Polysulfone/MMT Clay Mixed Matrix Membranes for Efficient Diclofenac Removal and Improved Antifouling Performance in Wastewater Treatment
Abstract
1. Introduction
2. Methodology
2.1. Chemicals and Reagents
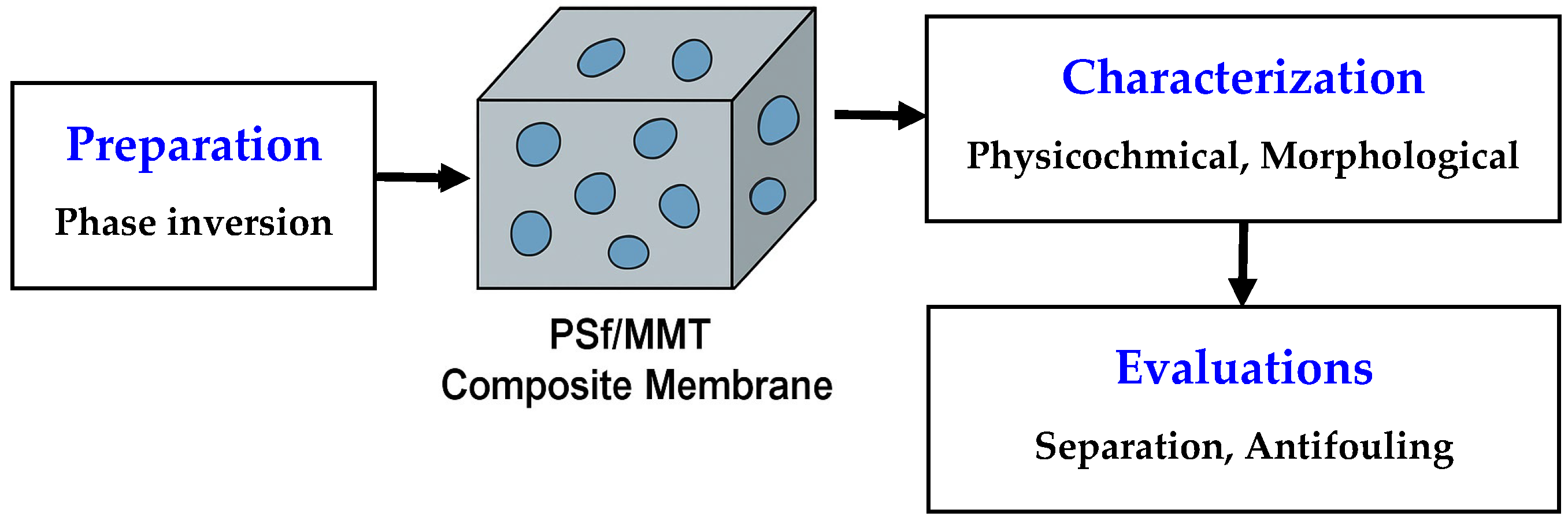
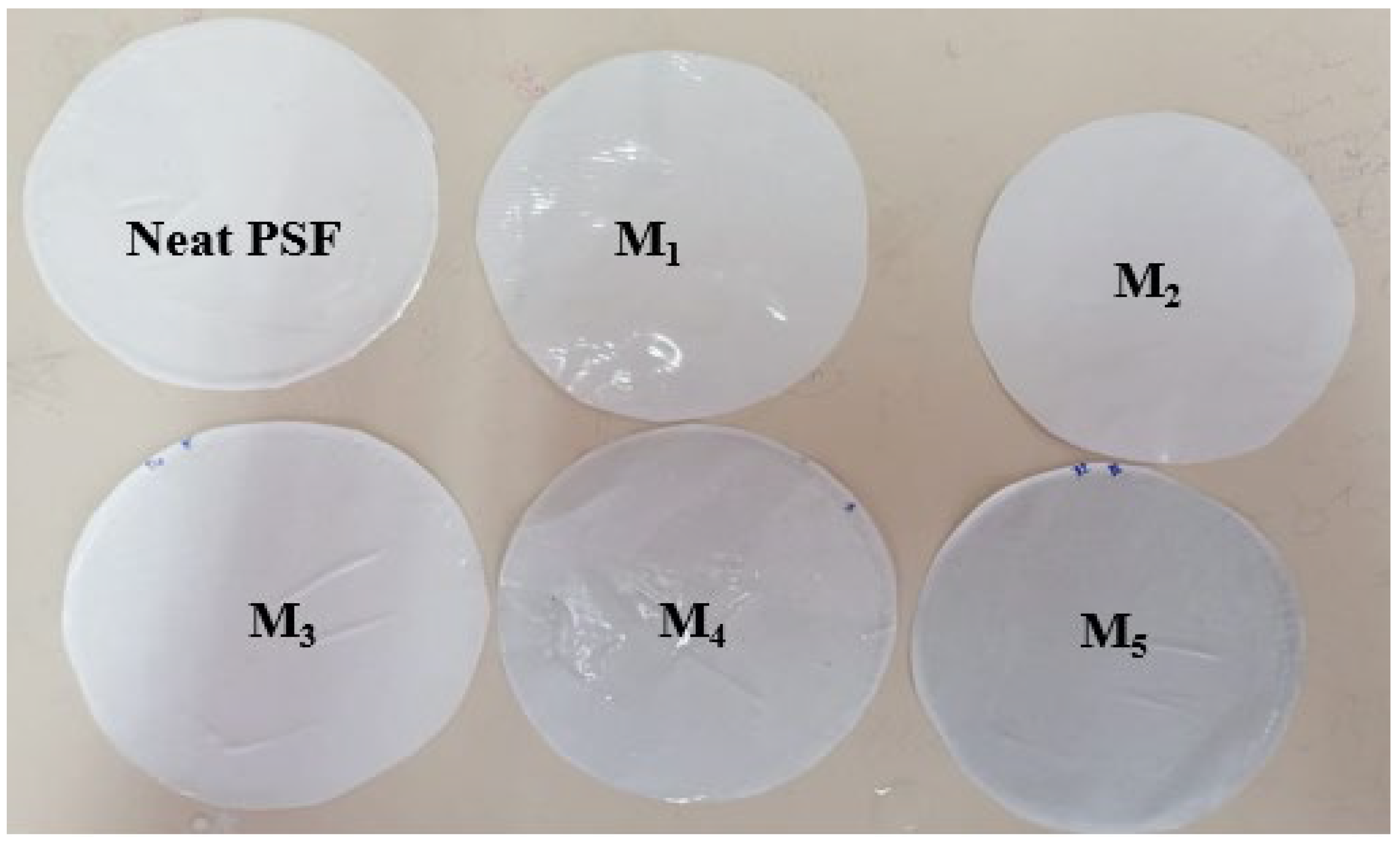
2.2. Membrane Preparation
2.3. Characterization Techniques
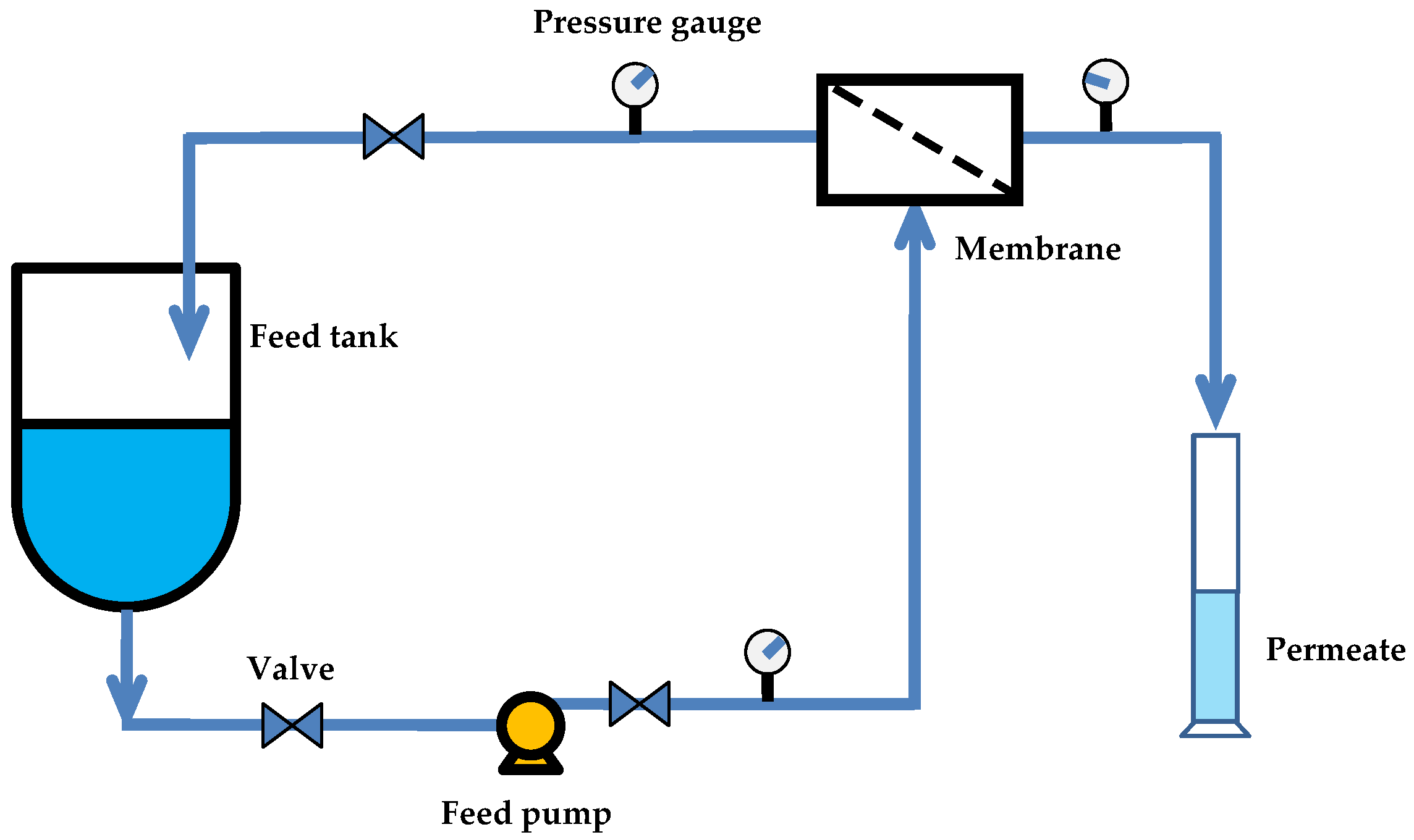
2.4. Filtration Tests
2.5. Fouling Studies Test
- Pure water filtration at 20 bar to obtain the initial pure water flux (Jw);
- BSA solution filtration;
- Cleaning the membrane with distilled water to remove reversible foulants, followed by measuring the recovered pure water flux (Jwa).
3. Results and Discussion
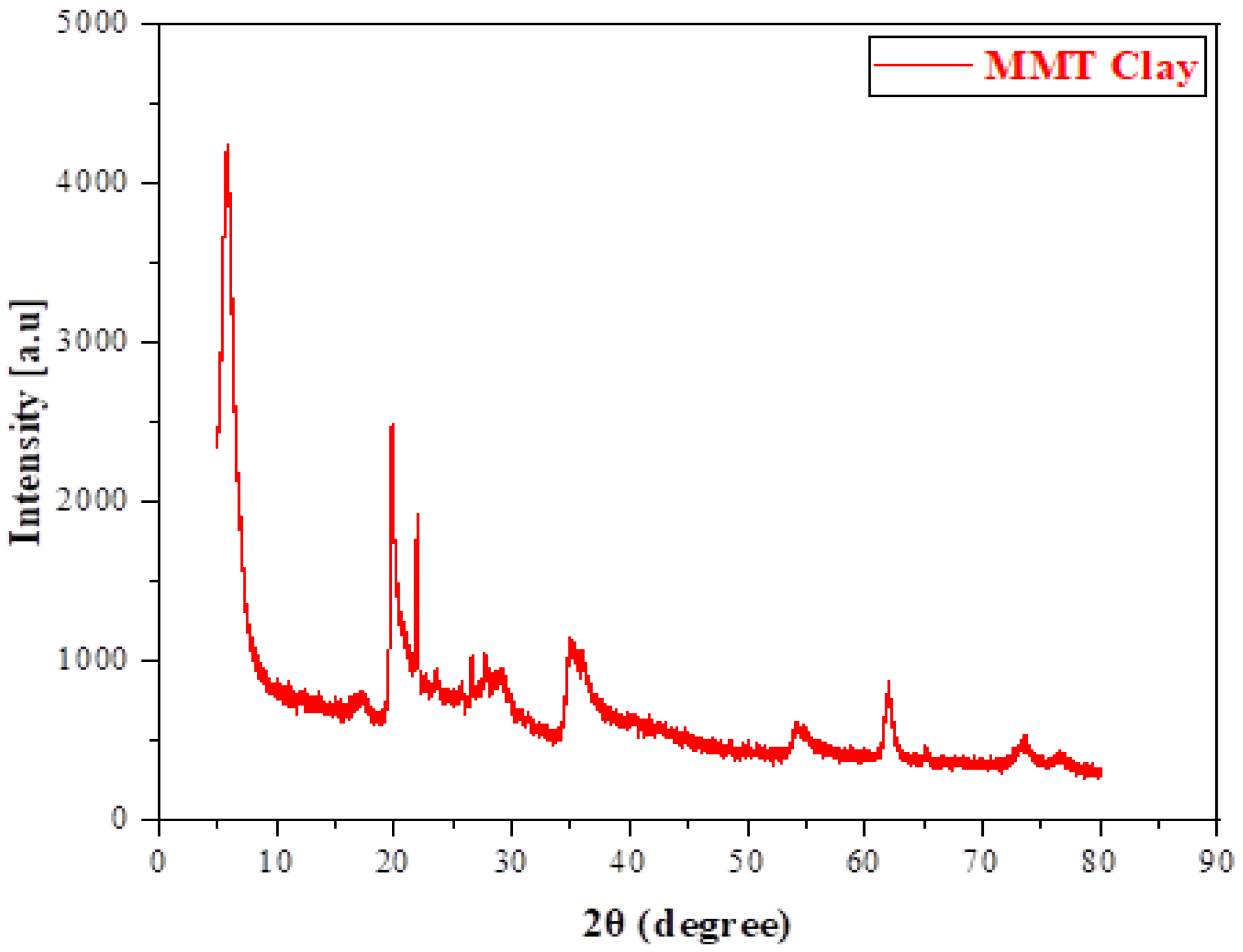
3.1. Characterization of Montmorillonite (MMT)
3.2. Membrane Characterization
3.2.1. XRD Analysis
3.2.2. FT-IR Studies
3.2.3. Morphological Studies
3.2.4. Chemical Resistance
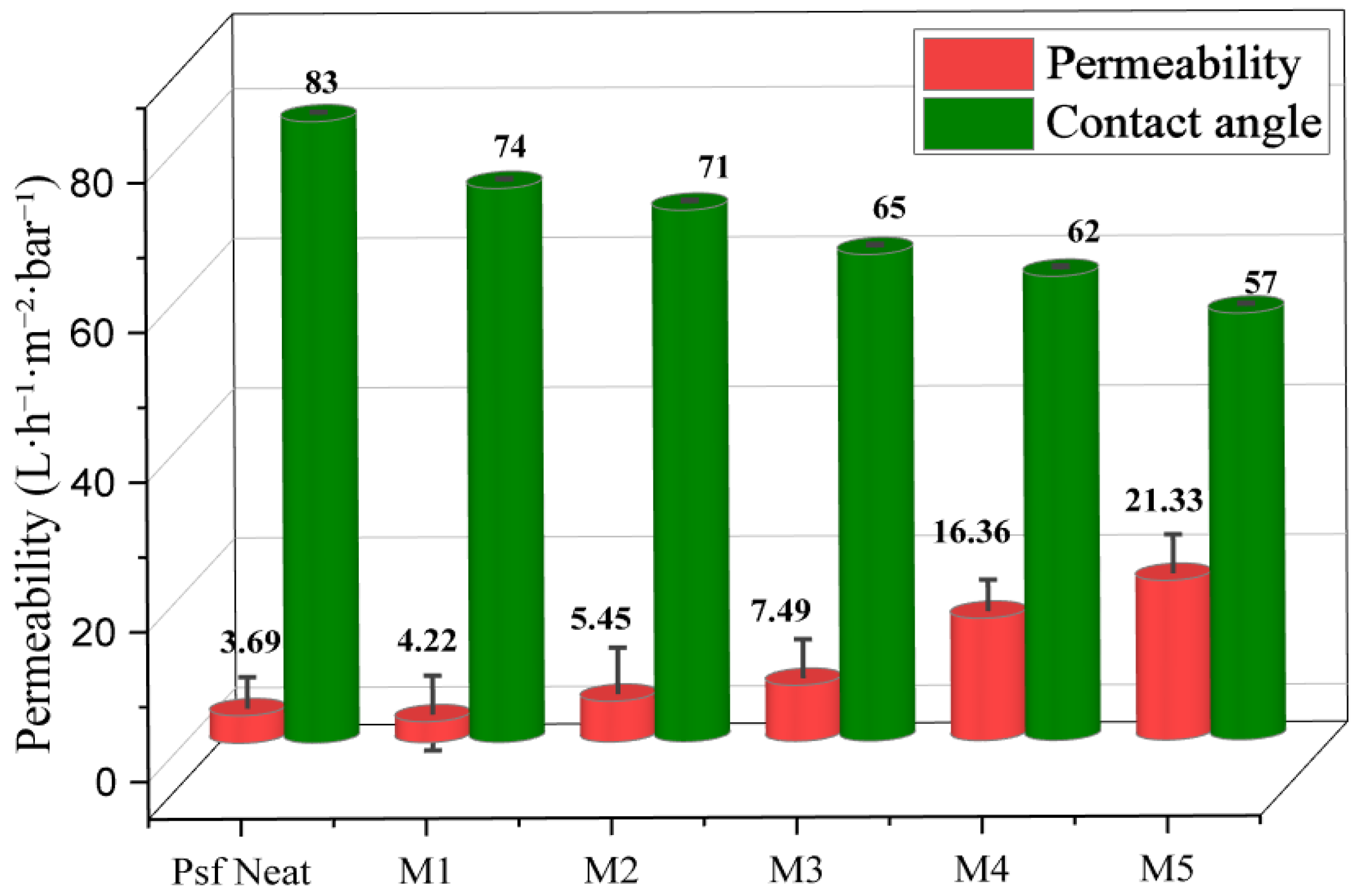
3.2.5. Hydrophilicity and Water Permeability
3.3. Application on DCF Removal, BSA Rejection, and Antifouling Properties
3.4. Diclofenac (DCF)Removal Mechanism by PSF/MMT Composite Membranes
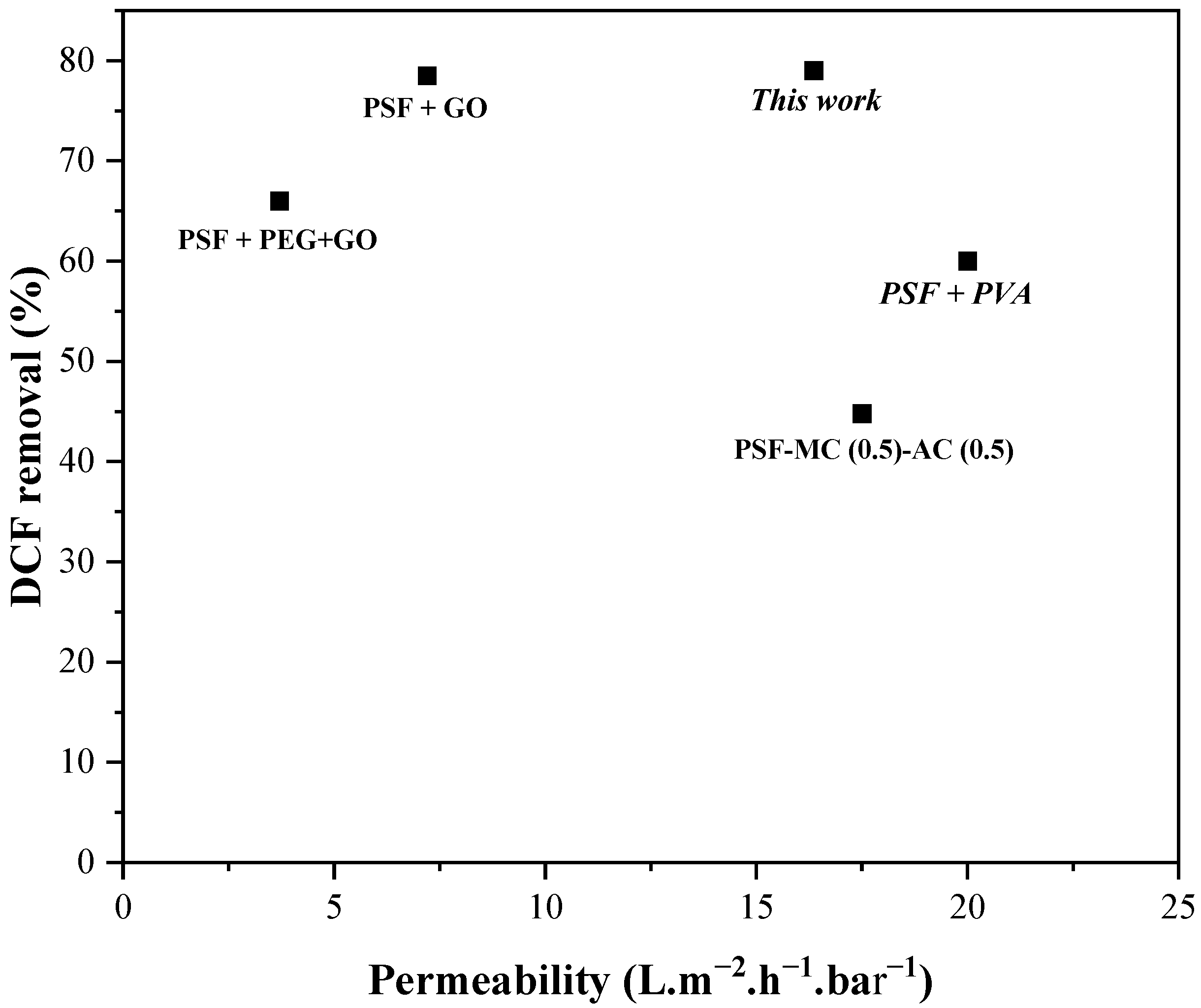
4. Comparative Analysis of Literature Data for DCF Removal Using PSF-Based Membranes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Afshar, N.R.; Fahmi, H. Impact of Climate Change on Water Resources in Iran. Int. J. Energy Water Resour. 2019, 3, 55–60. [Google Scholar] [CrossRef]
- Abbott, B.W.; Bishop, K.; Zarnetske, J.P.; Hannah, D.M.; Frei, R.J.; Minaudo, C.; Chapin, F.S.; Krause, S.; Conner, L.; Ellison, D.; et al. A Water Cycle for the Anthropocene. Hydrol. Process. 2019, 33, 3046–3052. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, Characterization, Activation and Environmental Applications of Engineered Biochar: A Review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fern, A.R.; Barcel, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.-V.N.; Nanda, S.; Nguyen, T.D. Optimization, Equilibrium, Adsorption Behavior and Role of Surface Functional Groups on Graphene Oxide-Based Nanocomposite towards Diclofenac Drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; de Pelegrini Soares, R.; Féris, L.A. Diclofenac Removal from Water by Adsorption Using Activated Carbon in Batch Mode and Fixed-Bed Column: Isotherms, Thermodynamic Study and Breakthrough Curves Modeling. J. Clean. Prod. 2018, 181, 145–154. [Google Scholar] [CrossRef]
- Badawy, A.S.; Xu, Y.; Ren, H.; Lu, Z. Diclofenac (DCF) as an Emerging Pollutant: Occurrence, Ecotoxicity, and Biodegradation Strategies. Ecotoxicol. Environ. Saf. 2025, 302, 118618. [Google Scholar] [CrossRef]
- Álvarez, S.; Ribeiro, R.S.; Gomes, H.T.; Sotelo, J.L.; García, J. Chemical Engineering Research and Design Synthesis of Carbon Xerogels and Their Application in Adsorption Studies of Caffeine and Diclofenac as Emerging Contaminants. Chem. Eng. Res. Des. 2014, 95, 229–238. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of Ibuprofen, Diclofenac, and Naproxen from Water Using Chitosan-Modified Waste Tire Crumb Rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P. Science of the Total Environment Determination of Pharmaceutical Residues in Drinking Water in Poland Using a New SPE-GC-MS (SIM) Method Based on Speedisk Extraction Disks and DIMETRIS Derivatization. Sci. Total Environ. 2015, 538, 402–411. [Google Scholar] [CrossRef]
- Salah, Z.; Aloulou, H.; Bhattacharyya, S.; Algieri, C.; Amar, R. Ben Potential Elimination of Diclofenac Sodium (DCF) from Aqueous Solution by Adsorption Using Orange Peel Waste-Based Activated Carbon. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2127–2143. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Pocostales, P.; Alvarez, P.; Oropesa, A. Diclofenac Removal from Water with Ozone and Activated Carbon. J. Hazard. Mater. 2009, 163, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Bethi, B.; Radhika, G.B.; Sonawane, S.H. Fundamentals of advanced oxidation processes (AOPs) for wastewater treatment: Challenges and opportunities. In Novel Approaches Towards Wastewater Treatment and Resource Recovery Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 209–220. [Google Scholar]
- Luque, S.; Gómez, D.; Álvarez, J.R. Industrial Applications of Porous Ceramic Membranes (Pressure-driven Processes). Membr. Sci. Technol. 2008, 13, 177–216. [Google Scholar]
- Sloboda Rigobello, E.; DI Bernardo Dantas, A.; DI Bernardo, L.; Vieira, E.M. Removal of Diclofenac by Conventional Drinking Water Treatment Processes and Granular Activated Carbon Filtration. Chemosphere 2013, 92, 184–191. [Google Scholar] [CrossRef]
- Vergili, I. Application of Nanofiltration for the Removal of Carbamazepine, Diclofenac and Ibuprofen from Drinking Water Sources. J. Environ. Manag. 2013, 127, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Nadour, M.; Boukraa, F.; Benaboura, A. Removal of Diclofenac, Paracetamol and Metronidazole Using a Carbon- Polymeric Membrane. J. Environ. Chem. Eng. 2019, 7, 103080. [Google Scholar] [CrossRef]
- Martins, W.; Val, P.; Vieira, S.; Fernandes, M. Diclofenac Removal from Water by Adsorption on Moringa Oleifera Pods and Activated Carbon: Mechanism, Kinetic and Equilibrium Study On E. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene Oxide Incorporated Thin Film Nanocomposite Nanofiltration Membrane for Enhanced Salt Removal Performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Salah, Z.; Bhattacharyya, S.; Cozzolino, V.; Algieri, C.; Calabrò, V.; Amar, R.B.; Chakraborty, S. Graphene Oxide-Polysulfone Nanocomposite Membranes for Diclofenac Removal. Emergent Mater. 2024, 8, 1671–1685. [Google Scholar] [CrossRef]
- Chai, P.V.; Law, J.Y.; Mahmoudi, E.; Mohammad, A.W. Development of Iron Oxide Decorated Graphene Oxide (Fe3O4/GO) PSf Mixed-Matrix Membrane for Enhanced Antifouling Behavior. J. Water Process Eng. 2020, 38, 101673. [Google Scholar] [CrossRef]
- Salah, Z.; Aloulou, H.; Aloulou, W.; Algieri, C. Development of Graphene Oxide Membrane on Flat Mud Ceramic Support for Methylene Blue Dyes Removal. ChemistrySelect 2025, 10, e202406142. [Google Scholar] [CrossRef]
- Jacob, L.; Joseph, S.; Varghese, L.A. Polysulfone/MMT Mixed Matrix Membranes for Hexavalent Chromium Removal from Wastewater. Arab. J. Sci. Eng. 2020, 45, 7611–7620. [Google Scholar] [CrossRef]
- Bitinis, N.; Hernández, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent Advances in Clay/Polymer Nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Ennaceri, H.; Mkpuma, V.O.; Moheimani, N.R. Nano-Clay Modified Membranes: A Promising Green Strategy for Microalgal Antifouling Filtration. Sci. Total Environ. 2023, 902, 166479. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.; Ha, S.; Shin, W.H.; Choi, J.; Mun, S.C.; Choi, Y.; Won, J.H. Clay-Mineral-Coated Separators for Lithium-Ion Batteries: Exploring the Relationships between Clay Mineral Morphology and Separator Performance. Adv. Sci. 2025, 12, e10779. [Google Scholar] [CrossRef]
- Hikosaka, M.Y.; Pulcinelli, S.H.; Santilli, C.V.; Dahmouche, K.; Craievich, A.F. Montmorillonite (MMT) Effect on the Structure of Poly (Oxyethylene)(PEO)–MMT Nanocomposites and Silica–PEO–MMT Hybrid Materials. J. Non-Cryst. Solids 2006, 352, 3705–3710. [Google Scholar] [CrossRef]
- Bin Ahmad, M.; Gharayebi, Y.; Salit, M.S.; Hussein, M.Z.; Shameli, K. Comparison of in Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposites. Int. J. Mol. Sci. 2011, 12, 6040–6050. [Google Scholar] [CrossRef]
- Olad, A.; Rashidzadeh, A. Preparation and Anticorrosive Properties of PANI/Na-MMT and PANI/O-MMT Nanocomposites. Prog. Org. Coat. 2008, 62, 293–298. [Google Scholar] [CrossRef]
- Anadão, P.; Sato, L.F.; Wiebeck, H.; Valenzuela-Díaz, F.R. Montmorillonite as a Component of Polysulfone Nanocomposite Membranes. Appl. Clay Sci. 2010, 48, 127–132. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Solati-Hashjin, M.; Moaddel, H. Novel Nanocomposite Proton Exchange Membranes Based on Nafion® and AMPS-Modified Montmorillonite for Fuel Cell Applications. J. Memb. Sci. 2010, 365, 286–293. [Google Scholar] [CrossRef]
- Chailuecha, C. Methanol Barrier Layers: Modified Membrane Electrode Assemblies for the Improvement of Direct Methanol Fuel Cell Performance; The University of Manchester: Manchester, UK, 2016; ISBN 1083569759. [Google Scholar]
- Ali, F.; Ullah, H.; Ali, Z.; Rahim, F.; Khan, F.; Zia, U.R. Polymer-Clay Nanocomposites, Preparations and Current Applications: A Review. Curr. Nanomater. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Aseri, N.S.; Lau, W.J.; Goh, P.S.; Hasbullah, H.; Othman, N.H.; Ismail, A.F. Preparation and Characterization of Polylactic Acid-Modified Polyvinylidene Fluoride Hollow Fiber Membranes with Enhanced Water Flux and Antifouling Resistance. J. Water Process Eng. 2019, 32, 100912. [Google Scholar] [CrossRef]
- Cervellere, M.R.; Qian, X.; Ford, D.M.; Carbrello, C.; Giglia, S.; Millett, P.C. Phase-Field Modeling of Non-Solvent Induced Phase Separation (NIPS) for PES/NMP/Water with Comparison to Experiments. J. Memb. Sci. 2021, 619, 118779. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a Novel Antifouling Mixed Matrix PES Membrane by Embedding Graphene Oxide Nanoplates. J. Memb. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Characterization of Surface-Modified Porous PVDF Hollow Fibers for Refinery Wastewater Treatment Using Microscopic Observation. Desalination 2011, 283, 206–213. [Google Scholar] [CrossRef]
- Moradi, G.; Rahimi, M.; Zinadini, S. Antifouling Nanofiltration Membrane via Tetrathioterephthalate Coating on Aniline Oligomers-Grafted Polyethersulfone for Efficient Dye and Heavy Metal Ion Removal. J. Environ. Chem. Eng. 2021, 9, 104717. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Fang, P.; Wang, D.; Dai, Y.; Wang, S.; Liew, K.M.; Xu, Z. Crystallization Behavior of Polypropylene/Polyamide 6/Montmorillonite Nanocomposites. Polym. Int. 2010, 59, 1303–1309. [Google Scholar] [CrossRef]
- Eversull, L.G.; Ferrell, R.E. Disordered Silica with Tridymite-like Structure in the Twiggs Clay. Am. Mineral. 2008, 93, 565–572. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, H.; Xiao, T.; Wu, Y. Application of Micro-FTIR Spectroscopy to Study Molecular Association of Adsorbed Water with Lignin. Int. J. Biol. Macromol. 2019, 131, 1038–1043. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-Assembly Processes in Hydrated Montmorillonite by FTIR Investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef]
- Madejová, J. FTIR Techniques in Clay Mineral Studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Madejova, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Anadão, P.; Sato, L.F.; Montes, R.R.; De Santis, H.S. Polysulphone/Montmorillonite Nanocomposite Membranes: Effect of Clay Addition and Polysulphone Molecular Weight on the Membrane Properties. J. Memb. Sci. 2014, 455, 187–199. [Google Scholar] [CrossRef]
- Madejová, J.; Barlog, M.; Jankovič, L.; Slaný, M.; Pálková, H. Comparative Study of Alkylammonium- and Alkylphosphonium-Based Analogues of Organo-Montmorillonites. Appl. Clay Sci. 2021, 200, 105894. [Google Scholar] [CrossRef]
- Bărdacă Urducea, C.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. [Google Scholar] [CrossRef] [PubMed]
- Bergaya, F.; Lagaly, G. Handbook of Clay Science; Newnes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, ISBN 0080993710. [Google Scholar]
- Kheirieh, S.; Asghari, M.; Afsari, M. Application and Modification of Polysulfone Membranes. Rev. Chem. Eng. 2018, 34, 657–693. [Google Scholar] [CrossRef]
- Anadão, P. Nanocomposite Filtration Membranes for Drinking Water Purification. In Water Purification; Elsevier: Amsterdam, The Netherlands, 2017; pp. 517–549. [Google Scholar]
- Alias, A.H.; Norizan, M.N.; Sabaruddin, F.A.; Asyraf, M.R.M.; Norrrahim, M.N.F.; Ilyas, A.R.; Kuzmin, A.M.; Rayung, M.; Shazleen, S.S.; Nazrin, A. Hybridization of MMT/Lignocellulosic Fiber Reinforced Polymer Nanocomposites for Structural Applications: A Review. Coatings 2021, 11, 1355. [Google Scholar] [CrossRef]
- Sand Chee, S.; Jawaid, M. The Effect of Bi-Functionalized MMT on Morphology, Thermal Stability, Dynamic Mechanical, and Tensile Properties of Epoxy/Organoclay Nanocomposites. Polymers 2019, 11, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, J.; Wang, Z.; Shi, F.; Liu, Q. Enhanced Separation Performance of PVDF/PVP-g-MMT Nanocomposite Ultrafiltration Membrane Based on the NVP-Grafted Polymerization Modification of Montmorillonite (MMT). Langmuir 2012, 28, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Wesén, E.V. Cellular Uptake of Amyloid Forming Proteins Related to Neurodegenerative Disease. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2020. [Google Scholar]
- Loske, L.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. 2D Nanocomposite Membranes: Water Purification and Fouling Mitigation. Membranes 2020, 10, 295. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An Introduction to Properties And. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; IntechOpen: London, UK, 2018; p. 1. [Google Scholar]
- Kaur, M.; Datta, M. Diclofenac Sodium Adsorption onto Montmorillonite: Adsorption Equilibrium Studies and Drug Release Kinetics. Adsorpt. Sci. Technol. 2014, 32, 365–388. [Google Scholar] [CrossRef]
- Khankhasaeva, S.T.; Badmaeva, S.V.; Ukhinova, M. V Adsorption of Diclofenac onto Fe2O3-Pillared Montmorillonite: Equilibrium, Kinetics and Thermodynamic Studies. J. Mol. Liq. 2023, 380, 121725. [Google Scholar] [CrossRef]
- Poorsharbaf Ghavi, F.; Raouf, F.; Dadvand Koohi, A. A Review on Diclofenac Removal from Aqueous Solution, Emphasizing on Adsorption Method. Iran. J. Chem. Chem. Eng. 2020, 39, 141–154. [Google Scholar]
- Bojnourd, F.M.; Pakizeh, M. Preparation and Characterization of a PVA/PSf Thin Film Composite Membrane after Incorporation of PSSMA into a Selective Layer and Its Application for Pharmaceutical Removal. Sep. Purif. Technol. 2018, 192, 5–14. [Google Scholar] [CrossRef]

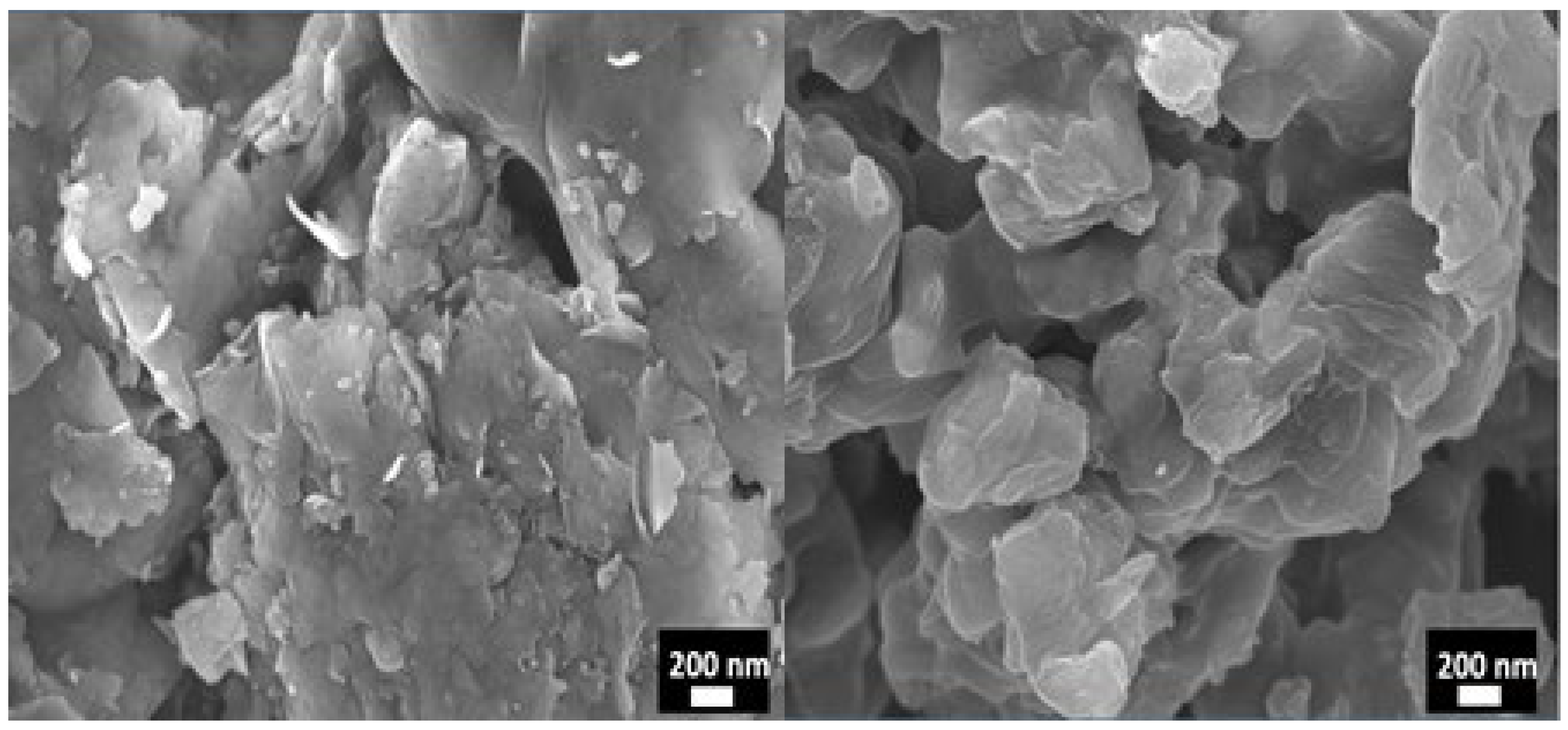
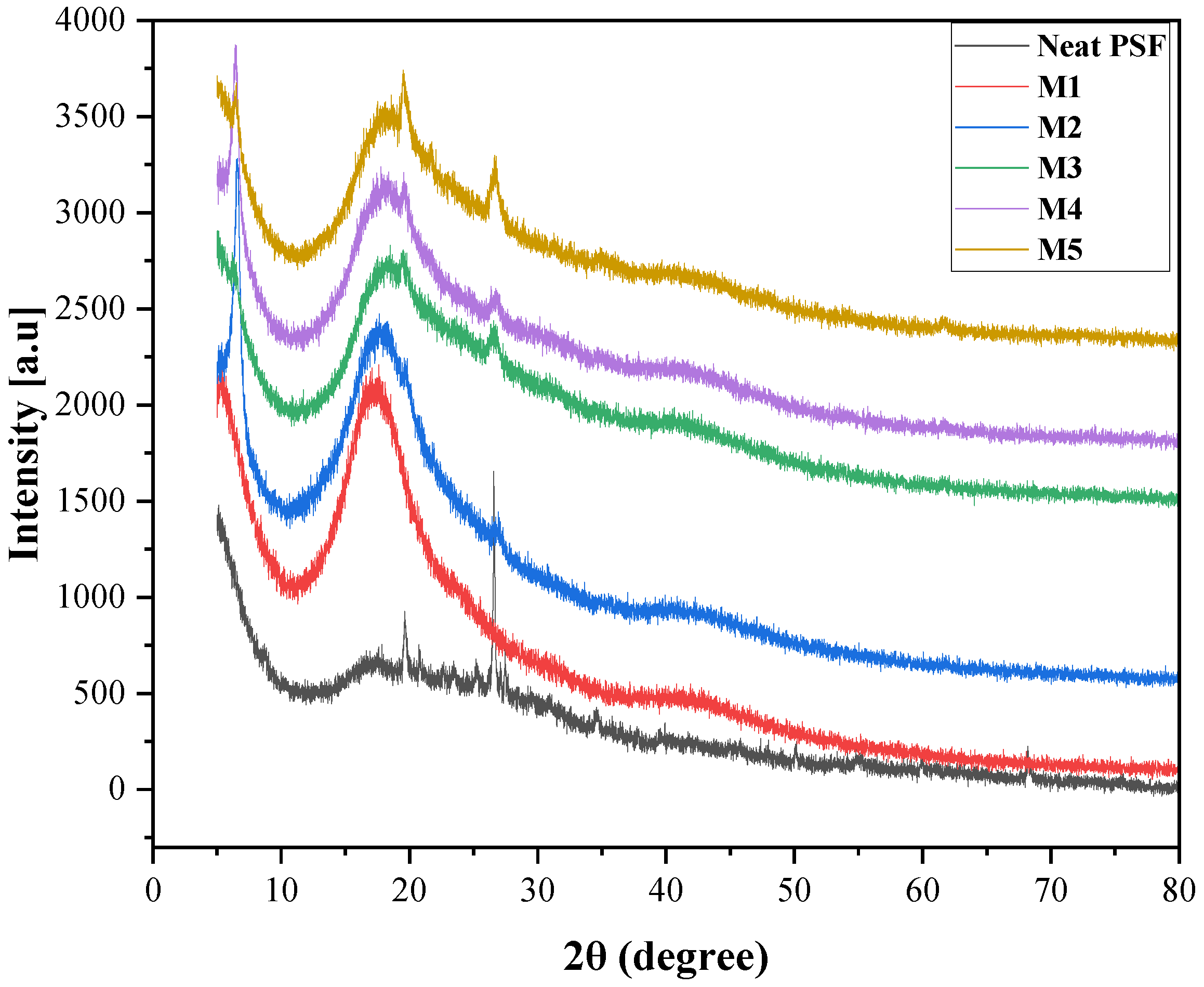
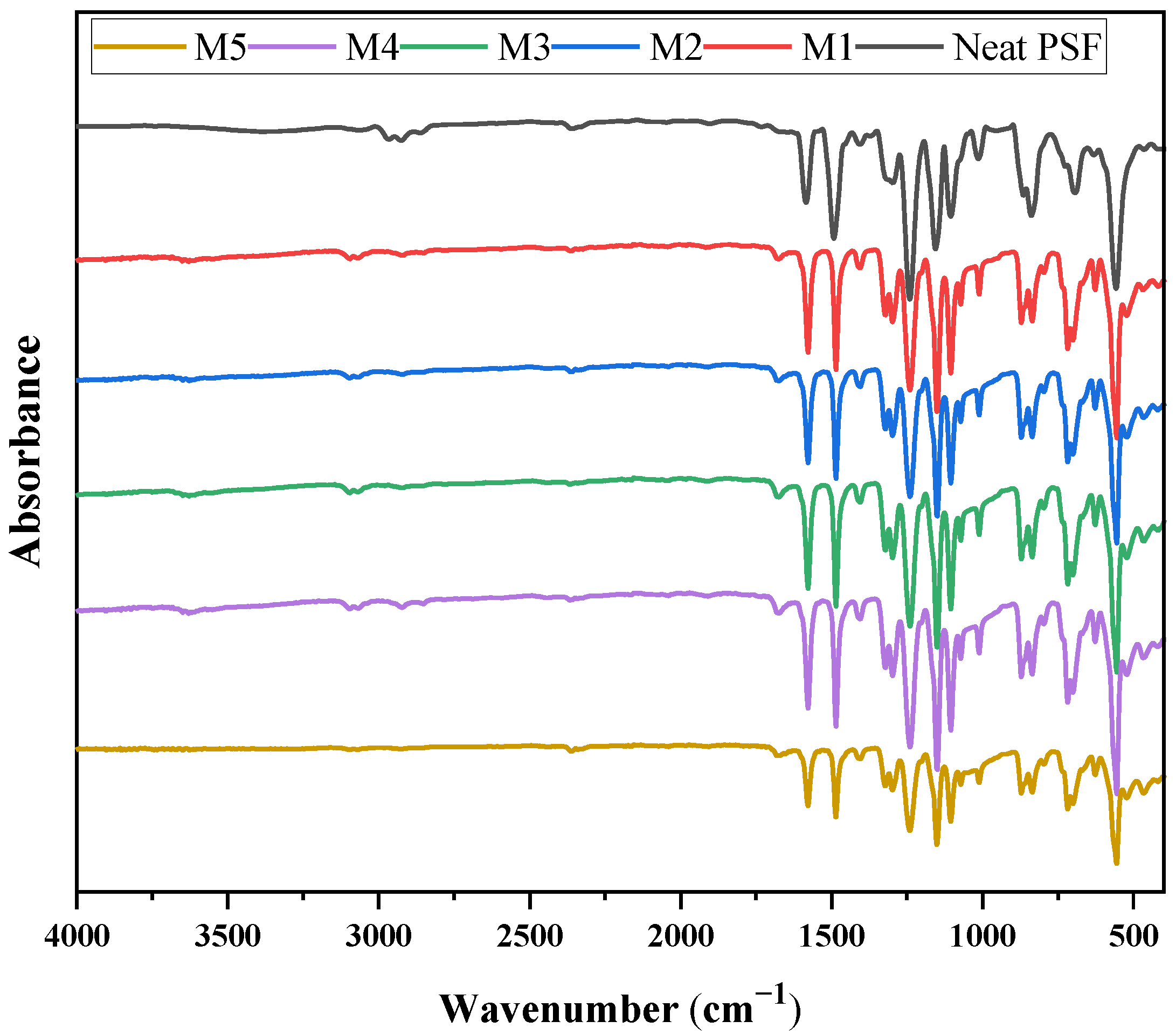
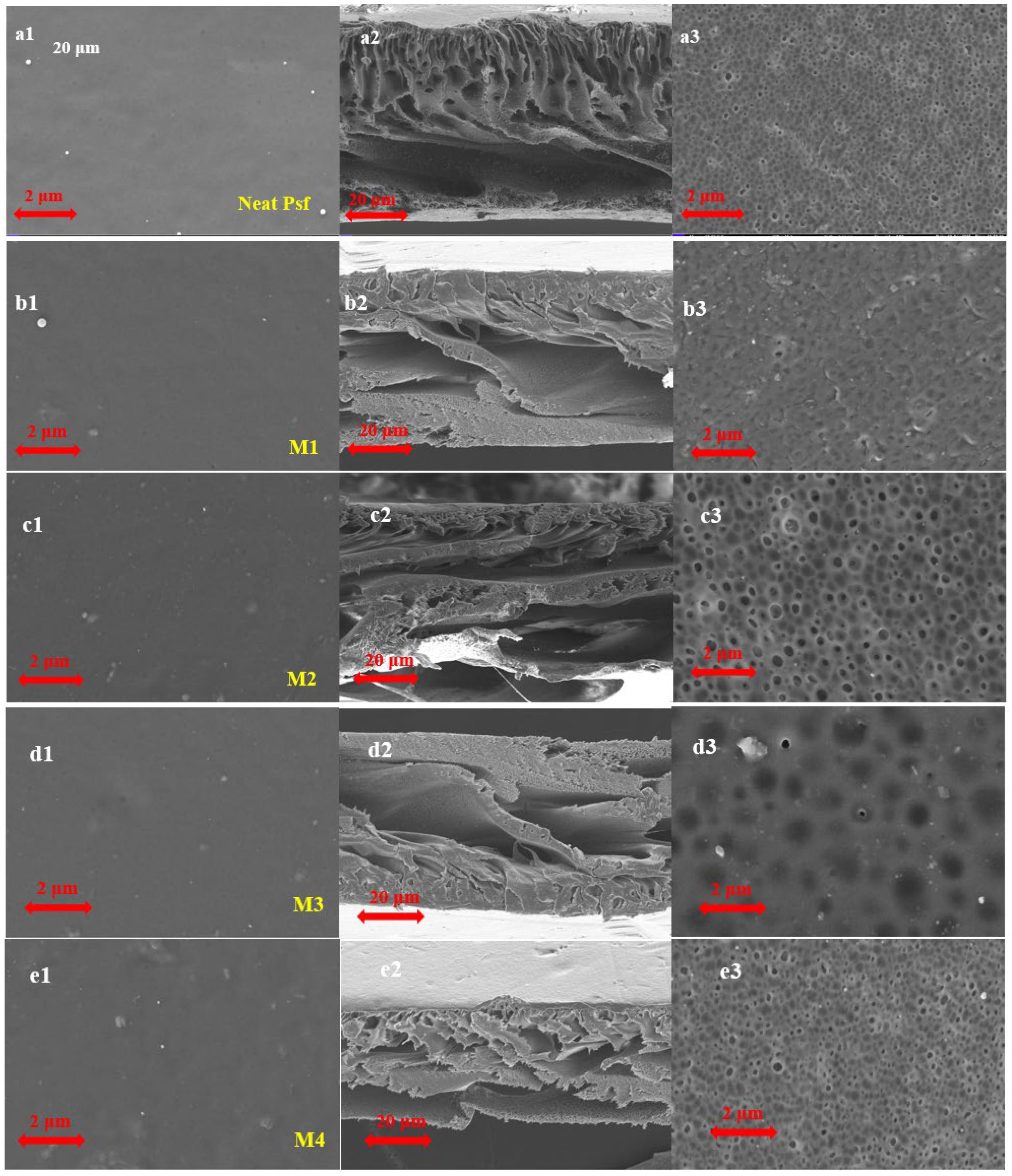





| Samples | PSF (wt%) | MMT (wt%) | NMP (wt%) |
|---|---|---|---|
| Neat PSF | 13 | 0.0 | 87.0 |
| M1(MMT-0.5%wt) | 13 | 0.5 | 86.5 |
| M2(MMT-1%wt) | 13 | 1.0 | 86.0 |
| M3(MMT-1.5%wt) | 13 | 1.5 | 85.5 |
| M4(MMT-2%wt) | 13 | 2.0 | 85.0 |
| M5(MMT-2.5%wt) | 13 | 2.5 | 84.5 |
| Membrane’s Types | Tensile Strain at Break (%) | Tensile Strength (MPa) | Young’s Module (MPa) |
|---|---|---|---|
| Neat PSF | 21.73 ± 1.18 | 3.97 ± 1.00 | 93.65 ± 1.04 |
| M1 (MMT-0.5%wt) | 29.43 ± 1.02 | 4.47 ± 1.05 | 70.08 ± 1.20 |
| M2 (MMT-1%wt) | 30.91 ± 1.17 | 3.61 ± 1.08 | 106 ± 1.02 |
| M3 (MMT-1.5%wt) | 37.45 ±1.35 | 3.44 ± 1.11 | 97.64 ± 1.02 |
| M4 (MMT-2%wt) | 38.40 ±1.20 | 3.80 ± 1.05 | 94.52 ± 1.16 |
| M5 (MMT-2.5%wt) | 35.95 ±1.08 | 3.50 ± 1.05 | 91.62 ± 1.05 |
| Membrane’s Types | Mean Pore Radius r (nm) | Porosity (%) |
|---|---|---|
| Neat PSF | 16.7 ± 0.6 | 74 ± 0.6 |
| M1 (MMT-0.5%wt) | 18.9 ± 0.4 | 80 ± 0.8 |
| M2 (MMT-1%wt) | 22.4 ± 0.8 | 86 ± 0.7 |
| M3 (MMT-1.5%wt) | 24.4 ±0.5 | 89 ± 0.9 |
| M4 (MMT-2%wt) | 19.0 ± 0.7 | 82 ± 0.6 |
| M5 (MMT-2.5%wt) | 21.0 ±0.6 | 85 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, Z.; Aloulou, H.; Algieri, C.; Dammak, L.; Ben Amar, R. Polysulfone/MMT Clay Mixed Matrix Membranes for Efficient Diclofenac Removal and Improved Antifouling Performance in Wastewater Treatment. Membranes 2025, 15, 344. https://doi.org/10.3390/membranes15110344
Salah Z, Aloulou H, Algieri C, Dammak L, Ben Amar R. Polysulfone/MMT Clay Mixed Matrix Membranes for Efficient Diclofenac Removal and Improved Antifouling Performance in Wastewater Treatment. Membranes. 2025; 15(11):344. https://doi.org/10.3390/membranes15110344
Chicago/Turabian StyleSalah, Zouhair, Hajer Aloulou, Catia Algieri, Lasaad Dammak, and Raja Ben Amar. 2025. "Polysulfone/MMT Clay Mixed Matrix Membranes for Efficient Diclofenac Removal and Improved Antifouling Performance in Wastewater Treatment" Membranes 15, no. 11: 344. https://doi.org/10.3390/membranes15110344
APA StyleSalah, Z., Aloulou, H., Algieri, C., Dammak, L., & Ben Amar, R. (2025). Polysulfone/MMT Clay Mixed Matrix Membranes for Efficient Diclofenac Removal and Improved Antifouling Performance in Wastewater Treatment. Membranes, 15(11), 344. https://doi.org/10.3390/membranes15110344







