Preparation of Am-MSN/PVDF Mixed Matrix Membranes for Enhanced Removal of Reactive Black 5
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Am-MSNs
2.3. Preparation of Am-MSN/PVDF Mixed Matrix Membranes
2.4. Characterization
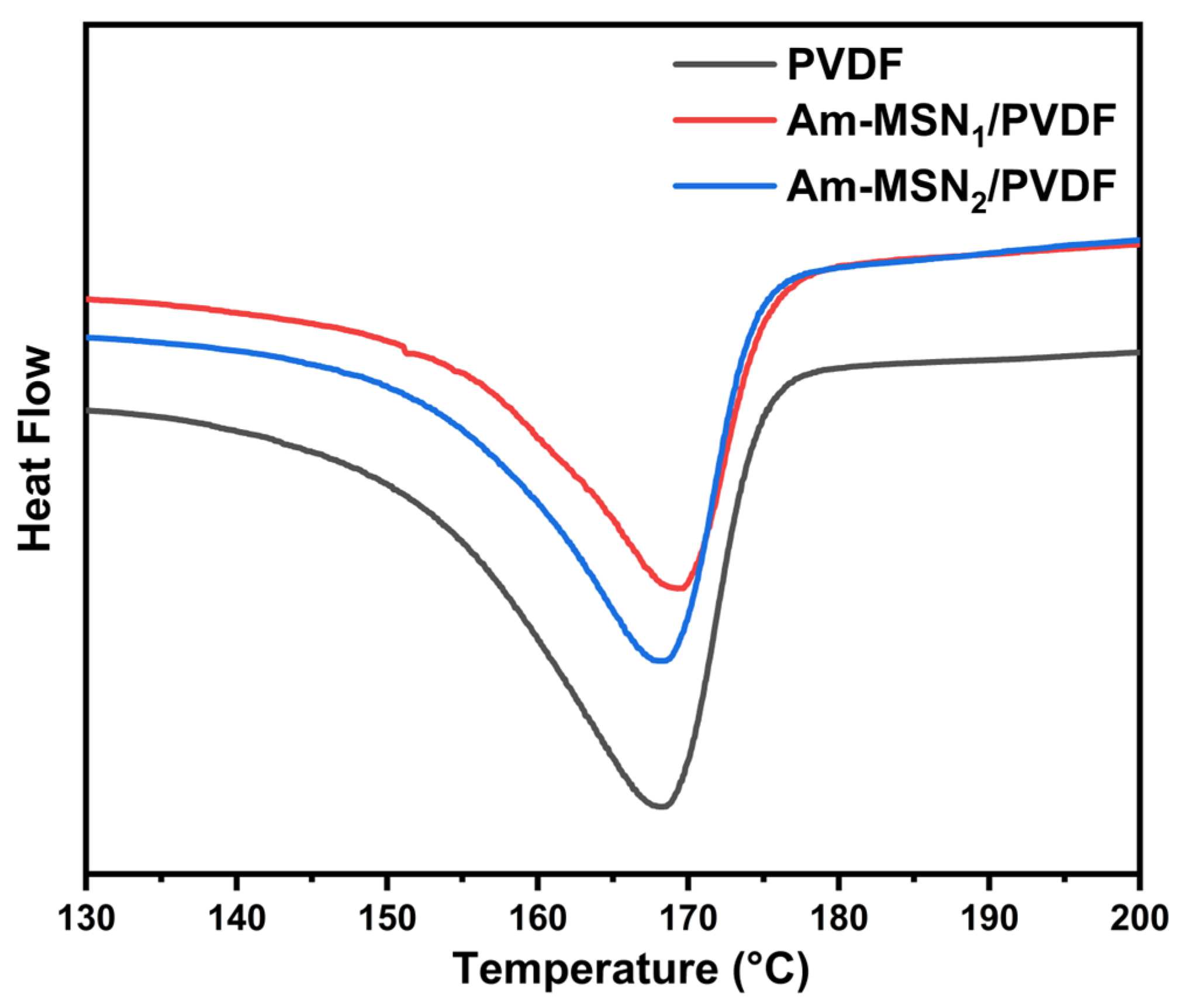
2.5. Crystallinity Test
2.6. Porosity Test
2.7. Dye Filtration Test
2.8. Static Adsorption Test
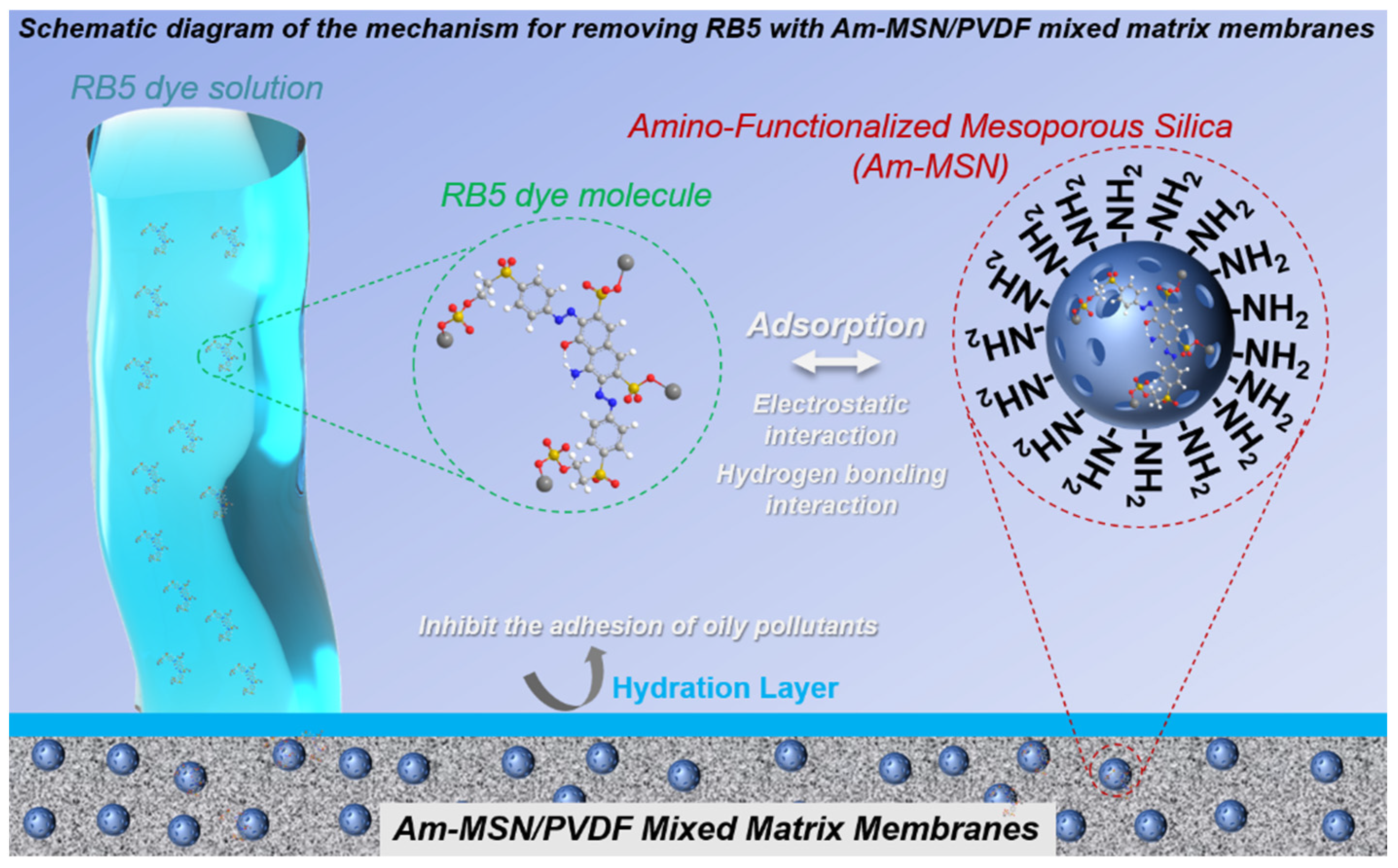
3. Results and Discussion
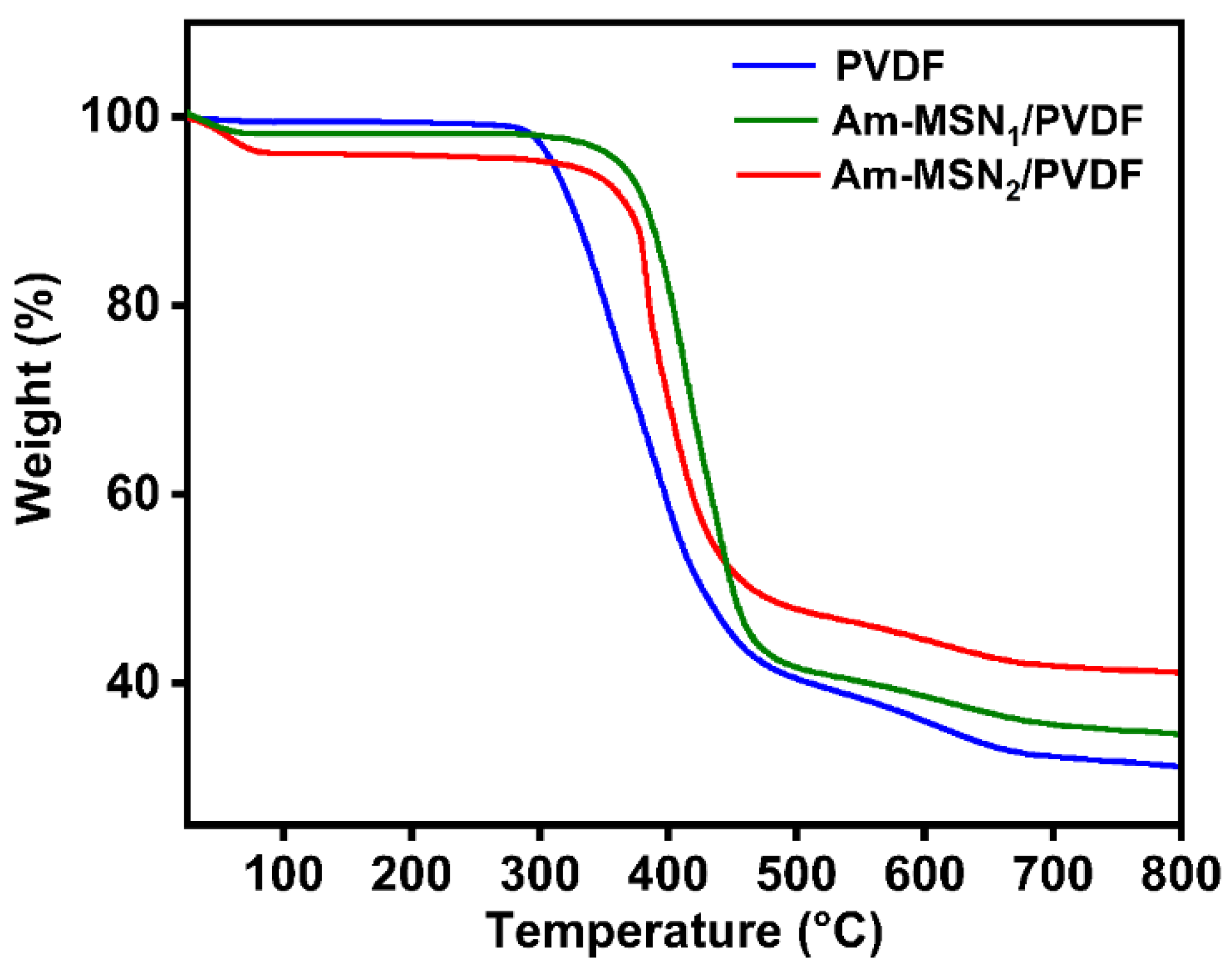
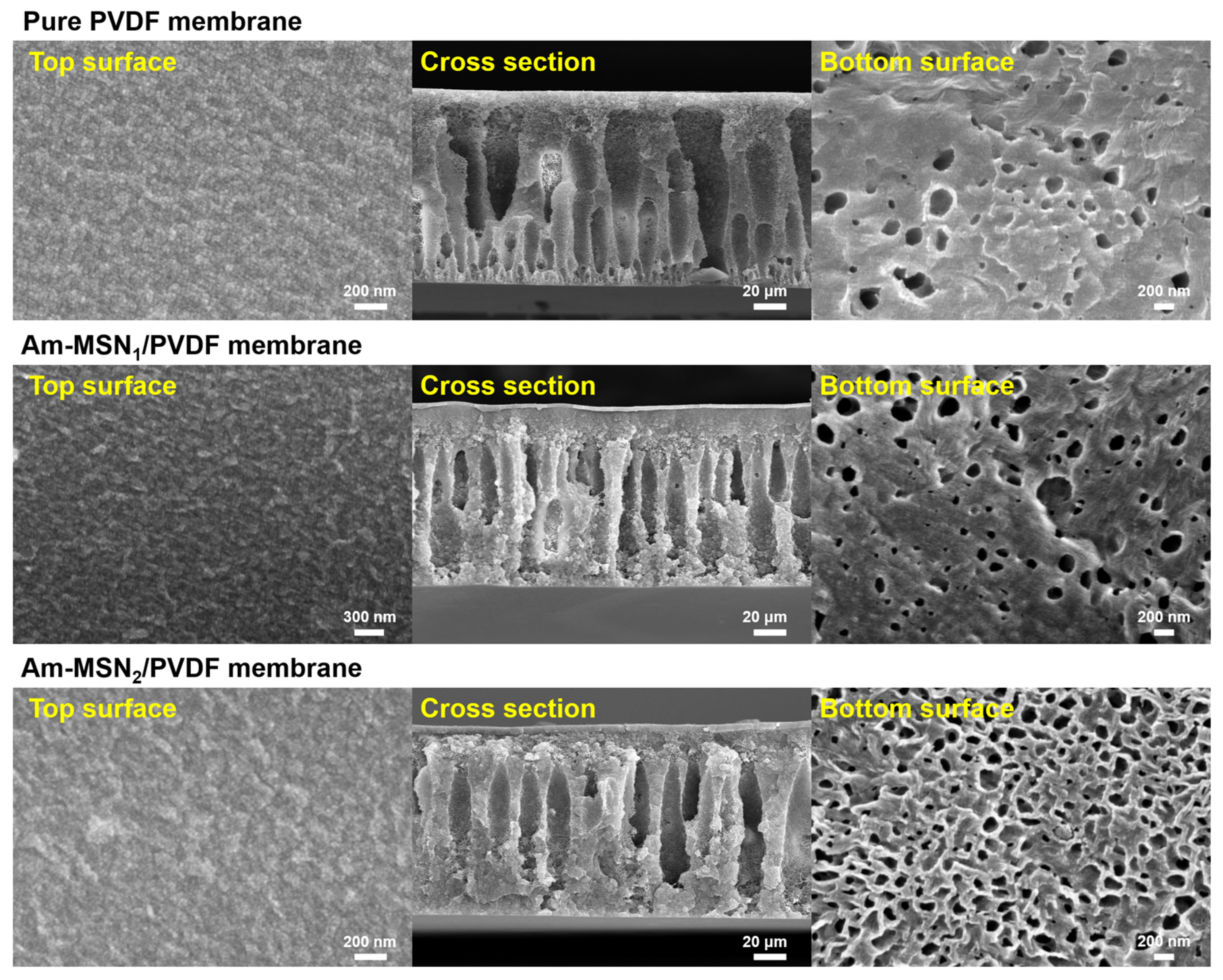
3.1. Surface Composition Analysis of Nanoparticles and Membranes
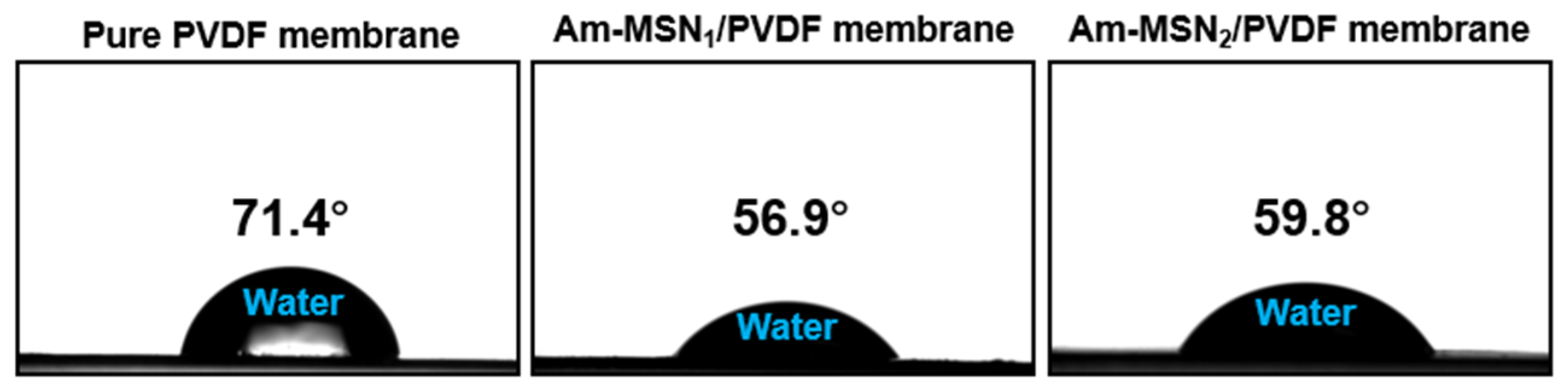
3.2. Wettability Analysis of Membranes
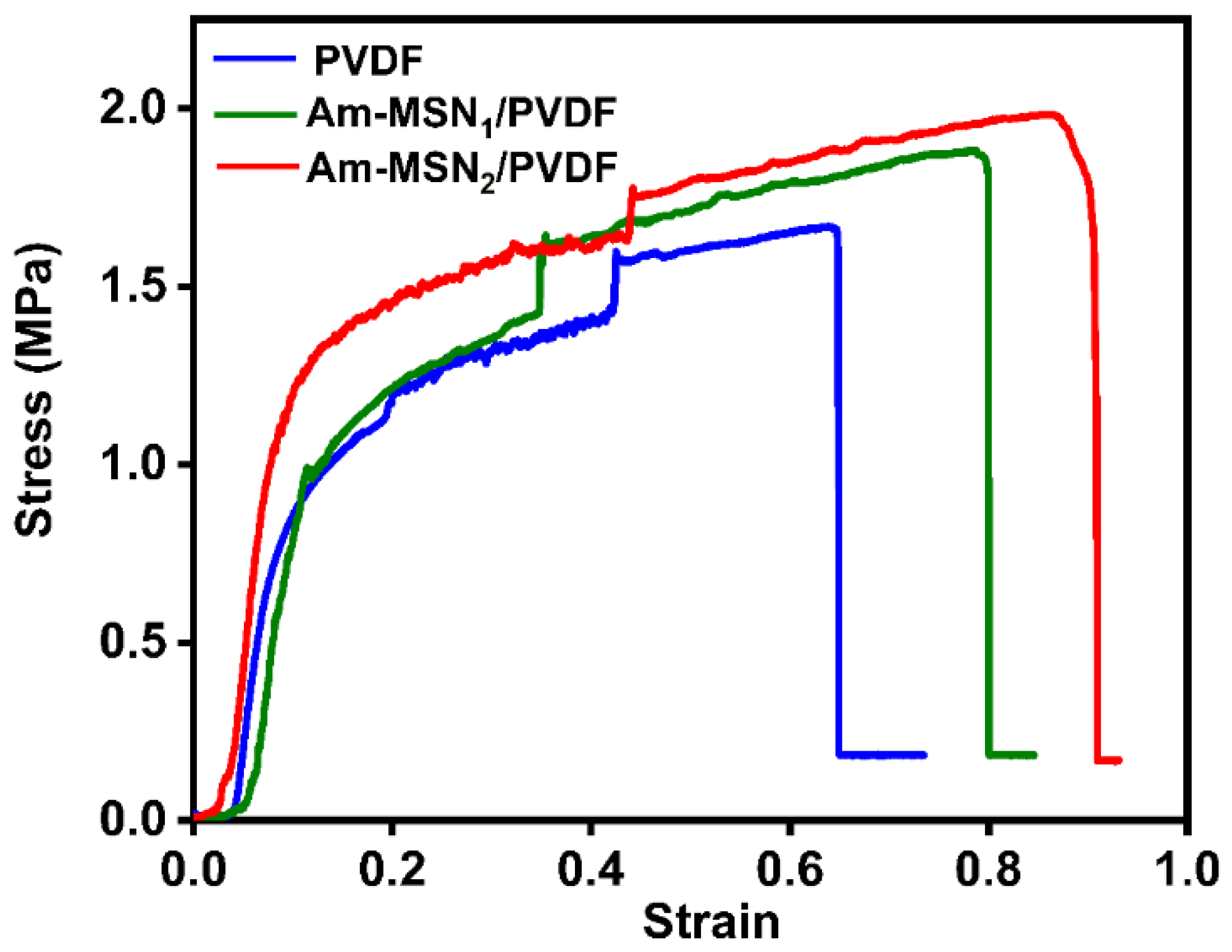
3.3. Mechanical Performance Analysis of Membranes
3.4. Separation Performance Analysis of Membranes
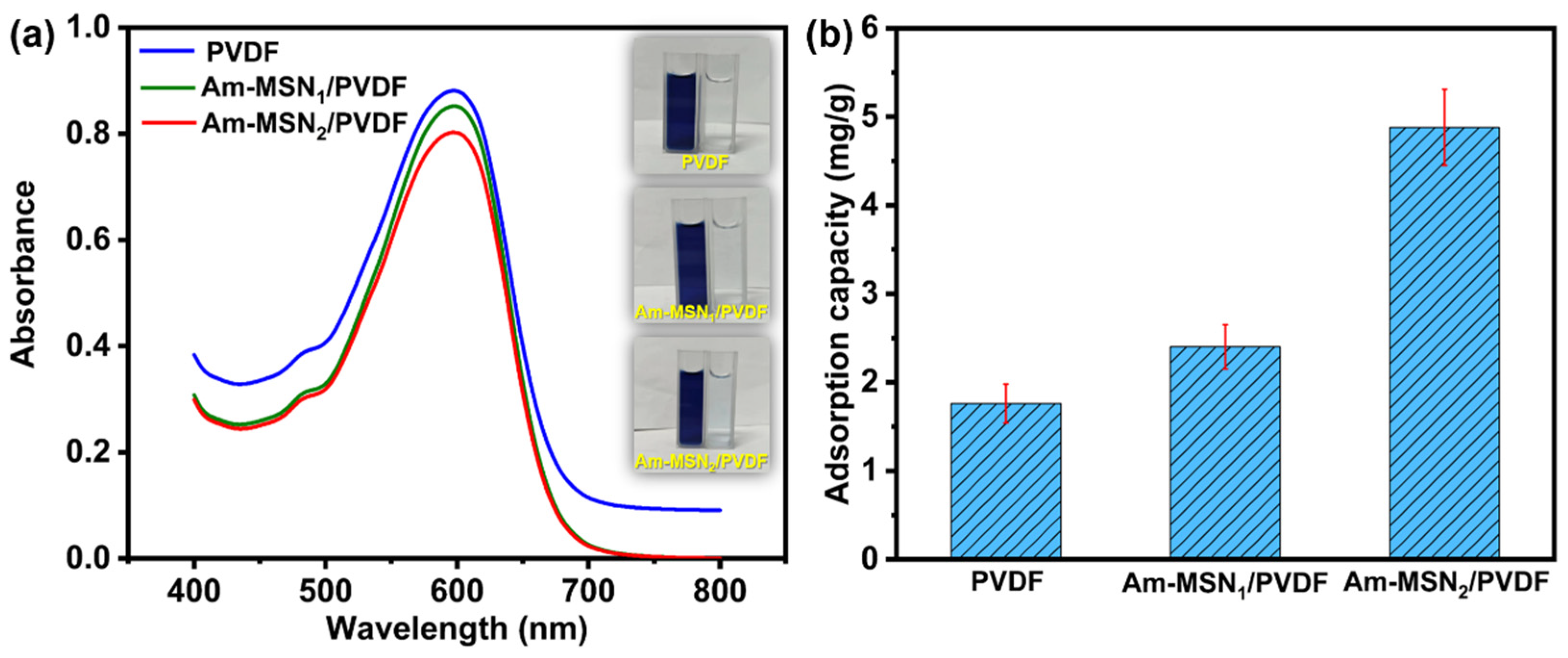
3.5. Static Adsorption Capacity Analysis of Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mi, Y.F.; Wang, N.; Qi, Q.; Yu, B.; Peng, X.D.; Cao, Z.H. A loose polyamide nanofiltration membrane prepared by polyether amine interfacial polymerization for dye desalination. Sep. Purif. Technol. 2020, 248, 117079. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, H.S.; Ravikant; Kumar, A.; Thomas, R.; Pradhan, D.K. Ferroelectric ceramic dispersion to enhance the β phase of polymer for improving dielectric and ferroelectric properties of the composites. Polym. Bull. 2021, 78, 5317–5336. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Macario, J.A. Removal of Different Dye Solutions: A Comparison Study Using a Polyamide NF Membrane. Membranes 2020, 10, 408. [Google Scholar] [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Lagiewka, J.; Jaksender, K.; Witt, K.; Malina, G.; Sabadash, V. Removal of Methylene Blue Dye from Aqueous Solutions Using Polymer Inclusion Membrane Containing Calix[4]pyrrole. Membranes 2024, 14, 92. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Ashraf, M.; Ullah, N. A critical review on the removal of anionic dyes by cross-linked resin: Recent progress, challenges and future perspective. Sep. Purif. Technol. 2024, 360, 131111. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Textile wastewater treatment by AOPs for brine reuse. Process Saf. Environ. 2017, 109, 420–428. [Google Scholar] [CrossRef]
- Dalvand, A.; Gholami, M.; Joneidi, A.; Mahmoodi, N.M. Dye Removal, Energy Consumption and Operating Cost of Electrocoagulation of Textile Wastewater as a Clean Process. Acta Hydrochim. Hydrobiol. 2011, 39, 665–672. [Google Scholar] [CrossRef]
- Muniasamy, S.K.; AlObaid, A.A.; Warad, I.; Ravindiran, G. Removal of Brilliant Green dye in aqueous solution using synthetic coagulation and flocculation technique. Desalin. Water Treat. 2023, 314, 231–240. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta A 2018, 192, 487–498. [Google Scholar] [CrossRef]
- Bogale, F.M.; Teffera, B.; Aragaw, T.A. Recent developments in integrated anaerobic/aerobic (A/O) process for textile industry wastewater treatment: A review. J. Hazard. Mater. Adv. 2024, 15, 100438. [Google Scholar] [CrossRef]
- Pan, K.; Qian, Z.; Guo, T.; Chen, Y.; Li, F.; Ding, M.; Li, J. Stability and performance of aerobic granular sludge for treatment of textile-dyeing wastewater. J. Water Process Eng. 2024, 63, 105401. [Google Scholar] [CrossRef]
- He, C.-C.; Hu, C.-Y.; Lo, S.-L. Evaluation of sono-electrocoagulation for the removal of Reactive Blue 19 passive film removed by ultrasound. Sep. Purif. Technol. 2016, 165, 107–113. [Google Scholar] [CrossRef]
- Soto, D.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Succinylated Starches for Dye Removal. Starch-Starke 2021, 73, 2000043. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Pan, S.; Zhang, L.; Yang, Z.; Liu, Y.; Zhang, P.; Zhang, Y.; Bi, M.; Sun, J.; et al. Engineering loose nanofiltration membranes through amion-functionalized nanodiamonds for efficient dye/salt separation. Chem. Eng. Sci. 2025, 304, 121065. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, W.; Fu, S.; Lin, X.; Lu, Q.; Liao, S.; Hu, H.; Zhang, M.; Zhou, C.; Wen, X.; et al. A novel Janus sponge fabricated by a green strategy for simultaneous separation of oil/water emulsions and dye contaminants. J. Hazard. Mater. 2022, 424, 127543. [Google Scholar] [CrossRef]
- Chen, T.; Xia, J.; Gu, J.; Lu, G.; Xue, Q.; Liu, C.; Yan, L.; Chen, T. Engineering Janus CNTs/OCS composite membrane at air/water interface for excellent dye molecules screening. Chem. Eng. J. 2021, 417, 127947. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane. Membranes 2020, 10, 47. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Zuo, Y.; Zhu, B.; Liu, X.; Jia, Y. Boron nitride modified PAN ultrafiltration membranes with im-proved water permeance and dye rejection performance. Desalin. Water Treat. 2024, 321, 100962. [Google Scholar] [CrossRef]
- Ao, C.; Zhong, S.; Zhang, B.; Xie, Y.; Pan, B.; Zhang, W.; Wu, M. Lanthanum hydroxide@cellulose membranes with tunable pore sizes for selective removal of dyes with the same charges. Int. J. Biol. Macromol. 2024, 278, 135002. [Google Scholar] [CrossRef]
- Zhou, L.-l.; Fang, Y.-x.; Ye, J.; Chen, M.; Yang, H.; Xu, Z.-l. Cross-section and pore size regulation of hydroxyurea-modified polyacrylonitrile membrane by NH2-CNT and Si(OEt)4 filler and heat treatment for dye removal application at medium temperature. Sep. Purif. Technol. 2023, 323, 124430. [Google Scholar] [CrossRef]
- Hassan, M.M.; Abudi, Z.N.; Al-Furaiji, M.H. Studying the performance of various polymeric ultrafiltration membranes in dye removal. Desalin. Water Treat. 2022, 280, 139–156. [Google Scholar] [CrossRef]
- Lagiewka, J.; Witt, K.; Gierszewska, M.; Zawierucha, I. Selective removal of organic dyes via polymer inclusion membrane containing a perbenzylated β-cyclodextrin derivative. J. Water Process Eng. 2024, 68, 106306. [Google Scholar] [CrossRef]
- Azzian, M.I.M.; Mohamad, S.F.; Hakimi, N.M.F.; Wan Salleh, W.N.; Ismail, N.H.; Ismail, A.F. Radiation-induced graft polymerization acrylamide on PVDF membrane for cation dye removal. Mater. Today Proc. 2024, 96, 55–61. [Google Scholar] [CrossRef]
- Zuo, J.-H.; Wei, C.; Cheng, P.; Yan, X.; Chen, Y.; Lang, W.-Z. Breakthrough the upperbond of permeability vs. tensile strength of TIPS-prepared PVDF membranes. J. Membr. Sci. 2020, 604, 118089. [Google Scholar] [CrossRef]
- Liu, F.; Abed, M.R.M.; Li, K. Hydrophilic modification of P(VDF-co-CTFE) porous membranes. Chem. Eng. Sci. 2011, 66, 27–35. [Google Scholar] [CrossRef]
- Zuo, J.-H.; Cheng, P.; Chen, X.-F.; Yan, X.; Guo, Y.-J.; Lang, W.-Z. Ultrahigh flux of polydopamine-coated PVDF membranes quenched in air via thermally induced phase separation for oil/water emulsion separation. Sep. Purif. Technol. 2018, 192, 348–359. [Google Scholar] [CrossRef]
- Veisi, P.; Seyed Dorraji, M.S.; Vatanpour, V.; Rasoulifard, M.H. Dimensional effect of ZnO-g-C3N4 heterostructures on hydrophilic and anti-fouling properties of the PVDF/PAN composite membrane: Dye rejection. J. Environ. Chem. Eng. 2023, 11, 110249. [Google Scholar] [CrossRef]
- Liu, H.; Xie, J.; Zhao, J.; Wang, R.; Qi, Y.; Lv, Z.; Yu, Y.; Sun, S. Construction of gradient SA-TiO2 hydrogel coated PVDF-g-IL fibre membranes with high hydrophilicity and self-cleaning for the efficient separation of oil-water emulsion and dye wastewater. J. Membr. Sci. 2024, 697, 122580. [Google Scholar] [CrossRef]
- Zhong, X.; Shi, Q.; Guo, Z. Synergistic Construction of Superhydrophilic PVDF Membranes by Dual Modification Strategies for Efficient Emulsion Separation. Small 2024, 20, 2402538. [Google Scholar] [CrossRef]
- Islam, S.S.; Jose, T.; Seikh, A.H.; Karim, M.R.; Alnaser, I.A.; Bose, S. Shear-aligned graphene oxide nanosheets incorporated PVDF composite membranes for selective dye rejection with high water flux. RSC Adv. 2024, 14, 27852–27861. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhang, Z.; Zhang, P.; Shi, Y.; Ding, P. Study on the improvement of PVDF flat ultrafiltration membrane with MWCNTs-OH as the additive and the influence of different MWCNTs-OH scales. Colloid Interface Sci. 2021, 43, 100433. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Yang, C.; Wang, X.; Wang, S.; Yin, J.; Du, Y.; Wu, D.; Hu, J.; Zhao, Q. Determination of Free Fatty Acids in Krill Oil during Storage Based on NH2-MMS. Foods 2024, 13, 2736. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Chen, L.; Zhong, Y.; Hu, F.; Wu, K.; Yin, X.; Wei, Y.; Ning, S. Phosphination of amino-modified mesoporous silica for the selective separation of strontium. J. Hazard. Mater. 2024, 467, 133741. [Google Scholar] [CrossRef]
- Peng, T.-q.; Wang, Y. Adsorption of uranium by amination mesoporous molecular sieve HMS, using response surface methodology. Sep. Sci. Technol. 2023, 58, 1731–1747. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Liou, T.-H.; Liu, W.-Y.; Hsu, C.-C.; Chiu, S.-E. Characterization of new modified mesostructured silica nanocomposites fabricated for effective removal of aromatic acids. Arab. J. Chem. 2023, 16, 105145. [Google Scholar] [CrossRef]
- Rita, S.; Eti, R.; Tetty, K. Aminopropyltrimethoxysilane (APTMS) modified nano silica as heavy metal iron (Fe) adsorbents in peat water. In Proceedings of the 17th International Conference on Ion Sources, Geneva, Switzerland, 15–20 October 2018. [Google Scholar]
- Hashemikia, S.; Hemmatineja, N.; Ahmadi, E.; Montazer, M. Optimization of tetracycline hydrochloride adsorption on amino modified SBA-15 using response surface methodology. J. Colloid Interface Sci. 2015, 443, 105–114. [Google Scholar] [CrossRef]
- Guo, J.; Chen, B.; Shen, Y.; Chen, C.; Yang, Y.; Yu, D. Morphological Modulation of Electrospun PVDF/PVP Nanofibers. J. Polym. Sci. 2025. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Cheng, Y.; Li, X.; Wei, Z. Multilayer-functionalized molecularly imprinted nanocomposite membranes for efficient acteoside separation. Microporous Mesoporous Mater. 2023, 348, 112345. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Shen, Y.; Tan, Q.; Sun, J.; Teng, J.; Lin, H. Innovative integration of porous materials and membranes: Achieving optimized pore orientation for enhanced performance. J. Membr. Sci. 2024, 689, 122158. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Tang, H.; Shi, H. PVDF-HFP/SiO2 composite solid electrolyte enhanced by supramolecular self-assembly of cyclodextrin. J. Energy Storage 2024, 88, 111461. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; He, B. Fabrication of nanoparticle array membranes by integrating semi-crystalline polymer self-assembly with NIPS for water treatment. Nanoscale Adv. 2024, 6, 3543–3552. [Google Scholar] [CrossRef]
- Darvishi, S.; Senses, E. Interfacial polymer architecture can control nanoparticle dispersion and rheological behavior of nanocomposites. Eur. Polym. J. 2023, 196, 112320. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, M.Z.; He, J.L.; Wu, D.Z.; Meng, J.W.; Ni, P.H. Effects of fluorinated SiO2 nanoparticles on the thermal and electrochemical properties of PP nonwoven/PVdF-HFP composite separator for Li-ion batteries. J. Membr. Sci. 2014, 455, 368–374. [Google Scholar] [CrossRef]
- Bhute, M.V.; Kondawar, S.B. Electrospun poly(vinylidene fluoride)/cellulose acetate/AgTiO2 nanofibers polymer electrolyte membrane for lithium ion battery. Solid State Ion. 2019, 333, 38–44. [Google Scholar] [CrossRef]
- Aksoy, Y.; Hasar, H. Fabrication of PVDF-HF membrane for bubble-free gas transfer via wet phase inversion. J. Appl. Polym. Sci. 2021, 138, 51405. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, X.; Zhang, Q.; Li, X.; Wei, Z. Constructing imprinted reticular structure in molecularly imprinted hybrid membranes for highly selective separation of acteoside. Sep. Purif. Technol. 2022, 298, 121572. [Google Scholar] [CrossRef]
- Mohammed, L.; Boating, B.; Mwemezi, M.; Hamenu, L.; Madzvamuse, A.; Nyarko, A.; Mohammed, M.; Oduro, W.; Agyenim, F.B.; Lee, Y.M.; et al. EMI-BF4 electrolyte and Al2O3/PVDF-HFP modified PE separator for high capacitance retention and cycle stability in supercapacitors. Korean J. Chem. Eng. 2022, 39, 3003–3011. [Google Scholar] [CrossRef]
- Lin, T.; Fan, D.; Wang, J.; Shi, J.; Ni, W.; Ding, M.; Li, Y.; Yang, Y.-B.; You, J. Synergism effect between internal and surface cubic-large-pores in the enhancement of separation performance in hierarchically porous membranes. Polymer 2023, 265, 125601. [Google Scholar] [CrossRef]
- Im, Y.M.; Palanisamy, G.; Thangarasu, S.; Oh, T.H. Fluoropolymer based porous membrane with Ti3C2Tx and nanocrystal-cellulose for bi-functional water treatment applications. J. Water Process Eng. 2024, 65, 105766. [Google Scholar] [CrossRef]
- Kong, F.; Chang, M.; Wang, Z. Comprehensive Analysis of Mechanical Properties of CB/SiO2/PVDF Composites. Polymers 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Janicijevic, A.; Filipovic, S.; Sknepnek, A.; Salevic-Jelic, A.; Jancic-Heinemann, R.; Petrovic, M.; Petronijevic, I.; Stamenovic, M.; Zivkovic, P.; Potkonjak, N.; et al. Structural, Mechanical, and Barrier Properties of the Polyvinylidene Fluoride-Bacterial Nanocellulose-Based Hybrid Composite. Polymers 2024, 16, 1033. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Geng, H.; Xu, F.; Zhang, M.; Ye, P.; Jiang, Y.; Wang, H. Endowing versatility and superamphiphobicity to composite coating via a bioinspired strategy. Chem. Eng. J. 2023, 455, 140772. [Google Scholar] [CrossRef]
- Yan, J.; Xiao, C.; Wang, C. Robust preparation of braid-reinforced hollow fiber membrane covered by PVDF nanofibers and PVDF/SiO2 micro/nanospheres for highly efficient emulsion separation. Sep. Purif. Technol. 2022, 298, 121593. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, S.; Nayak, S.K. Study of Nonisothermal Crystallization Kinetics of Unstretched and Uniaxially Stretched Electroactive PVDF Composite Films. Macromol. Chem. Phys. 2023, 224, 2200326. [Google Scholar] [CrossRef]
- Teng, L.; Yue, C.; Zhang, G. Epoxied SiO2 nanoparticles and polyethyleneimine (PEI) coated polyvinylidene fluoride (PVDF) membrane for improved oil water separation, anti-fouling, dye and heavy metal ions removal capabilities. J. Colloid Interface Sci. 2023, 630, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Zeinalipour, N.; Kerachian, M.A.; Heidari, A.A. Preparation of high-performance PVDF mixed matrix membranes incorporated with PVDF-g-PMMA copolymer and GO@SiO2 nanoparticles for dye rejection applications. J. Water Process Eng. 2022, 46, 102560. [Google Scholar] [CrossRef]
- Jozwiak, T.; Filipkowska, U.; Walczak, P. The Use of Aminated Wheat Straw for Reactive Black 5 Dye Removal from Aqueous Solutions as a Potential Method of Biomass Valorization. Energies 2022, 15, 6257. [Google Scholar] [CrossRef]
- Peng, H.; Minic, A.; Banjerdteerakul, K.; Li, K. COF-300/PVDF adsorbents with aligned microchannels for fast removal of polycyclic aromatic hydrocarbons (PAHs). Chem. Eng. J. 2023, 465, 142901. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Zienkiewicz-Strzalka, M.; Kusmierek, K.; Skrzypczynska, K.; Swiatkowski, A. Effect of the SBA-15 N-functionalization on the adsorption of organic contaminants. Desalin. Water Treat. 2023, 312, 39–49. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, J.; Wang, J.; Lin, J.; Reheman, A. Excellent adsorption ability of Hg(II) by poly(o-phenylenediamine) modified mesoporous materials. Desalin. Water Treat. 2023, 298, 61–74. [Google Scholar] [CrossRef]

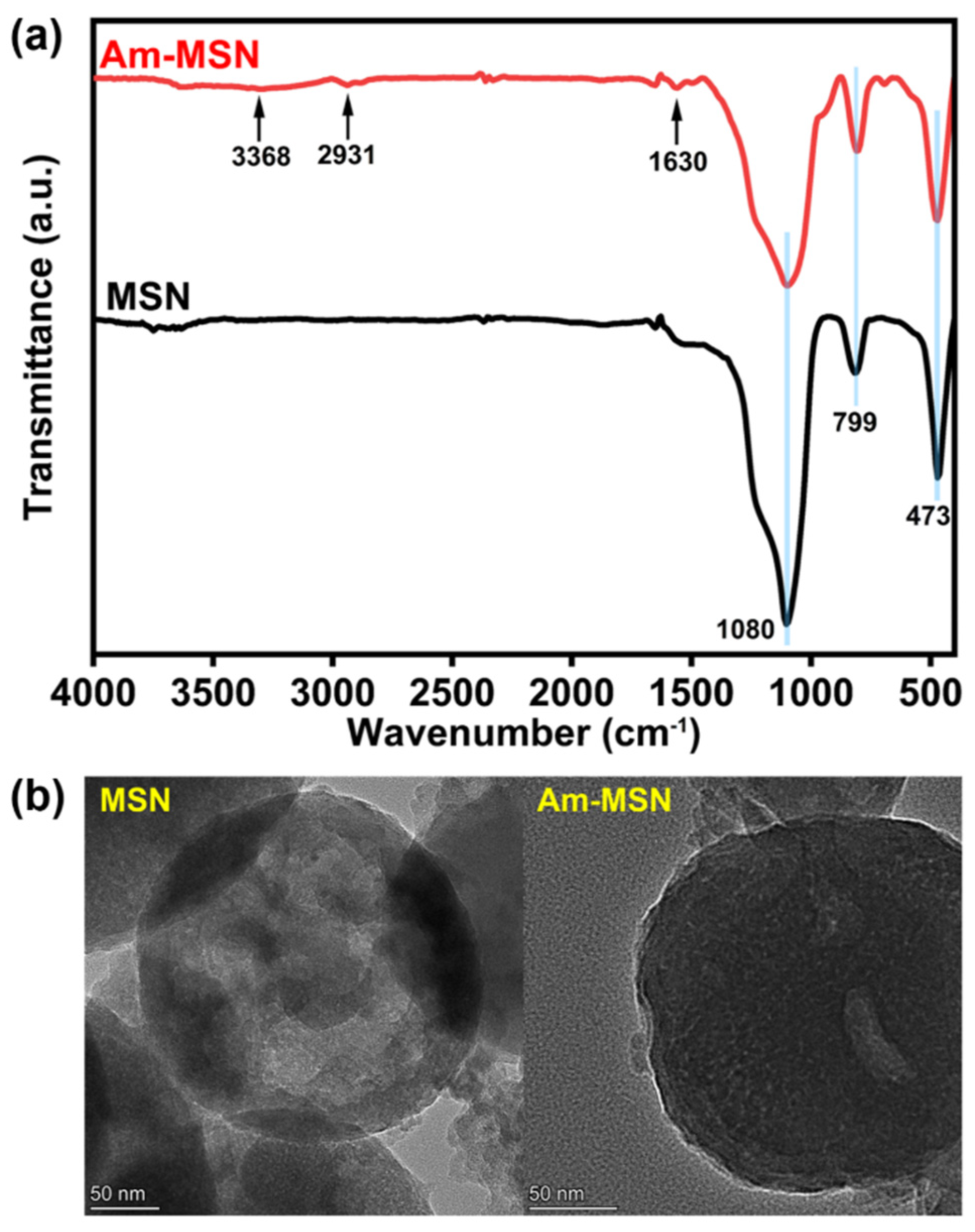
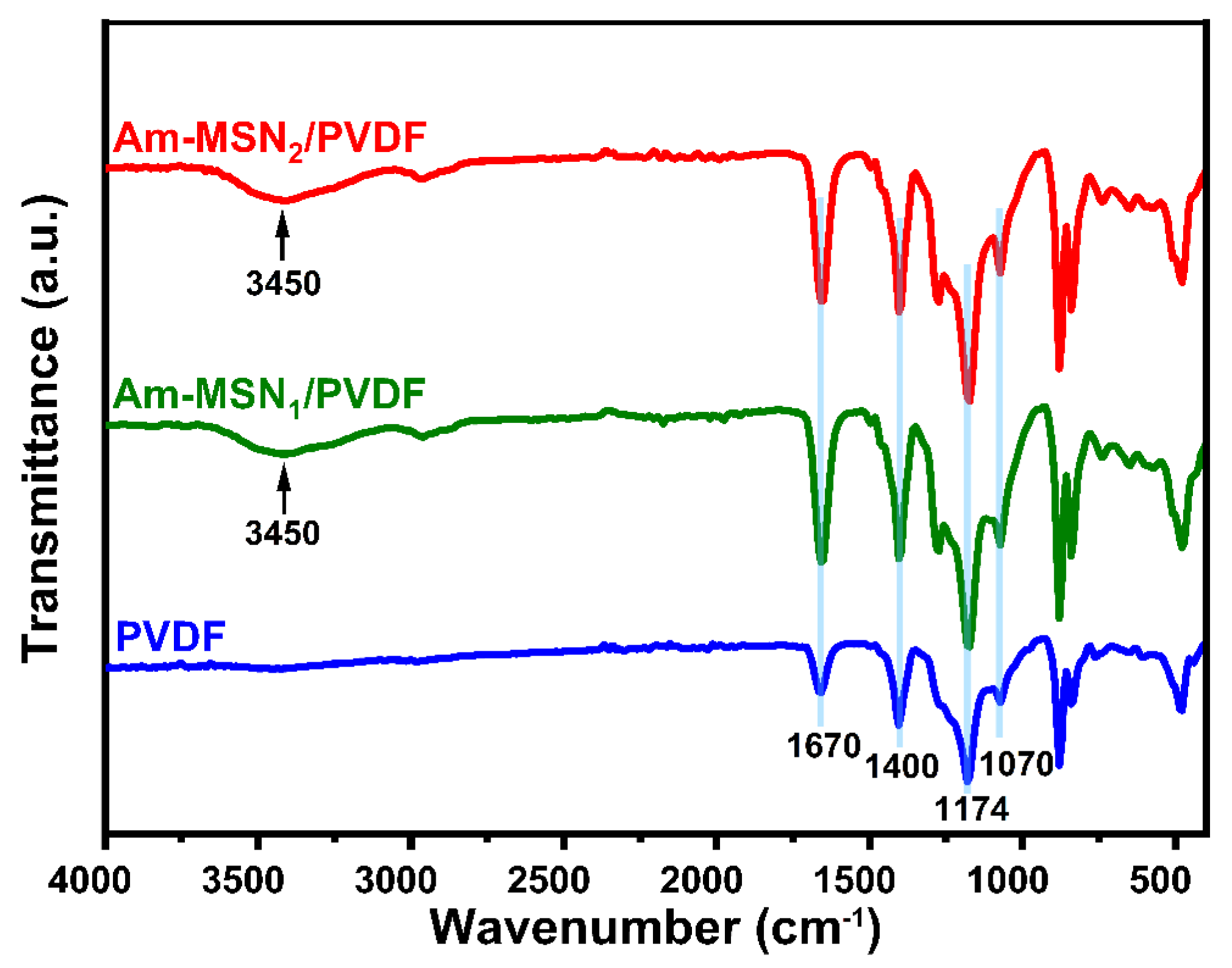
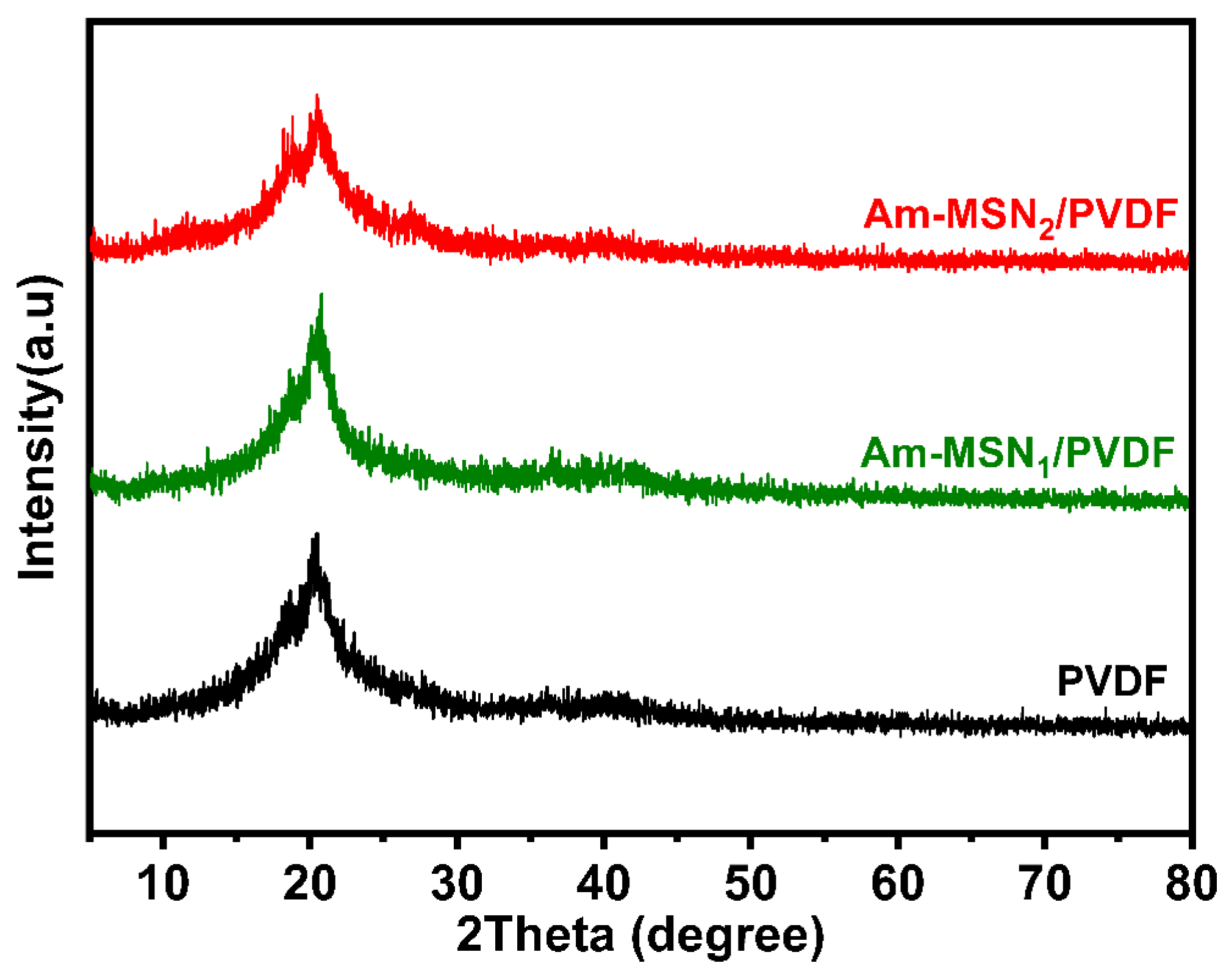

| Membrane Samples | PVDF (g) | PVP (g) | DMF (g) | Am-MSN (g) |
|---|---|---|---|---|
| PVDF | 11 | 4 | 35 | 0 |
| Am-MSN1/PVDF | 11 | 4 | 35 | 0.3 |
| Am-MSN2/PVDF | 11 | 4 | 35 | 0.6 |
| Sample | Melting Temperature (°C) | Crystallinity (%) |
|---|---|---|
| Pure PVDF | 167.8 | 45.2 |
| Am-MSN1/PVDF | 168.5 | 47.3 |
| Am-MSN2/PVDF | 169.3 | 48.1 |
| Membranes | PVDF | Am-MSN1/PVDF | Am-MSN2/PVDF |
|---|---|---|---|
| Porosity (%) | 35.8 | 69.7 | 68.7 |
| Sample | C | N | O | F | Si | Total | Si/F |
|---|---|---|---|---|---|---|---|
| Pure PVDF | 55.94 | 1.86 | 3.04 | 38.15 | 0 | 100.00 | 0 |
| Am-MSN1/PVDF | 52.59 | 3.30 | 3.75 | 39.77 | 0.60 | 100.00 | 1.51 |
| Am-MSN2/PVDF | 53.83 | 2.54 | 4.12 | 38.59 | 0.91 | 100.00 | 2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Lu, M.; Cai, J.; Lan, R.; Zeng, X.; Zhou, C. Preparation of Am-MSN/PVDF Mixed Matrix Membranes for Enhanced Removal of Reactive Black 5. Membranes 2025, 15, 42. https://doi.org/10.3390/membranes15020042
Zuo J, Lu M, Cai J, Lan R, Zeng X, Zhou C. Preparation of Am-MSN/PVDF Mixed Matrix Membranes for Enhanced Removal of Reactive Black 5. Membranes. 2025; 15(2):42. https://doi.org/10.3390/membranes15020042
Chicago/Turabian StyleZuo, Jihao, Mengkang Lu, Jinting Cai, Ruopeng Lan, Xinjuan Zeng, and Cailong Zhou. 2025. "Preparation of Am-MSN/PVDF Mixed Matrix Membranes for Enhanced Removal of Reactive Black 5" Membranes 15, no. 2: 42. https://doi.org/10.3390/membranes15020042
APA StyleZuo, J., Lu, M., Cai, J., Lan, R., Zeng, X., & Zhou, C. (2025). Preparation of Am-MSN/PVDF Mixed Matrix Membranes for Enhanced Removal of Reactive Black 5. Membranes, 15(2), 42. https://doi.org/10.3390/membranes15020042






