Microfiltration of Post-Fermentation Broths: Long-Term Studies on the Use of Modules with Polymeric Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Post-Fermentation Broths
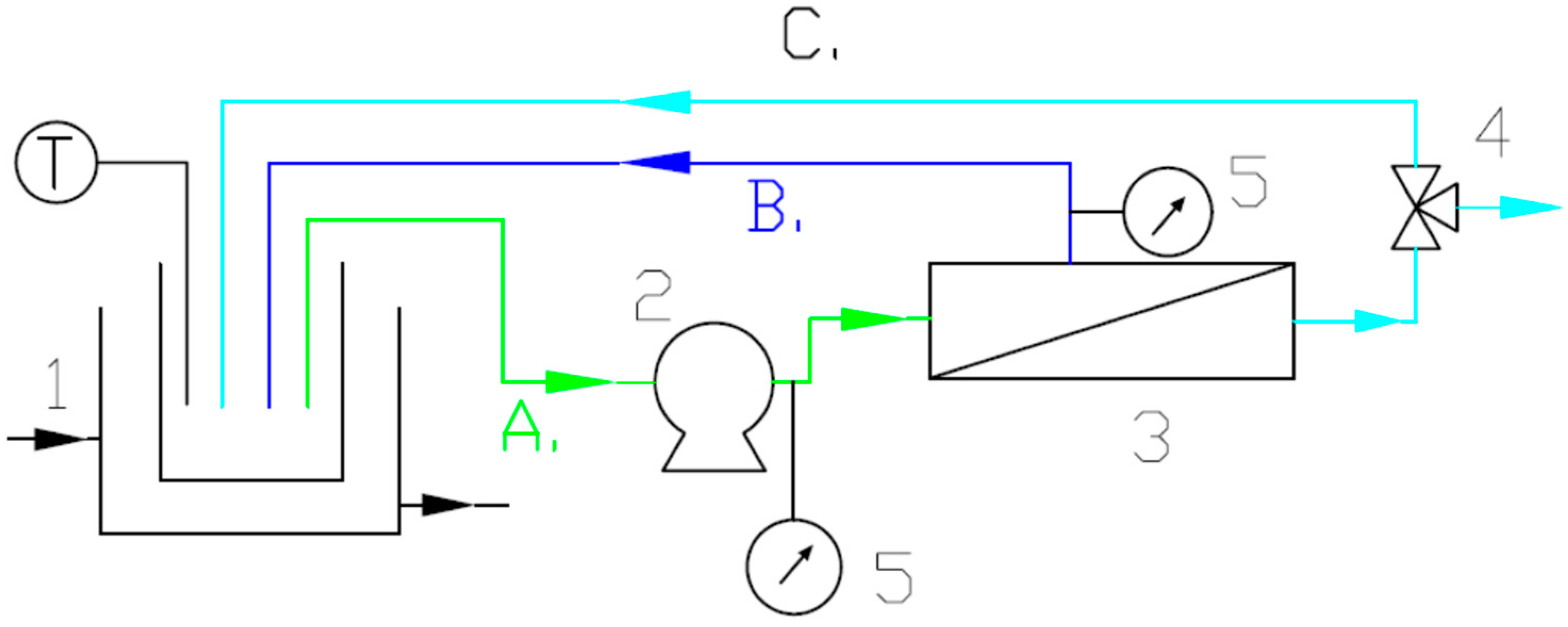
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Membranes Cleaning
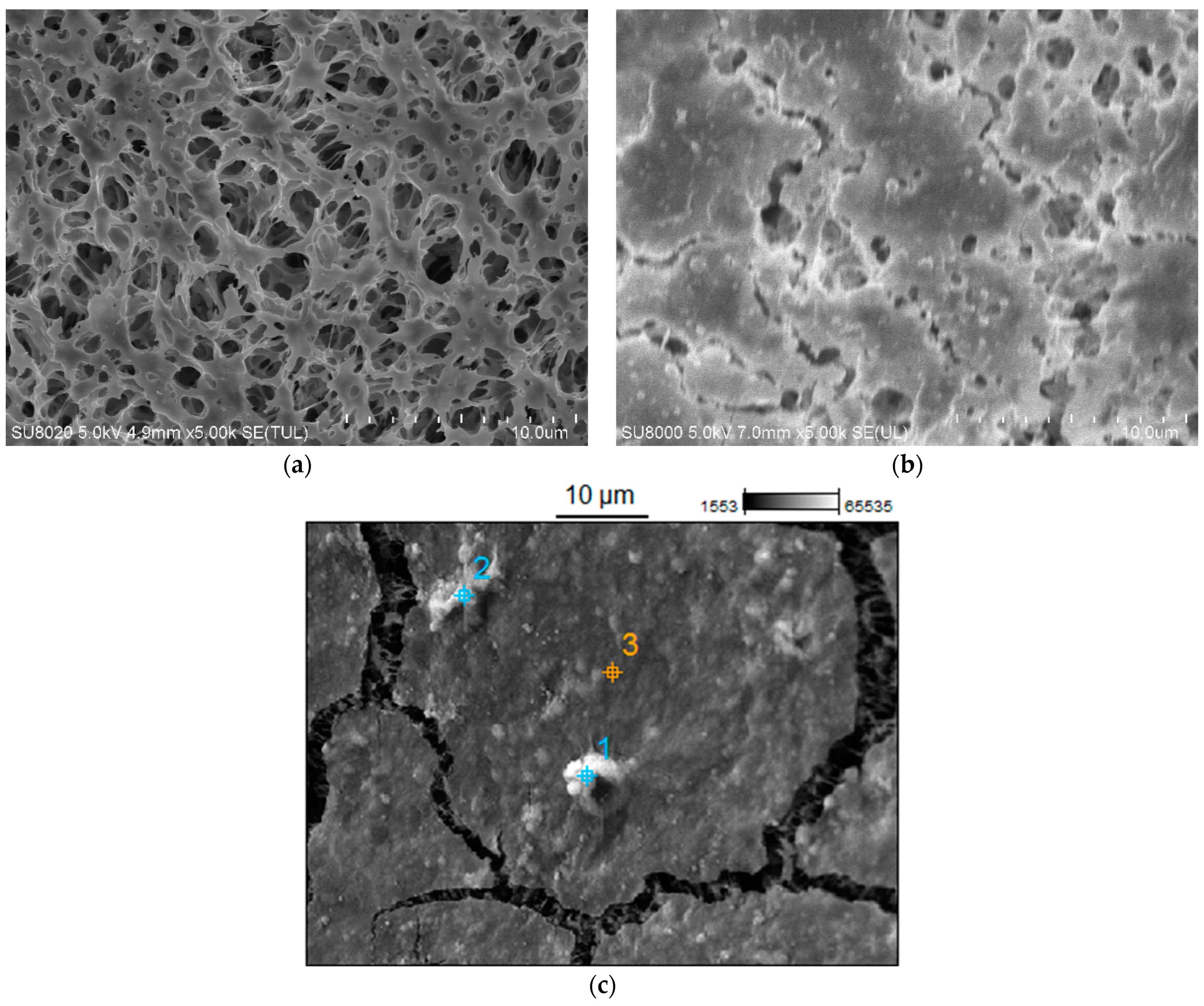
2.5. Analysis of Fouling Mechanism
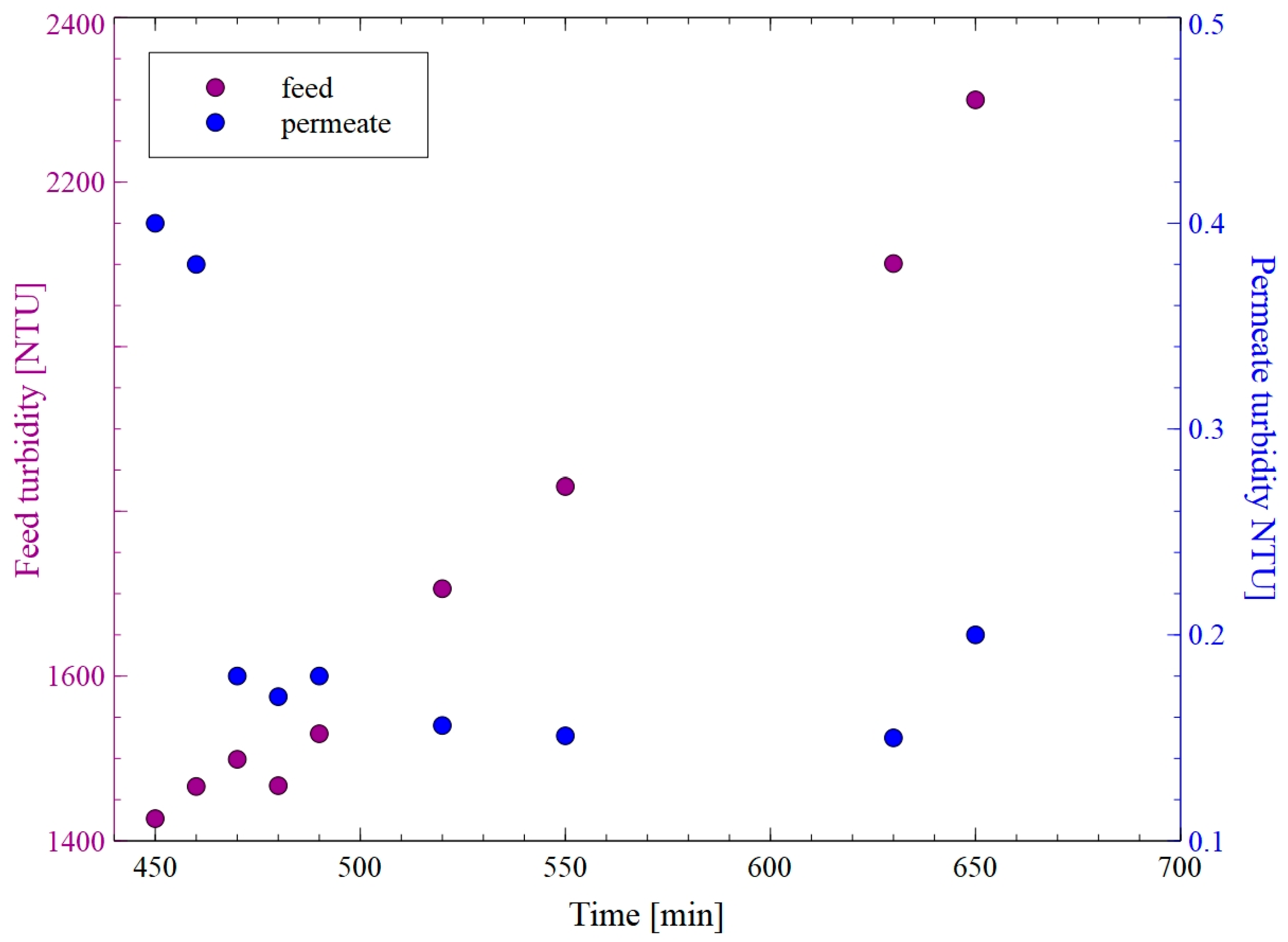
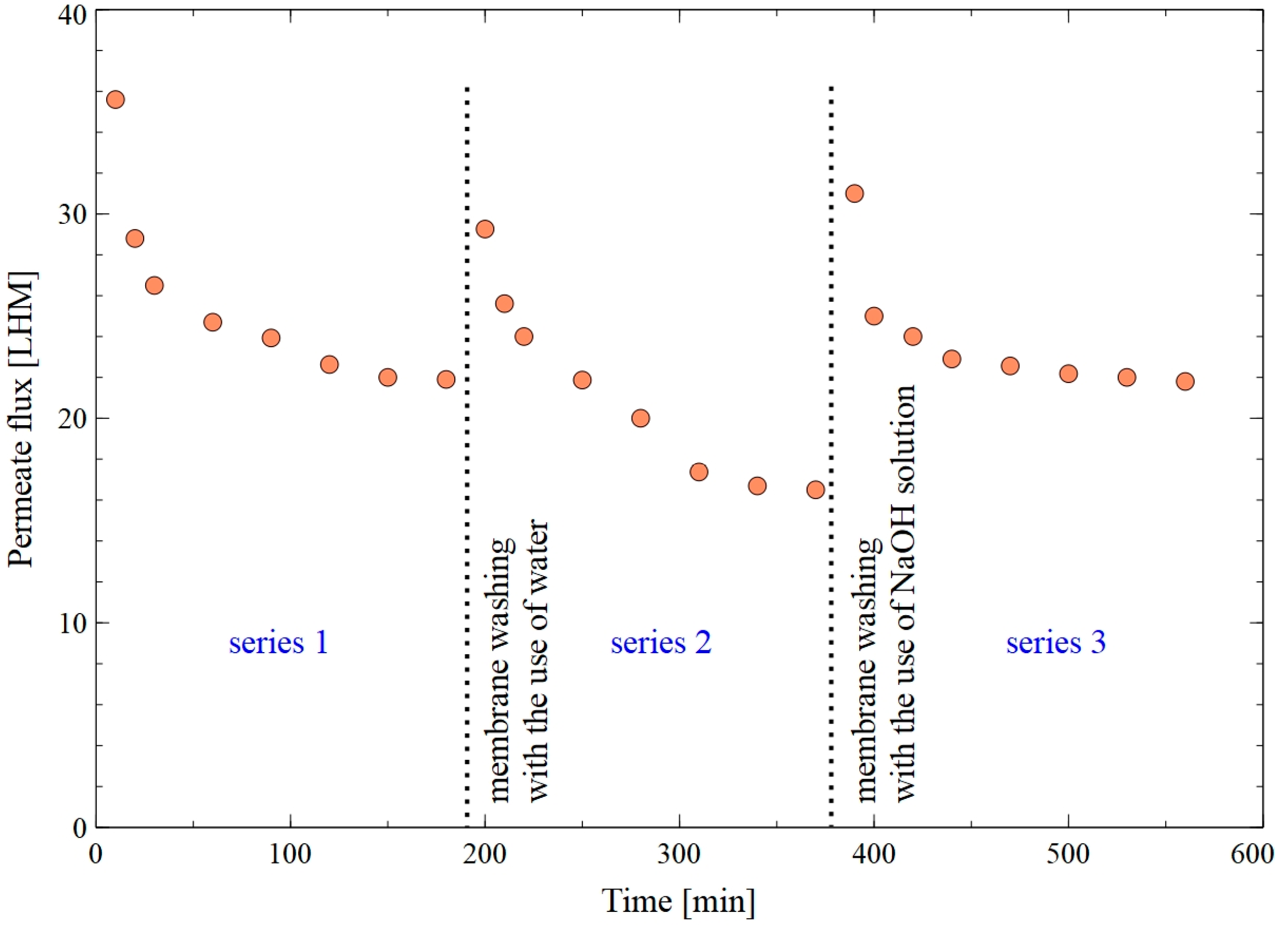
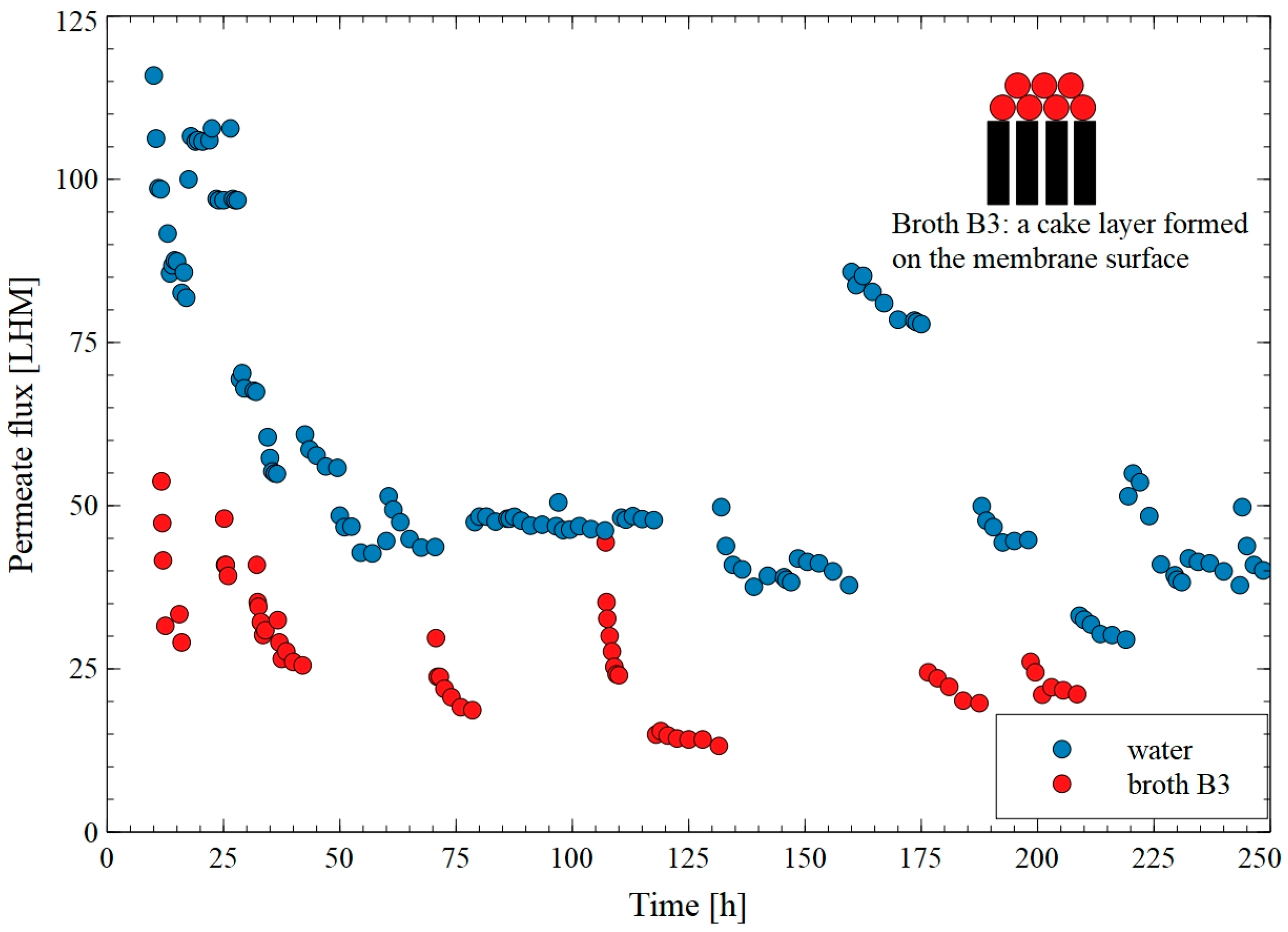
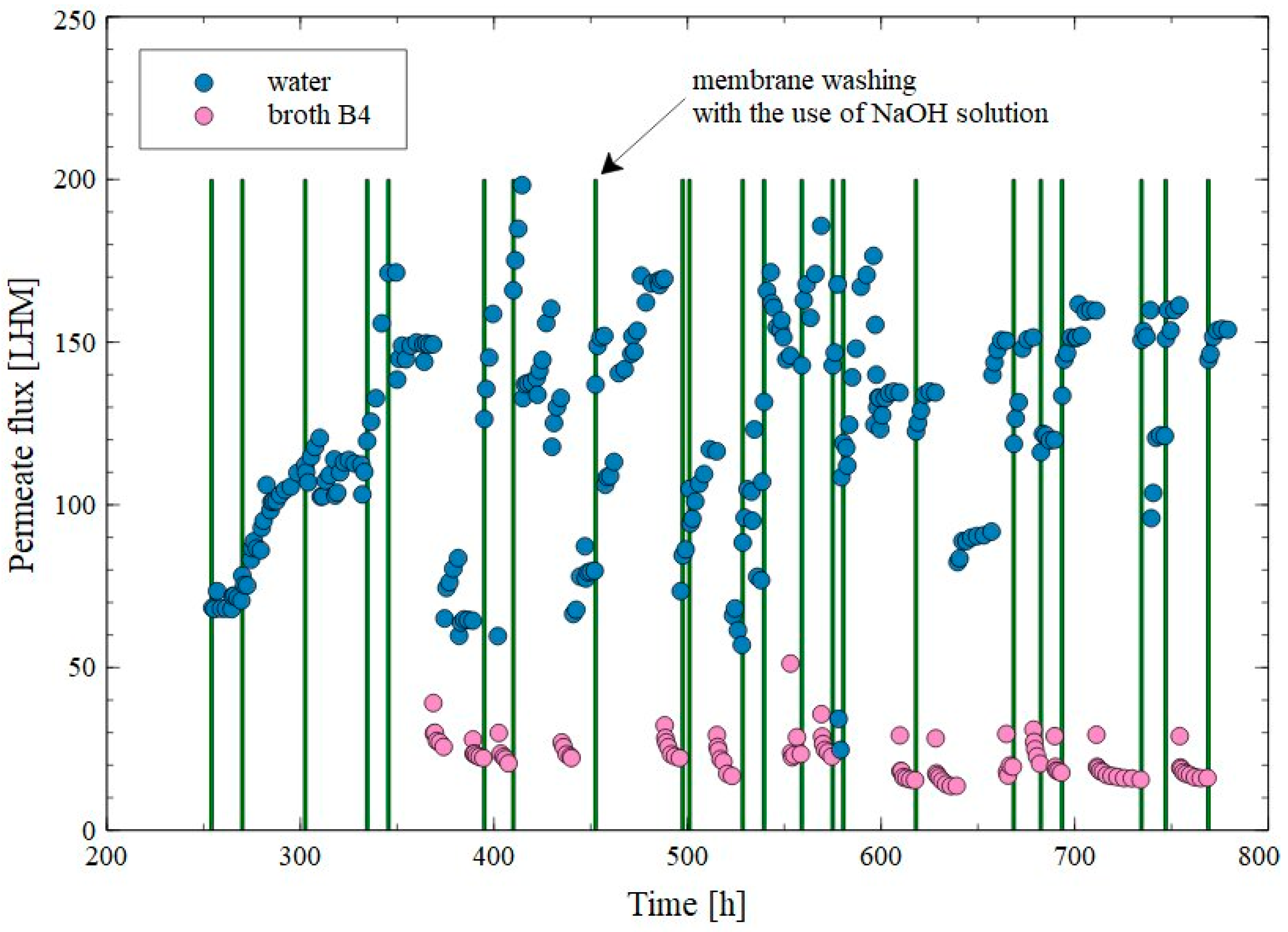
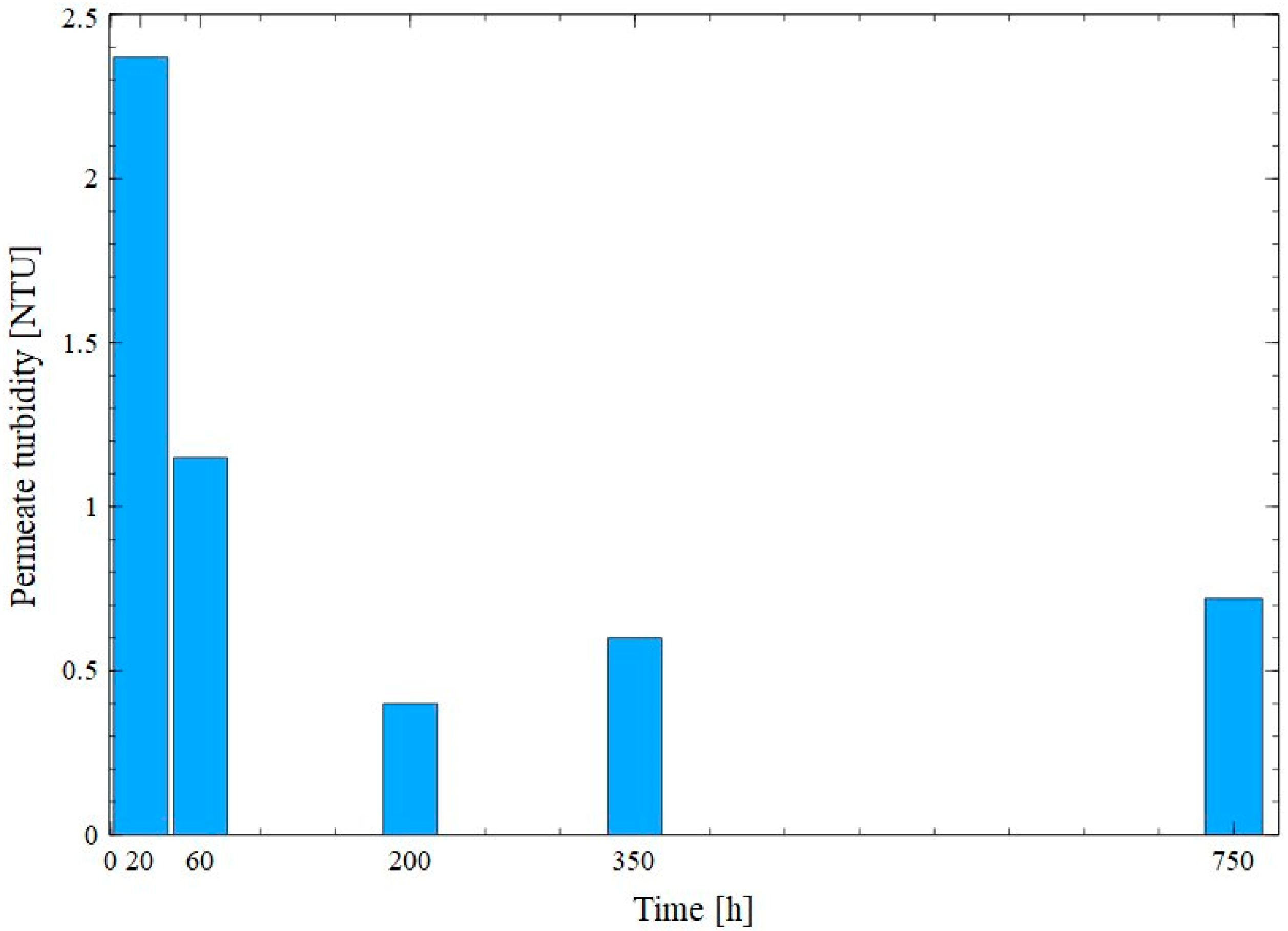
3. Results and Discussion
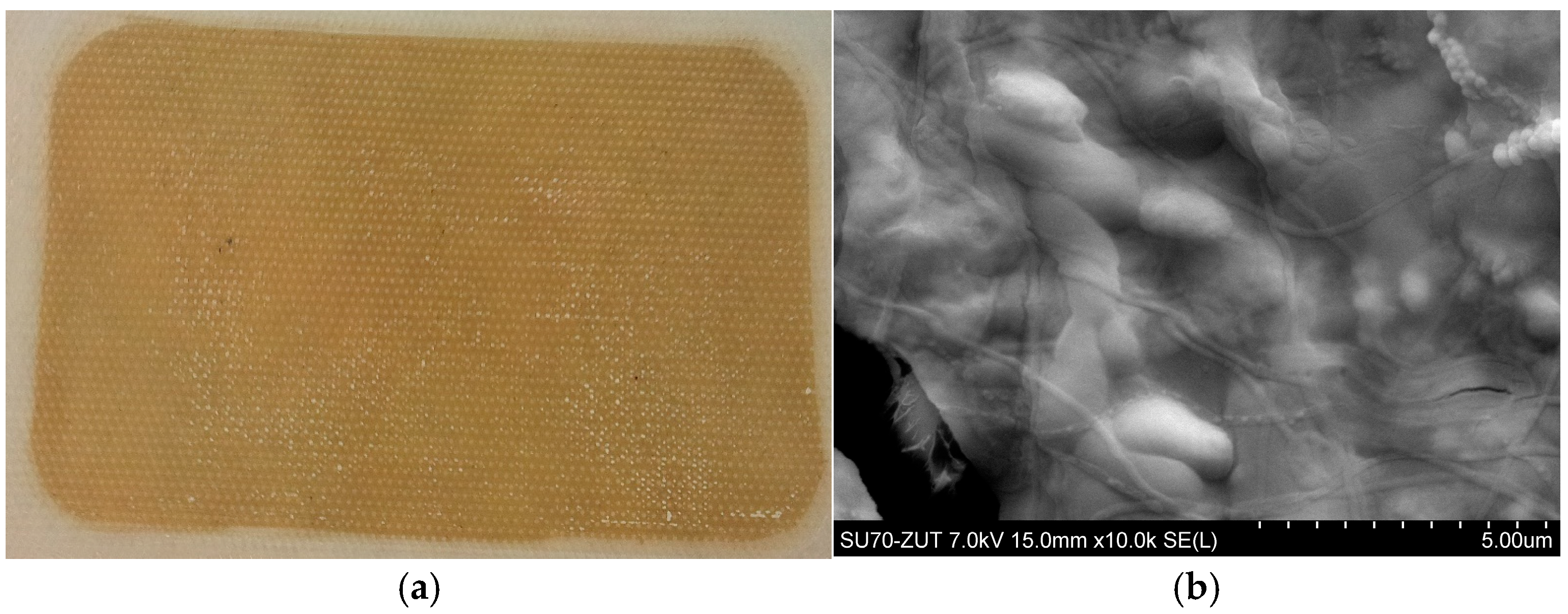
3.1. Flat-Sheet Membrane
3.2. Capillary Module
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Fermentation Broth | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation |
|---|---|---|---|---|
| B1 broth | 0.8767 | 0.8553 | 0.8292 | 0.8667 * |
| B2 broth | 0.5415 | 0.5589 | 0.5746 | 0.6004 * |
| MF Period | MF Time [min] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation |
|---|---|---|---|---|---|
| series 1 | 0–180 | 0.7361 | 0.7666 | 0.7955 | 0.8467 * |
| series 2 | 200–370 | 0.9286 | 0.9432 | 0.9546 | 0.9687 * |
| series 3 | 390–560 | 0.5808 | 0.6025 | 0.6243 | 0.6675 * |
| MF Period | MF Time [h] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation |
|---|---|---|---|---|---|
| series 1 | 11.6–12.5 | 0.9921 * | 0.9971 * | 0.9992 * | 0.9957 * |
| series 2 | 25.2–26.0 | 0.6011 | 0.6106 | 0.6203 | 0.6402 * |
| series 3 | 32.1–34.0 | 0.7333 | 0.7481 | 0.7619 | 0.7869 * |
| series 4 | 36.7–42.0 | 0.5841 | 0.5958 | 0.6074 | 0.6320 * |
| series 5 | 70.7–78.5 | 0.7454 | 0.7767 | 0.8061 | 0.8581 * |
| series 6 | 107.0–110.0 | 0.8376 | 0.8672 | 0.8967 | 0.9387 * |
| series 7 | 118.0–131.5 | 0.8600 | 0.8615 | 0.8627 | 0.8637 * |
| series 8 | 176.5–187.5 | 0.8535 | 0.8542 | 0.8546 | 0.8548 * |
| series 9 | 198.5–208.5 | 0.5573 | 0.5571 | 0.5566 | 0.7548 * |
| MF Period | MF Time [h] | Complete Blocking | Standard Blocking | Intermediate Blocking | Cake Formation |
|---|---|---|---|---|---|
| series 1 | 368.7–374.0 | 0.5788 | 0.6074 | 0.6363 | 0.6937 * |
| series 2 | 389.1–394.5 | 0.4783 | 0.4917 | 0.5053 | 0.5356 * |
| series 3 | 402.5–407.5 | 0.7869 | 0.8062 | 0.8252 | 0.8612 * |
| series 4 | 435.0–440.0 | 0.9360 | 0.9399 | 0.9435 | 0.9501 * |
| series 5 | 488.1–496.0 | 0.7707 | 0.7904 | 0.8091 | 0.8432 * |
| series 6 | 515.1–523.0 | 0.9061 | 0.9239 | 0.9381 | 0.9568 * |
| series 7 | 553.1–558.5 | 0.8399 | 0.8274 | 0.8745 | 0.8889 * |
| series 8 | 569.1–574.5 | 0.6717 | 0.7013 | 0.7307 | 0.7863 * |
| series 9 | 609.7–617.5 | 0.6104 | 0.6430 | 0.6473 | 0.6480 * |
| series 10 | 628.2–639.0 | 0.4910 | 0.5416 | 0.5928 | 0.6905 * |
| series 11 | 664.7–668.0 | 0.8987 | 0.8829 | 0.8677 | 0.8905 * |
| series 12 | 678.7–682.0 | 0.8989 | 0.9178 | 0.9348 | 0.9626 * |
| series 13 | 689.7–693.0 | 0.6209 | 0.6374 | 0.6550 | 0.6928 * |
| series 14 | 711.2–734.0 | 0.6394 | 0.6839 | 0.7295 | 0.7400 * |
| series 15 | 754.2–768.5 | 0.6181 | 0.6551 | 0.6936 | 0.7719 * |
References
- Janković, T.; Straathof, A.J.J.; Kiss, A.A. Eco-Efficient Downstream Processing of 1,3-Propanediol Applicable to Various Fermentation Processes. Process Biochem. 2024, 143, 210–224. [Google Scholar] [CrossRef]
- Sadare, O.O.; Ejekwu, O.; Moshokoa, M.F.; Jimoh, M.O.; Daramola, M.O. Membrane Purification Techniques for Recovery of Succinic Acid Obtained from Fermentation Broth during Bioconversion of Lignocellulosic Biomass: Current Advances and Future Perspectives. Sustainability 2021, 13, 6794. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Zhao, H.; Gao, M.; Wang, Q. The Performance of Microfiltration Process for Purifying Lactic Acid in the Fermented Broth of Kitchen Waste. Membranes 2023, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Tomczak, W. Microfiltration of Post-Fermentation Broth with Backflushing Membrane Cleaning. Chem. Pap. 2015, 69, 544–552. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Chen, H.; Yang, F.; Wang, Q.; Zheng, T.; Zhou, R.; Wu, C.; Jin, Y. Fouling Mechanisms in the Clarification of 1,3-Propanediol Fermentation Broths by Membrane Processes. Membranes 2025, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ni, J.; Huang, W.; Zhang, J. Separation of Hyaluronic Acid from Fermentation Broth by Tangential Flow Microfiltration and Ultrafiltration. Sep. Purif. Technol. 2006, 52, 29–38. [Google Scholar] [CrossRef]
- Kujundzic, E.; Greenberg, A.R.; Fong, R.; Moore, B.; Kujundzic, D.; Hernandez, M. Biofouling Potential of Industrial Fermentation Broth Components during Microfiltration. J. Membr. Sci. 2010, 349, 44–55. [Google Scholar] [CrossRef]
- Sikder, J.; Pereira, C.; Palchoudhury, S.; Vohra, K.; Basumatary, D.; Pal, P. Synthesis and Characterization of Cellulose Acetate-Polysulfone Blend Microfiltration Membrane for Separation of Microbial Cells from Lactic Acid Fermentation Broth. Desalination 2009, 249, 802–808. [Google Scholar] [CrossRef]
- Wojciech, B.; Celińska, E.; Dembczyński, R.; Szymanowska, D.; Nowacka, M.; Jesionowski, T.; Grajek, W. Cross-Flow Microfiltration of Fermentation Broth Containing Native Corn Starch. J. Membr. Sci. 2013, 427, 118–128. [Google Scholar] [CrossRef]
- Nie, C.; Luan, W.; Chen, X.; Li, L.; Wei, K.; Qiu, M.; Fan, Y. Comparison of Ceramic Microfiltration and Ultrafiltration Membranes for the Clarification of Simulated Sebacic Acid Fermentation Broth. J. Environ. Chem. Eng. 2023, 11, 109820. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Cross-Flow Microfiltration of Glycerol Fermentation Broths with Citrobacter Freundii. Membranes 2020, 10, 67. [Google Scholar] [CrossRef]
- Carrère, H.; Blaszkow, F.; Balmann, H.R.D. Modelling the Clarification of Lactic Acid Fermentation Broths by Cross-Flow Microfiltration. J. Membr. Sci. 2001, 186, 219–230. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane Technologies for Lactic Acid Separation from Fermentation Broths Derived from Renewable Resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef]
- Wang, F.; Tarabara, V.V. Pore Blocking Mechanisms during Early Stages of Membrane Fouling by Colloids. J. Colloid. Interface Sci. 2008, 328, 464–469. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Liao, C.-Y.; Tung, K.-L. Analysis of Particle Fouling during Microfiltration by Use of Blocking Models. J. Membr. Sci. 2007, 287, 287–293. [Google Scholar] [CrossRef]
- Tran, M.L.; Chen, Y.-S.; Juang, R.-S. Fouling Analysis in One-Stage Ultrafiltration of Precipitation-Treated Bacillus subtilis Fermentation Liquors for Biosurfactant Recovery. Membranes 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Lin, H.J.; Leung, K.T.; Schraft, H.; Liao, B.Q. Structure of Cake Layer in a Submerged Anaerobic Membrane Bioreactor. J. Membr. Sci. 2011, 374, 110–120. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Castillo, C.; Brouwer-Hanzens, A.H.; Harmsen, D.J.H.; Cornelissen, E.R.; Van Der Kooij, D. Quantitative Assessment of the Efficacy of Spiral-Wound Membrane Cleaning Procedures to Remove Biofilms. Water Res. 2012, 46, 6369–6381. [Google Scholar] [CrossRef] [PubMed]
- Sanawar, H.; Bucs, S.S.; Pot, M.A.; Zlopasa, J.; Farhat, N.M.; Witkamp, G.-J.; Kruithof, J.C.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Pilot-Scale Assessment of Urea as a Chemical Cleaning Agent for Biofouling Control in Spiral-Wound Reverse Osmosis Membrane Elements. Membranes 2019, 9, 117. [Google Scholar] [CrossRef]
- WuDunn, D.; Squeri, A.; Vu, J.; Dhingra, A.; Coffman, J.; Lee, K. Effect of Inner Diameter, Filter Length, and Pore Size on Hollow Fiber Filter Fouling during Perfusion Cell Culture. Biotechnol. Prog. 2024, 40, e3440. [Google Scholar] [CrossRef]
- Zhou, Z.; He, X.; Zhou, M.; Meng, F. Chemically Induced Alterations in the Characteristics of Fouling-Causing Bio-Macromolecules–Implications for the Chemical Cleaning of Fouled Membranes. Water Res. 2017, 108, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The Anaerobic Co-Digestion of Food Waste and Cattle Manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, Full-Scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Xue, Q.; Lim, Y.J.; Wang, R. Chemically Robust Hollow Fiber Thin-Film Composite Membranes Based on Polyurea Selective Layers for Nanofiltration under Extreme pH Conditions. J. Membr. Sci. 2026, 738, 124818. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Hou, M.; Li, Q.; Che, Y. Hydrophilic Modification of Polytetrafluoroethylene (PTFE) Capillary Membranes with Chemical Resistance by Constructing Three-Dimensional Hydrophilic Networks. Polymers 2024, 16, 1154. [Google Scholar] [CrossRef]
- Sano, T.; Ito, H.; Ishida, K.; Sato, A.; Kawagoshi, Y. Mechanical Durability and Fouling Development of Flat-Sheet Membranes in a Submerged Membrane Bioreactor. Jpn. J. Water Treat. Biol. 2022, 58, 71–81. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, R.; Gao, S.; Chang, H.; Van Der Bruggen, B.; Tian, J. Anti-Drying Nanofiltration (NF) Membranes Constructed on PTFE Microfiltration (MF) Substrate via Novel Interfacial Polymerization. J. Membr. Sci. 2021, 638, 119721. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.M.; Khan, S.A.; Hossain, M.D. Research and Application of Polypropylene: A Review. Discov. Nano 2024, 19, 2. [Google Scholar] [CrossRef]
- Wu, Q.; Li, D.; Liu, J.; Long, S.; Huang, Y.; Li, X. Antifouling PTFE Hollow Fiber Microfiltration Membrane with a Double-Defense Mechanism. Nano Lett. 2025, 25, 7081–7088. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance Properties and Applications of Polytetrafluoroethylene (PTFE)—A Review. Adv. Compos. Hybrid. Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, W.; Yu, S.; Zhu, Y.; Zhang, R.; Li, L. Optimization of Cleaning Conditions on Polytetrafluoroethylene (PTFE) Microfiltration Membrane Used in Treatment of Oil-Field Wastewater. RSC Adv. 2015, 5, 104960–104971. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Ultrafiltration for Separation of Glycerol Solution Fermented by Bacteria. Pol. J. Chem. Technol. 2013, 15, 115–120. [Google Scholar] [CrossRef]
- Tomczak, W.; Grubecki, I.; Gryta, M. The Use of NaOH Solutions for Fouling Control in a Membrane Bioreactor: A Feasibility Study. Membranes 2021, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Hermia, J. Constant pressure blocking filtration laws-application to power-law non-newtonian fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Tomczak, W. Fouling of the Nanofiltration Membrane NF270 Used for Separation of Fermentation Broths: Impact of Feed Pretreatment Process. Processes 2023, 11, 817. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Dahiya, D.; Kumar, M.; Pugazhenthi, G.; Vasanth, D. Separation of Bacteria Kocuria Rhizophila from Fermentation Broth by Cross-Flow Microfiltration Using Inexpensive Tubular Ceramic Membrane. Arab. J. Sci. Eng. 2022, 47, 5767–5776. [Google Scholar] [CrossRef]
- Han, Q.; Lay, H.T.; Li, W.; Chew, J.W. Effect of Initial Particle Deposition Rate on Cake Formation during Dead-End Microfiltration. J. Membr. Sci. 2021, 618, 118672. [Google Scholar] [CrossRef]
- Graves, K.; Rozeboom, G.; Heng, M.; Glatz, C. Broth Conditions Determining Specific Cake Resistance during Microfiltration of Bacillus subtilis. Biotechnol. Bioeng. 2006, 94, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, N.; Tomimatsu, K.; Date, K.; Iritani, E. Yeast Cell Cake Characterization in Alcohol Solution for Efficient Microfiltration. Membranes 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Arkhangelsky, E.; Bazarbayeva, A.; Kamal, A.; Kim, J.; Inglezakis, V.; Gitis, V. Tangential Streaming Potential, Transmembrane Flux, and Chemical Cleaning of Ultrafiltration Membranes. Sep. Purif. Technol. 2021, 258, 118045. [Google Scholar] [CrossRef]
- Huang, J.; Luo, J.; Chen, X.; Feng, S.; Wan, Y. How Do Chemical Cleaning Agents Act on Polyamide Nanofiltration Membrane and Fouling Layer? Ind. Eng. Chem. Res. 2020, 59, 17653–17670. [Google Scholar] [CrossRef]
- Qi, L.; Hu, Y.; Chai, Q.; Wang, Q. Enhanced Filtration Performance and Anti-Biofouling Properties of Antibacterial Polyethersulfone Membrane for Fermentation Broth Concentration. J. Ind. Eng. Chem. 2019, 72, 346–353. [Google Scholar] [CrossRef]




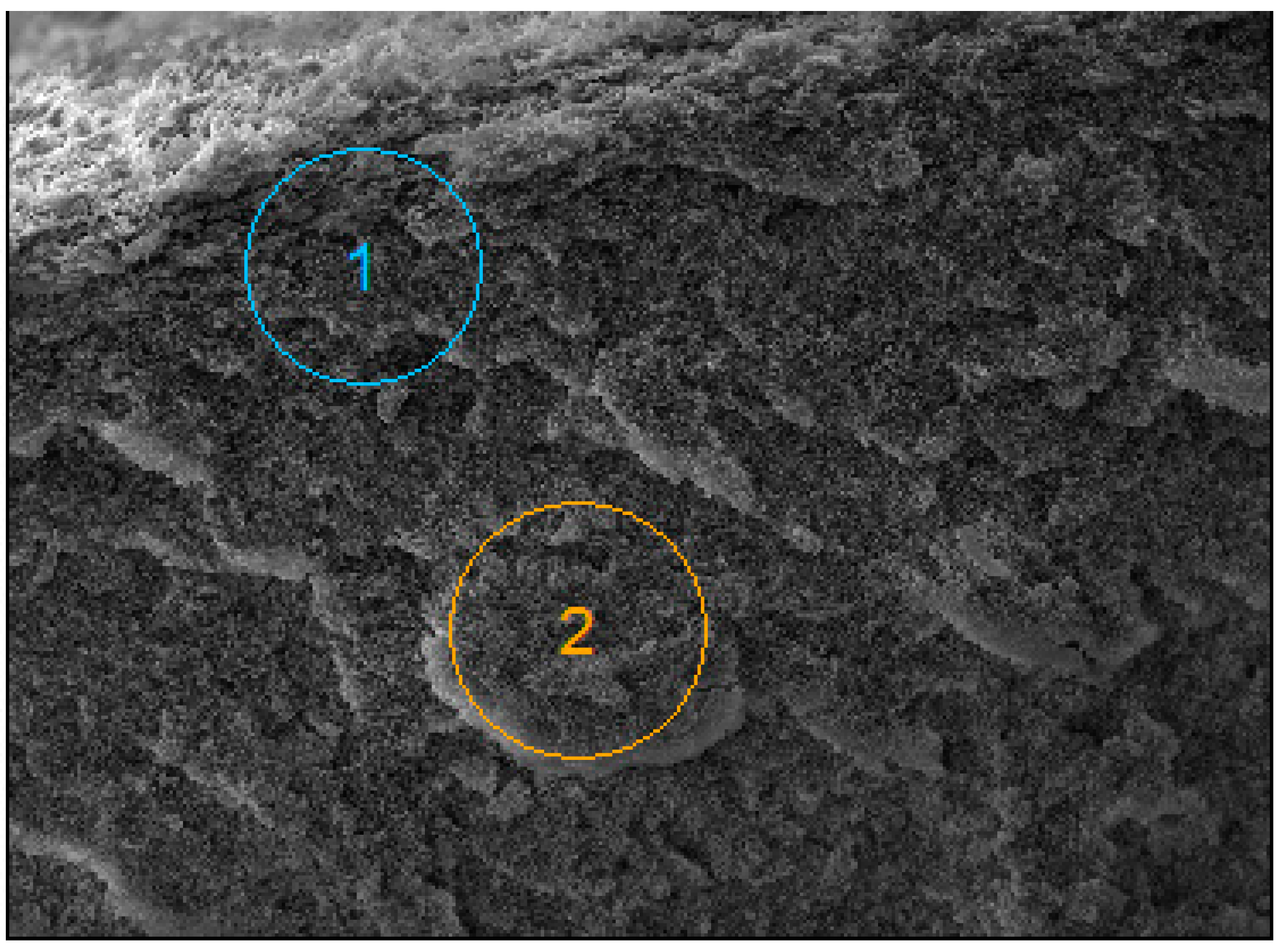
| Component [g/L] | B1 | B3 | B2 | B4 |
|---|---|---|---|---|
| 1,3-propanediol | 3.51 | 7.97 | 2.65 | 10.98 |
| glycerol | 5.23 | 2.87 | 5.56 | 0.25 |
| lactic acid | 0.69 | 2.48 | 0.77 | 1.67 |
| acetic acid | 0.81 | 1.52 | 0.92 | 2.63 |
| succinic acid | 0.31 | 0.78 | 0.33 | 1.43 |
| Cl− | 0.04 | 0.10 | 0.04 | 0.04 |
| PO43− | 2.31 | 2.39 | 2.51 | 2.04 |
| SO42− | 1.42 | 1.75 | 1.54 | 1.53 |
| NH4+ | 0.57 | 0.58 | 0.59 | 0.56 |
| K+ | 1.67 | 2.10 | 1.83 | 1.36 |
| Na+ | 0.39 | 2.10 | 0.56 | 2.63 |
| Ca2+ | 0.02 | 0.05 | 0.03 | 0.03 |
| Mg2+ | 0.05 | 0.04 | 0.04 | 0.03 |
| pH | 5.55 | 6.69 | 5.21 | 7.02 |
| Step | Cleaning Agent | Additional Information |
|---|---|---|
| 1 | water | After MF of the broth (3–4 h), the membrane module was flushed with water (3 L passed through the system). Subsequently, the module was supplied with distilled water, and the water flux was determined. |
| 2 | 1% NaOH solution | A total of 2 L of the 1% NaOH solution was recirculated for 10 min, after which it was flushed with 3 L of water. Then, the system was fed with distilled water, and MF was carried out to determine the water flux. |
| Point | C | N | O | Mg | Si | K | Fe |
|---|---|---|---|---|---|---|---|
| 1 | 47.80 | - | 43.20 | 0.51 | 4.45 | 2.46 | 1.28 |
| 2 | 36.59 | 7.87 | 44.50 | 0.18 | 9.88 | 0.98 | - |
| 3 | 68.13 | - | 27.23 | 0.52 | 4.12 | - | - |
| Point | C | O | Na | Mg | Si | Cl | Na |
|---|---|---|---|---|---|---|---|
| 1 | 83.72 | 8.35 | 2.09 | 0.10 | 0.35 | 3.20 | 2.18 |
| 2 | 99.10 | - | 0.38 | 0.09 | 0.09 | 0.08 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. Microfiltration of Post-Fermentation Broths: Long-Term Studies on the Use of Modules with Polymeric Membranes. Membranes 2025, 15, 345. https://doi.org/10.3390/membranes15110345
Tomczak W, Gryta M. Microfiltration of Post-Fermentation Broths: Long-Term Studies on the Use of Modules with Polymeric Membranes. Membranes. 2025; 15(11):345. https://doi.org/10.3390/membranes15110345
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2025. "Microfiltration of Post-Fermentation Broths: Long-Term Studies on the Use of Modules with Polymeric Membranes" Membranes 15, no. 11: 345. https://doi.org/10.3390/membranes15110345
APA StyleTomczak, W., & Gryta, M. (2025). Microfiltration of Post-Fermentation Broths: Long-Term Studies on the Use of Modules with Polymeric Membranes. Membranes, 15(11), 345. https://doi.org/10.3390/membranes15110345







