Stable Ti3C2 MXene-Based Nanofiltration Membrane Prepared by Bridging for Efficient Dye Wastewater Treatment
Abstract
1. Introduction
2. Experimental
2.1. Materials
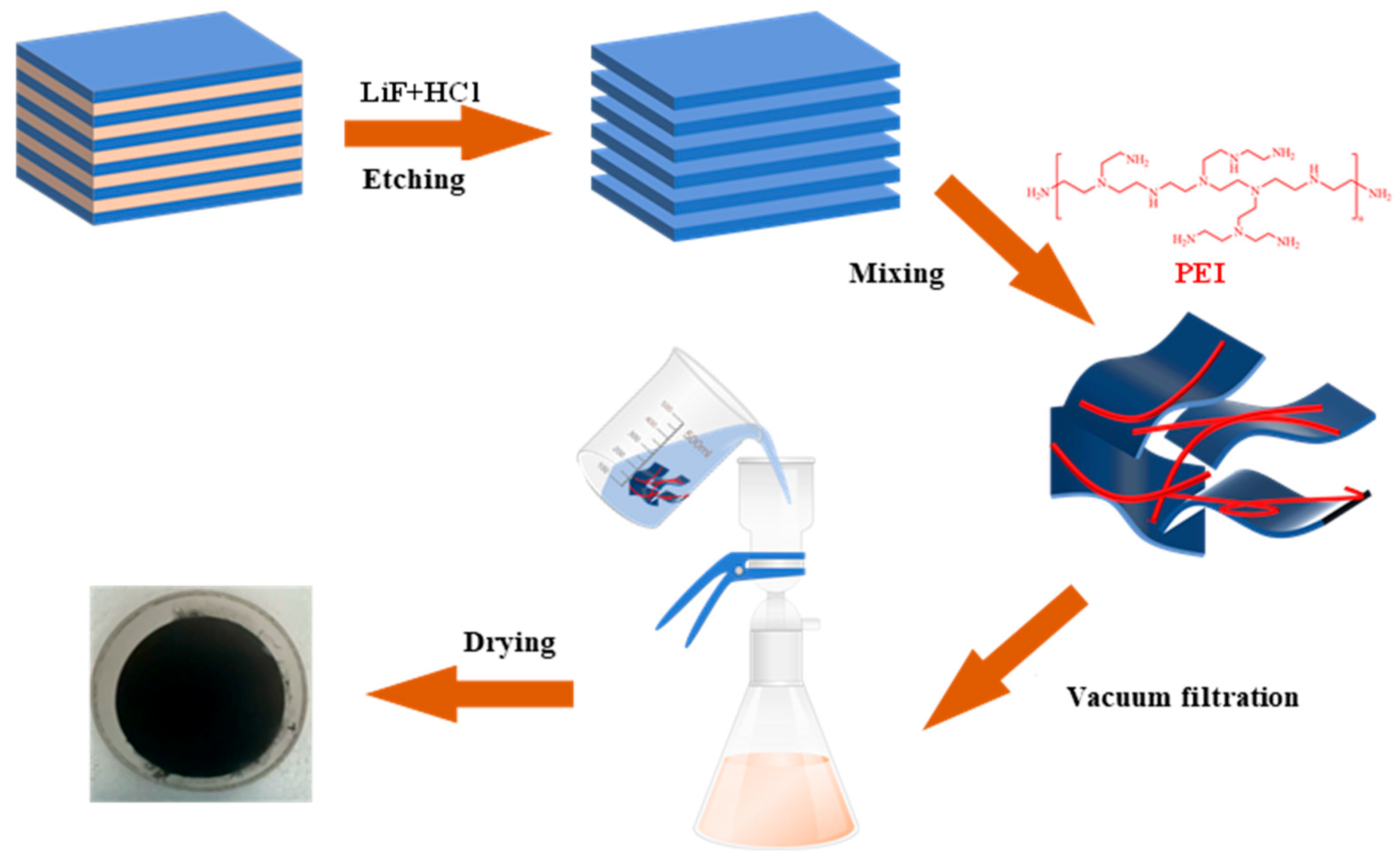
2.2. Preparation of Ti3C2
2.3. Preparation of Ti3C2 Membranes
2.4. Instrument
2.5. Separation Performance of the Ti3C2 Membrane
3. Results
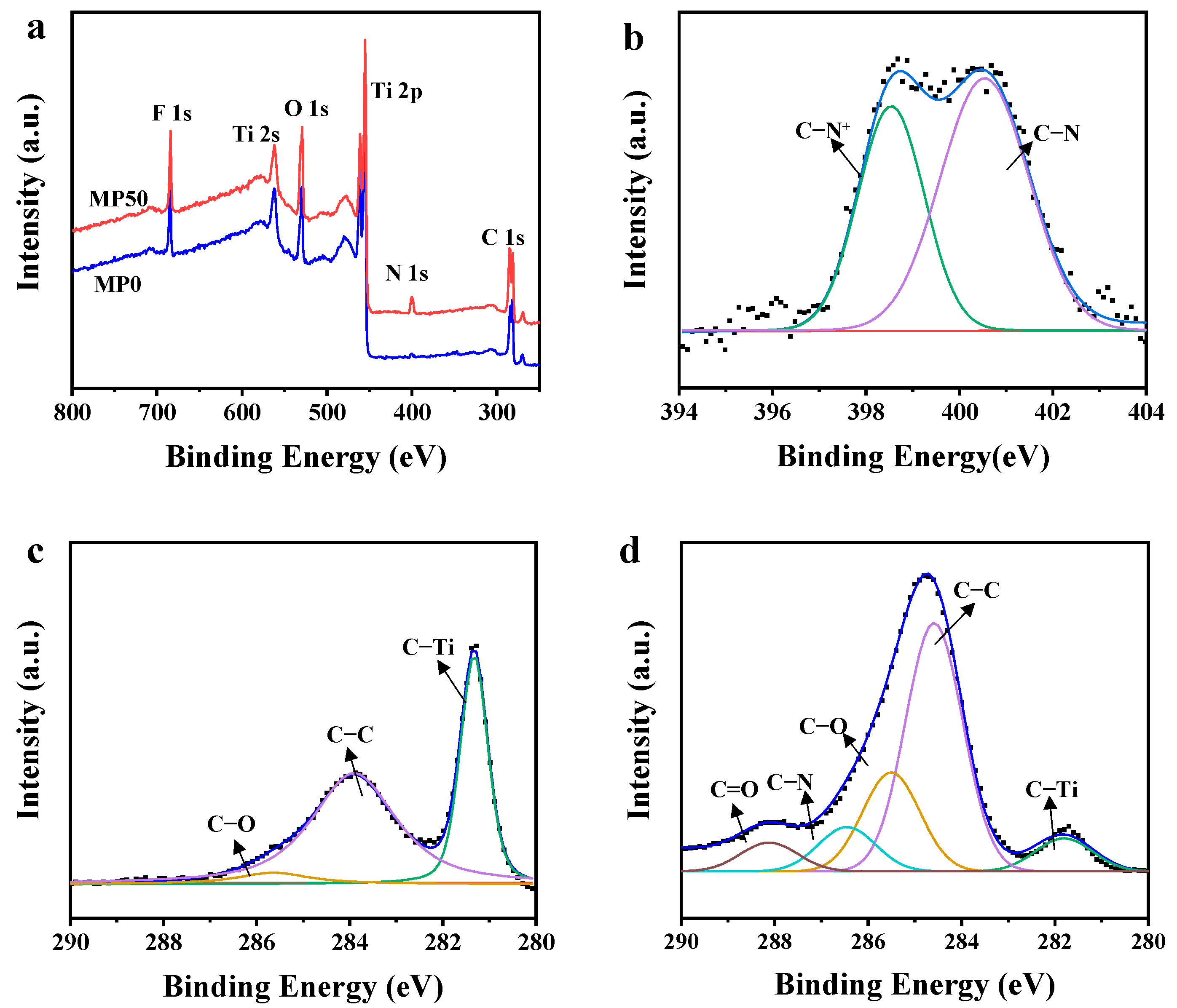
3.1. Characterization of Ti3C2
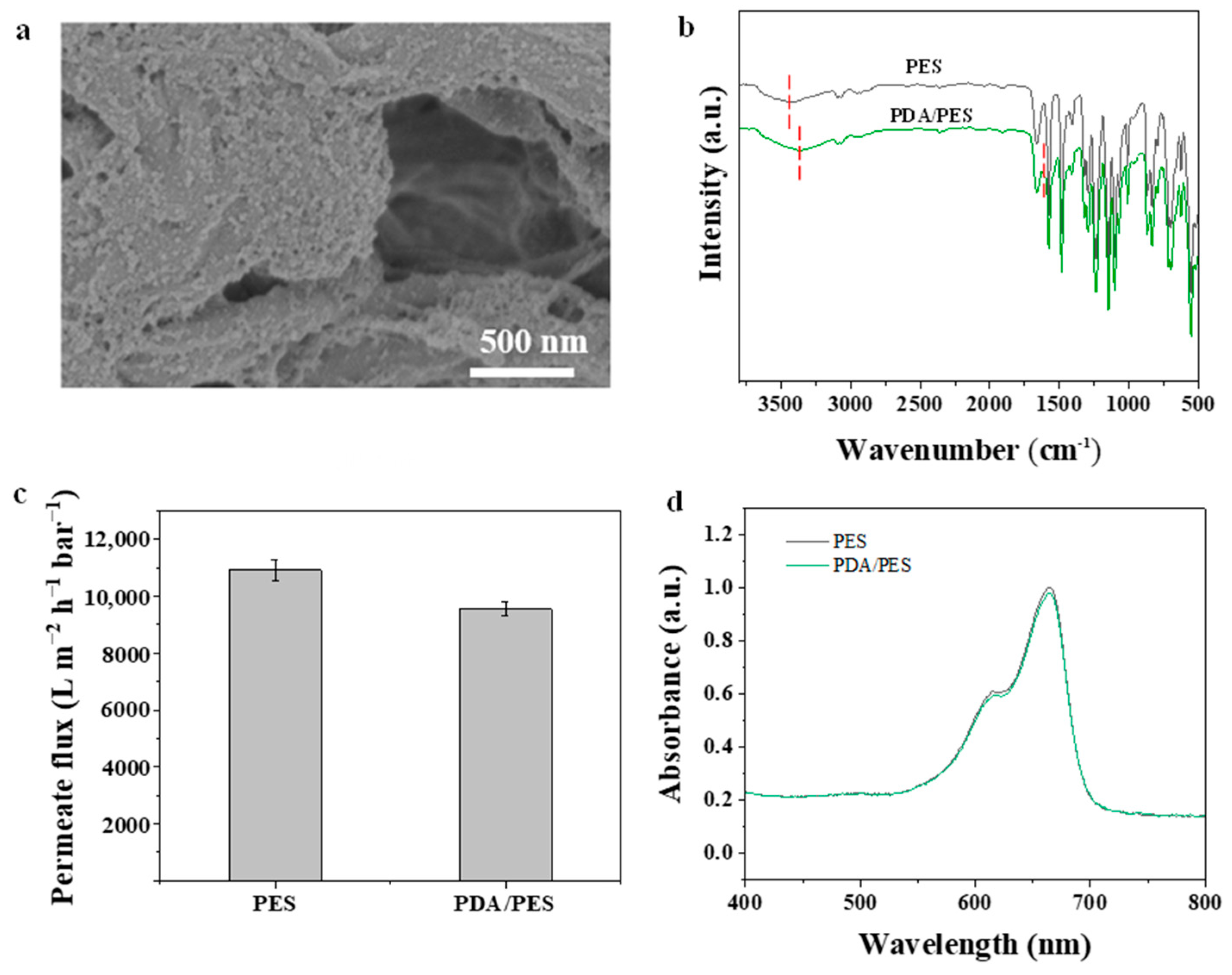
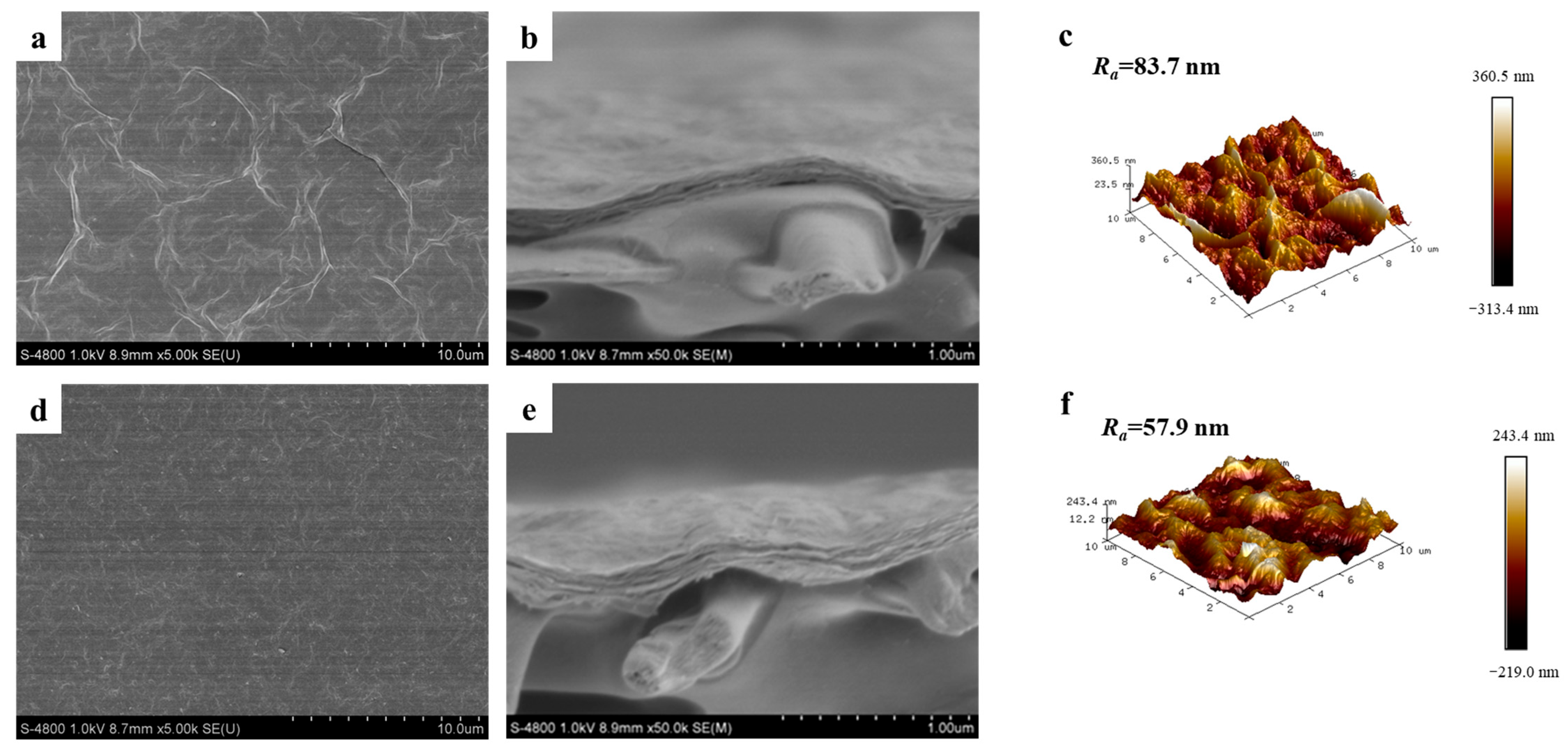
3.2. Characterization of Membranes
3.3. Performance of Ti3C2-Based Membranes
3.4. Long-Term Stability of Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, H.; Yu, L.; Yang, L.; Yao, Y.; Shen, G.; Wang, Y.; Li, B.; Meng, J.; Miao, M.; Zhi, C. Graphene oxide composites for dye removal in textile, printing and dyeing wastewaters: A review. Environ. Chem. Lett. 2025, 23, 165–193. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, Z.; Xu, L.; Liu, T.; Fan, Y.; Jin, L.; Dong, R.; Yi, Y.; Li, Y. Waste textile reutilization via a scalable dyeing technology: A strategy to enhance dyestuffs degradation efficiency. Adv. Fiber Mater. 2022, 4, 1595–1608. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Chi, M.; Eygen, G.V.; Guan, K.; Matsuyama, H. Comprehensive review of nanofiltration membranes for efficient resource recovery from textile wastewater. Chem. Eng. J. 2025, 506, 160132. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, J.; Jin, J.; Wang, Y.; Zhang, Y.; Van der Bruggen, B. Polymers of intrinsic microporosity for membrane-based precise separations. Prog. Mater. Sci. 2024, 144, 101285. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, P.; Wang, Z.; Huang, J.; Fu, Y.; Hu, W. Recent advances in regulation methods for selective separation and precise control of two-dimensional (2D) lamellar membranes. Adv. Colloid Interfac. 2024, 334, 103330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, Y.; Xie, L.; Liu, Q.; Li, J.; Zhang, B. A loose polyethersulfone hybrid nanofiltration membrane incorporated with polyethyleneimine-decorated silica nanoparticles for highly-efficient dye/salt separation. Chem. Eng. J. 2024, 487, 150643. [Google Scholar] [CrossRef]
- Baig, N.; Usman, J.; Abba, S.I.; Benaafi, M.; Aljundi, I.H. Fractionation of dyes/salts using loose nanofiltration membranes: Insight from machine learning prediction. J. Clean. Prod. 2023, 418, 138193. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Liu, Z.; Chen, R.; Wang, H.; Wang, K.; Zhao, H. Electroneutral nanofiltration membranes from interfacial polymerization of fluorinated activated ester. Sep. Purif. Technol. 2025, 363, 132084. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Elimelech, M.; Wang, Y. Scalable catalytic nanofiltration membranes for advanced water treatment. Nat. Water 2025, 3, 1038–1047. [Google Scholar] [CrossRef]
- Pan, Y.; Tu, C.; Li, X.; Gao, L.; Xu, B.; Zhang, W.-H.; Meng, H. Facile fabrication of COF nanofiltration membranes via acetic acid-catalyzed interfacial polymerization. J. Membr. Sci. 2025, 732, 124269. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Huang, J.; Chen, X.; Feng, S.; Wan, Y.; Luo, J. Strengthening nanofiltration membrane: Strategies for enhanced antifouling performance. Chem. Eng. J. 2025, 508, 160964. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, N.K.; Osuji, C.O. Fouling resistant nanofiltration membranes from self-assembled quaternary ammonium monomers. J. Membr. Sci. 2025, 727, 124101. [Google Scholar] [CrossRef]
- Xie, Y.; Ren, J.; Liu, P.; Zheng, J.; Mai, Z.; Liu, Y.; Zhu, X.; Li, X.; Xu, D.; Liang, H. Fabrication of green xylose-based nanofiltration membrane with enhanced performance and chlorine resistance. Desalination 2025, 593, 118243. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-dimensional-material membranes: A new family of high-performance separation membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef]
- Zhao, J.; He, G.; Chen, Y.; Dong, S.; Jiang, Z.; Jin, W. Engineering high-flux 2D separation membranes: Fundamentals, strategies, and future directions. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Xing, C.; Han, J.; Pei, X.; Zhang, Y.; He, J.; Huang, R.; Li, S.; Liu, C.; Lai, C.; Shen, L.; et al. Tunable graphene oxide nanofiltration membrane for effective dye/salt separation and desalination. ACS Appl. Mater. Interfaces 2021, 13, 55339–55348. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. High-performance and stable two-dimensional MXene-polyethyleneimine composite lamellar membranes for molecular separation. ACS Appl. Mater. Interfaces 2022, 14, 10237–10245. [Google Scholar] [CrossRef]
- Diao, Z.; Zhuang, S.; Yan, J.; Xu, Y.; Wu, Y.; Pan, J.; Yan, M. Recognition and separation of artemisinin by two-dimensional GO/MXene laminar imprinted composite membranes constructed based on self-supporting technology. J. Membr. Sci. 2024, 702, 122795. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Q.; Sim, A.; Yu, J.; Duan, Y.; Poon, S.; Liu, B.; Han, Q.; Urban, J.J.; Sedlak, D.; et al. Superselective removal of lead from water by two-dimensional MoS2 nanosheets and layer-stacked membranes. Environ. Sci. Technol. 2020, 54, 12602–12611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jian, M.; Meng, N.; Ji, Y.; Bai, X.; Wu, L.; Yang, H.; Tan, C.; Li, H. Recent progress in developing 2D MOFs/COFs/Zeolites nanosheets membranes for water purification. Sep. Purif. Technol. 2024, 337, 126404. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, Y.; Liu, Y.; Shen, Z. Defect remediation of mxene membranes facilitated by intercalation and coordination processes for enhanced organic pollutant removal. ACS Appl. Nano Mater. 2025, 8, 18070–18079. [Google Scholar] [CrossRef]
- Li, Z.; Fan, J.; Wang, L.; Yang, X.; Guo, L.; Chen, H.; Gong, D.; Yang, G.; Xu, Q.; Zou, S.; et al. Two-dimensional lamellar stacking COF membrane with charge repulsion effect for ions separation. J. Membr. Sci. 2024, 699, 122645. [Google Scholar] [CrossRef]
- Qiu, M.; Shen, Z.; Xia, Q.; Li, X.; Huang, H.; Wang, Y.; Liu, Y.; Wang, Y. Metal-polyphenol cross-linked titanium carbide membranes with stable interlayer spacing for efficient wastewater treatment. J. Colloid Interface Sci. 2022, 628, 649–659. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Zou, L.; Yang, W.; Li, X.; Yang, Y.; Jiang, D. Facile MXene-templated thin film composite membranes for enhanced nanofiltration performance. Sep. Purif. Technol. 2025, 362, 131680. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Wang, M.; Ren, Y.-X.; Li, S.; Liu, X.-Y.; Cao, Y.; Wu, X.-Q.; Hai, G.; Jiang, Z.; Li, D.-S. MXene-intercalated covalent organic framework membranes for high-flux nanofiltration. J. Membr. Sci. 2024, 701, 122755. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, Z.; Xu, Y.; Zhang, Y.; He, L.; Li, P.; Xiong, L.; Ding, L.; Wei, Y.; Wang, H. Supported MXene/GO composite membranes with suppressed swelling for metal ion sieving. Membranes 2021, 11, 621. [Google Scholar] [CrossRef]
- Helal, M.I.; Sinopoli, A.; Gladich, I.; Tong, Y.; Alfahel, R.; Gomez, T.; Mahmoud, K.A. Understanding the swelling behavior of Ti3C2Tx MXene membranes in aqueous media. J. Mater. Chem. A 2024, 12, 30729–30742. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.-K.; Wang, H. Self-crosslinked MXene (Ti3C2Tx) membranes with good antiswelling property for monovalent metal ion exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, Y.; Zhou, H.; Zhu, S.; Yang, J. Nanocellulose-intercalated MXene NF membrane with enhanced swelling resistance for highly efficient antibiotics separation. Sep. Purif. Technol. 2023, 305, 122425. [Google Scholar] [CrossRef]
- Yang, R.; Wang, H.; Hua, J.; Li, J. Intercalation of sodium carboxymethyl cellulose with MXene membrane layer spacing regulation and monovalent/divalent ion separation. Sep. Purif. Technol. 2025, 354, 128812. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Li, S.; Cui, L.; Zhuang, Y.; Xing, W.; Jing, W. Assembly of multidimensional MXene-carbon nanotube ultrathin membranes with an enhanced anti-swelling property for water purification. J. Membr. Sci. 2021, 623, 119075. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion sieving by a two-dimensional Ti3C2Tx alginate lamellar membrane with stable interlayer spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Huang, R.; He, J.; Sun, Y.; Qin, Y.; Shen, L. Stabilizing MXene-based nanofiltration membrane by forming analogous semi-interpenetrating network architecture using flexible poly(acrylic acid) for effective wastewater treatment. J. Membr. Sci. 2022, 648, 120360. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, S.; Chen, J.; Zhang, Z.; Qi, G.; Liu, Z.-Q. Self-assembly enabled nano-intercalation for stable high-performance MXene membranes. J. Membr. Sci. 2021, 635, 119464. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene materials for designing advanced separation membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Q.; Hao, S.; Zhao, J.-K.; Jia, Z.-Q.; Tan, H.-W.; Yang, Y.; Hou, L.-A. Exfoliated MXene/poly-melamine-formaldehyde composite membranes for removal of heavy metals and organics from aqueous solutions. J. Hazard. Mater. 2024, 463, 132866. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Huang, Y.; Wang, H.; Gao, Y.; Zhao, M.; Tu, L.; Xue, L.; Gao, C. Polyethyleneimine (PEI) based positively charged thin film composite polyamide (TFC-PA) nanofiltration (NF) membranes for effective Mg2+/Li+ separation. Desalination 2023, 565, 116814. [Google Scholar] [CrossRef]
- Xie, J.; Su, F.; Fan, L.; Mu, Z.; Wang, H.; He, Z.; Zhang, W.; Yao, D.; Zheng, Y. Robust and stretchable Ti3C2Tx MXene/PEI conductive composite dual-network hydrogels for ultrasensitive strain sensing. Compos. Part A-Appl. S. 2024, 176, 107833. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, W.; Li, Y.; Zhu, W.; Chen, X.; Hao, H.; Yu, M.; Huang, Y. Dual-bioinspired fabrication of Janus Micro/nano PDA-PTFE/TiO2 membrane for efficient oil-water separation. Sep. Purif. Technol. 2024, 330, 125201. [Google Scholar] [CrossRef]
- Li, Z.-K.; Wei, Y.; Gao, X.; Ding, L.; Lu, Z.; Deng, J.; Yang, X.; Caro, J.; Wang, H. Antibiotics Separation with MXene Membranes Based on Regularly Stacked High-Aspect-Ratio Nanosheets. Angew. Chem. Int. Ed. 2020, 59, 9751–9756. [Google Scholar] [CrossRef]
- Zhao, X.; Che, Y.; Mo, Y.; Huang, W.; Wang, C. Fabrication of PEI modified GO/MXene composite membrane and its application in removing metal cations from water. J. Membr. Sci. 2021, 640, 119847. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Xue, J.; Wang, H. Enhanced antipressure ability through graphene oxide membrane by intercalating g-C3N4 nanosheets for water purification. AIChE J. 2019, 65, e16699. [Google Scholar] [CrossRef]
- Ruiz-Torres, C.A.; Kang, J.; Kang, K.M.; Cho, K.M.; Nam, Y.T.; Byon, C.; Chang, Y.-Y.; Kim, D.W.; Jung, H.-T. Graphene-based ultrafast nanofiltration membrane under cross-flow operation: Effect of high-flux and filtered solute on membrane performance. Carbon 2021, 185, 641–649. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, S.; Qi, F.; Liu, R.; Xiao, T.; Hu, J.; Li, K.; Wang, R.; Min, Y. Direct deposition of two-dimensional MXene nanosheets on commercially available filter for fast and efficient dye removal. J. Hazard. Mater. 2020, 384, 121367. [Google Scholar] [CrossRef]
- Zhu, X.; Lou, M.; Chen, J.; Fang, X.; Huang, S.; Li, F. MXene/ZIF-L co-stacking membranes with high water permeation for solute-tailored selectivity. Appl. Surf. Sci. 2023, 625, 157194. [Google Scholar] [CrossRef]
- Gu, S.; Ma, Y.; Zhang, T.; Yang, Y.; Xu, Y.; Li, J. MXene nanosheet tailored bioinspired modification of a nanofiltration membrane for dye/salt separation. ACS EST Water 2023, 3, 1756–1766. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Li, Y.; Dai, R.; Zhou, H.; Li, X.; Wang, Z. Aramid nanofiber membranes reinforced by MXene nanosheets for recovery of dyes from textile wastewater. ACS Appl. Nano Mater. 2021, 4, 6328–6336. [Google Scholar] [CrossRef]




| Membrane | Ti3C2 Loading (mg) | PEI Loading (mg) | PEI/Ti3C2 Ratio (%) |
|---|---|---|---|
| MP0 | 1.0 | 0 | 0 |
| MP25 | 1.0 | 0.25 | 25 |
| MP50 | 1.0 | 0.50 | 50 |
| MP75 | 1.0 | 0.75 | 75 |
| MP100 | 1.0 | 1.0 | 100 |
| Membrane | Water Permeance (Lm−2 h−1 bar−1) | Dye Rejection (%) | Reference |
|---|---|---|---|
| MP50 | 112.3 | AB8GX:99.9% MB: 99.5% CR: 97.8% | This work |
| MCE/MXene | 44.97 | MeB: 100.0% | [45] |
| MXene/ZIF-L | 287.3 | CR: 99.2% | [46] |
| PAA-MXene | 516.3 | AB8GX: 99.5% | [33] |
| MXene/PDA/PEI | 38.4 | CR: 99.7% | [47] |
| 21%-Ag@MXene | 420 | RB: 79.9% | [48] |
| MXene-ANF | 195.3 | AB: 99.1% | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qiu, M. Stable Ti3C2 MXene-Based Nanofiltration Membrane Prepared by Bridging for Efficient Dye Wastewater Treatment. Membranes 2025, 15, 343. https://doi.org/10.3390/membranes15110343
Zhang Y, Qiu M. Stable Ti3C2 MXene-Based Nanofiltration Membrane Prepared by Bridging for Efficient Dye Wastewater Treatment. Membranes. 2025; 15(11):343. https://doi.org/10.3390/membranes15110343
Chicago/Turabian StyleZhang, Yu, and Ming Qiu. 2025. "Stable Ti3C2 MXene-Based Nanofiltration Membrane Prepared by Bridging for Efficient Dye Wastewater Treatment" Membranes 15, no. 11: 343. https://doi.org/10.3390/membranes15110343
APA StyleZhang, Y., & Qiu, M. (2025). Stable Ti3C2 MXene-Based Nanofiltration Membrane Prepared by Bridging for Efficient Dye Wastewater Treatment. Membranes, 15(11), 343. https://doi.org/10.3390/membranes15110343






