Integrated Purification Systems for the Removal of Disinfectants from Wastewater
Abstract
1. Introduction
2. Materials and Methods
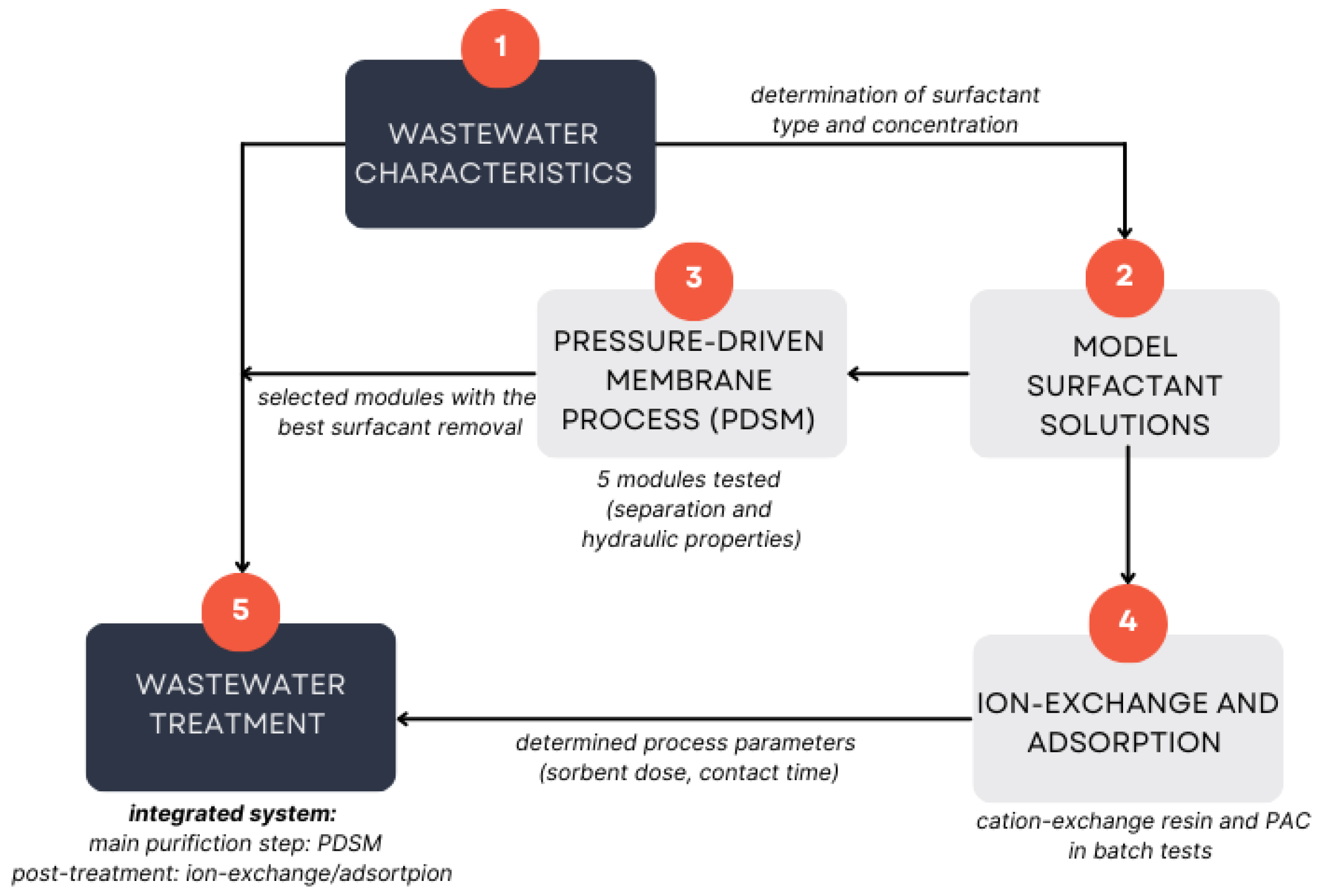
2.1. Research Plan and Scope
2.2. Wastewater
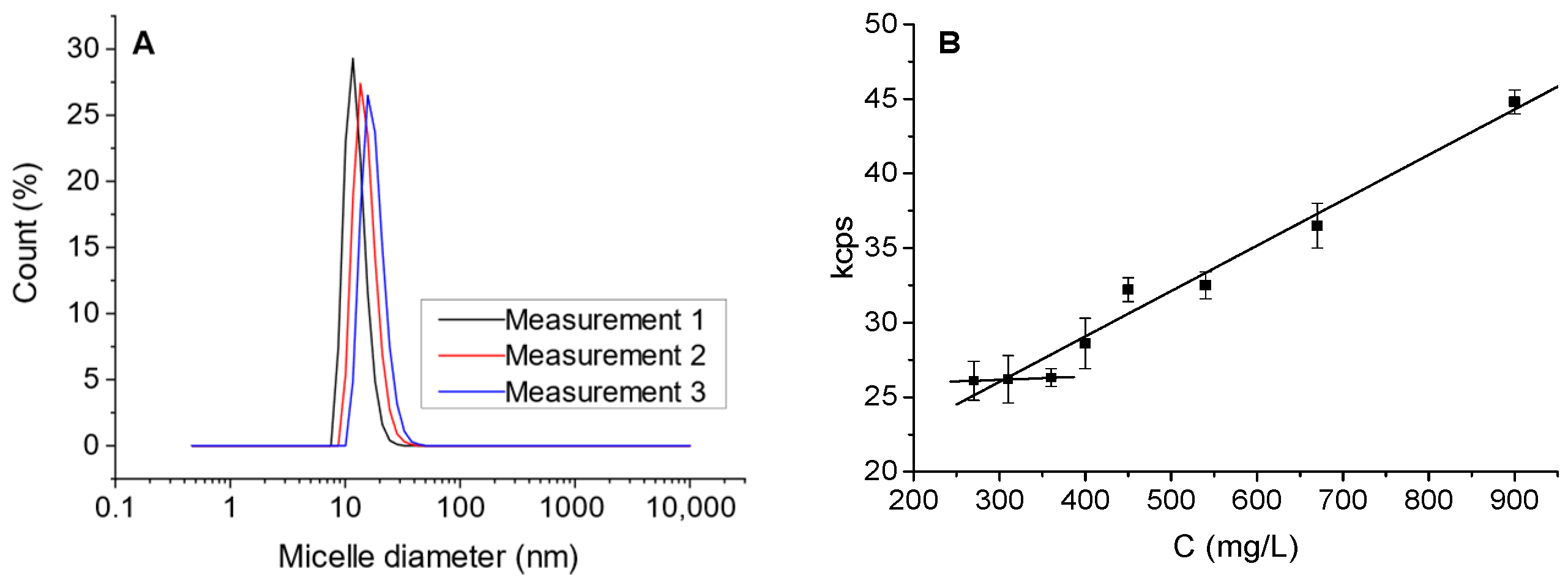
2.3. Model Solutions
2.4. Membrane Separation
2.5. Ion Exchange and Adsorption
3. Results
3.1. Model Solutions
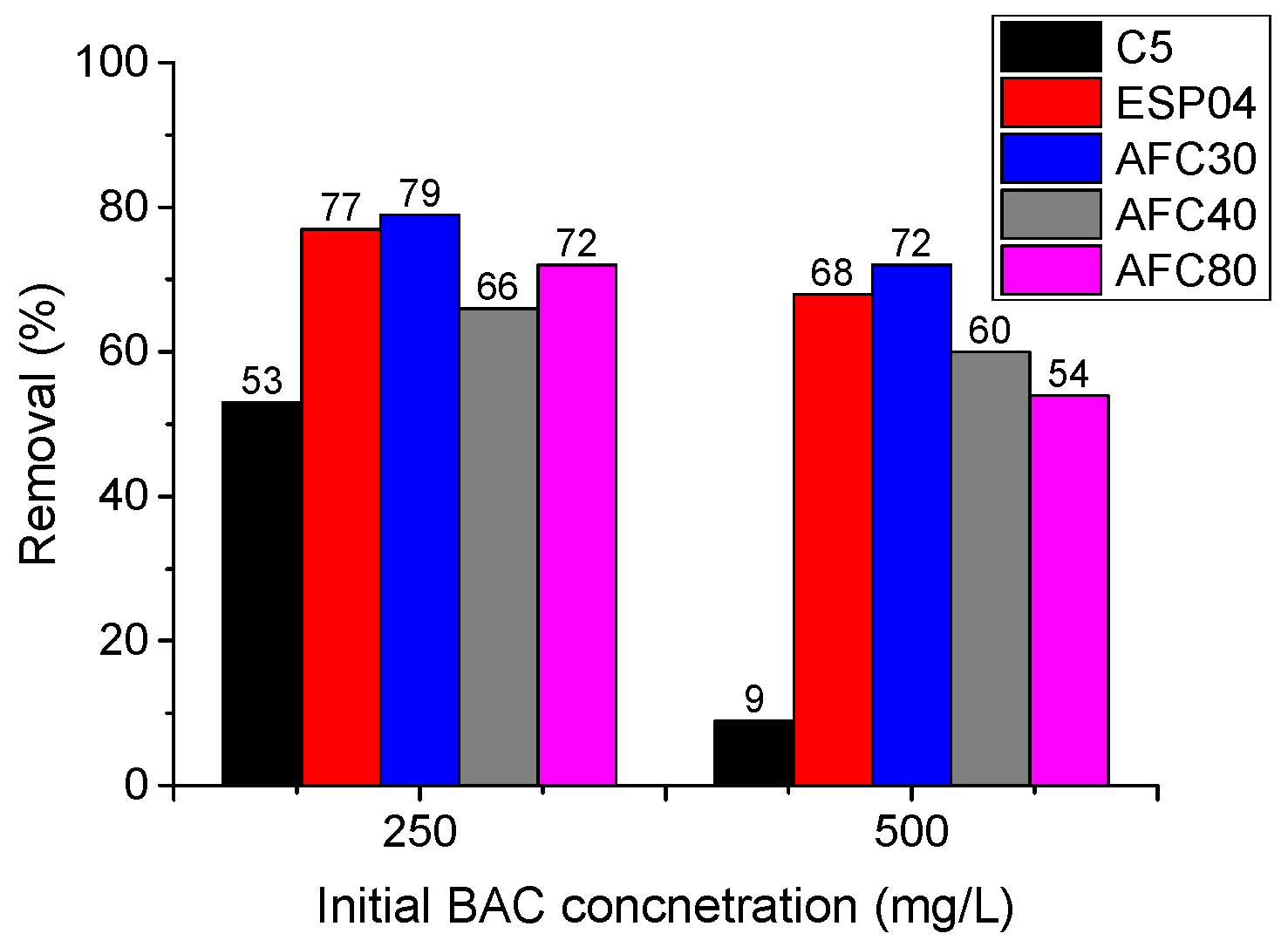
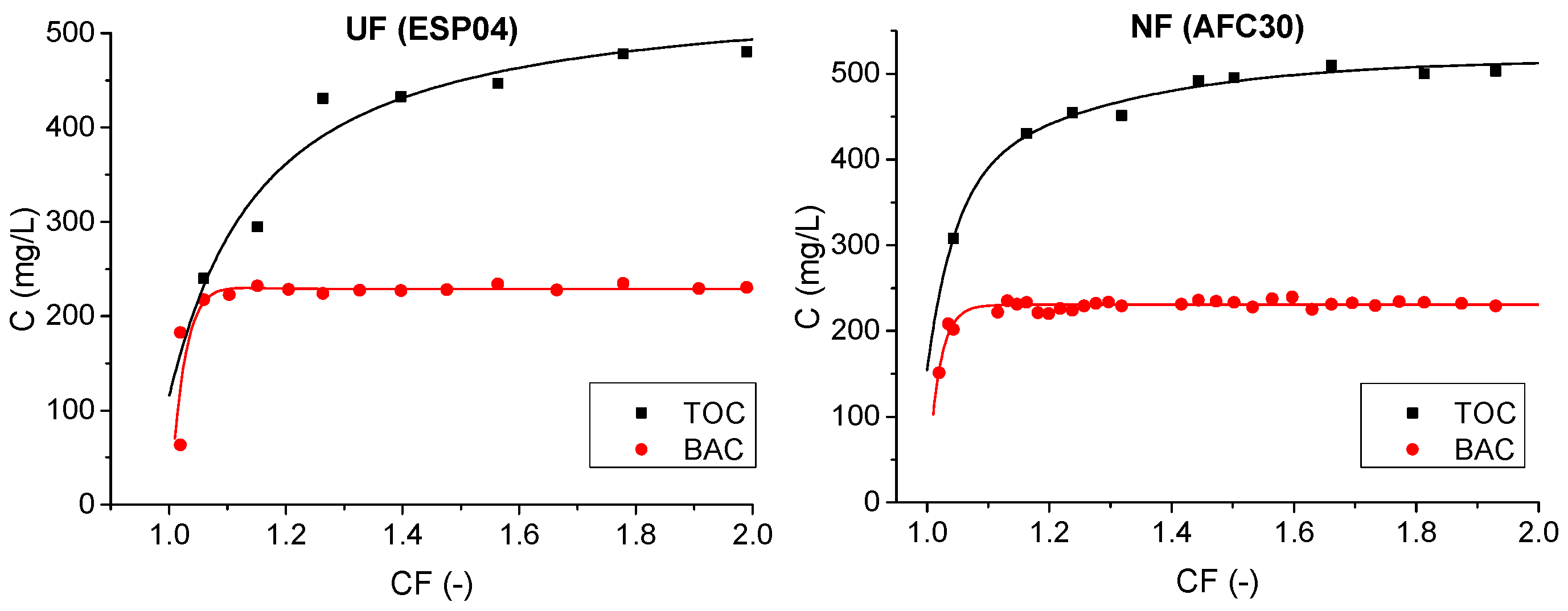
3.1.1. Pressure-Driven Membrane Processes
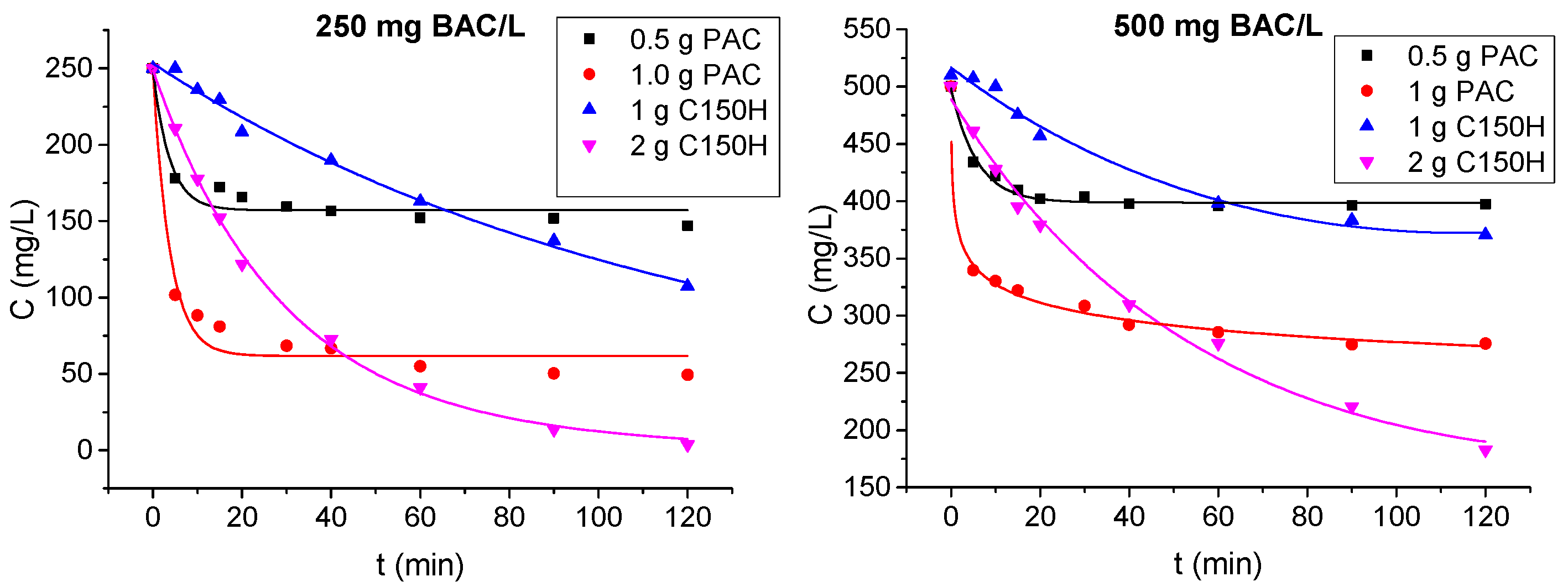
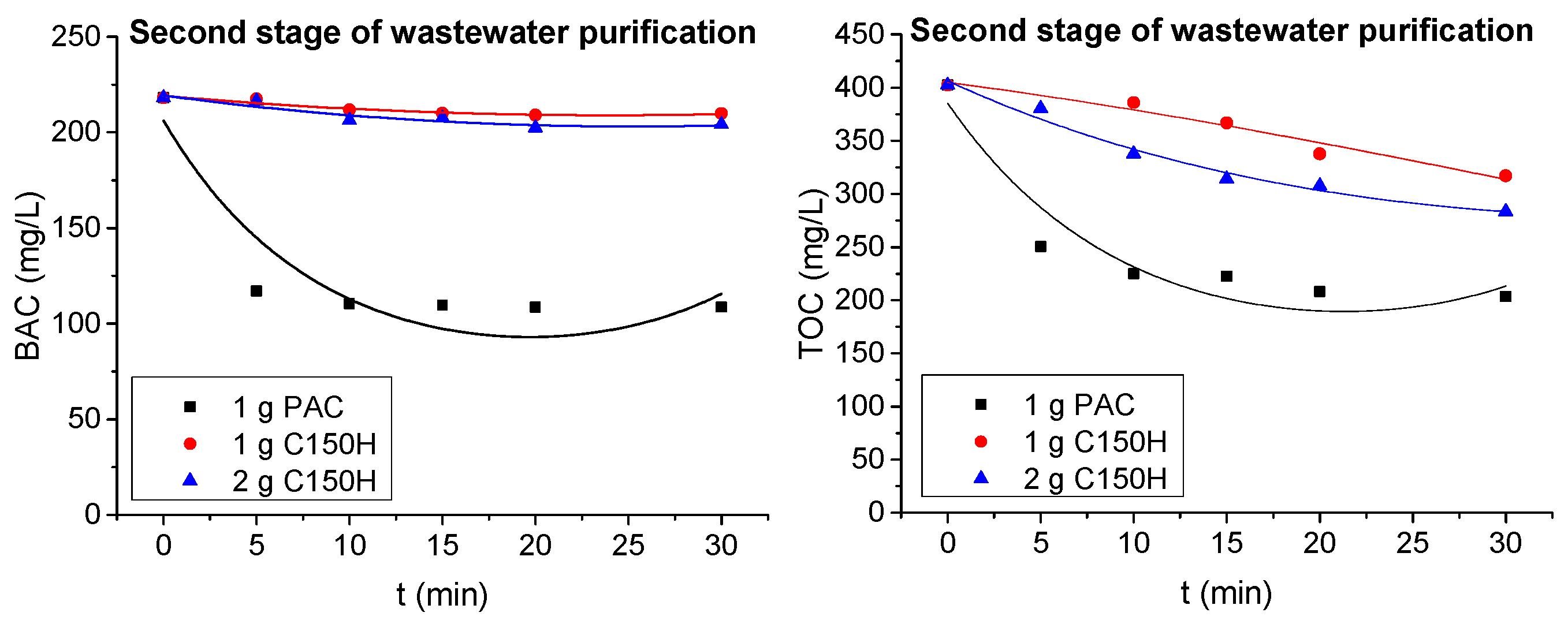
3.1.2. Ion Exchange and Adsorption Tests
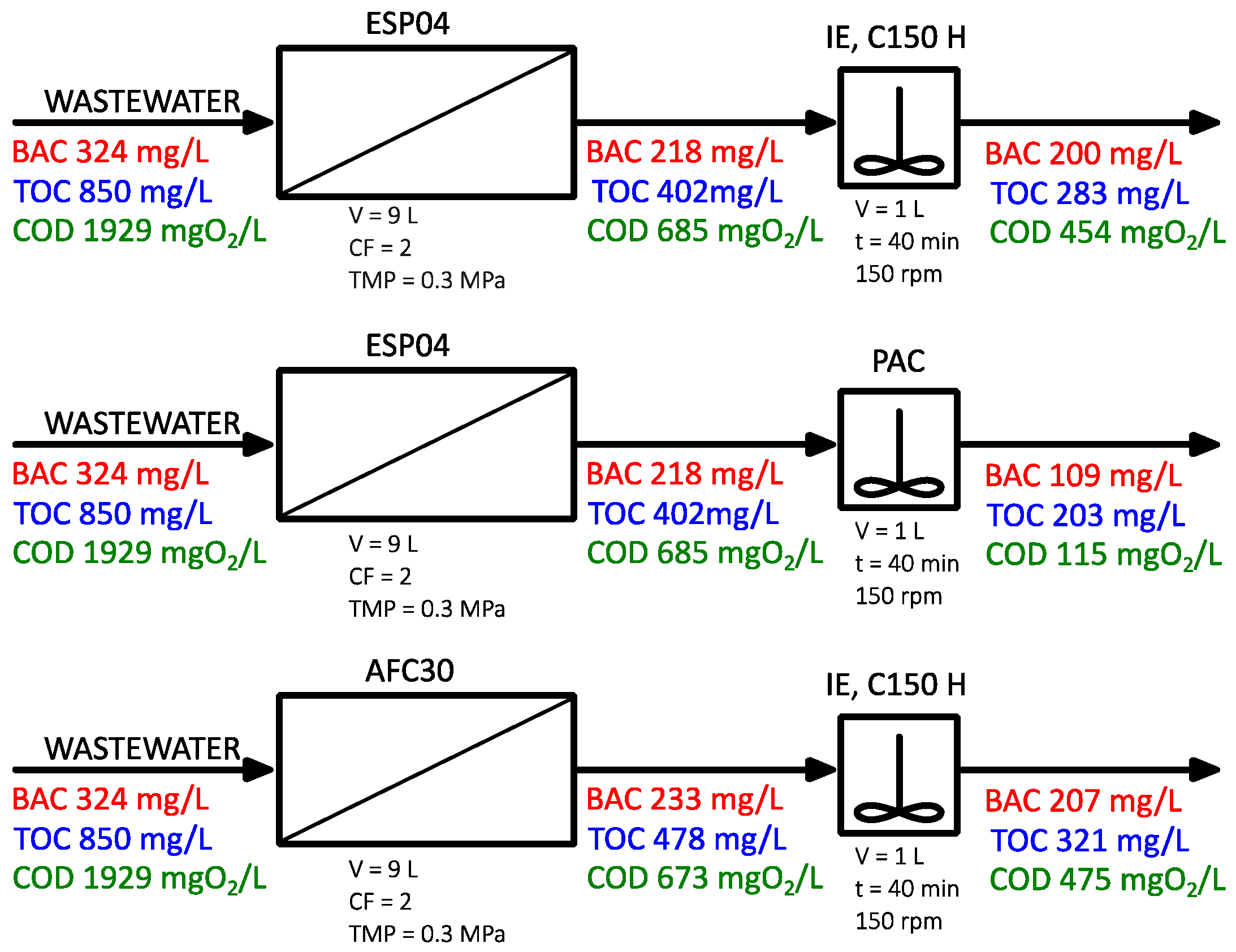
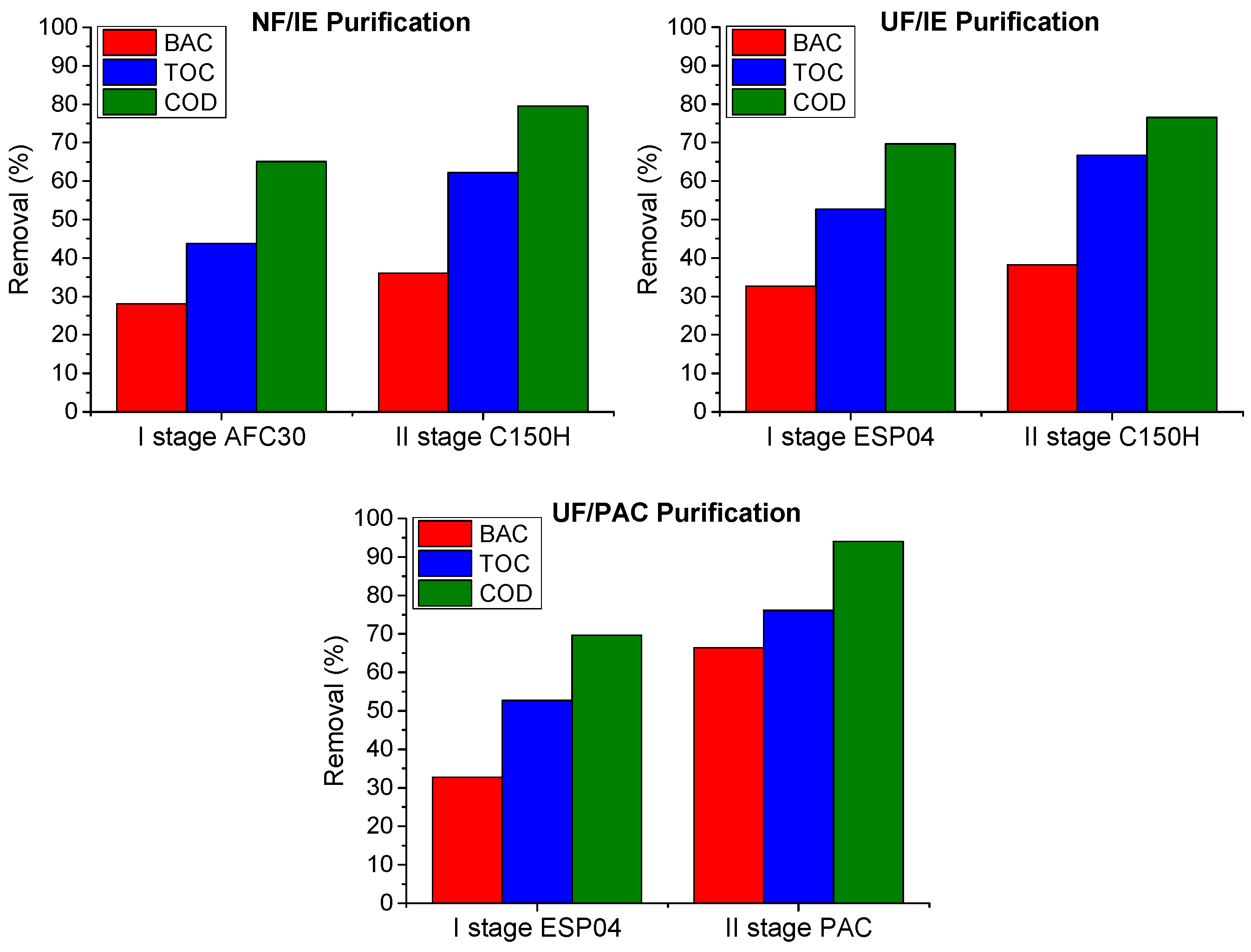
3.2. Wastewater
4. Conclusions
- The ultrafiltration (UF) and nanofiltration (NF) modules partially removed cationic surfactant benzalkonium chloride (BAC) from the model solutions. The most effective modules (ESP04 and AFC30) achieved separation efficiencies ranging from 68% to 79%.
- The preliminary adsorption/ion exchange tests on model solutions proved differences in the removal kinetics of BAC. Adsorption on PAC occurred rapidly, reaching equilibrium within minutes, making it suitable for pretreatment or situations requiring short contact times. Ion exchange was less dynamic, requiring extended contact times to reach full capacity; however, the total exchange capacity of the resin was higher than that of activated carbon.
- The efficiency of pollutant removal from wastewater by means of PDMPs was much lower compared to model solutions due to its more complexity. The ultrafiltration module (ESP04) reduced COD, TOC, and BAC by 70, 55, and 30%, respectively. The use of PAC in the post-treatment process improved removal ratios up to 94, 76, and 66%, respectively.
- The integrated purification system proved to be effective in decreasing the concentration of organic compounds in wastewater. However, it should be noted that the surfactant concentration was reduced to a much lesser extent compared to model solutions, indicating that other contaminants present in wastewater are preferentially removed.
- Simple systems based on easy-to-operate membrane processes are preferred. However, for complex wastewater, the two-stage approach remains a practical and effective solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, J.; Ranjan, A.; Chauhan, A.; Biswas, R.; Rajput, V.D.; Sushkova, S.; Mandzhieva, S.; Minkina, T.; Jindal, T. Surfactant pollution, an emerging threat to ecosystem: Approaches for effective bacterial degradation. J. Appl. Microbiol. 2022, 133, 1229–1244. [Google Scholar] [CrossRef]
- Nunes, R.F.; Teixeira, A.C.S.C. An overview on surfactants as pollutants of concern: Occurrence, impacts and persulfate-based remediation technologies. Chemosphere 2022, 300, 134507. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Shibaev, A.V.; Molchanov, V.S.; Philippova, O.E.; Khokhlov, A.R. Cationic surfactants as disinfectants against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 6645. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and bio-derived Surfactants versus microbial biosurfactants in the cosmetic industry: An overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- McDonnell, G. Sterilization and disinfection. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 529–548. [Google Scholar]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Vaishnavi, S.N.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green surfactants (biosurfactants): A petroleum-free substitute for sustainability─Comparison, applications, market, and future prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Abo-Riya, M.A.; Reda, L.M.; Tantawy, A.H.; Metwally, A.M. Novel cationic copolymeric surfactants bearing imidazole moiety as petro-dispersing/petro-collecting agents: Synthesis, surface activity and characterization. J. Mol. Liq. 2022, 364, 120057. [Google Scholar] [CrossRef]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021, 108, 142–145. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. List N: Disinfectants for Coronavirus (COVID-19). 2024. Available online: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2 (accessed on 6 December 2024).
- Business Research Insights. Cationic Surfactant Market, 2024. Business Research Insights. Available online: https://www.businessresearchinsights.com/market-reports/cationic-surfactant-market-108201 (accessed on 9 December 2024).
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Li, X.; Brownawell, B.J. Quaternary ammonium compounds in urban estuarine sediment environments—A class of contaminants in need of increased attention? Environ. Sci. Technol. 2010, 44, 7561–7568. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Zhang, C.; Valsaraj, K.T.; Constant, W.D.; Roy, D. Aerobic biodegradation kinetics of four anionic and nonionic surfactants at sub- and supra-critical micelle concentrations (CMCs). Water Res. 1999, 33, 115–124. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Basiry, D.; Entezari Heravi, N.; Uluseker, C.; Kaster, K.M.; Kommedal, R.; Pala-Ozkok, I. The effect of disinfectants and antiseptics on co- and cross-selection of resistance to antibiotics in aquatic environments and wastewater treatment plants. Front. Microbiol. 2022, 13, 1050558. [Google Scholar] [CrossRef]
- Kruszelnicka, I.; Ginter-Kramarczyk, D.; Wyrwas, B.; Idkowiak, J. Evaluation of surfactant removal efficiency in selected domestic wastewater treatment plants in Poland. J. Environ. Health Sci. Eng. 2019, 17, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Rawat, N.; Ghosh, P. Removal and recovery of a cationic surfactant from its aqueous solution by foam fractionation. J. Environ. Chem. Eng. 2020, 8, 103555. [Google Scholar] [CrossRef]
- Arslan, A.; Topkaya, E.; Bingöl, D.; Veli, S. Removal of anionic surfactant sodium dodecyl sulfate from aqueous solutions by O3/UV/H2O2 advanced oxidation process: Process optimization with response surface methodology approach. Sustain. Environ. Res. 2018, 28, 65–71. [Google Scholar] [CrossRef]
- Panizza, M.; Delucchi, M.; Cerisola, G. Electrochemical degradation of anionic surfactants. J. Appl. Electrochem. 2005, 35, 357–361. [Google Scholar] [CrossRef]
- Wu, S.H.; Pendleton, P. Adsorption of anionic surfactant by activated carbon: Effect of surface chemistry, ionic strength, and hydrophobicity. J. Colloid Interface Sci. 2001, 243, 306–315. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption Technique for the removal of organic pollutants from water and wastewater. In Organic Pollutants—Monitoring, Risk and Treatment; Intech: Rijeka, Croatia, 2013. [Google Scholar]
- Carter, K.E.; Farrell, J. Removal of perfluorooctane and perfluorobutane sulfonate from water via carbon adsorption and ion exchange. Sep. Sci. Technol. 2010, 45, 762–767. [Google Scholar] [CrossRef]
- Schuricht, F.; Borovinskaya, E.S.; Reschetilowski, W. Removal of perfluorinated surfactants from wastewater by adsorption and ion exchange—Influence of material properties, sorption mechanism and modelling. J. Environ. Sci. 2017, 54, 160–170. [Google Scholar] [CrossRef]
- Schouten, N.; van der Ham, L.G.J.; Euverink, G.-J.W.; de Haan, A.B. Selection and evaluation of adsorbents for the removal of anionic surfactants from laundry rinsing water. Water Res. 2007, 41, 4233–4241. [Google Scholar] [CrossRef]
- Korzenowski, C.; Martins, M.B.O.; Bernardes, A.M.; Ferreira, J.Z.; Duarte, E.C.N.F.; De Pinho, M.N. Removal of anionic surfactants by nanofiltration. Desalination Water Treat. 2012, 44, 269–275. [Google Scholar] [CrossRef]
- Kertész, S.; László, Z.; Horváth, Z.H.; Hodúr, C. Analysis of nanofiltration parameters of the removal of an anionic detergent. Desalination 2008, 221, 303–311. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Iqbal, M.M.; Shoaib, M.; Park, D.R.; Lee, K.H.; Shin, S.; Khan, S.; Yeom, I.T. Removal of ZnO nanoparticles from natural waters by coagulation-flocculation process: Influence of surfactant type on aggregation, dissolution, and colloidal stability. Sustainability 2019, 11, 17. [Google Scholar] [CrossRef]
- Dereszewska, A.; Cytawa, S.; Tomczak-Wandzel, R.; Medrzycka, K. The Effect of anionic surfactant concentration on activated Sludge condition and phosphate release in biological treatment plant. Pol. J. Environ. Stud. 2015, 24, 83–91. [Google Scholar] [CrossRef]
- Reynolds, L.; Blok, J.; de Morsier, A.; Gerike, P.; Wellens, H.; Bontinck, W.J. Evaluation of the toxicity of substances to be assessed for biodegradability. Chemosphere 1987, 16, 2259–2277. [Google Scholar] [CrossRef]
- Conidi, D.; Andalib, M.; Andres, C.; Bye, C.; Umble, A.; Dold, P. Modeling quaternary ammonium compound inhibition of biological nutrient removal activated sludge. Water Sci. Technol. 2019, 79, 41–50. [Google Scholar] [CrossRef]
- Nguyen, L.A.T.; Schwarze, M.; Schomäcker, R. Adsorption of non-ionic surfactant from aqueous solution onto various ultrafiltration membranes. J. Membr. Sci. 2015, 493, 120–133. [Google Scholar] [CrossRef]
- Nguyen, L.A.T. Adsorption of Non-Ionic Surfactants onto Ultrafiltration Membranes in Aqueous and Organic Solutions. Doctoral Dissertation, Technische Universität Berlin, Berlin, Germany, 2015. [Google Scholar]
- Yakout, S.M.; Nayl, A.A. Removal of cationic surfactant (CTAB) from aqueous solution onto activated carbon obtained from corncob. Carbon Sci. Technol. 2009, 2, 107–116. [Google Scholar]
- Kaya, Y.; Gönder, Z.B.; Vergili, I.; Barlas, H. Removal of cetyltrimethylammonium bromide and sodium dodecylether sulfate by granular activated carbon. J. Sci. Ind. Res. 2008, 67, 249–253. [Google Scholar]
- Gustafson, R.L.; Lirio, J.A. Adsorption of organic ions by anion exchange resins. Environ. Ind. Eng. Chem. Prod. Res. Dev. 1968, 7, 116–120. [Google Scholar] [CrossRef]
- Yang, W.-B.; Xia, M.; Li, A.; Yang, L.; Zhang, Q. Mechanism and behaviour of surfactant adsorption onto resins with different matrices. React. Funct. Polym. 2007, 67, 609–616. [Google Scholar] [CrossRef]
- Kudlek, E.; Bohdziewicz, J.; Dudziak, M. Influence of water matrix on the retention of pharmaceuticals by high-pressure membrane filtration. Ecol. Chem. Eng. A 2015, 22, 469–479. [Google Scholar] [CrossRef]
- Otero-Fernández, A.; Díaz, P.; Otero, J.A.; Ibáñez, R.; Maroto-Valiente, A.; Palacio, L.; Prádanos, P.; Carmona, F.J.; Hernández, A. Morphological, chemical and electrical characterization of a family of commercial nanofiltration polyvinyl alcohol coated polypiperazineamide membranes. Eur. Polym. J. 2020, 126, 109544. [Google Scholar] [CrossRef]
- Klimonda, A.; Kowalska, I. Removal of quaternary ammonium compounds in ion exchange process. E3S Web Conf. 2019, 116, 34. [Google Scholar] [CrossRef]
- Kaleta, J. Evaluation of efectibeness of adsorption process in removal of surfactants from water. Environ. Prot. Eng. 2010, 36, 103–112. [Google Scholar]


| Symbol | Type | Material | Salt Removal, % | Molecular Weight Cut-off, kDa | Pore Size, nm | Deionized Water Flux, L/m2h * | Hydrophilicity ** |
|---|---|---|---|---|---|---|---|
| AFC80 | RO/NF | PA | 80 NaCl | <0.2 [40] | 0.38 ± 0.24 [41] | 5 | hydrophilic |
| AFC40 | NF | PA | 40 CaCl2 | 0.2 [40] | 0.44 ± 0.07 [41] | 15 | hydrophilic |
| AFC30 | NF | PA | 53 CaCl2 | 0.2 [40] | 0.51 ± 0.10 [41] | 16 | hydrophilic |
| ESP04 | UF | modified PES | - | 4 | - | 48 | moderately hydrophilic |
| C5 | UF | TiO2 | - | 5 | - | 46 | highly hydrophilic |
| Parameter | Average Value |
|---|---|
| pH | 10.24 ± 0.95 |
| Conductivity, µS/cm | 119 ± 18 |
| Turbidity, NTU | 0.8 ± 0.2 |
| BAC, mg/L | 324 ± 23 |
| COD, mg O2/L | 1929 ± 135 |
| TOC, mg C/L | 850 ± 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimonda, A.; Kowalska, I. Integrated Purification Systems for the Removal of Disinfectants from Wastewater. Membranes 2025, 15, 43. https://doi.org/10.3390/membranes15020043
Klimonda A, Kowalska I. Integrated Purification Systems for the Removal of Disinfectants from Wastewater. Membranes. 2025; 15(2):43. https://doi.org/10.3390/membranes15020043
Chicago/Turabian StyleKlimonda, Aleksandra, and Izabela Kowalska. 2025. "Integrated Purification Systems for the Removal of Disinfectants from Wastewater" Membranes 15, no. 2: 43. https://doi.org/10.3390/membranes15020043
APA StyleKlimonda, A., & Kowalska, I. (2025). Integrated Purification Systems for the Removal of Disinfectants from Wastewater. Membranes, 15(2), 43. https://doi.org/10.3390/membranes15020043







