Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 2: Countermeasures and Applications
Abstract
1. Introduction
- The removal of natural organic matter (NOM), such as humic substances, through coagulation, ultrafiltration (UF), and activated carbon (AC);
- The removal of oils (hydrocarbons or silicone-based) and greases (O&G) using coagulation and AC.
2. Pretreatment
2.1. Adsorption Process
| Adsorbents and Process | Comments | Reference |
|---|---|---|
| AC | Granular activated carbon (GAC) | [23,24,25,26] |
| Powdered activated carbon (PAC) | [28,29,30,31] | |
| Fibrous activated carbon (FAC) | [27,32] | |
| AC Material | Bituminous AC | [33,34,35] |
| Peat-based AC | [36] | |
| Others | Polyamide (PA) | [37] |
| Synthetic adsorbents | [26] | |
| Integrated Process | Coagulation + multi-media filter (MMF) + GAC | [33] |
| Coagulation/PAC + MF/UF | [38] | |
| GAC + UF | [39] | |
| UF + GAC | [36] | |
| NF + GAC | [40] | |
| Membrane bioreactor (MBR) + GAC | [41] | |
| Advanced oxidation process (AOP) + GAC | [42] |
- When raw water has high turbidity or suspended solids, pretreatment with coagulation/filtration or MF/UF is used to prevent AC clogging.
- For feed water with high TOC concentrations, integrated processes—including MBR, NF, and AOP—are utilized to extend AC lifespan by removing organic compounds. For example, alum clarification applied to secondary effluent treatment has been shown to increase TOC removal in solids contact clarifiers to 30–45%, leading to a prolonged carbon life [33].
2.2. Specialty Water Filters
2.3. Oxidation and AOPs
2.4. Antifoulnants or Dispersants
- Polymer compounds having a carbonyl group and a structure including a nitrogen atom bonded to a carbonyl carbon atom, such as PVP and polyacrylamide [80];
- Chemicals including organic amines having two or more nitrogen atoms and five or more carboxyl groups or four or more phosphate groups, e.g., ethylene diamine tetra (methylene phosphonic acid) [81];
- Polymer compounds having a carboxyl group and a sulfonic acid group [82].
3. Detection and Prediction of LMWOC Fouling and Identification of Foulants
3.1. Quick Prediction of LMWOC Fouling Potential
3.2. Detection and Prediction of LMWOC Fouling
- Preliminary evaluation of raw water: Bench-scale tests (tests with small elements);
- Preliminary evaluation of pretreatment processes: Bench-scale tests (a flat sheet membrane test might be acceptable for screening purposes);
- The final test of pretreatment: Pilot tests (4-inch or 8-inch elements);
- Detection of fouling during plant operation: Canary tests (small elements).
3.3. Identification of Foulants
4. Cleaning of LMWOCs-Fouled RO/NF Membranes
4.1. Special Cleaning Agents for LMWOCs Fouling
4.2. Timing of Cleaning
- Normalized permeate flow has decreased by 10% since startup or last cleaning.
- Normalized salt passage has increased by 10% since startup or last cleaning.
- Normalized pressure drop from feed to concentrate has increased by 15% since startup or last cleaning.
- The concentration of leachables from plant construction materials decreases over time to a level that does not interfere with the RO operation.
- Measures are taken to prevent problematic substances from entering the plant, such as fouling caused by hydraulic fluid leaks and installing a GAC filter.
5. Operational Methods
5.1. High pH Operations
- Increase in negative charge of membrane: Increase in hydrophilicity (contact angle) and swelling of membrane;
- Electrostatic interactions: Decrease in sorption of organic compounds;
- Hydrolysis of certain organic compounds: Phthalates;
- Increase in solubility of organics: Hydrocarbons, fatty acids, etc.
5.2. Low pH Operation
6. Low Fouling RO/NF Membranes and In Situ Treatment
6.1. Membrane Surface Modification
6.2. Pore Size Reduction
7. Applications for Enhancing RO/NF Performance and Rejuvenation
7.1. Application as a Performance Enhancer
- The flow rate reduction must be as low as possible.
- Targeted boron and micropollutant rejections must be increased as much as possible.
- The performance of modified BWRO membranes needs to be compared with SWRO membranes as a reference.
- The ability to be immobilized in the membrane.
7.1.1. Tannic Acid (TA)
7.1.2. Other LMWOCs as Performance Enhancers
- Improvement of salt rejection by plugging micro defects;
- Improving the rejection of monovalent cations at low concentrations;
- Improvement of salt rejection at low and high pH.
| Targets | Rejection Enhancer | Comments | Reference |
|---|---|---|---|
| Improvement of inorganic and organic rejections | |||
| Na+ ion | Quaternary ammonium | Under low TDS conditions | [298] |
| Salt rejection | Cationic organic compounds | Asymmetric polyamide membrane | [295] |
| Urea, IPA | Polyalkylene glycol | PEG (MW 2000–6000) | [300] |
| Salt rejection | Polyalkylene glycol | PEG (MW 1000–10,000), low pH performance | [301] |
| TOC | Polyalkylene glycol | Improve TOC rejection at high pH (>9.5) | [302] |
| Salt rejection | Polyalkylene glycol | Under low TDS conditions, i.e., second pass RO | [299] |
| IPA | Polyalkylene glycol + LBL PEC | After PEG treatment, an LBL PEC treatment | [303] |
| As(III) | Aramide dendrimers | With oligoethylene glycol chains, for NF membrane | [230] |
| Boron | Surfactants | Cationic, nonionic, and anionic surfactants | [266] |
| Boron | Pyrogallol derivatives | Pyrogallol and/or a pyrogallol derivative: MW < 500 | [304] |
| Boron | Higher alkylamines | Decylamine and dodecylamine | [305,306,307] |
| Boron | 4-Nitrobenzenesulfonyl chloride | Hydrolyzed to 4-Nitrobenzenesulfonic acid | [308] |
| Urea | MPD | Carbodiimide chemistry is used to attach a diamine | [309] |
| Urea | MPD, 1,8-diaminooctane, etc. | Carbodiimide chemistry and effect of amines | [310] |
| Improvement of Micropollutant rejection | |||
| NDMA | Tryptophan | Model LMW foulant in municipal wastewater | [292] |
| NDMA | Alkylamines | Hexylamine, decylamine, dodecylamine, etc. | [311,312] |
| Improve chlorine tolerance | |||
| Chlorine | Diphenylamine | First, a membrane is treated with sodium peroxide. | [313] |
- PEG-based: PEG (MW 1000–10,000) and Tween 80 (ethoxylated surfactant);
- Cationic surfactant with long alkyl chains: CTAB and dodecylamine;
- NBS: SES method.
7.2. Application as an RJA
- Two mechanisms are proposed to explain the rejuvenation process: “surface treatment”, which is, in effect, a surface coating on the membrane, and “hole plugging”.
- Membrane rejuvenation typically increases salt rejection to at least 94%.
- The key to successful application is to clean the RO membranes thoroughly.
- When salt rejection is below 75%, successful rejuvenation is unlikely.
- PT-B treatment should be conducted at a pH less than 5.0 [272].
- PT-A is generally durable.
- PT-B wears away with time.
- Cleaning treatments may remove PT-A and will readily remove PT-B.
- It is usually very effective when salt rejection is above 80%.
7.2.1. TA and Polyphenols as RJAs
7.2.2. Other RJAs
7.2.3. Multicomponent RJAs
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2B3T | Two-bed, three-tower pure water system |
| 2CP | 2-Chlorophenol |
| 2NP | 2-Nitrophenol |
| 3,5-DABA | 3,5-Diaminobenzoic acid |
| 4CP | 4-Chlorophenol |
| 4NP | 4-Nitrophenol |
| AC | Activated carbon |
| AE | Alkyl ethoxylate |
| AEO | Alcohol ethoxylate |
| AOM | Algal organic matter |
| AOP | Advanced oxidation process |
| APA | Aromatic polyamide |
| APEO | Alkylphenol ethoxylate |
| APT | Aminopentane |
| ATD | Anti-telescoping device |
| ATP | Adenosine triphosphate |
| ATR | Attenuated total reflection |
| BAC | Benzalkonium chloride |
| BPA | Bisphenol A |
| BSA | Bovine serum albumin |
| BTEX | Benzene, Toluene, Ethylbenzene, and Xylenes |
| BWRO | Brackish water reverse osmosis |
| CA | Cellulose acetate |
| CAPB | Cocamidopropyl betaine |
| CAR | Carboxen |
| CEB | Chemical-enhanced backwash |
| CF | Cartridge filter |
| CIP | Cleaning in place |
| CMC | Critical micelle concentration |
| CMCL | Carboxymethyl cellulose |
| COD | Chemical oxygen demand |
| CTA | Cellulose triacetate |
| CTAB | Cetyltrimethylammonium bromide |
| CTAC | Cetyltrimethylammonium chloride |
| CTBD | Cooling tower blowdown |
| DAF | Dissolved air flotation |
| DBP | Disinfection byproduct |
| DCC | Dichloroisocyanurate |
| DCHP | Dicyclohexyl phthalate |
| DCP | 2,4-Dichlorophenol |
| DEHP | Di-2-ethylhexyl phthalate |
| DEP | Diethyl phthalate |
| DMAc | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DMP | Dimethyl phthalate |
| DMSO | Dimethyl sulfoxide |
| DnBP | Dibutyl phthalate |
| DNP | 2, 4-Dinitrophenol |
| DO | Dissolved oxygen |
| DOC | Dissolved organic carbon |
| DOM | Dissolved organic matter |
| DOP | Dioctyl phthalate |
| DP | Differential pressure |
| DTAB | Dodecyltrimethylammonium bromide |
| DVB | Divinylbenzene |
| E260 | UV absorbance at 260 nm |
| ED | Electrodialysis |
| EDTA | Ethylenediaminetetraacetic acid |
| EEM | Excitation/emission matrix |
| EfOM | Effluent organic matter |
| EG | Ethylene glycol |
| EPS | Extracellular polymeric substances |
| ESCA | Electron spectroscopy for chemical analysis |
| FAC | Fibrous activated carbon |
| GFPD | Flat panel display |
| FRP | Fiber-reinforced plastic |
| FT-IR | Fourier transform infrared spectroscopy |
| GAC | Granular activated carbon |
| GC | Gas Chromatography |
| gfd | Gallons/ft2/day |
| GMA | Glycidyl methacrylate |
| GWRS | Groundwater Replenishment System |
| HA | Humic acid |
| HEM | n-Hexane Extractable Material |
| HLB | Hydrophilic–Lipophilic Balance |
| HPM | High-pressure membrane |
| HPO | Hydrophobic |
| HS-SPME | Headspace solid-phase microextraction |
| ICP | Inductively coupled plasma |
| ICR | Information Collection Rule |
| IEX | Ion exchange |
| IPA | Isopropanol |
| IR | Infrared |
| LAS | Linear alkyl benzene sulfonate |
| LbL | Layer-by-layer |
| LC-OCD | Liquid chromatography—organic carbon detection |
| LES | Lauryl ether sulfate |
| LMH | L/m2/h |
| LMW | Low molecular weight |
| LMWOCs | Low-molecular-weight organic compounds |
| log Dow | Logarithm of the pH-dependent n-octanol/water distribution coefficient |
| log p | Logarithm of the octanol-water partition coefficient |
| LOI | Loss on ignition |
| LP | Low pressure |
| LPM | Low-pressure membrane |
| MBR | Membrane bioreactor |
| MF | Microfiltration |
| MeOH | Methanol |
| MFI | Modified fouling index |
| MMF | Multi-media filter |
| MPD | m-phenylenediamine |
| MS | Membrane softening |
| MTCw | Water mass transfer coefficients |
| MW | Molecular weight |
| MWCO | Molecular weight cut-off |
| NBS | 4-nitrobenzenesulfonyl chloride |
| NDMA | N-nitrosodimethylamine |
| NF | Nanofiltration |
| NMP | N-methyl-2-pyrrolidinone |
| NOM | Natural organic matter |
| NP | Nitrophenol |
| OCWD | Orange County Water District |
| ORP | Oxidation–reduction potential |
| O&G | Oil and grease |
| O&M | Operation and maintenance |
| PA | Polyamide |
| PAC | Powdered activated carbon |
| PALS | Positron annihilation lifetime spectroscopy |
| PAN | Polyacrylonitrile |
| PASP | Polyaspartic acid |
| PDMS | Polydimethylsiloxane |
| PEC | Polyelectrolyte complex |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PEO | Polyethylene oxide |
| PES | Polyether sulfone |
| PFAS | Perfluoroalkyl substances |
| PFOS | Perfluorooctane sulfonate |
| PG | Propylene glycol |
| PHE | Phenol |
| pKa | Acid dissociation constant |
| PMSP | Poly(1-(trimethylsilyl)-1-propyne) |
| POE | Polyoxyethylene octyl phenyl ether |
| polyDADMAC | Polydiallyldimethylammonium chloride |
| PP | Polypropylene |
| PPA | Piperazine polyamide |
| PPCPs | Pharmaceuticals and personal care products |
| PPO | Polyphenylene oxide |
| PSF | Polysulfone |
| PSS-Na | Polystyrene sulfonate |
| PT-A | Polyvinyl methyl ether |
| PT-B | Tannic acid |
| PTFE | Polytetrafluoroethylene |
| PV | Pervaporation |
| PVA | Polyvinyl alcohol |
| PVAM | Polyvinylamidine |
| PVDF | Polyvinylidene fluoride |
| PVME | Polyvinyl methyl ether |
| PVP | Polyvinylpyrrolidone |
| RJA | Rejuvenation agent |
| RBSMT | Rapid bench-scale membrane test |
| RO | Reverse osmosis |
| SBS | Sodium bisulfite |
| SDBD | Sodium dodecyl benzenesulfonate |
| SDGs | Sustainable development goals |
| SDI | Silt density index |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscopy |
| SES | Swelling–embedding–shrinking |
| SGPW | Shale gas-produced water |
| SMPs | Soluble microbial products |
| SO | Sodium oleate |
| SOC | Synthetic organic compound |
| STPP | Sodium tripolyphosphate |
| SULP | Super ultra-low pressure |
| SUVA | Specific UV absorbance |
| SWRO | Seawater reverse osmosis |
| TBP | Tributyl phosphate |
| TCP | 2,4,6-trichlorophenol |
| TDS | Total dissolved solids |
| TEP | Transparent exopolymer particles |
| TFC | Thin-film composite |
| TFN | Thin-film nanocomposite |
| TGA | Thermo gravimetric analysis |
| THM | Trihalomethane |
| TMC | Trimesoyl chloride |
| TMP | Transmembrane pressure |
| TOC | Total organic carbon |
| TPI | Transphilic |
| TSP | Trisodium phosphate |
| UF | Ultrafiltration |
| ULP | Ultra-low pressure |
| UPW | Ultrapure water |
| USEPA | US Environmental Protection Agency |
| XAD | Polymeric adsorbent resin |
| WBMWD | West Basin Municipal Water District |
| WF-21 | Water Factory 21 |
| WOCS | Weathered oil-contaminated seawater |
References
- Loeb, S.; Selover, E. Sixteen months of field experience on the Coalinga pilot plant. Desalination 1967, 2, 75–80. [Google Scholar] [CrossRef]
- McCutchan, J.W.; Johnson, J.S. Reverse Osmosis at Coalinga, California. J. AWWA 1970, 62, 346–353. [Google Scholar] [CrossRef]
- Oron, G. Marginal-water application in arid zones. GeoJournal 1987, 15, 259–266. [Google Scholar] [CrossRef]
- Glueckstern, P.; Nadav, N.; Priel, M. Desalination of marginal water: Environmental and cost impact: Part 1: The effect on long-range regional development Part 2: Case studies of desalinated water vs. local desalination of marginal brackish water. Desalination 2001, 138, 157–163. [Google Scholar] [CrossRef]
- Redondo, J. Combined Systems Tackle Marginal Resources. Int. Desalin. Water Reuse Q. 2001, 11, 37–45. [Google Scholar]
- Gerard, R.; Hachisuka, H.; Hirose, M. New membrane developments expanding the horizon for the application of reverse osmosis technology. Desalination 1998, 119, 47–55. [Google Scholar] [CrossRef]
- Inoue, T.; Sugita, K.; Isaka, H.; Fusaoka, Y. Low Fouling RO Membranes. Membrane 2002, 27, 209–212. [Google Scholar] [CrossRef]
- Maeda, Y. Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 1: Fundamentals and Mechanism. Membranes 2024, 14, 221. [Google Scholar] [CrossRef]
- Burns and Roe Industrial Services Corporation. Reverse Osmosis Technical Manual; PB80-186950/XAB, OWRT-C-80155-S(8530), DI-14-34-0001-8530; Burns and Roe Industrial Services Corporation: Paramus, NJ, USA, 1979. [Google Scholar]
- AWWA. Reverse Osmosis and Nanofiltration, 2nd ed.; American Water Works Assoc.: Denver, CO, USA, 2007; Volume 46. [Google Scholar]
- DuPont. FilmTec™ Reverse Osmosis Membranes Technical Manual, Form No. 45-D01504-en, Rev. 16; DuPont: Wilmington, DE, USA, 2023. [Google Scholar]
- Byrne, W. Reverse Osmosis: A Practical Guide for Industrial Users, 2nd ed.; Tall Oaks Publishing, Inc.: Littleton, CO, USA, 2002. [Google Scholar]
- Kucera, J. Reverse Osmosis: Industrial Processes and Applications, 2nd ed.; Scrivener Publishing/John Wiley & Sons: Beverly, MA, USA, 2015. [Google Scholar]
- Van Der Bruggen, B.; Vandecasteele, C. Flux decline during nanofiltration of organic components in aqueous solution. Environ. Sci. Technol. 2001, 35, 3535–3540. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Braeken, L.; Vandecasteele, C. Flux decline in nanofiltration due to adsorption of organic compounds. Sep. Purif. Technol. 2002, 29, 23–31. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Braeken, L.; Vandecasteele, C. Evaluation of parameters describing flux decline in nanofiltration of aqueous solutions containing organic compounds. Desalination 2002, 147, 281–288. [Google Scholar] [CrossRef]
- Boussu, K.; Kindts, C.; Vandecasteele, C.; Van der Bruggen, B. Applicability of nanofiltration in the carwash industry. Sep. Purif. Technol. 2007, 54, 139–146. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Mulyawan, R.; Muarif, A. A Review Of Reverse Osmosis Membrane Fouling: Formation and Control. Int. J. Eng. Sci. Inf. Technol. 2021, 1, 110–115. [Google Scholar] [CrossRef]
- Sisay, E.J.; Al-Tayawi, A.N.; László, Z.; Kertész, S. Recent Advances in Organic Fouling Control and Mitigation Strategies in Membrane Separation Processes: A Review. Sustainability 2023, 15, 13389. [Google Scholar] [CrossRef]
- Maeda, Y. Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes 2022, 12, 170. [Google Scholar] [CrossRef]
- Mohamed Nageeb, R. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In Organic Pollutants; Rashed, M.N., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 7. [Google Scholar]
- Nickerson, G.; McClain, B.; Bukay, M. Investigation of Severe Flux Decline in a Two-Pass CA/Thin-Film Composite Membrane RO System. Ultrapure Water 1994, 11, 26–33. [Google Scholar]
- Eriksson, P.; Taylor, B.; Scarth, I. Nanofiltration Followed by Reverse Osmosis of Cooling Water Blowdown to Achieve Zero Liquid Discharge—IWC-03-38. In Proceedings of the 64th International Water Conference, Pittsburgh, PA, USA, 19–23 October 2003; pp. 420–432. [Google Scholar]
- Arai, N.; Hitotsuyanagi, N. Evaluation Method of Feed Water to Reverse Osmosis Membrane and Operation Control Method of Water Treatment Equipment. Japanese Unexamined Patent Application Publication No. JP2004188387A, 8 July 2004. [Google Scholar]
- Hitotsuyanagi, N.; Arai, N.; Masudo, M. Influence of Trace Organic Matter on RO Membrane Performance in Industrial Wastewater Reclamation. In Proceedings of the 38th Japan Society on Water Environment Annual Meeting, Sapporo, Japan, 17–19 March 2004. [Google Scholar]
- Koizumi, M.; Furuichi, M. Water Treating Apparatus. Japanese Unexamined Patent Application Publication No. JPH04354581A, 8 December 1992. [Google Scholar]
- Shon, H.K.; Vigneswaran, S.; Ngo, H.H.; Kim, D.; Park, N.; Jang, N.; Kim, I.T.S. Characterisation of Effluent Organic Matter (EFOM)Of Fouled Nanofilter (NF) Membranes. In Proceedings of the Fifth International Membrane Science & Technology Conference, Sydney, Australia, 10–14 November 2003. [Google Scholar]
- Shon, H.k.; Vigneswaran, S.; Ngo, H.H.; Aim, R.B. Low Pressure Nanofiltration with Adsorption As Pretreatment In Tertiary Wastewater Treatment for Reuse. In Proceedings of the Fifth International Membrane Science & Technology Conference, Sydney, Australia, 10–14 November 2003. [Google Scholar]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Ngo, H.H. Effect of pretreatment on the fouling of membranes: Application in biologically treated sewage effluent. J. Membr. Sci. 2004, 234, 111–120. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Ngo, H.H. The effect of pretreatment to ultrafiltration of biologically treated sewage effluent: A detailed effluent organic matter (EfOM) characterization. Water Res. 2004, 38, 1933–1939. [Google Scholar] [CrossRef]
- Suzuki, M. Application of Fiber Adsorbents in Water Treatment. Water Sci. Technol. 1991, 23, 1649–1658. [Google Scholar] [CrossRef]
- Darji, J.D.; Ballard, P.T. Treatment of Secondary Wastewater for Reverse Osmosis Systems. In Proceedings of the International Water Conference, Pittsburgh, PA, USA, 11–13 October 1993; p. IWC-93-18. [Google Scholar]
- Geselbracht, J.; Freeman, S. Polyamide RO Membranes Help Reclaim Water. Water Environ. Technol. 1995, 7, 18–20. [Google Scholar]
- Freeman, S.D.N.; Crook, J. An Update on Membrane Water Reuse Projects. In Proceedings of the AWWA Membrane Technology Conference, Reno, NV, USA, 13–16 August 1995; pp. 665–698. [Google Scholar]
- Ikuno, N. Treatment Method for Biologically Treated Water-Containing Water. Japanese Unexamined Patent Application Publication No. JP2005058934A, 10 March 2005. [Google Scholar]
- Masudo, M.; Hitotsuyanagi, N. Method and Apparatus for Treating Water and Method for Analyzing Contaminant of Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication No. JP2003275760A, 30 September 2003. [Google Scholar]
- Katsu, Y.; Kojima, Y. Method for Treating Organic Waste Water. Japanese Unexamined Patent Application Publication No. JPH0760249A, 7 March 1995. [Google Scholar]
- Wang, D.; Tong, F.; Aerts, P. Application of the combined ultrafiltration and reverse osmosis for refinery wastewater reuse in Sinopec Yanshan Plant. Desalin. Water Treat. 2011, 25, 133–142. [Google Scholar] [CrossRef]
- Takeda, A.; Miyamaru, H. Wastewater Treatment Method, Wastewater Treatment Apparatus, and Wastewater Recovery System. Japanese Unexamined Patent Application Publication No. JP2007313445A, 6 December 2007. [Google Scholar]
- Gur-Reznik, S.; Katz, I.; Dosoretz, C.G. Removal of dissolved organic matter by granular-activated carbon adsorption as a pretreatment to reverse osmosis of membrane bioreactor effluents. Water Res. 2008, 42, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Hitotsuyanagi, N. Surfactant-Containing Waste Water Treatment Method. Japanese Unexamined Patent Application Publication No. JP2007260494A, 11 October 2007. [Google Scholar]
- Haberkamp, J.; Ruhl, A.S.; Ernst, M.; Jekel, M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration. Water Res. 2007, 41, 3794–3802. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Fang, H.H.P. Applications of Porous Resin Sorbents in Industrial Wastewater Treatment and Resource Recovery. Crit. Rev. Environ. Sci. Technol. 2003, 33, 363–389. [Google Scholar] [CrossRef]
- Goto, T.; Hirai, M.; Al-Hinai, H.; Yamada, H.; Iwahashi, H.; Matsuo, I. Designing and Performance of Full SWRO Plant in the Middle East. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Singapore, 11–16 September 2005. [Google Scholar]
- Mitsubishi Chemical Corporation. DIAION Technical Manual, Synthetic Adsorbents. Mitsubishi Chemical Corporation: Tokyo, Japan, 2018. [Google Scholar]
- Robinson, K. RO Pretreatment; Cartridge Filtration, An Operational Overview. In Proceedings of the AMTA/AWWA Membrane Technology Conference, Knoxville, TN, USA, 20–23 February 2023. [Google Scholar]
- Nordham, D.J. Oil-Fouling Evaluation of Membranes and Components of a Navy Shipboard RO Desalination System. Ultrapure Water 1999, 16, 18–28. [Google Scholar]
- Subramani, A.K.; Schlicher, R.; Long, J.; Yu, J.; Lehman, S.; Jacangelo, J.G. Recovery optimization of membrane processes for treatment of produced water with high silica content. Desalin. Water Treat. 2011, 36, 297–309. [Google Scholar] [CrossRef]
- D’Angelo, P.; Abbott, K. A New Approach to Organic Removal for Make-up and Wastewater Treatment in Membrane Systems. Ultrapure Water 2002, 19, 16–21. [Google Scholar]
- Case Study: Protecting Reverse Osmosis via Membrane Pre-Treatment System. 2017. Available online: https://mycelx.com/wp-content/uploads/2020/09/MYCELX_CaseStudy_PetrochemPreRO_USA.pdf (accessed on 22 January 2025).
- Oda, N.; Matsushita, N.; Hayakawa, K. Water Treatment Method and Water Treatment Apparatus. Japanese Unexamined Patent Application Publication No. JP2005329334A, 2 December 2005. [Google Scholar]
- Tsuyumoto, M.; Karakane, H.; Maeda, Y.; Tsugaya, H. Development of polyion complex hollow fiber membrane for separation of water-ethanol mixtures. Desalination 1991, 80, 139–158. [Google Scholar] [CrossRef]
- Maeda, Y.; Tsuyumoto, M.; Karakane, H.; Tsugaya, H. Separation of Water-Ethanol Mixture by Pervaporation through Hydrolyzed Polyacrylonitrile Hollow Fiber Membranes. Polym. J. 1991, 23, 501–511. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater-A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Sugita, K.; Kitanaka, A.; Taniguchi, M.; Uemura, T. Development of MBR+RO system for reuse of sewage and wastewater. In Proceedings of the 45th Japan Annual Technical Conference on Sewerage, Yokohama, Japan, 22–24 July 2008; pp. 497–499. [Google Scholar]
- Hu, J.Y.; Shan, J.H. Control of RO/NF organic fouling by monitoring and modification of organic polarity of feed water. Water Supply 2008, 8, 467–472. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lim, J.; Lee, S.; Lee, C.; Hong, S. Cold-cathode X-ray irradiation pre-treatment for fouling control of reverse osmosis (RO) in shale gas produced water (SGPW) treatment. Chem. Eng. J. 2019, 374, 49–58. [Google Scholar] [CrossRef]
- Okuma, N.; Sumiyoshi, M.; Nakaoka, A. Treatment for Waste Launder Water. Japanese Unexamined Patent Application Publication No. JPS5995989A, 2 June 1984. [Google Scholar]
- Song, W.; Ravindran, V.; Koel, B.E.; Pirbazari, M. Nanofiltration of natural organic matter with H2O2/UV pretreatment: Fouling mitigation and membrane surface characterization. J. Membr. Sci. 2004, 241, 143–160. [Google Scholar] [CrossRef]
- Williams, M.; Deshmukh, R.; Bhattacharyya, D. Separation of hazardous organics by reverse osmosis membranes. Environ. Prog. 1990, 9, 118–125. [Google Scholar] [CrossRef]
- Fujii, K.; Matsuo, I. Water Purifying Method by Ozone Resistant Membrane. Japanese Unexamined Patent Application Publication No. JPH10309577A, 24 November 1997. [Google Scholar]
- Stanford, B.; Pisarenko, A.; Snyder, S. A Cost-Benefit Analysis of the Energy Requirements for UV/Peroxide and Ozone Pre-Oxidation for Organic Fouling Control in RO Membranes. In Proceedings of the 16th Annual Water Reuse & Desalination Research Conference, San Diego, CA, USA, 4–5 June 2012. [Google Scholar]
- Vatankhah, H.; Murray, C.C.; Brannum, J.W.; Vanneste, J.; Bellona, C. Effect of pre-ozonation on nanofiltration membrane fouling during water reuse applications. Sep. Purif. Technol. 2018, 205, 203–211. [Google Scholar] [CrossRef]
- Szczuka, A.; Berglund-Brown, J.P.; Chen, H.K.; Quay, A.N.; Mitch, W.A. Evaluation of a Pilot Anaerobic Secondary Effluent for Potable Reuse: Impact of Different Disinfection Schemes on Organic Fouling of RO Membranes and DBP Formation. Environ. Sci. Technol. 2019, 53, 3166–3176. [Google Scholar] [CrossRef]
- Yacouba, Z.A.; Mendret, J.; Lesage, G.; Zaviska, F.; Brosillon, S. Removal of organic micropollutants from domestic wastewater: The effect of ozone-based advanced oxidation process on nanofiltration. J. Water Process Eng. 2021, 39, 101869. [Google Scholar] [CrossRef]
- Hitotsuyanagi, N.; Arai, N. Method and Apparatus for Water Treatment. Japanese Unexamined Patent Application Publication No. JP2005230731A, 2 September 2005. [Google Scholar]
- Arai, N.; Hitotsuyanagi, N. Water Treatment Method and Water Treatment Apparatus. Japanese Unexamined Patent Application Publication No. JP2005230774A, 2 September 2005. [Google Scholar]
- Pisarenko, A.N.; Yan, D.; Snyder, S.A.; Stanford, B.D. Comparing Oxidative Organic Fouling Control in RO Membrane Applications. IDA J. Desalin. Water Reuse 2011, 3, 45–49. [Google Scholar] [CrossRef]
- Stanford, B.D.; Pisarenko, A.N.; Holbrook, R.D.; Snyder, S.A. Preozonation Effects on the Reduction of Reverse Osmosis Membrane Fouling in Water Reuse. Ozone Sci. Eng. 2011, 33, 379–388. [Google Scholar] [CrossRef]
- Pisarenko, A.N.; Yan, D.; Snyder, S.A.; Stanford, B.D. Comparison of UV, UV/Peroxide, Ozone, and Ozone/Peroxide for Organic Fouling Control in RO Membrane Applications. In Proceedings of the AWWA Membrane Technology Conference, Long Beach, CA, USA, 28–31 March 2011. [Google Scholar]
- Sarp, S.; Chon, K.; Kim, I.S.; Cho, J. Advanced treatment of membrane bioreactor (MBR) effluents for effective wastewater reclamation. Water Sci. Technol. 2011, 63, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. UV/AOP a key part of the groundwater replenishment system. In Proceedings of the WEFTEC 2011, Los Angeles, CA, USA, 15–19 October 2011; pp. 3516–3525. [Google Scholar]
- Backlund, P. Degradation of aquatic humic material by ultraviolet light. Chemosphere 1992, 25, 1869–1878. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef]
- Srisukphun, T.; Chiemchaisri, C.; Urase, T.; Yamamoto, K. Fouling mechanism of reverse osmosis membrane in textile wastewater reuse plant. In Proceedings of the IWA International Conference on Membranes for Water and Wastewater Treatment, Harrogate, UK, 15–17 May 2007. [Google Scholar]
- Sweity, A.; Ronen, Z.; Herzberg, M. Induced organic fouling with antiscalants in seawater desalination. Desalination 2014, 352, 158–165. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Li, Y. Humic Acid fouling mitigation by antiscalant in reverse osmosis system. Environ. Sci. Technol. 2010, 44, 5153–5158. [Google Scholar] [CrossRef]
- Fujii, A.; Kawakatsu, T. Dispersant for Water Treatment and Water Treatment Method. U.S. Patent Application Publication No. US20170107126A1, 20 April 2017. [Google Scholar]
- Ishii, K.; Ikuno, N. Water Treatment Chemical for Membrane, and Membrane Treatment Method. U.S. Patent Application Publication No. US20200129928A1, 30 April 2020. [Google Scholar]
- Ishii, K. Water Treatment Chemical for Membranes and Membrane Treatment Method. U.S. Patent Application Publication No. US20210339205A1, 4 November 2021. [Google Scholar]
- Kronmiller, D. RO Permeate Water Flux Enhancement. Ultrapure Water 1993, 10, 37–40. [Google Scholar]
- Kronmiller, D.L.; Netwig, C.L. Method of Improving the Reverse Osmosis Dewatering of an Aqueous Caffine Stream. U.S. Patent 5,525,234, 11 June 1996. [Google Scholar]
- Takagishi, T.; Kuroki, N. Interaction of polyvinylpyrrolidone with methyl orange and its homologs in aqueous solution: Thermodynamics of the binding equilibria and their temperature dependences. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 1889–1901. [Google Scholar] [CrossRef]
- Molyneux, P.; Frank, H.P. The Interaction of Polyvinylpyrrolidone with Aromatic Compounds in Aqueous Solution. Part I. Thermodynamics of the Binding Equilibria and Interaction Forces1. J. Am. Chem. Soc. 1961, 83, 3169–3174. [Google Scholar] [CrossRef]
- Maruthamuthu, M.; Sobhana, M. Hydrophobic interactions in the binding of polyvinylpyrrolidone. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 3159–3167. [Google Scholar] [CrossRef]
- Shimpo, C.; Hayakawa, K.; Imai, K.; Ishi, K. Innovative approach to preventing RO fouling caused by humic substance. In Proceedings of the Singapore International Water Week 2018, Singapore, 8–12 July 2018. [Google Scholar]
- Kiso, Y.; Sugiura, Y.; Kitao, T.; Nishimura, K. Effects of hydrophobicity and molecular size on rejection of aromatic pesticides with nanofiltration membranes. J. Membr. Sci. 2001, 192, 1–10. [Google Scholar] [CrossRef]
- Williams, M.E.; Hestekin, J.A.; Smothers, C.N.; Bhattacharyya, D. Separation of Organic Pollutants by Reverse Osmosis and Nanofiltration Membranes: Mathematical Models and Experimental Verification. Ind. Eng. Chem. Res. 1999, 38, 3683–3695. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Madadi, M.R. Separation of Phenolic Compounds by Low Pressure Composite Membranes: Mathematical Model and Experimental Results. In New Membrane Materials and Processes for Separation; Sirkar, K.K., Lloyd, D.R., Eds.; American Institute of Chemical Engineers: New York, NY, USA, 1988; pp. 139–157. [Google Scholar]
- Arsuaga, J.M.; Sotto, A.; López-Muñoz, M.J.; Braeken, L. Influence of type and position of functional groups of phenolic compounds on NF/RO performance. J. Membr. Sci. 2011, 372, 380–386. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chiang, P.C.; Chang, E.E. Reduction of disinfection by-products precursors by nanofiltration process. J. Hazard. Mater. 2006, 137, 324–331. [Google Scholar] [CrossRef]
- Macintosh, P.D.; Fane, A.G.; Papazoglou, D. Reclamation of Contaminated Ground Waters by a Multiple Membrane Process. In Proceedings of the 3rd International Conference on Membranes in Drinking and Industrial Water Production, Mulheim an der Ruhr, Germany, 22–26 September 2002; p. 525. [Google Scholar]
- Poy, F.L. Fouling Effects of Tri-n-Butylphosphate on Reverse Osmosis Performance and Techniques for Performance Recovery; DPST-87-387, DE91005116/XAB, AC09-89SR18035; Savannah River Lab.: Aiken, SC, USA, 1987. [Google Scholar]
- Hasson, D.; Limoni-Relis, B.; Semiat, R.; Tob, P. Fouling of RO membranes by phthalate esters contamination. Desalination 1996, 105, 13–20. [Google Scholar] [CrossRef]
- Cartwright, P.S. Industrial Wastewater Treatment with Membranes—A United States Perspective. Water Sci. Technol. 1992, 25, 373–390. [Google Scholar] [CrossRef]
- Saavedra, A.; Bertoni, G.; Fajner, D.; Sarti, G.C. Reverse osmosis treatment of process water streams. Desalination 1991, 82, 249–266. [Google Scholar] [CrossRef]
- Ang, W.S.; Tiraferri, A.; Chen, K.L.; Elimelech, M. Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J. Membr. Sci. 2011, 376, 196–206. [Google Scholar] [CrossRef]
- Hodgkiess, T.; Hanbury, W.T.; Law, G.B.; Al-Ghasham, T.Y. Effect of hydrocarbon contaminants on the performance of RO membranes. Desalination 2001, 138, 283–289. [Google Scholar] [CrossRef]
- Wilbert, M.C.; Pellegrino, J.; Zydney, A. Bench-scale testing of surfactant-modified reverse osmosis/nanofiltration membranes. Desalination 1998, 115, 15–32. [Google Scholar] [CrossRef]
- Ishida, K.P.; Bold, R.; Phipps, D. Identification and Evaluation of Unique Chemicals for Optimum Membrane Compatibility and Improved Cleaning Efficiency; Orange County Water District Fountain: Valley, CA, USA, 2005. [Google Scholar]
- Kawakatsu, T.; Izawa, S.; Hitotsuyanagi, N.; Orita, N. Regeneration of RO membranes fouled with nonionic surfactants and polyethylene glycols by novel cleaning method. In Proceedings of the ICOM2005, Seoul, Republic of Korea, 21–26 August 2005. [Google Scholar]
- Bellona, C.L.; Wuertle, A.; Xu, P.; Drewes, J.E. Evaluation of a bench-scale membrane fouling protocol to determine fouling propensities of membranes during full-scale water reuse applications. In Proceedings of the 7th IWA World Congress on Water Reclamation and Reuse, Brisbane, Australia, 20–25 September 2009. [Google Scholar]
- Ukai, N.; Suzuki, H.; Nakashoji, H.; Yoshioka, S.; Eda, M. System and Method to Prevent Chemical Fouling on Reverse Osmosis Membrane. U.S. Patent Application Publication No. US20160046509A1, 18 February 2016. [Google Scholar]
- Knoell, T.; Patel, M.; Dunivin, W.; Owens, E. From Qualification to Selection: Orange County’s Groundwater Replenishment System RO Membrane Procurement Process. In Proceedings of the AMTA/AWWA Membrane Technology Conference, San Antonio, TX, USA, 25–28 February 2013. [Google Scholar]
- Knoell, T.; Shu, J.; Coker, S.; Majamaa, K.; Patel, M.; Dunivin, W. Establishment of an Application Center to Enhance RO Membrane Performance for Municipal Water Reuse. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015. [Google Scholar]
- Choi, S.I.; Lee, S.; Hong, S.; Kim, H.; Park, C. Evaluation of organic matter fouling potential by membrane fouling index. Water Supply 2007, 7, 27–33. [Google Scholar] [CrossRef]
- Ando, M.; Ishihara, S.; Iwahori, H.; Tada, N. Peculiar or unexpected behavior of silt density index of pretreated seawater for RO desalination. In Proceedings of the IDA World Congress, Paradise Island, Bahamas, 28 September–3 October 2003; p. BAH03-071. [Google Scholar]
- Escobar, L.; Sellerberg, W.; Sanchez, D.; Pastrana, F.; Wachinski, A. Detailed Analysis of the Silt Density Index (SDI) on Desalination and Wastewater Reuse Applications for Reverse Osmosis Evaluation. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 7–12 November 2009; p. IDAWC/DB09-074. [Google Scholar]
- Handley, G.J.; Owens, E. Silt Density Index: A Fresh Perspective on an Old Index. In Proceedings of the AMTA/AWWA Membrane Technology Conference & Exposition, Las Vegas, NV, USA, 10–14 March 2014. [Google Scholar]
- Yoo, H.Y.; Lee, Y.S.; Oh, H.K.; Kim, J.-O. Silt density index as a fouling propensity parameter of various membrane materials using dissolved organic matter. J. Water Process Eng. 2021, 44, 102391. [Google Scholar] [CrossRef]
- Jönsson, C.; Jönsson, A.-S. Influence of the membrane material on the adsorptive fouling of ultrafiltration membranes. J. Membr. Sci. 1995, 108, 79–87. [Google Scholar] [CrossRef]
- Arai, N.; Hitotsuyanagi, N.; Furuichi, M.; Sawada, S. Evaluation of RO Membrane Feed Water by Modified MF Method. In Proceedings of the 68th Annual Meeting of SCEJ, Tokyo, Japan, 23–25 March 2003; p. R320. [Google Scholar]
- Bartels, C.R.; Franks, R. Understanding RO Membrane Fouling at Wastewater Treatment Plants. In Proceedings of the AWWA/AMTA Membrane Technology Conference, Glendale, AZ, USA, 27 February–1 March 2012. [Google Scholar]
- Darton, T.; Annunziata, U.; del Vigo Pisano, F.; Gallego, S. Membrane autopsy helps to provide solutions to operational problems. Desalination 2004, 167, 239–245. [Google Scholar] [CrossRef]
- López, S.G.; del Vigo Pisano, F.; Muñoz, J.S. Membrane autopsies: Helps to provide solutions to operational problems in reverse osmosis plants. Water Supply 2005, 5, 129–135. [Google Scholar] [CrossRef]
- Tasaka, K.; Katsura, T.; Iwahori, H.; Kamiyama, Y. Analysis of RO elements operated at more than 80 plants in Japan. Desalination 1994, 96, 259–272. [Google Scholar] [CrossRef]
- Sasaki, T.; Hachisuka, H.; Ikeda, K. New Low Fouling RO Membrane. In Proceedings of the 9th MRC (Membrane Research Circle of Food) Autumn Meeting, Osaka, Japan, 13–14 November 1997. (In Japanese). [Google Scholar]
- Hachisuka, H.; Nakamura, T.; Ikeda, K. Method for Analyzing Contamination of Separation Membrane. Japanese. Unexamined Patent Application Publication No. JPH11197472A, 27 July 1999. [Google Scholar]
- Martinez, C.; Gomez, V.; Pocurull, E.; Borrull, F. Characterization of organic fouling in reverse osmosis membranes by headspace solid phase microextraction and gas chromatography-mass spectrometry. Water Sci. Technol. 2015, 71, 117–125. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z. Complex fouling and cleaning-in-place of a reverse osmosis desalination system. Desalination 2001, 141, 15–22. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Arai, N. Method for Evaluating Supply Water to Reverse Osmosis Membrane and Method of Managing Operation of Water Treatment Apparatus. Japanese Unexamined Patent Application Publication No. JP2005106516A, 21 April 2005. [Google Scholar]
- Martínez, C.; Gómez, V.; Borrull, F.; Pocurull, E. Headspace-solid phase microextraction: Useful technique to characterize volatile and semi-volatile organic compounds in water reuse applications. Desalin. Water Treat. 2016, 57, 23176–23184. [Google Scholar] [CrossRef]
- Khan, M.T.; de O. Manes, C.-L.; Aubry, C.; Gutierrez, L.; Croue, J.P. Kinetic Study of Seawater Reverse Osmosis Membrane Fouling. Environ. Sci. Technol. 2013, 47, 10884–10894. [Google Scholar] [CrossRef]
- Li, H.; Yu, P.; Luo, Y. Fouling mechanisms and primary foulant constituents in reverse osmosis membrane reclamation of a petrochemical secondary effluent. Desalin. Water Treat. 2015, 54, 3200–3210. [Google Scholar] [CrossRef]
- LG. Membrane Cleaning. In LG Water Solutions, Technical Service Bulletin 113; LG Chem, Ltd.: Seoul, Republic of Korea, 2023. [Google Scholar]
- Hydranautics. Foulants and Cleaning Procedures for composite polyamide RO/NF Membrane Elements. In Technical Service Bulletin 107; Hydranautics: Oceanside, CA, USA, 2020. [Google Scholar]
- Toray. Operation, Maintenance, and Handling Manual; Toray: Tokyo, Japan, 2024. [Google Scholar]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Samieirad, S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 2010, 257, 80–86. [Google Scholar] [CrossRef]
- Ang, W.S.; Yip, N.Y.; Tiraferri, A.; Elimelech, M. Chemical cleaning of RO membranes fouled by wastewater effluent: Achieving higher efficiency with dual-step cleaning. J. Membr. Sci. 2011, 382, 100–106. [Google Scholar] [CrossRef]
- Kimura, K.; Ogawa, N.; Watanabe, Y. Permeability decline in nanofiltration/reverse osmosis membranes fed with municipal wastewater treated by a membrane bioreactor. Water Sci. Technol. 2013, 67, 1994–1999. [Google Scholar] [CrossRef]
- Ruiz, S.G.; Ramírez, J.A.L.; Zerrouk, M.H.; Alonso, J.M.Q. Optimization of the Sequence of Washing Reverse Osmosis Membranes Used for Seawater Desalination. Chem. Biochem. Eng. 2017, 31, 21–32. [Google Scholar] [CrossRef]
- Amjad, Z.; Workman, K.R.; Castete, D.R. Considerations in Membrane Cleaning. In Reverse Osmosis: Membrane Technology, Water Chemistry and Industrial Applications; Amjad, Z., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1993. [Google Scholar]
- Jefferies, M.; Freeman, D. Extreme Membrane Cleaning: How Far Can You Bend the Rules? In Proceedings of the IDA World Congress on Desalination and Water Reuse, Dubai, United Arab Emirates, 7–12 November 2009; p. DB09-319. [Google Scholar]
- Sehn, P. Long-term experiences with reverse osmosis membranes in three different process water plants. VGB PowerTech 2012, 92, 80–83. [Google Scholar]
- Kawakatsu, T.; Hitotsuyanagi, N. Washing Method of Reverse Osmosis Membrane Device. Japanese Unexamined Patent Application Publication No. JP2005028329A, 3 February 2005. [Google Scholar]
- Ishii, K.; Kawakatsu, T. Agent, Liquid, and Method for Cleaning Reverse Osmosis Membrane. U.S. Patent Application Publication No. US20180257038A1, 13 September 2018. [Google Scholar]
- Yoda, K.; Shimura, K. Method for Washing Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication No. JP2018122205A, 9 August 2018. [Google Scholar]
- Yoda, K.; Shimura, K. Method for Washing RO Membrane. Japanese Unexamined Patent Application Publication No. JP2018122206A, 9 August 2018. [Google Scholar]
- Toma, H.; Suehiro, S.; Inami, R.; Sato, K.; Hara, M. Desalination Plant of Factory Waste Water by Reverse Osmosis Membrane. J. Ind. Water 1991, 25–33. [Google Scholar]
- Hiramatsu, T. In-house Developed Wastewater Reclamation System by RO Method. J. Water Re-Use Technol. 1991, 17, 38–42. [Google Scholar]
- Izawa, S.; Kawakatsu, T. Regeneration of reverse osmosis membranes fouled with surfactants. In Proceedings of the 70th SCEJ Annual Meeting, Nagoya, Japan, 22–24 March 2005; p. H123. [Google Scholar]
- Izawa, S.; Kawakatsu, T. Effect of Detergent Composition on Surfactant-fouled Reverse Osmosis Membrane. In Proceedings of the 71st SCEJ Annual Meeting, Tokyo, Japan, 28–30 March 2006; p. N123. [Google Scholar]
- Kawakatsu, T.; Orita, N.; Hitotsuyanagi, N. Detergent for selectively permeable film and method for cleaning. U.S. Patent Application Publication No. US20070015680A1, 18 January 2007. [Google Scholar]
- Jung, Y.-J.; Kiso, Y.; Yamada, T.; Shibata, T.; Lee, T.-G. Chemical cleaning of reverse osmosis membranes used for treating wastewater from a rolling mill process. Desalination 2006, 190, 181–188. [Google Scholar] [CrossRef]
- Sehn, P. Data Normalization as a Tool to Evaluate the Field Performance of Reverse Osmosis Membranes. In Proceedings of the 7th WISA-MTD Symposium and Workshop, Mabalingwe Nature Reserve, Bela-Bela, South Africa, 18–20 March 2007. [Google Scholar]
- Ikuno, N.; Kawakatsu, T.; Izawa, S. Method for Washing Permeable Membrane. Japanese Unexamined Patent Application Publication No. JP2005224671A, 25 August 2005. [Google Scholar]
- Izawa, S.; Kawakatsu, T. Detergent for Permselective Membrane and Washing Method. Japanese Unexamined Patent Application Publication No. JP2006159062A, 22 June 2006. [Google Scholar]
- Ishii, K.; Honda, Z. Separation Specificity Recovering Method of Membrane. Japanese Unexamined Patent Application Publication No. JPS531178A, 7 January 1978. [Google Scholar]
- Freeman, S.; Harvey, G. As Easy As 1, 2, 3. Ind. Wastewater 1999, 7, 30–34. [Google Scholar]
- Farooque, M.; Al-amoudi, A.; Hassan, A. Chemical Cleaning Experiments for Performance Restoration of NF Membranes Operated on Seawater Feed. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Manama, Bahrain, 8–13 March 2002. [Google Scholar]
- Freeman, S.; Harvey, G.; Adams, L. Eight Years and Two Billion Gallons Later: An Update on a Triple Membrane Plant. In Proceedings of the AWWA Membrane Technology Conference, Phoenix, AZ, USA, 6–9 March 2005. [Google Scholar]
- Li, H.; Yu, P.; Luo, Y. Oxidative cleaning of reverse osmosis membranes during reclamation of steel wastewater. Desalin. Water Treat. 2016, 57, 5687–5699. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Hayakawa, K.; Aoki, T. Method for Washing Reverse Osmosis Membrane, and Washing Solution. Japanese Unexamined Patent Application Publication No. JP2013248547A, 12 December 2013. [Google Scholar]
- Fremont, H.A.; Agar, R.C.; Bray, J.W.; Marquart, G.W. Membrane Cleaning Process. U.S. Patent 4,740,308, 26 April 1988. [Google Scholar]
- Li, H.; Yu, P.; Luo, Y. Oxidative Cleaning of Reverse Osmosis Membrane Used in the Steel Industry Wastewater Reclamation. Procedia Eng. 2012, 44, 567–570. [Google Scholar] [CrossRef]
- Aoki, T.; Kawakatsu, T.; Hayakawa, K. Agent and Method for Cleaning Permeable Membranes. U.S. Patent Application Publication No. US20150045276A1, 12 February 2015. [Google Scholar]
- Ishii, K.; Kawakatsu, T. Reverse Osmosis Membrane Cleaning Agent, Cleaning Liquid, and Cleaning Method. International Patent Publication WO2017017994A1, 2 February 2017. [Google Scholar]
- Louie, J.S.; Pinnau, I.; Reinhard, M. Effects of surface coating process conditions on the water permeation and salt rejection properties of composite polyamide reverse osmosis membranes. J. Membr. Sci. 2011, 367, 249–255. [Google Scholar] [CrossRef]
- Zwack, R.R.; Christenson, R.M. Method of Cleaning Membrane Filter. U.S. Patent 4,136,025, 23 January 1979. [Google Scholar]
- Tsukamoto, T.; Kojima, Y. Removal of Contaminant of Membrane. Japanese Unexamined Patent Application Publication No. JPS5551406A, 15 April 1980. [Google Scholar]
- Maki, M.L.; Katsura, T.; Tasaka, K.; Ionue, K. External Pressurizing Capillary Ultrafiltration Module for High-Purity Water Systems. Ultrapure Water 1993, 10, 20. [Google Scholar]
- Moch, I.; Hamida, A.B.; Pohland, H.W. New Technology to Control Biofouling. In Proceedings of the IDA World Congress on Desalination, Abu Dhabi, United Arab Emirates, 18–24 November 1995; pp. 59–72. [Google Scholar]
- Hamida, A.B.; Moch, I. Controlling biological fouling in open sea intake RO plants without continuous chlorination. Desalination Water Reuse 1996, 6, 40–45. [Google Scholar]
- Glater, J.; McCutchan, J.W.; McCray, S.B.; Zachariah, M.R. The Effect of Halogens on the Performance and Durability of Reverse-Osmosis Membranes. In Synthetic Membranes; ACS Symposium Series; Turbak, A.F., Ed.; American Chemical Society: Washington, DC, USA, 1981; Volume 153, pp. 171–190. [Google Scholar]
- Jons, S.D.; Stutts, K.J.; Ferritto, M.S.; Mickols, W.E. Treatment of Composite Polyamide Membranes to Improve Performance. U.S. Patent Application Publication No. US5876602A, 2 March 1999. [Google Scholar]
- Obara, T.; Hirose, M. Production of Multiple Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication No. JP2000334280A, 5 December 2000. [Google Scholar]
- Acker, C.L.; Colvin, C.K.; Mariñas, B.J.; Lozier, J.C. Assessing the Integrity of Reverse Osmosis Spiral-Wound Membrane Elements with Biological and Non-Biological Surrogate Indicators. In Proceedings of the AWWA Membrane Technology Conference, San Antonio, TX, USA, 4–7 March 2001. [Google Scholar]
- Yu, J.; Baek, Y.; Yoon, H.; Yoon, J. New disinfectant to control biofouling of polyamide reverse osmosis membrane. J. Membr. Sci. 2013, 427, 30–36. [Google Scholar] [CrossRef]
- Malki, M. The Use of Chlorine Dioxide as a RO Membrane Cleaning Aid for Natural and Synthetic Organic Foulants. In Proceedings of the AMTA/AWWA Membrane Technology Conference & Exposition, West Palm Beach, FL, USA, 11–15 March 2018. [Google Scholar]
- Oh, J.; Maeda, Y.; Ko, Y.; Wang, L. Practical Approaches to Mitigate Reverse Osmosis (RO) Fouling and Reduce O&M Cost in Wastewater Treatment. In Proceedings of the AWWA/AMTA Membrane Technology Conference, West Palm Beach, FL, USA, 4–7 March 2024. [Google Scholar]
- Patel, M.; Owens, E. Developing RO Cleaning Strategies based on Minimizing Energy and CIP Costs. In Proceedings of the AMTA Annual Conference & Exposition, San Diego, CA, USA, 12–15 July 2010. [Google Scholar]
- Carney, M. 3.4-The beverage industry. In Membranes for Industrial Wastewater Recovery and Re-Use; Judd, S., Jefferson, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 159–170. [Google Scholar]
- Ko, Y.; Wang, L.; Wu, T.; Maeda, Y. Application of LG NanoH2O™ Second Generation Thin-Film Nanocomposite Membranes for Wastewater Treatment in a Steel Plant. In Proceedings of the IDA World Congress, Sydney, Australia, 9–13 October 2022. [Google Scholar]
- Won, W.; Owens, E. Comparison of RO Membrane Performance: From Qualification Testing to Full Scale Application at West Basin MWD. In Proceedings of the AMTA Annual Meeting, San Diego, CA, USA, 12–15 July 2010. [Google Scholar]
- Majamaa, K.; Aerts, P.E.M.; Groot, C.; Paping, L.L.M.J.; van den Broek, W.; van Agtmaal, S. Industrial water reuse with integrated membrane system increases the sustainability of the chemical manufacturing. Desalin. Water Treat. 2010, 18, 17–23. [Google Scholar] [CrossRef]
- Knoell, T.; Won, W.; Owens, E. Time To Replace Your RO Membranes: Lessons From Two Premier California Water Recyclers. In Proceedings of the AMTA/SEDA Joint Conference & Exposition, Miami Beach, FL, USA, 18–21 July 2011. [Google Scholar]
- Ekowati, Y.; Msuya, M.; Salinas Rodriguez, S.G.; Veenendaal, G.; Schippers, J.C.; Kennedy, M.D. Synthetic organic polymer fouling in municipal wastewater reuse reverse osmosis. J. Water Reuse Desalin. 2014, 4, 125–136. [Google Scholar] [CrossRef]
- Franks, R.N.; Bartels, C.R.; Davis, M.; Shin, D. Design and Operation of an Integrated Membrane System for the Reclamation of Industrial Wastewater. In Proceedings of the AMTA/AWWA Membrane Technology Conference, New Orleans, LA, USA, 25–28 February 2019. [Google Scholar]
- Moftah, K. For water treatment, consider high-pH reverse osmosis. Chem. Eng. 2003, 110, 62–69. [Google Scholar]
- McBride, D.; Mukhopadhyay, D. 450 ppm Silica Sustained in Innovative Reverse Osmosis Technology. In Proceedings of the 57th Annual International Water Conference, Pittsburgh, PA, USA, 21–23 October 1996; pp. 145–152. [Google Scholar]
- Magara, Y.; Tabata, A.; Kohki, M.; Kawasaki, M.; Hirose, M. Development of boron reduction system for sea water desalination. Desalination 1998, 118, 25–33. [Google Scholar] [CrossRef]
- Kawasaki, M.; Hirose, M.; Ohara, T.; Kimura, S. Simulation of Boron Reduction System for Reverse Osmosis Sea Water Desalination Unit. Membrane 1999, 24, 296–303. [Google Scholar] [CrossRef]
- Hitotsuyanagi, N.; Furuichi, M.; Murakami, Y.; Ishii, Y.; Kagi, S. Treatment of organic waste liquid. Japanese Unexamined Patent Application Publication No. JPS61181590A, 14 August 1986. [Google Scholar]
- Redondo, J.A.; Lomax, I. Experiences with the pretreatment of raw water with high fouling potential for reverse osmosis plant using FILMTEC membranes. Desalination 1997, 110, 167–182. [Google Scholar] [CrossRef]
- Franks, R.; Bartels, C.; Anit, A. RO Membrane Performance when Reclaiming Produced Water from the Oil Extraction Process. In Proceedings of the IDA World Congress 2009 on Desalination and Water Reuse, Dubai, United Arab Emirates, 7–12 November 2009; p. IDAWC/DB09-194. [Google Scholar]
- Alzahrani, S.; Mohammad, A.W.; Hilal, N.; Abdullah, P.; Jaafar, O. Identification of foulants, fouling mechanisms and cleaning efficiency for NF and RO treatment of produced water. Sep. Purif. Technol. 2013, 118, 324–341. [Google Scholar] [CrossRef]
- Lester, A.; Muddasani, S. Zero Liquid Discharge (ZLD) System–An Advanced Water Reuse System for Oil & Gas Refinery. In Proceedings of the 83rd Annual International Water Conference, Orlando, FL, USA, 6–10 November 2022; p. IWC 22-48. [Google Scholar]
- Holmes-Farley, S.R.; Reamey, R.H.; McCarthy, T.J.; Deutch, J.; Whitesides, G.M. Acid-base behavior of carboxylic acid groups covalently attached at the surface of polyethylene: The usefulness of contact angle in following the ionization of surface functionality. Langmuir 1985, 1, 725–740. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M.V. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Franks, R.; Bartels, C.; Nagghappan, L. Performance of a reverse osmosis system when reclaiming high pH-high temperature wastewater. In Proceedings of the AWWA Membrane Technology Conference, Memphis, TN, USA, 15–18 March 2009. [Google Scholar]
- Ang, W.S.; Elimelech, M. Fatty acid fouling of reverse osmosis membranes: Implications for wastewater reclamation. Water Res. 2008, 42, 4393–4403. [Google Scholar] [CrossRef]
- van’t Hul, J.P.; Racz, I.G.; Reith, T. Application of reverse osmosis for the re-use of process water and the reduction of waste water volume in textile washing processes subsequent to reactive dyeing processes. In Proceedings Colloquium “Entwicklungen zur Produkt-und Wasserruckgewinnung aus Industrieabwassern”; Technische Universitat Berlin: Berlin, Germany, 1998; pp. 169–183. [Google Scholar]
- Wolfe, N.L.; Steen, W.C.; Burns, L.A. Phthalate ester hydrolysis: Linear free energy relationships. Chemosphere 1980, 9, 403–408. [Google Scholar] [CrossRef]
- Tao, F.; Curtice, S.; Hobbs, R.; Sides, J.L.; Wieser, J.; Dyke, C.; Tuohey, D.; Pilger, P.F. Reverse osmosis process successfully converts oil field brine into freshwater. Oil Gas J. 1993, 91, 88–91. [Google Scholar]
- Brinck, J.; Jönsson, A.S.; Jönsson, B.; Lindau, J. Influence of pH on the adsorptive fouling of ultrafiltration membranes by fatty acid. J. Membr. Sci. 2000, 164, 187–194. [Google Scholar] [CrossRef]
- Into, M.; Jönsson, A.-S.; Lengdén, G. Reuse of industrial wastewater following treatment with reverse osmosis. J. Membr. Sci. 2004, 242, 21–25. [Google Scholar] [CrossRef]
- Ikuno, N. Treatment Method and Treatment Apparatus for Organic Substance-Containing Wastewater. Japanese Unexamined Patent Application Publication No. JP2005081268A, 31 March 2005. [Google Scholar]
- Ikuno, N. Method and Apparatus for Treating Organic Material-Containing Waste Water. Japanese Unexamined Patent Application Publication No. JP2005169372A, 30 June 2005. [Google Scholar]
- Mullett, M.; Fornarelli, R.; Ralph, D. Nanofiltration of Mine Water: Impact of Feed pH and Membrane Charge on Resource Recovery and Water Discharge. Membranes 2014, 4, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Idil Mouhoumed, E.; Szymczyk, A.; Schäfer, A.; Paugam, L.; La, Y.H. Physico-chemical characterization of polyamide NF/RO membranes: Insight from streaming current measurements. J. Membr. Sci. 2014, 461, 130–138. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Probing the nano- and micro-scales of reverse osmosis membranes—A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Fernández, J.F.; Jastorff, B.; Störmann, R.; Stolte, S.; Thöming, J. Thinking in Terms of Structure-Activity-Relationships (T-SAR): A Tool to Better Understand Nanofiltration Membranes. Membranes 2011, 1, 162–183. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of Polyamide Nanofiltration and Reverse Osmosis Membranes by Hypochlorite. Environ. Sci. Technol. 2012, 46, 852–859. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Boussu, K.; Kindts, C.; Vandecasteele, C.; Van der Bruggen, B. Surfactant fouling of nanofiltration membranes: Measurements and mechanisms. Chemphyschem 2007, 8, 1836–1845. [Google Scholar] [CrossRef]
- Ochi, H.; Chiba, T. Method for Treating Waste Water Containing Surfactant and Waste Water Treatment System. Japanese Unexamined Patent Application Publication No. JP2006247576A, 21 September 2006. [Google Scholar]
- Ikuno, N.; Maeda, Y. Method and Apparatus for Treating Cationic Surfactant-Containing Waste Water. Japanese Unexamined Patent Application Publication No. JP2015009230A, 19 January 2015. [Google Scholar]
- Kawaguchi, T.; Tamura, K. Membrane Separation and Treatment of Aqueous Solution Containing Nonionic Surface Active Agent. Japanese Unexamined Patent Application Publication No. JPS59179188A, 11 October 1984. [Google Scholar]
- Reinhard, M.; Goodman, N.L.; McCarty, P.L.; Argo, D.G. Removing Trace Organics by Reverse Osmosis Using Cellulose Acetate and Polyamide Membranes. J.-Am. Water Work. Assoc. 1986, 78, 163–174. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Kim, I.-C.; Lee, K.-H. Dyeing process wastewater treatment using fouling resistant nanofiltration and reverse osmosis membranes. Desalination 2006, 192, 246–251. [Google Scholar] [CrossRef]
- Hachisuka, H.; Ikeda, K. Composite Reverse Osmosis Membrane Having a Separation Layer with Polyvinyl Alcohol Coating and Method of Reverse Osmotic Treatment of Water Using the Same. U.S. Patent 6,177,011, 23 January 2001. [Google Scholar]
- Inoue, T.; Isaka, H.; Sugita, K. Composite Semipermeable Membrane and Method for Manufacturing the Same. Japanese Unexamined Patent Application Publication No. JP2003200026A, 15 July 2003. [Google Scholar]
- Zhang, Q.; Zhang, C.; Xu, J.; Nie, Y.; Li, S.; Zhang, S. Effect of poly(vinyl alcohol) coating process conditions on the properties and performance of polyamide reverse osmosis membranes. Desalination 2016, 379, 42–52. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, K.; Yan, F.; Shi, Y.; Yu, P.; Yu, S.; Li, S.; Gao, C. Enhancing the performance of aromatic polyamide reverse osmosis membrane by surface modification via covalent attachment of polyvinyl alcohol (PVA). J. Membr. Sci. 2016, 501, 209–219. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kawakatsu, T. Method for Hydrophilizing Reverse Osmosis Membrane. International Patent Publication WO2014098059A1, 26 June 2014. [Google Scholar]
- Kawakatsu, T. Anti-Contamination Processing Method for Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication No. JP2015229159A, 21 December 2015. [Google Scholar]
- Uemura, T.; Fujimaki, H.; Kurihara, M. Composite semipermeable membrane. Japanese Unexamined Patent Application Publication No. JPS62266103A, 18 November 1987. [Google Scholar]
- Zhou, Y.; Yu, S.; Gao, C.; Feng, X. Surface modification of thin film composite polyamide membranes by electrostatic self deposition of polycations for improved fouling resistance. Sep. Purif. Technol. 2009, 66, 287–294. [Google Scholar] [CrossRef]
- Maeda, Y.; Kai, M. Recent Progress in Pervaporation Membranes for Water/Ethanol Separation. In Pervaporation Membrane Separation Processes; Huang, R.Y.M., Ed.; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1991; pp. 391–435. [Google Scholar]
- Iseki, Y.; Honda, Z.; Ase, T. New Dual Separating Membrane and Treatment of Waste Water Using the Membrane. Japanese Unexamined Patent Application Publication No. JPH05253454A, 5 October 1993. [Google Scholar]
- Ishigami, T.; Amano, K.; Fujii, A.; Ohmukai, Y.; Kamio, E.; Maruyama, T.; Matsuyama, H. Fouling reduction of reverse osmosis membrane by surface modification via layer-by-layer assembly. Sep. Purif. Technol. 2012, 99, 1–7. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Shirakawa, N.; Terada, M.; Yui, N. Formation of polyelectrolyte complex and its adsorption properties. J. Appl. Polym. Sci. 1980, 25, 1645–1653. [Google Scholar] [CrossRef]
- Tanaka, Y.; Osawa, M. Reverse Osmosis Membrane, Reverse Osmosis Membrane Device, and Method of Hydrophilizing Reverse Osmosis Membrane. Japanese Unexamined Patent Application Publication JP2009101335A, 14 May 2009. [Google Scholar]
- Zhang, Y.; Wan, Y.; Guo, M.; Pan, G.; Shi, H.; Yao, X.; Liu, Y. Surface modification on thin-film composite reverse osmosis membrane by cation complexation for antifouling. J. Polym. Res. 2019, 26, 68. [Google Scholar] [CrossRef]
- Saenz de Jubera, A.M.; Gao, Y.; Moore, J.S.; Cahill, D.G.; Marinas, B.J. Enhancing the performance of nanofiltration membranes by modifying the active layer with aramide dendrimers. Environ. Sci. Technol. 2012, 46, 9592–9599. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Li, W.; Xing, W.; Wang, Y. Depositing lignin on membrane surfaces for simultaneously upgraded reverse osmosis performances: An upscalable route. AIChE J. 2017, 63, 2221–2231. [Google Scholar] [CrossRef]
- Boussu, K.; Vandecasteele, C.; Van der Bruggen, B. Relation between membrane characteristics and performance in nanofiltration. J. Membr. Sci. 2008, 310, 51–65. [Google Scholar] [CrossRef]
- Fujioka, T.; Nghiem, L.D. Modification of a polyamide reverse osmosis membrane by heat treatment for enhanced fouling resistance. Water Supply 2013, 13, 1553–1559. [Google Scholar] [CrossRef]
- Izumi, A.; Edogawa, K.; Uemura, T. Production of Semipermeable Composite Membrane. Japanese Unexamined Patent Application Publication No. JPS63287507A, 24 November 1988. [Google Scholar]
- Obara, T.; Hirose, M. Reverse Osmotic Membrane Module and Treatment of Sea Water. Japanese Unexamined Patent Application Publication No. JPH1119493A, 26 January 1999. [Google Scholar]
- Wood, J.; Arba, J. The Use of Hot Water for Sanitization of RO Membranes in Ultrapure Water Systems. In Proceedings of the Fifteenth Annual Membrane Technology/Separations Planning Conference, Newton, MA, USA, 29 October 1997. [Google Scholar]
- Eriksson, P.K. Hot Water Disinfection Effect on Polyamide RO Elements. In Proceedings of the IDA World Congress, Dubai, United Arab Emirates, 20–24 October 2019. [Google Scholar]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Higgins, M.; Price, W.E.; Henderson, R.K.; Nghiem, L.D. Effect of heat treatment on fouling resistance and the rejection of small and neutral solutes by reverse osmosis membranes. Water Supply 2015, 15, 510–516. [Google Scholar] [CrossRef]
- Fujioka, T.; Ishida, K.P.; Shintani, T.; Kodamatani, H. High rejection reverse osmosis membrane for removal of N-nitrosamines and their precursors. Water Res. 2018, 131, 45–51. [Google Scholar] [CrossRef]
- Sato, Y.; Tamura, M. Tannic Acid as an Important Tool to Improve Quality Standards of Commercial RO/NF Membranes. Kagaku Kogaku Ronbunshu 2008, 34, 493–498. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshikawa, H. Modification Method for Reverse Osmosis Membrane, Reverse Osmosis Membrane, and Processing Method for Water Containing Boron. Japanese Unexamined Patent Application Publication No. JP2016155067A, 1 September 2016. [Google Scholar]
- Cadotte, J.E. Treatment of Composite Polyamide Membranes via Substitution with Amine Reactive Reagents. U.S. Patent 4,960,517, 2 October 1990. [Google Scholar]
- Yasuda, T.; Adachi, Y.; Mitsui, S.; Shimura, H.; Ogawa, T. Composite Semi-Permeable Membrane. U.S. Patent Application Publication No. US20230001363A1, 5 January 2023. [Google Scholar]
- Liu, Y.; Hong, Z.; Lin, S.; Liu, M.; Yu, S.; Gao, C. Surface room-temperature treatment of polyamide-based reverse osmosis membrane by diazotization reagent: Perm-selectivity regulation and mechanism. J. Membr. Sci. 2023, 684, 121901. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Li, H.; Lu, P.; Yu, Y.; Zhang, X.; Wang, Y.; Xia, J.; He, D.; Li, Y. Diazotized polyamide membranes on commercial polyethylene textile with simultaneously improved water permeance, salt rejections and anti-fouling. Desalination 2023, 549, 116307. [Google Scholar] [CrossRef]
- Cadotte, J.E.; Schmidt, D.L. Method of Improving Membrane Properties via Reaction of Diazonium Compounds or Precursors. U.S. Patent 4,888,116, 19 December 1989. [Google Scholar]
- Inoue, T. Compound Semi-Permeable Membrane and its Manufacturing Method. Japanese Unexamined Patent Application Publication No. JP2006021094A, 26 January 2006. [Google Scholar]
- Mitsuhata, T.; Tomioka, H.; Sasaki, T. Production Method of Composite Semi-Permeable Membrane. Japanese Unexamined Patent Application Publication No. JP2008260009A, 30 October 2008. [Google Scholar]
- Mitsuhata, T.; Sasaki, T.; Iwai, K. Production Method of Composite Semi-Permeable Membrane. Japanese Unexamined Patent Application Publication No. JP2009255075A, 5 November 2009. [Google Scholar]
- Ogawa, T.; Kimura, M.; Sasaki, T. Separation Membrane Element and Method for Producing Composite Semipermeable Membrane. U.S. Patent Application Publication No. US20130126419A1, 23 May 2013. [Google Scholar]
- Maeda, Y.; Tsuyumoto, M.; Karakane, H.; Tsugaya, H. Separation of Water-Acetic Acid Mixture by Pervaporation through Aromatic Polymer Membranes. In Proceedings of the Fifth International Conference on Pervaporation Processes in the Chemical Industry, Heidelberg, Germany, 11–15 March 1991; pp. 31–44. [Google Scholar]
- Maeda, Y.; Magara, K.; Tsugaya, H. Permselective Membrane of Polyacrylonitrile Copolymer and Process for Producing the Same. U.S. Patent Application Publication No. US5554292A, 10 September 1996. [Google Scholar]
- Wilbert, M.C. Enhancement of Membrane Fouling Resistance Through Surface Modification; PB97-157473/XAB, Water Treatment Technology-22; Water Treatment Engineering and Research Group, Bureau of Reclamation: Denver, CO, USA, 1997. [Google Scholar]
- Lee, H.; Kim, S. Factors affecting the removal of isopropyl alcohol by reverse osmosis membranes for ultrapure water production. Desalin. Water Treat. 2015, 54, 916–922. [Google Scholar] [CrossRef]
- Rydzewski, J. Identification of Critical Contaminants by Applying an Understanding of Different TOC Measuring Technologies. Ultrapure Water 2002, 19, 20–27. [Google Scholar]
- Godec, R. Measurement and Removal of Trace Levels of Urea. Ultrapure Water 2003, 20, 27–35. [Google Scholar]
- Redondo, J.; Busch, M.; De Witte, J.-P. Boron removal from seawater using FILMTECTM high rejection SWRO membranes. Desalination 2003, 156, 229–238. [Google Scholar] [CrossRef]
- Agenson, K.O.; Oh, J.-I.; Urase, T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Membr. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Altalyan, H.N.; Jones, B.; Bradd, J.; Nghiem, L.D.; Alyazichi, Y.M. Removal of volatile organic compounds (VOCs) from groundwater by reverse osmosis and nanofiltration. J. Water Process Eng. 2016, 9, 9–21. [Google Scholar] [CrossRef]
- Maeda, Y. Recent Progress in RO Elements for High-Purity Water Production. Ultrapure Water 2001, 18, 20–28. [Google Scholar]
- Weerakoon, D.; Bansal, B.; Padhye, L.P.; Rachmani, A.; James Wright, L.; Silyn Roberts, G.; Baroutian, S. A critical review on current urea removal technologies from water: An approach for pollution prevention and resource recovery. Sep. Purif. Technol. 2023, 314, 123652. [Google Scholar] [CrossRef]
- Muianga, C.; Roney, N.; Przybyla, J.; Carlson-Lynch, H.; Citra, M.; Heit, C. Toxicological Profile for N-Nitrosodimethylamine (NDMA); Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2023. [Google Scholar]
- Steinle-Darling, E.; Zedda, M.; Plumlee, M.H.; Ridgway, H.F.; Reinhard, M. Evaluating the impacts of membrane type, coating, fouling, chemical properties and water chemistry on reverse osmosis rejection of seven nitrosoalklyamines, including NDMA. Water Res. 2007, 41, 3959–3967. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Toward Improved Boron Removal in RO by Membrane Modification: Feasibility and Challenges. Environ. Sci. Technol. 2011, 45, 3613–3620. [Google Scholar] [CrossRef]
- Childress, A.E.; Deshmukh, S.S. Effect of humic substances and anionic surfactants on the surface charge and performance of reverse osmosis membranes. Desalination 1998, 118, 167–174. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Allred, J.; Knoell, T.; Jeong, B.-H. Modeling the effects of fouling on full-scale reverse osmosis processes. J. Membr. Sci. 2008, 314, 33–49. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Li, F.; Zhang, X. Influence of surfactant fouling on rejection of trace nuclides and boron by reverse osmosis. Desalination 2016, 377, 47–53. [Google Scholar] [CrossRef]
- DuPont. Posttreatments for Permasep Permeators. In Permasep Engineering Manual, Bulletin 508; DuPont: Wilmingtom, DE, USA, 1982. [Google Scholar]
- Chen, C.; Ganci, J.B. Tannin Treatment for Nonporous Semipermeable Membranes. U.S. Patent 3,886,066, 27 May 1975. [Google Scholar]
- Lamendola, M.F.; Tua, A. Desalination of Sea Water by Reverse Osmosis: The Malta Experience. In Proceedings of the International Workshop, Membranes in Drinking Water Production, Paris, France, 27–29 March 1995; pp. 153–159. [Google Scholar]
- Lamendola, M.F.; Tua, A. Desalination of seawater by reverse osmosis, the Malta experience. Desalin. Water Reuse 1995, 5, 18–22. [Google Scholar]
- Applegate, L.E. Sea Water Desalination Process. European Patent Application No. EP0082705A1, 29 June 1983. [Google Scholar]
- Sasaki, T.; Henmi, M.; Inoue, T. Method for Manufacturing Semipermeable Membrane. Japanese Unexamined Patent Application Publication No. JP2003117360A, 22 April 2003. [Google Scholar]
- Sato, Y.; Tamura, M. Method and Device for Modifying Separation Membrane, and Separation Membrane Modified by the Method. Japanese Unexamined Patent Application Publication No. JP2006223963A, 31 August 2006. [Google Scholar]
- Sato, Y.; Tamura, M. Method and Device for Treating/Preserving Separation Membrane. Japanese Unexamined Patent Application Publication No. JP2007000790A, 11 January 2007. [Google Scholar]
- Sato, Y.; Tamura, M. Method and Device for Enhancing Performance of Separation Membrane, and Separation Membrane Treated by the Method. Japanese Unexamined Patent Application Publication No. JP2007000801A, 11 January 2007. [Google Scholar]
- Sato, Y.; Tamura, M. Method for Manufacturing Separation Membrane. Japanese Unexamined Patent Application Publication No. JP2007000828A, 11 January 2007. [Google Scholar]
- Sato, Y.; Tamura, M. Reforming Method and Apparatus for Separation Membrane. Japanese Unexamined Patent Application Publication No. JP2007111607A, 10 May 2007. [Google Scholar]
- Mitrouli, S.T.; Karabelas, A.J.; Isaias, N.P.; Sioutopoulos, D.C.; Al Rammah, A.S. Reverse Osmosis Membrane Treatment Improves Salt-Rejection Performance. IDA J. Desalin. Water Reuse 2010, 2, 22–34. [Google Scholar] [CrossRef]
- Cadotte, J.E.; Walker, D.R. Polyamide Membranes Useful for Water Softening. U.S. Patent 4,765,897, 23 August 1988. [Google Scholar]
- Cadotte, J.E.; Walker, D.R. Novel Water Softening Membranes. U.S. Patent 4,812,270, 14 March 1989. [Google Scholar]
- Cadotte, J.E.; Walker, D.R. Novel water Softening Process Using Membranes. U.S. Patent 4,824,574, 25 April 1989. [Google Scholar]
- Sato, Y.; Tamura, M. Method and Device for Modifying Separation Membrane, and Separation Membrane Modified by the Method. Japanese Unexamined Patent Application Publication No. JP2006224048A, 31 August 2006. [Google Scholar]
- Sato, Y.; Tsuru, T.; Nakao, S.-I. Model Analysis of Separation Performance of Commercial Nanofiltration Membranes Improved by Tannic Acid. J. Chem. Eng. Jpn. 2009, 42, 95–106. [Google Scholar] [CrossRef]
- Sato, Y.; Tamura, M. Sterilization Method and Apparatus of Separation Membrane, and Separation Membrane Treated by This Method. Japanese Unexamined Patent Application Publication No. JP2007160173A, 28 June 2007. [Google Scholar]
- Sato, Y.; Tamura, M. Method for Operating Separation Membrane and Apparatus. Japanese Unexamined Patent Application Publication No. JP2007167713A, 5 July 2007. [Google Scholar]
- Sato, Y.; Tamura, M. Preventing Oxidative Damage in Commercial RO/NF Membranes by Tannic Acid Treatment. Kagaku Kogaku Ronbunshu 2008, 34, 499–504. [Google Scholar] [CrossRef]
- Sato, Y.; Tamura, M. Method and Apparatus for Modifying Separation Membrane, and Separation Membrane Modified by The Method. Japanese Unexamined Patent Application Publication No. JP2007167725A, 5 July 2007. [Google Scholar]
- Moch, I., Jr. Hollow Fiber Permeators and Reverse Osmosis. Desalin. Water Reuse 1993, 3, 30–37. [Google Scholar]
- Xu, P.; Drewes, J.E.; Kim, T.-U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Henderson, R.K.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. Effects of membrane fouling on N-nitrosamine rejection by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2013, 427, 311–319. [Google Scholar] [CrossRef]
- Fujioka, T.; Aizawa, H.; Kodamatani, H. Fouling substances causing variable rejection of a small and uncharged trace organic chemical by reverse osmosis membranes. Environ. Technol. Innov. 2020, 17, 100576. [Google Scholar] [CrossRef]
- Fujioka, T.; Kodamatani, H.; Aizawa, H.; Gray, S.; Ishida, K.P.; Nghiem, L.D. Role of membrane fouling substances on the rejection of N-nitrosamines by reverse osmosis. Water Res. 2017, 118, 187–195. [Google Scholar] [CrossRef]
- Mickols, W.E.; Krauss, R.C.; Niu, Q.J.; Gaupp, C.L. Modified Membrane. International Patent Publication No. WO2007133362A1, 22 November 2007. [Google Scholar]
- Nitta, K.; Matsui, Y.; Morino, M. Treatment of Semipermeable Membrane. Japanese Unexamined Patent Application Publication JPH03245828A, 1 November 1991. [Google Scholar]
- Nakagawa, Y.; Edogawa, K.; Kurihara, M.; Tonomura, T. Solute Separation and Transport Characteristics Through Polyether Composite (PEC)-1000 Reverse-Osmosis Membranes. In Reverse Osmosis and Ultrafiltration; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 281, pp. 187–199. [Google Scholar]
- Lesan, R.; Tomaschke, J. A comparison of different classes of spiral-wound membrane elements at low concentration feeds. Ultrapure Water 1990, 7, 18–22. [Google Scholar]
- Nakagawa, Y.; Murakishi, H.; Uemura, T.; Ooto, K.; Hirai, M. Treatment of Cross-Linked Polyamide-Based Reverse Osmotic Membrane. Japanese Unexamined Patent Application Publication No. JPH022827A, 8 January 1990. [Google Scholar]
- Kawakatsu, T.; Tsujinaka, N. Pure Water Production Method and Apparatus. Japanese Unexamined Patent Application Publication JP2008161818A, 17 July 2008. [Google Scholar]
- Kawakatsu, T. Agent and Process for Increasing Rejection of Nanofiltration Membrane or Reverse Osmosis Membrane, Nanofiltration Membrane or Reverse Osmosis Membrane, Process for Water Treatment and Apparatus for Water Treatment. U.S. Patent Application No. US20090266764A1, 29 October 2009. [Google Scholar]
- Anzai, S.; Kawakatsu, T. Water Treatment Apparatus and Water Treatment Method. International Publication No. JPWO2008059824A1, 22 May 2008. [Google Scholar]
- Kawakatsu, T.; Tsujinaka, N. Water Treatment Apparatus and Water Treatment Method. Japanese Unexamined Patent Application Publication JP2008132421A, 12 June 2008. [Google Scholar]
- Hayakawa, K.; Kawakatsu, T. Method for Improving Inhibition Rate of Permeable Membrane, Permeable Membrane with Improved Inhibition Rate, Permeable Membrane Treatment Method and Device. Japanese Unexamined Patent Application Publication JP2008238121A, 9 October 2008. [Google Scholar]
- Kawakatsu, T. Blocking Rate Improver of Reverse Osmosis Membrane, and Blocking Rate Improvement Method. Japanese Unexamined Patent Application Publication No. JP2018047406A, 29 March 2018. [Google Scholar]
- Shultz, S.; Freger, V. In situ modification of membrane elements for improved boron rejection in RO desalination. Desalination 2018, 431, 66–72. [Google Scholar] [CrossRef]
- Shultz, S.; Bass, M.; Semiat, R.; Freger, V. Modification of polyamide membranes by hydrophobic molecular plugs for improved boron rejection. J. Membr. Sci. 2018, 546, 165–172. [Google Scholar] [CrossRef]
- Stolov, M.; Keisar, O.; Cohen, Y.; Freger, V. Elucidating the Effect of Aliphatic Molecular Plugs on Ion-Rejecting Properties of Polyamide Membranes. ACS Appl. Mater. Interfaces 2022, 14, 13335–13343. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, X.; Zhou, Y.; Shen, H.; Cao, X.; Zhang, P.; Gao, C. High boron removal polyamide reverse osmosis membranes by swelling induced embedding of a sulfonyl molecular plug. J. Membr. Sci. 2020, 597, 117716. [Google Scholar] [CrossRef]
- Habib, S.; Weinman, S.T. Modification of polyamide reverse osmosis membranes for the separation of urea. J. Membr. Sci. 2022, 655, 120584. [Google Scholar] [CrossRef]
- Habib, S.; Wilkins, M.A.; Weinman, S.T. Emerging investigator series: Post-synthesis modification of reverse osmosis membranes for the enhanced separation of small neutral molecules. Environ. Sci. Water Res. Technol. 2023, 9, 2836–2846. [Google Scholar] [CrossRef]
- Fujioka, T.; Osako, M.; Tanabe, S.; Kodamatani, H.; Shintani, T. Plugging nonporous polyamide membranes for enhanced rejection of small contaminants during advanced wastewater treatment. Sep. Purif. Technol. 2020, 253, 117490. [Google Scholar] [CrossRef]
- Morović, S.; Drmić, K.M.; Babić, S.; Košutić, K. Maximizing N-Nitrosamine Rejection via RO Membrane Plugging with Hexylamine and Hexamethylenediamine. Nanomaterials 2024, 14, 1117. [Google Scholar] [CrossRef]
- Kwon, S.; Jee, K.Y.; Lee, Y.T. Surface modification of reverse osmosis membrane with diphenylamine for improved chlorine and fouling resistance. Membr. J. 2013, 23, 439–449. [Google Scholar] [CrossRef]
- Kawakatsu, T. Inhibition-Ratio Improver of Nano Filtration Membrane or Reverse Osmosis Membrane, Improvement Method of Inhibition-Ratio, Nano Filtration Membrane or Reverse Osmosis Membrane, Water Treatment Method, and Water Treatment Apparatus. Japanese Unexamined Patent Application Publication JP2007289922A, 8 November 2007. [Google Scholar]
- Hirose, M. Composite Reverse Osmosis Membrane and Method for Producing the Same. U.S. Patent US6709590B1, 23 March 2004. [Google Scholar]
- Koo, J.-Y.; Hong, S. Composite Polyamide Reverse Osmosis Membrane Showing High Boron Rejection and Method of Producing the Same. U.S. Patent Application Publication No. US20070227966A1, 4 October 2007. [Google Scholar]
- Maugin, T. Fate of Reverse Osmosis (RO) Membranes During Oxidation by Disinfectants Used in Water Treatment: Impact on Membrane Structure and Performances; King Abdullah University of Science and Technology: Thuwal, Kingdom of Saudi Arabia, 2013. [Google Scholar]
- Yoshikawa, H.; Nakamura, Y. Operational Method for Separation Membrane, Modification Method for Separation Membrane, and Separation Membrane. Japanese Unexamined Patent Application Publication No. JP2016155074A, 1 September 2016. [Google Scholar]
- Lagartos, A.; Santos, R.; Son, Y.S.; Lee, S.; Maeda, Y.; Park, J.S. Possible Consequences of Iodine Effect on Reverse Osmosis Membranes and Solution to Remediate and Prevent Them. In Proceedings of the XII Congreso Internacional de Aedyr, Toledo, Spain, 23–25 October 2018; p. AedyrTOL18-TOL18-12. [Google Scholar]
- García, N.P.; Rodriguez, J.; Vigo, F.D.; Chesters, S.P. 20 Years of Data from 500 Seawater Membrane Autopsies. In Proceedings of the IDA 2022 World Congress, Sydney, Australia, 9–13 October 2022. [Google Scholar]
- Sundaram, R.; Natarajan, M.; Ragunathan, R. Operational Experience of Open Sea Intake-1 MGD SWRO Desalination Plant in South India. In Proceedings of the IDA World Congress on Desalination and Water Reuse, Manama, Bahrain, 8–13 March 2002. [Google Scholar]
- Ebrahim, S.; Malik, A. Membrane fouling and cleaning at DROP. Desalination 1987, 66, 201–221. [Google Scholar] [CrossRef]
- da Silva, M.K.; Ambrosi, A.; dos Ramos, G.M.; Tessaro, I.C. Rejuvenating polyamide reverse osmosis membranes by tannic acid treatment. Sep. Purif. Technol. 2012, 100, 1–8. [Google Scholar] [CrossRef]
- Pan, G.; Zhao, M.; Wan, Y.; Zhao, G.; Yu, H.; Tang, G.; Zhang, Y.; Liu, Y. One-step rejuvenation for prolonging running lifespan of nanofiltration membranes. Adv. Membr. 2024, 4, 100094. [Google Scholar] [CrossRef]
- Wilf, M.; Guleckstern, P. Restoration of commercial reverse osmosis membranes under field conditions. Desalination 1985, 54, 343–350. [Google Scholar] [CrossRef]
- Sato, Y.; Tamura, M. Method and Device for Recovering Performance of Separation Membrane, and Separation Membrane Processed by the Method. Japanese Unexamined Patent Application Publication JP2006224049A, 31 August 2006. [Google Scholar]
- Buijs, P.J.; Schippers, J.C.; Baas, K.J. Restoring membrane rejection after chlorination damage: A long term solution. In Proceedings of the Euromed 2008, Desalination for Clean Water and Energy, Dead Sea, Jordan, 9–13 November 2008. [Google Scholar]
- McDonald, M.; Mila, I.; Scalbert, A. Precipitation of Metal Ions by Plant Polyphenols: Optimal Conditions and Origin of Precipitation. J. Agric. Food Chem. 1996, 44, 599–606. [Google Scholar] [CrossRef]
- Raval, H.D.; Modi, R.; Dave, K.; Raviya, M. Selectivity enhancement of oxidative degraded reverse osmosis membrane by chitosan–tannic acid treatment. J. Appl. Polym. Sci. 2022, 139, e52643. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yamamura, H.; Kurihara, M. Treatment of Semipermeable Membrane. Japanese Unexamined Patent Application Publication No. JPS5811005A, 21 January 1983. [Google Scholar]
- Nakagawa, Y.; Oto, K.; Kurihara, M. Membrane Treatment Method for Semipermeable Membranes. U.S. Patent 4,634,531, 6 January 1987. [Google Scholar]
- Fujiwara, K.; Goto, S. Method for Recovering Performance of Permselective Membrane. Japanese Unexamined Patent Application Publication No. JP2008086945A, 17 April 2008. [Google Scholar]
- Sugita, K.; Kubo, H.; Kodera, K. Rejection Rate Improving Method of Composite Semipermeable Membrane. Japanese Unexamined Patent Application Publication No. JP2015016448A, 29 January 2015. [Google Scholar]
- Sugita, K.; Taniguchi, M.; Takabatake, H. Rejection rate Improving Method of Semipermeable Membrane. Japanese Unexamined Patent Application Publication No. JP2016128142A, 14 July 2016. [Google Scholar]
- Valls, S.T.; Palazon, J.A.R.; Panovic, D. Recuperation of Spiral Aromatic Polyamide Reverse Osmosis Membranes Consists of Successive Citric Acid Polyvinyl Methyl Ether and Tannic Acid Solutions at Relatively Low Pressure. Spanish Patent Application No. ES2212905A1, 1 August 2004. [Google Scholar]
- Mitrouli, S.T.; Karabelas, A.J.; Isaias, N.P.; Al Rammah, A.S. Application of hydrophilic macromolecules on thin film composite polyamide membranes for performance restoration. Desalination 2011, 278, 105–116. [Google Scholar] [CrossRef]
- Isaias, N.P.; Karabelas, A.J.; Mitrouli, S.T. Application of Rejection Enhancing Agents (REAs) that Do Not Have Cloud Point Limitations on Desalination Membranes. U.S. Patent Application Publication No. US20120080058A1, 5 April 2012. [Google Scholar]
- Makkar, H.P.S.; Blümmel, M.; Becker, K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef]
- Kawakatsu, T. Anti-Fouling and Rejuvenation Technologies for Reverse Osmosis Membrane. In Proceedings of the 15th IUMRS-International Conference in Asia, Fukuoka, Japan, 24–30 August 2014. [Google Scholar]
- Nakamura, T.; Kawakatsu, T.; Hayakawa, K.; Matsumoto, K.; Matoba, Y.; Ishii, K. Innovative Chemicals for Deteriorated RO Membranes. In Proceedings of the IDA World Congress on Desalination and Water Reuse, San Diego, CA, USA, 30 August–4 September 2015; p. IDAWC15-Nakamura_51420. [Google Scholar]
- Aoki, T.; Kawakatsu, T. Method for Improving Rejection of Permeable Membrane and Permeable Membrane. International Publication No. JPWO2011040354A1, 7 April 2011. [Google Scholar]
- Kawakatsu, T.; Aoki, T. Method for Improving Blocking Rate of Permeable Membrane and Treating Agent for Improving Blocking Rate, and Permeable Membrane. Japanese Unexamined Patent Application Publication JP2012187468A, 4 October 2012. [Google Scholar]




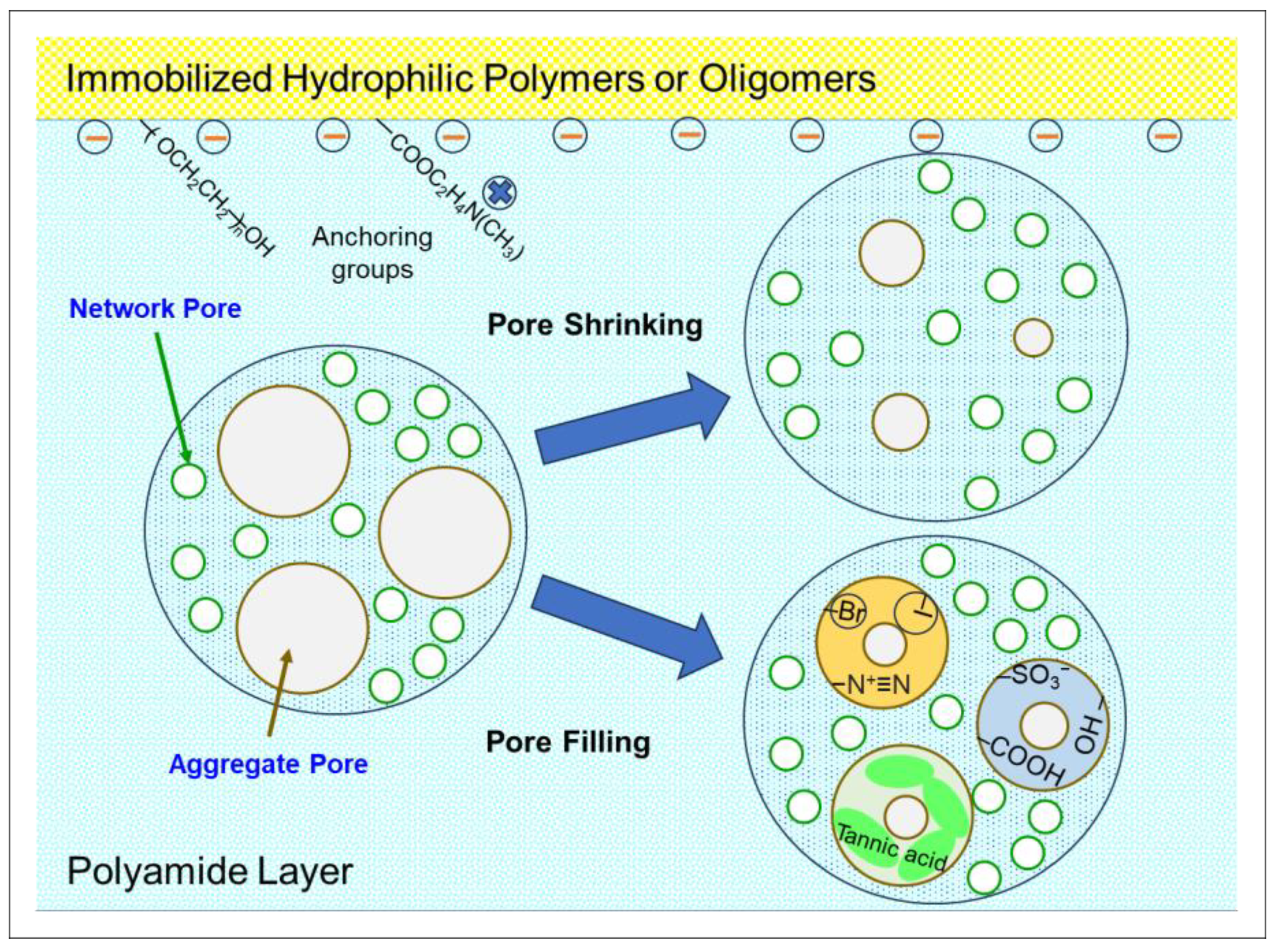
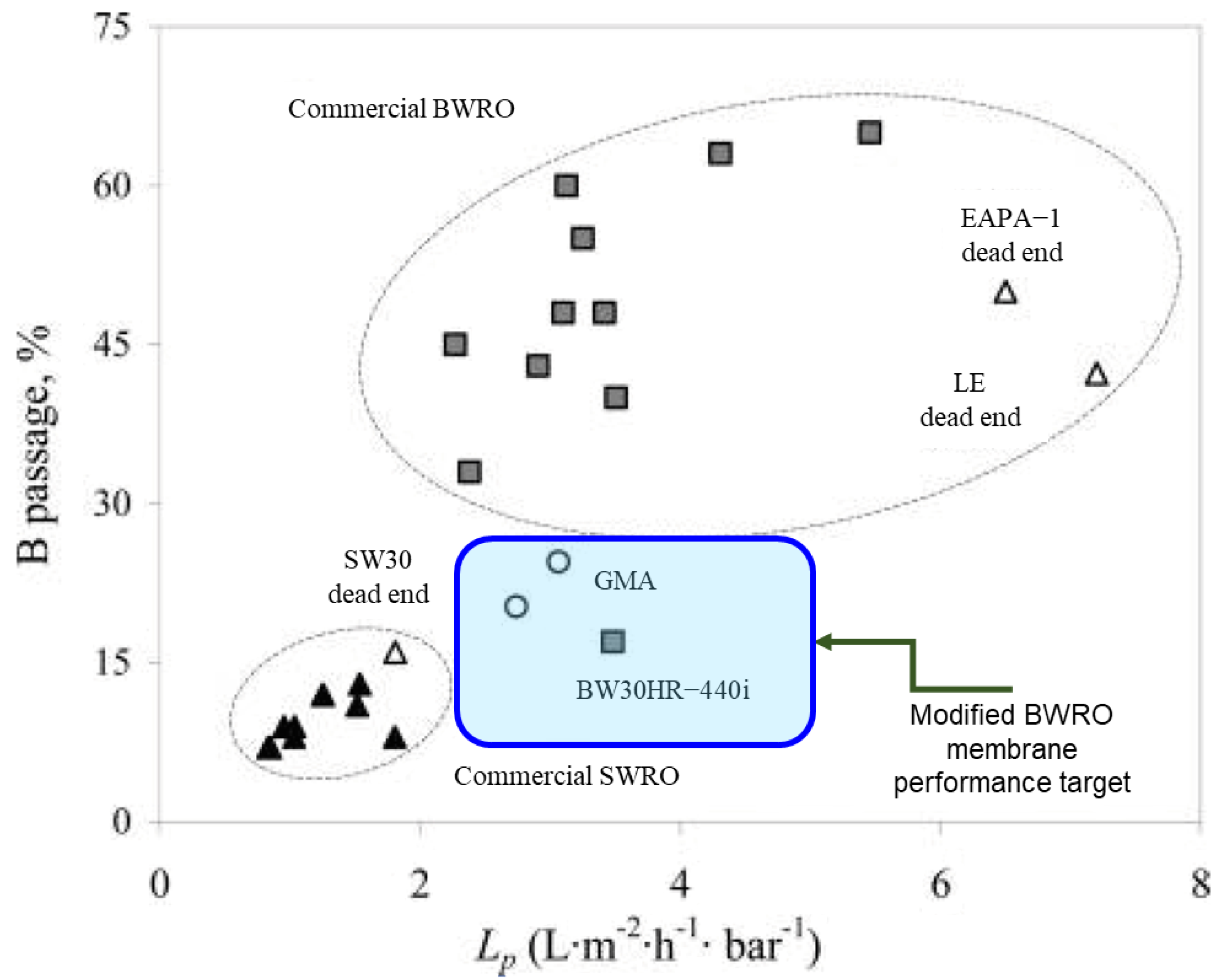
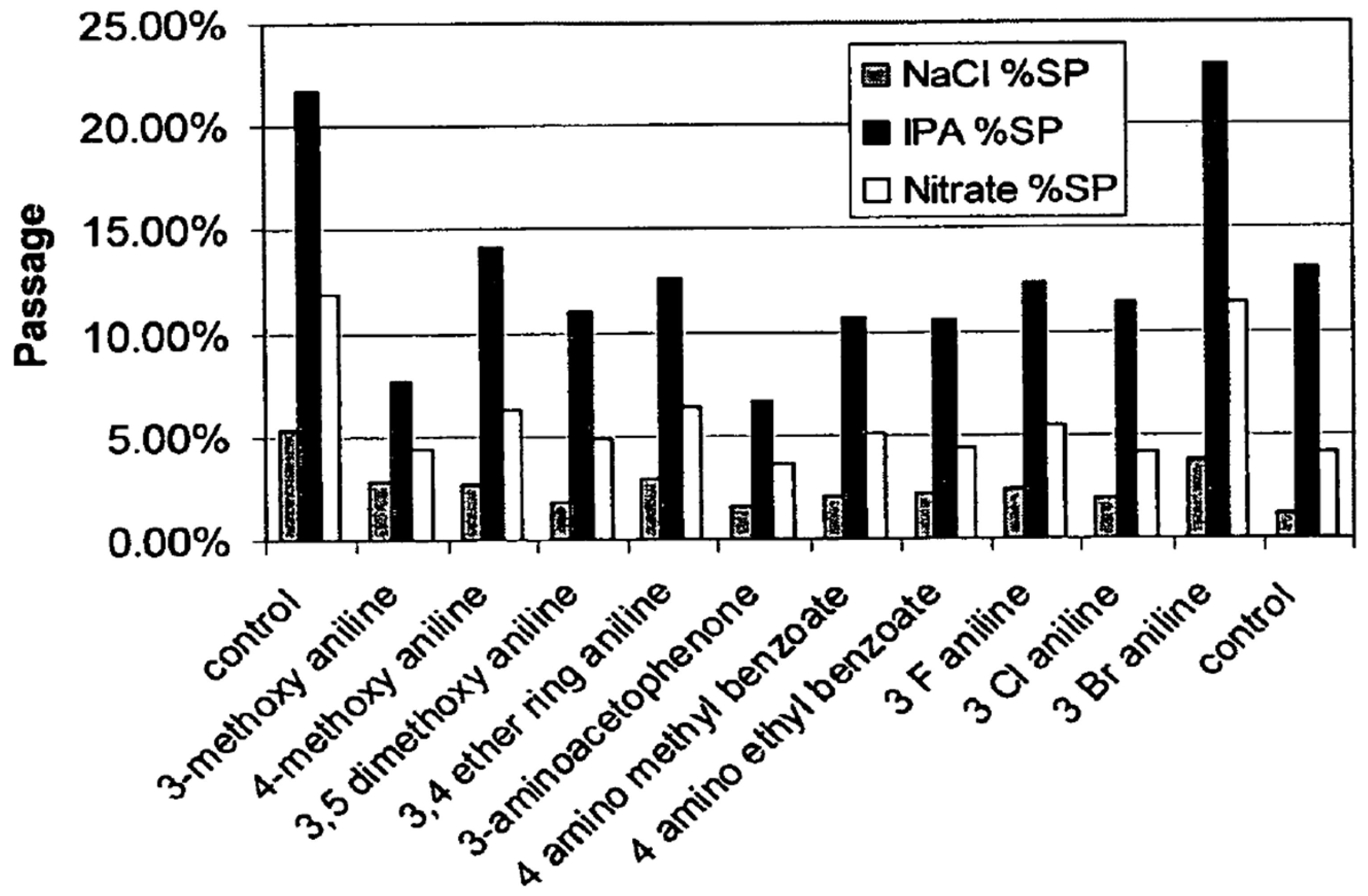

| Corrective and Preventive Measures | Examples | |
|---|---|---|
| Pretreatment | Adsorbents | Activated carbon (AC) and synthetic adsorbents |
| Filters | Organic/oil removal filters | |
| Oxidation | Ozone, Advanced oxidation process (AOP), NaOCl, etc. | |
| Antifoulants | Polyvinylpyrrolidone (PVP), anionic polyvinyl alcohol (PVA), etc. | |
| Detection | Prediction | Bench or pilot tests and coupon tests with polyamide (PA) membrane |
| Identification | GC-MS (extraction or pyrolysis) | |
| CIP | Organic CIP | Lower alcohols, butyl cellosolve, ethylene glycol (EG)/alcohol, etc. |
| Oxidative CIP | Nitric acid, high pH NaOCl, dichloroisocyanurate, etc. | |
| Operation | High pH | Negative membrane charge and swelling |
| Low pH | Positive membrane charge | |
| Low fouling membranes | Tight membranes | Seawater RO membranes |
| Posttreatment | Heat treatment and nitrous acid (diazonium coupling) | |
| Surface modification | Polyvinyl alcohol (PVA), polyelectrolyte complex (PEC), etc. | |
| Oxidant | Organics | Membrane | Comments | Ref. |
|---|---|---|---|---|
| NaOCl | MBR EfOM | APA RO | NaOCl: 10 mg/L; Retention time: 30 min. | [57] |
| KMnO4 | Surface water and EfOM | AG (RO) and HL (NF) | KMnO4: 0.45–0.8 mg/L | [58] |
| X-ray | Produced water | SWC-5 | Cold-cathode X-ray: 20–30 kV/0.1–03 mA | [59] |
| H2O2/UV | Nonionic surfactant | Polybenzimidazole | H2O2: 100 mg/L; UV: 0.07 W·min/cm2 | [60] |
| H2O2/UV | Groundwater | NF70 | H2O2: 1–2 mM; UV (254 nm); TOC: 3.5 mg/L | [61] |
| Ozone (O3) | Phenolics | NF40 and FT30 | Chlorophenols, O2 containing 2% O3 | [62] |
| Ozone (O3) | Surface water | APA NF | O3: 3 ppm; Feed COD: 2–4 ppm | [63] |
| Ozone (O3) | MBR EfOM | ESPA-2 | Pilot test (MBR—O3—RO); O3: 1.5–2 ppm | [64] |
| Ozone (O3) | EfOM | NF90 | O3: 0.2 and 0.4 mg O3/mg DOC | [65] |
| Ozone (O3) | MBR EfOM | ESPA-DHR | O3: 0.8 mg O3/mg DOC | [66] |
| Ozone (O3) | MBR EfOM | NF90 | Gas ozone concentration: 5 g O3/Nm3 | [67] |
| O3 or H2O2/O3 | NA | NTR-759 (RO) | Ozonation at high pH | [68] |
| O3 or H2O2/O3 | NA | ES-10 (RO) | O3 dosing regulation (UV absorbance) | [69] |
| O3 or H2O2/O3 | NOM, MBR EfOM | ESPA-2 | O3: 1.5, 3, and 6 mg/L | [70,71,72] |
| O3 or H2O2/O3 | MBR EfOM | NE90 | Ozonation at high pH; O3: 3, 6, and 9 mg/L | [73] |
| Organic Compound | Molecular Structure | CAS No. | Fouling | MW | Log P | Reference |
|---|---|---|---|---|---|---|
| Phenolic Derivatives | ||||||
| 2,4-Dinitrophenol | C6H3(OH)(NO2)2 | 51-28-5 | S | 184.11 | 1.67 | [90] |
| 2,4,6-Trichlorophenol | C6H2Cl3OH | 88-06-2 | S | 197.45 | 3.69 | [90,91] |
| 2,4-Dicholrophenol | C6H4Cl2O | 120-83-2 | S | 163.0 | 3.06 | [90] |
| Phenol | C6H5OH | 108-95-2 | W | 94.1 | 1.46 | [90,91,92] |
| 2-Fluorophenol | C6H4FOH | 367-12-4 | W | 112.1 | 1.76 | [90] |
| 2-Nitrophenol | C6H5NO3 | 88-75-5 | M | 139.1 | 1.79 | [90] |
| 3-Nitrophenol | C6H5NO3 | 554-84-7 | S | 139.1 | 2.00 | [92] |
| 2-Chlorophenol | C6H4ClOH | 95-57-8 | M | 128.5 | 2.15 | [90] |
| Catechol | C6H4(OH)2 | 120-80-9 | W | 110.1 | 0.88 | [92] |
| Resorcinol | C6H4(OH)2 | 108-46-3 | W | 110.1 | 0.8 | [93] |
| Pyrogallol | C6H3(OH)3 | 87-66-1 | M | 126.1 | 0.23 | [92] |
| Phloroglucinol | C6H3(OH)3 | 108-73-6 | W | 126.1 | 0.16 | [93] |
| 3-Hydroxybenzonic acid | HOC6H4CO2H | 99-96-7 | M | 138.1 | 1.58 | [93] |
| Bisphenol A | (CH3)2C(C6H4OH)2 | 80-05-7 | S | 228.3 | 3.64 | [94] |
| Nonionic Surfactant | ||||||
| Triton-X 35 | C14H22O(C2H4O)3 | 9036-19-5 | S | 340 | 4.31 | |
| Triton-X 45 | C14H22O(C2H4O)4 | 2315-63-1 | S | 382.5 | 4.04 | |
| Plasticizer | ||||||
| Tributyl Phosphate | (C4H9)3PO4 | 126-73-8 | S | 266.3 | 4.0 | [95] |
| Dimethyl terephthalate | C6H4(COOCH3)2 | 120-61-6 | S | 194.2 | 2.25 | [23] |
| Dibutyl Phthalate | C6H4(COOC4H9)2 | 84-74-2 | S | 278.3 | 4.61 | [23,96] |
| Other Aromatics | ||||||
| Alkylated diphenylamine | C20H27N | 68921-45-9 | S | 281.43 | 5.2 | [97] |
| Diphenylamine | (C6H5)2NH | 122-39-4 | S | 169.22 | 3.29 | [97] |
| Cumene hydroperoxide | C6H5C(CH3)2OOH | 80-15-9 | S | 152.19 | 2.41 | [98] |
| Benzene | C6H6 | 71-43-2 | W | 78.11 | 1.99 | [90] |
| Aliphatic Organics | ||||||
| Methanol | CH3OH | 67-56-1 | W | 32 | −0.63 | |
| Ethanol | C2H5OH | 64-17-5 | W | 46 | −0.14 | |
| 2-Propanol | CH3CH(OH)CH3 | 67-63-0 | W | 60 | 0.05 | |
| Ethylene glycol | C2H4(OH)2 | 107-21-1 | W | 62.07 | −1.36 | |
| Propylene glycol | CH3CHOHCH2OH | 57-55-6 | W | 76.1 | −0.92 | |
| Acetic acid | CH3COOH | 64-19-7 | W | 60 | −0.17 | [91] |
| n-Octanoic acid | CH3(CH2)6COOH | 124-07-2 | M | 144.21 | 3.05 | [99] |
| n-Hexane | C6H14 | 110-54-3 | W | 86.18 | 3.9 | [100] |
| Glucose | C6H12O6 | 50-99-7 | W | 180.2 | −3.24 | |
| Sucrose | C12H22O11 | 57-50-1 | W | 342.3 | −3.7 | |
| CIP Solution | Components and Comments | Reference |
|---|---|---|
| Alkaline-based Cleaner | ||
| Pressurized cleaning | A part of the alkaline solution is passed through fouled membranes. | [138] |
| Alkaline cleaner | An aliphatic or aromatic amide-containing cleaner. | [139] |
| Alkaline cleaner | Periodical change from an amide cleaner to no amide-containing one. | [140,141] |
| Organic Cleaner | ||
| Isopropyl alcohol | IPA | [135] |
| Methanol | 30% MeOH | [23] |
| Butyl cellosolve | 10% butyl cellosolve (ethylene glycol monobutyl ether) | [142,143] |
| Polyol/alcohol | A mixture of Polyols (such as EG and PG) and alcohols (e.g., MeOH) | [103,144,145,146] |
| Organic Solutions | 50% MeOH or 10% ethylene glycol monobutyl ether | [147] |
| Oxidative Cleaner | ||
| Nitric acid | High-concentration nitric acid | [118,137,148] |
| Nitric acid | Three step cleaning: alkaline (pH > 12) + nitric acid (pH < 1) + alkaline (pH > 12) | [149] |
| Polyol + nitric acid | Two-step cleaning: Nitric acid cleaning followed by EG cleaning | [150] |
| High pH NaOCl | Cleaning solution containing oxidizing substances with higher pH. | [151,152,153,154,155] |
| High pH NaOCl | NaOCl concertation < 10 mg/L as free chlorine and pH > 10 | [156] |
| H2O2 + NaOCl | An inorganic peroxide cleaning and rinsing with an alkali metal or alkaline earth metal hypohalite. | [157] |
| Oxidizing agent | An oxidizing agent, followed by cleaning with an alkaline detergent | [136] |
| Dichloroisocyanurate | Flow was restored, but there was a negative effect on salt rejection. | [137] |
| H2O2 | Alkaline H2O2 (pH12) | [155,158] |
| Chloramines | Chloramine compounds, e.g., monochlorosulfamic acid at high pH | [159] |
| Components | MW | Applications |
|---|---|---|
| Neutral organic and inorganic compounds | ||
| IPA | 60.1 | UPW [254] |
| Urea | 60.06 | UPW [255,256] |
| Boron: B(OH)3 | 61.83 | UPW and seawater desalination [257] |
| Arsenite, As(III): H3AsO3 | 125.9 | Drinking water treatment |
| Micropollutants | ||
| NDMA | 74.08 | Municipal wastewater reuse |
| 1,4-dioxane | 88.11 | Municipal wastewater reuse |
| Trihalomethanes (THMs) | 119.37 (CHCl3) | Drinking water treatment (DBP removal) |
| Haloalkane, haloalkene, etc. | Drinking water (volatile organic compounds) [258,259] | |
| Purpose | Polyphenols | Treatment Method and Comments | Reference |
|---|---|---|---|
| RO | |||
| Rej. enhancer | Tannic acid (TA) | Hot water treatment with TA; | [273] |
| Ca, silica, IPA | Polyphenol (gallnut) | Treatment in low pH (1–5), flushing with water; | [274,275] |
| Rej. enhancer | Polyphenol (gallnut) | Pressurized treatment and improving rejections at LP; | [276] |
| Rej. enhancer | Polyphenol (gallnut) | Optimize treatment conditions (temperature, duration); | [277] |
| Rej. enhancer | Polyphenol (gallnut) | Effect of polyphenol (gallnut) lot; | [278] |
| Rej. enhancer | Hydrolyzed tannin | Combination of PVME and TA; | [279] |
| NF | |||
| Rej. enhancer | TA | Treatment with a strong mineral acid followed by TA; | [280,281,282] |
| NaCl, Ca, silica | Tannins | Treatment with various tannins | [240,283,284] |
| Other applications (improve oxidant tolerance) | |||
| Anti-oxidant | Polyphenol (gallnut) | Intermittent on-line TA (5–20 mg/L) treatment | [285,286,287] |
| Anti-oxidant | Polyphenol (gallnut) | TA treatment with a reducing agent (sodium bisulfite, SBS) | [288] |
| Solutes | Rejection Enhancer | Flow (L/m2/h) | Rejection (%) | Passage Decrease (%) | Pressure (MPa) | Membrane | Ref. |
|---|---|---|---|---|---|---|---|
| IPA | Blank | Relative 1.0 | 95 | – | – | ES10 | [275] |
| TA (gallnut) | Relative 0.95 | 96.5 | 30 | – | |||
| Blank | 44.5 | 77.8 | – | 0.75 | ES20 | [314] | |
| PEG 4000 | 26.0 | 87.9 | 46 | ||||
| Blank | 34.2 | 92.3 | – | 0.75 | ES20 | [303] | |
| Sulfonated PEG4000 + LBL | 25.4 | 96.7 | 57 | ||||
| Boron | Blank (12 h run) | Relative 0.81 | 55.7 | – | 1.2 | LE | [266] |
| Sodium dodecylbenzene sulfonate | Relative 0.42 | 68.2 | 28 | ||||
| Tween-80 | Relative 0.56 | 73.7 | 40.6 | ||||
| CTAB | Relative 0.6 | 70.5 | 33.4 | ||||
| Blank | 41.7 | 45.1 | – | – | ES20 | [304] | |
| pyrogallol | 41.7 | 53.7 | 15.7 | ||||
| Blank | Relative 1.0 | 84 | – | 5.5 | TM820V | ||
| Propyl gallate | Relative 0.955 | 88 | 25 | ||||
| Blank | Pure water 110 | 74 | – | 5.5 | SWC4B | [306] | |
| Dodecylamine (C12) | Pure water 45 | 87 | 50 | ||||
| Blank | 34 | 88 | – | 5.5 | SW30 | [308] | |
| NBS | 14 | 93.1 | 58 | ||||
| Urea | Blank | 42.7 | 15.4 | – | 0.75 | ES20 | [314] |
| PEG 4000 | 29.1 | 32.2 | 19.9 | ||||
| PEG 7100 | 17.5 | 39.2 | 28.1 | ||||
| Blank (22 °C) | 142 | 18 | – | 1.59 | XLE | [309] | |
| MPD (Carbodiimide chemistry) | 76 | 37 | 23 | ||||
| Blank (78 °C heat treat) | 60 | 47 | 35 | ||||
| MPD (78 °C heat treat) | 30 | 55 | 45 | ||||
| NDMA | Blank | 3.5 LMH/bar | 42 | – | 0.42 | ESPAB | [311] |
| Dodecylamine (MW: 185.4)) | 1.2 LMH/bar | 82 | 69 | 1.25 | |||
| Dodecanamide (MW: 199.3) | 2.3 LMH/bar | 64 | 38 | 0.65 | |||
| 1,2-Epoxydodecane (MW: 184.3) | 3.5 LMH/bar | 60 | 31 | 0.42 |
| Rejection Enhancer | Flow (L/m2/h) | Boron Rej. (%) | Passage Decrease (%) | Pressure (MPa) | Membrane | Ref. |
|---|---|---|---|---|---|---|
| LMWOC Rejection Enhancer | ||||||
| Blank | Relative 1.0 | 84 | – | 5.5 | TM820V | [304] |
| Propyl gallate | Relative 0.955 | 88 | 25 | |||
| Blank | Pure water 110 | 74 | – | 5.5 | SWC4B | [306] |
| Dodecylamine (C12) | Pure water 45 | 87 | 50 | |||
| Blank | 34 | 88 | – | 5.5 | SW30 | [308] |
| NBS | 14 | 93.1 | 58 | |||
| Bromine and Iodine Treatment | ||||||
| Blank | 25 | 84 | – | 5.5 | – | [315] |
| Bromine | 25 | 92 | 50 | 6.6 | ||
| Blank | – | 78 | – | 6.0 | SWC5 | [241] |
| Stabilized Bromine | – | 96 | 82 | |||
| Blank | 30.6 | 86 | – | 5.5 | – | [316] |
| Br (NaOCl 100 ppm + 10 ppm NaBr) | 20.1 | 93.6 | 54 | |||
| I (NaOCl 100 ppm + 20 ppm KI) | 25.9 | 95 | 64 | |||
| Iodine chloride (ICl), 3 ppm | 16.5 | 97 | 79 | |||
| HOI | Relative 1.0 | 91.96 | – | 5.5 | SW30HR | [317] |
| HOI (0.013 mM·h contact) | 0.7 | 95.33 | 42 | |||
| HOI (0.032 mM·h contact) | 0.6 | 98.11 | 76 | |||
| Case | Type | Conc. (mg/L) | pH | P (MPa) | Pre- Clean | Memb. | Type | Before | After | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow (LMH) | Rej. (%) | Flow (LMH) | Rej. (%) | |||||||||
| 1 | TA | 1 | – | 2.76 | AC&AL | TFC PA | Old | 109% * | 84 | 98.8% * | 90.9 | [325] |
| 2 | Gallnut | 100 | – | Yes | No | ES10 | New | – | 80 | – | 98 | [283] |
| 2 | Gallnut | 100 | – | No | No | ES10 | New | – | 80 | – | 80 | |
| 3 | Gallnut | 100 | 2.2 | Yes | AC&AL | ES10 | Old | – | 92 | – | 99.1 | [326] |
| 3 | Gallnut | 100 | 2.2 | Yes | No | ES10 | Old | – | 92 | – | 93 | |
| 4 | Digallic | 240 | 4.0 | 0.29 | AL | BW30 | New | 65.5 | 85.6 | 37.4 | 97.3 | [323] |
| Compounds | Conc. mg/L | I or Pres. (MPa) | Before | After | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Flow (LMH) | NaCl Rej. (%) | Silica Rej. (%) | Flow (LMH) | NaCl Rej. (%) | Silica Rej. (%) | ||||
| TRITON X-405 | 1000 | I; 16 h | 76.7 | 99.5 | 98 | 40.8 | 99.6 | 99 | [332] |
| CTAC | 1000 | I; 16 h | 76.7 | 99.5 | 98 | 19.6 | 99.1 | 99.2 | |
| Flow (LMH) | NaCl Rej. (%) | Boron Rej. (%) | Flow (LMH) | NaCl Rej. (%) | Boron Rej. (%) | ||||
| PEG(1000) | 15 | 0.45 | 46.3 | 99.5 | 89.1 | 44.6 | 99.6 | 89.5 | [333] |
| PEG(8000) | 15 | 0.45 | 46.3 | 99.5 | 89.1 | 37.1 | 99.7 | 92.3 | |
| PEG(50,000) | 15 | 0.45 | 46.3 | 99.5 | 89.1 | 36.3 | 99.7 | 92.5 | |
| PEG(8000) | 1 | 0.45 | 46.3 | 99.5 | 89.1 | 42.1 | 99.6 | 91.0 | |
| PEG(8000) | 15 | I; 1 h | 46.3 | 99.5 | 89.1 | 42.1 | 99.6 | 90.1 | |
| PEG(8000)—Before CIP | – | – | 28 m3/d | 99.0 | 85 | – | – | – | [334] |
| PEG(8000)—After CIP | 15 | 0.45 | 32 m3/d | 98.6 | 84 | 28 m3/d3 | 99.3 | 91 | |
| PEG(8000)—pH 12 | 15 | 0.45 | 28 m3/d | 99.0 | 85 | 22 m3/d | 99.2 | 90 | |
| PEG(8000)—No CIP | 15 | 0.45 | 28 m3/d | 99.0 | 85 | 25 m3/d | 99.2 | 87 | |
| RJAs | Before | After | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | Flow (LMH) | NaCl Rej. (%) | IPA Rej. (%) | Flow (LMH) | NaCl Rej. (%) | IPA Rej. (%) | |
| 3,5-DABA | – | – | 49.6 | 89.2 | – | 33.8 | 94.5 | – | [341] |
| 3,5-DABA | APT | – | 50.0 | 88.4 | – | 34.6 | 95.1 | – | |
| 3,5-DABA | APT | PVAM | 49.6 | 88.1 | – | 34.2 | 96.1 | – | |
| Arginine | – | – | 37.9 | 91.7 | 78.1 | 37.1 | 94.3 | 84.6 | [342] |
| Arginine | Aspartame | – | 37.9 | 91.2 | 78.3 | 36.7 | 94.7 | 85.0 | |
| Arginine | Aspartame | TA | 37.5 | 92.3 | 78.5 | 33.3 | 98.6 | 90.2 | |
| Arginine | Aspartame | Mimosa | 38.3 | 90.8 | 77.6 | 33.8 | 97.7 | 91.2 | |
| – | – | TA | 37.5 | 92.0 | 78.7 | 35.8 | 94.6 | 83.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, Y. Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 2: Countermeasures and Applications. Membranes 2025, 15, 94. https://doi.org/10.3390/membranes15030094
Maeda Y. Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 2: Countermeasures and Applications. Membranes. 2025; 15(3):94. https://doi.org/10.3390/membranes15030094
Chicago/Turabian StyleMaeda, Yasushi. 2025. "Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 2: Countermeasures and Applications" Membranes 15, no. 3: 94. https://doi.org/10.3390/membranes15030094
APA StyleMaeda, Y. (2025). Fouling of Reverse Osmosis (RO) and Nanofiltration (NF) Membranes by Low Molecular Weight Organic Compounds (LMWOCs), Part 2: Countermeasures and Applications. Membranes, 15(3), 94. https://doi.org/10.3390/membranes15030094





