
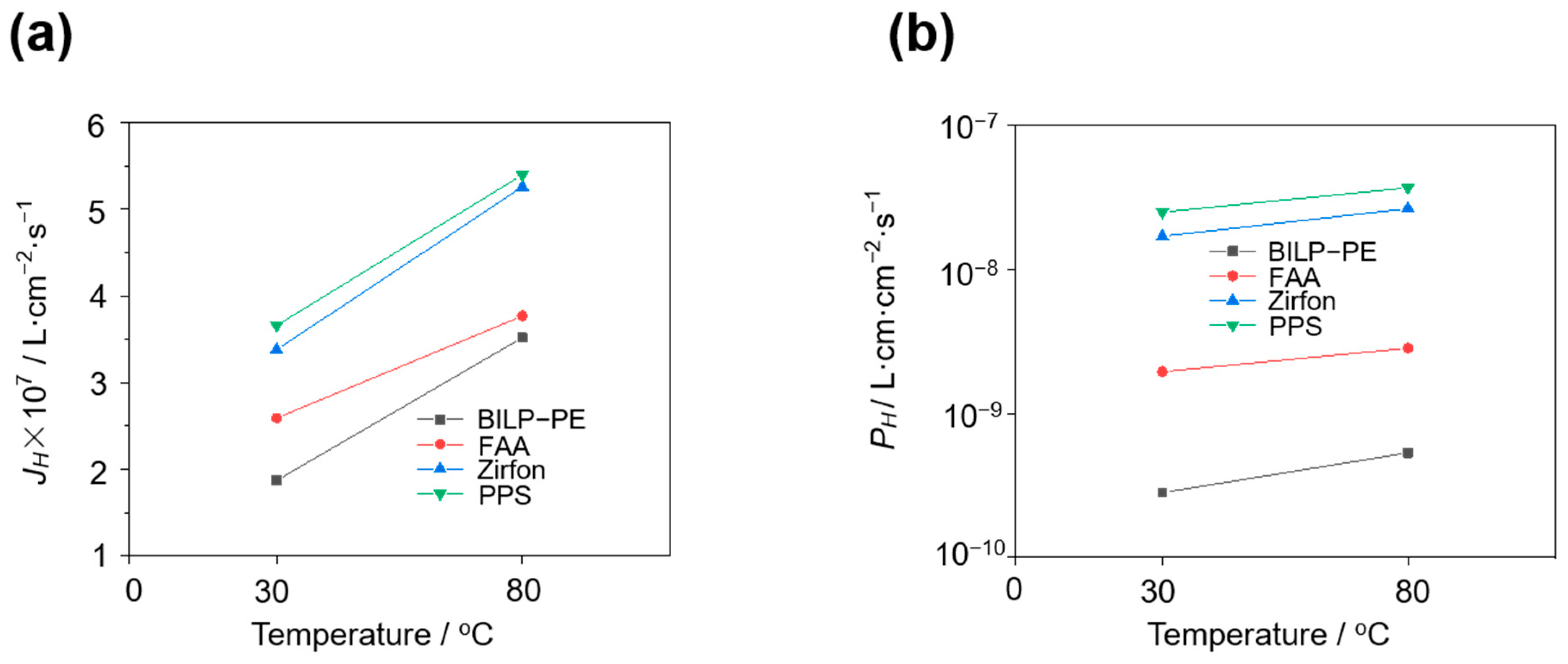
3.4. Effect of Temperature
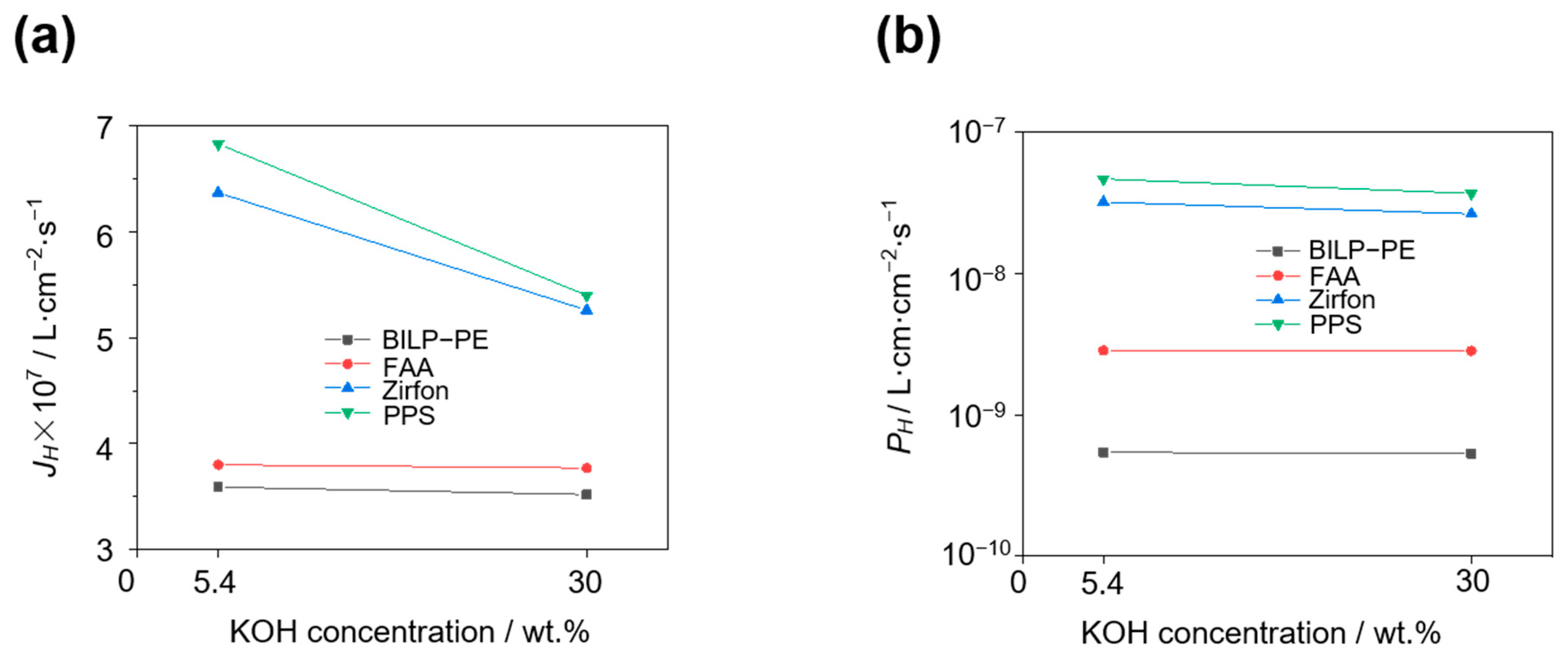
3.5. Effect of KOH Concentration
3.6. Position of HOR Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Cho, W.-C.; Jannasch, P.; Stojadinovic, J.; Li, Q.; Aili, D.; Jensen, J.O. Separators and Membranes for Advanced Alkaline Water Electrolysis. Chem. Rev. 2024, 124, 6393–6443. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Min, L.; Xu, W.; Wang, Y.; Zhang, W. Anion Exchange Membranes Based on Poly(aryl piperidinium) Containing Both Hydrophilic and Hydrophobic Side Chains. Ind. Eng. Chem. Res. 2022, 61, 14232–14241. [Google Scholar] [CrossRef]
- Depuydt, S.; Van der Bruggen, B. Green Synthesis of Cation Exchange Membranes: A Review. Membranes 2024, 14, 23. [Google Scholar] [CrossRef]
- Liu, L.; Ma, H.; Khan, M.; Hsiao, B.S. Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review. Membranes 2024, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Park, J.-S. Effect of Anion-Conducting Electrolytes in Pore-Filling Membranes on Performance and Durability in Water Electrolysis. Membranes 2024, 14, 265. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Zhang, J.; Li, G.; Xu, W.; Wang, Y.; Zhang, W. A rolling strategy for layered double hydroxide membrane to boost alkaline water electrolysis. J. Membr. Sci. 2025, 720, 123778. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.-S. Enhanced Performance and Durability of Pore-Filling Membranes for Anion Exchange Membrane Water Electrolysis. Membranes 2024, 14, 269. [Google Scholar] [CrossRef]
- Schuldt, K.; Lillepärg, J.; Pohlmann, J.; Brinkmann, T.; Shishatskiy, S. Permeance of Condensable Gases in Rubbery Polymer Membranes at High Pressure. Membranes 2024, 14, 66. [Google Scholar] [CrossRef]
- Jung, J.; Kim, G.; Gim, G.; Park, C.; Lee, J. Determination of Gas Permeation Properties in Polymer Using Capacitive Electrode Sensors. Sensors 2022, 22, 1141. [Google Scholar] [CrossRef]
- Gode, P.; Lindbergh, G.; Sundholm, G. In-situ measurements of gas permeability in fuel cell membranes using a cylindrical microelectrode. J. Electroanal. Chem. 2002, 518, 115–122. [Google Scholar] [CrossRef]
- Bensmann, B.; Hanke-Rauschenbach, R.; Sundmacher, K. In-situ measurement of hydrogen crossover in polymer electrolyte membrane water electrolysis. Int. J. Hydrogen Energy 2014, 39, 49–53. [Google Scholar] [CrossRef]
- Park, E.J.; Komini Babu, S.; Kim, Y.S. Gas Permeability Test Protocol for Ion-Exchange Membranes. Front. Energy Res. 2022, 10, 945654. [Google Scholar] [CrossRef]
- Kim, S.; Nguyen, B.T.D.; Ko, H.; Kim, M.; Kim, K.; Nam, S.; Kim, J.F. Accurate evaluation of hydrogen crossover in water electrolysis systems for wetted membranes. Int. J. Hydrogen Energy 2021, 46, 15135–15144. [Google Scholar] [CrossRef]
- Stähler, M.; Stähler, A.; Scheepers, F.; Carmo, M.; Lehnert, W.; Stolten, D. Impact of porous transport layer compression on hydrogen permeation in PEM water electrolysis. Int. J. Hydrogen Energy 2020, 45, 4008–4014. [Google Scholar] [CrossRef]
- Zhang, J.; Gasteiger, H.A.; Gu, W. Electrochemical Measurement of the Oxygen Permeation Rate through Polymer Electrolyte Membranes. J. Electrochem. Soc. 2013, 160, F616–F622. [Google Scholar] [CrossRef]
- Schalenbach, M.; Lueke, W.; Stolten, D. Hydrogen Diffusivity and Electrolyte Permeability of the Zirfon PERL Separator for AlkalineWater Electrolysis. J. Electrochem. Soc. 2016, 163, F1480–F1488. [Google Scholar] [CrossRef]
- Jones, S.F.; Evans, G.M.; Galvin, K.P. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Janssen, L.J.J.; van Stralen, S.J.D. Bubble behaviour on and mass transfer to an oxygen-evolving transparent nickel electrode in alkaline solution. Electrochim. Acta 1981, 26, 1011–1022. [Google Scholar] [CrossRef]
- Song, C.; Min, L.; Zhang, W.; Xu, L.; Wang, Y. A benzimidazole-linked polymer membrane in alkaline water electrolysis. J. Membr. Sci. 2023, 683, 121883. [Google Scholar] [CrossRef]
- Matsushima, H.; Kiuchi, D.; Fukunaka, Y. Measurement of dissolved hydrogen supersaturation during water electrolysis in a magnetic field. Electrochim. Acta 2009, 54, 5858–5862. [Google Scholar] [CrossRef]
- Brauns, J.; Schoenebeck, J.; Kraglund, M.R.; Aili, D.; Hnat, J.; Zitka, J.; Mues, W.; Jensen, J.O.; Bouzek, K.; Turek, T. Evaluation of Diaphragms and Membranes as Separators for Alkaline Water Electrolysis. J. Electrochem. Soc. 2021, 168, 014510. [Google Scholar] [CrossRef]
- Sakai, T.; Takenaka, H.; Wakabayashi, N.; Kawami, Y.; Torikai, E. Gas Permeation Properties of Solid Polymer Electrolyte (SPE) Membranes. J. Electrochem. Soc. 1985, 132, 1328. [Google Scholar] [CrossRef]
- Broka, K.; Ekdunge, P. Oxygen and hydrogen permeation properties and water uptake of Nafion® 117 membrane and recast film for PEM fuel cell. J. Appl. Electrochem. 1997, 27, 117–123. [Google Scholar] [CrossRef]




| Membranes | Thickness (μm) | IEC (meq·g−1) | jl (mA·cm−2) | JH × 107 (L·cm−2·s−1) | PH × 1010 (L·cm·cm−2·s−1) |
|---|---|---|---|---|---|
| PPS Zirfon | 500 | — | 4.65 | 5.40 | 367 |
| 680 | — | 4.53 | 5.26 | 263 | |
| FAA BILP-PE | 75.0 | 1.20–1.40 | 3.25 | 3.77 | 28.3 |
| 15.1 | — | 3.03 | 3.52 | 5.32 |
| Electrolytic Current Densities/mA·cm−2 | V1 vs. SCE/V | V2 vs. SCE/V | Phenomena on Nickel Plates |
|---|---|---|---|
| 10 | −0.10 | 0.23 | No significant change |
| 20 | −0.56 | 0.32 | No significant change |
| 40 | −1.09 | 0.47 | Bubbles are produced |
| 50 | −1.11 | 0.55 | A large number of bubbles are produced |
| 60 | −1.38 | 0.72 | A large number of bubbles are produced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Song, C.; Xu, L.; Wang, Y.; Zhang, W. In-Situ Measurement of Gas Permeability for Membranes in Water Electrolysis. Membranes 2025, 15, 147. https://doi.org/10.3390/membranes15050147
Li S, Song C, Xu L, Wang Y, Zhang W. In-Situ Measurement of Gas Permeability for Membranes in Water Electrolysis. Membranes. 2025; 15(5):147. https://doi.org/10.3390/membranes15050147
Chicago/Turabian StyleLi, Shuaimin, Chuan Song, Li Xu, Yuxin Wang, and Wen Zhang. 2025. "In-Situ Measurement of Gas Permeability for Membranes in Water Electrolysis" Membranes 15, no. 5: 147. https://doi.org/10.3390/membranes15050147
APA StyleLi, S., Song, C., Xu, L., Wang, Y., & Zhang, W. (2025). In-Situ Measurement of Gas Permeability for Membranes in Water Electrolysis. Membranes, 15(5), 147. https://doi.org/10.3390/membranes15050147







