Green and Sustainable Clay Ceramic Membrane Preparation and Application to Textile Wastewater Treatment for Color Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
- −
- −
- Almond shells. They were collected locally from agricultural and food waste. After washing and drying, they were ground into powder using an electric grinder and passed through a 33-mesh sieve.
- −
- Lime. It was locally sourced and obtained by calcining limestone in a traditional kiln.
2.2. Preparation of MK Membranes
2.3. Membrane Characterization
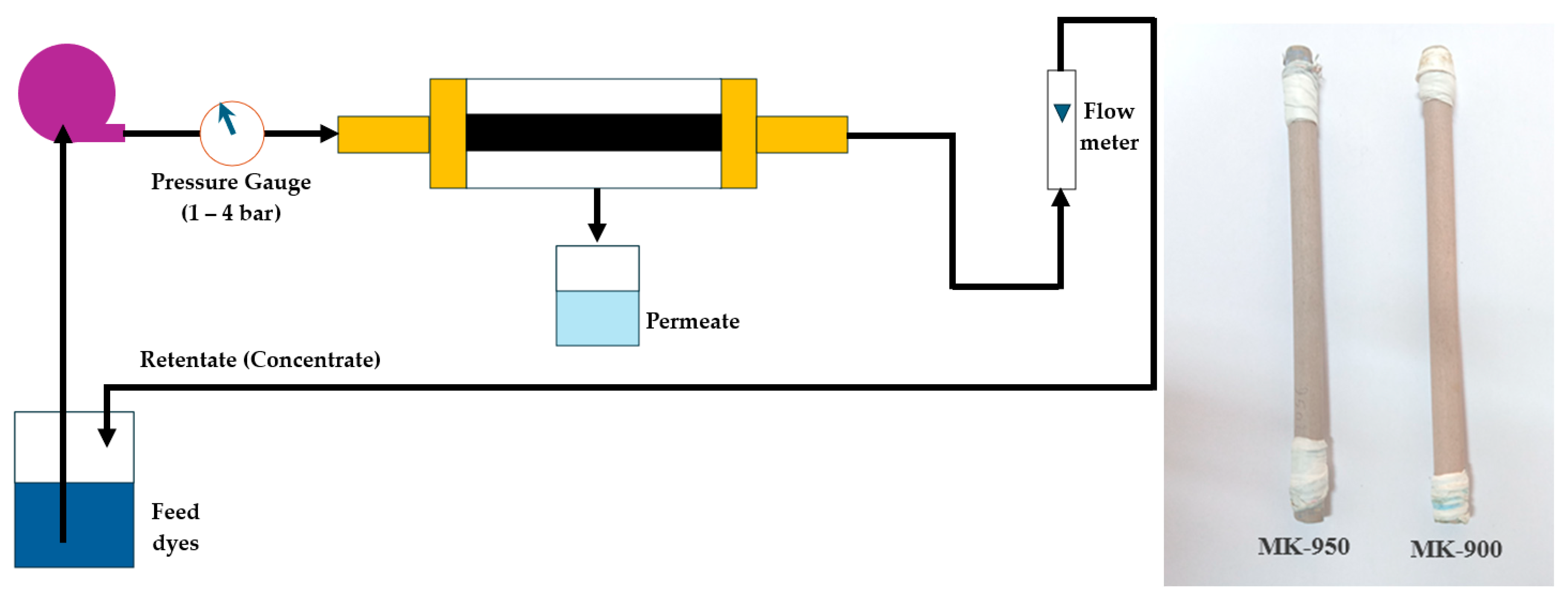
2.4. Filtration Performances of MK Membranes
2.4.1. Water Permeability
2.4.2. Application to Dye Removal from Textile Wastewater
2.5. Membrane Fouling
2.6. Membrane Regeneration
3. Results
3.1. Characterization of the Raw Materials
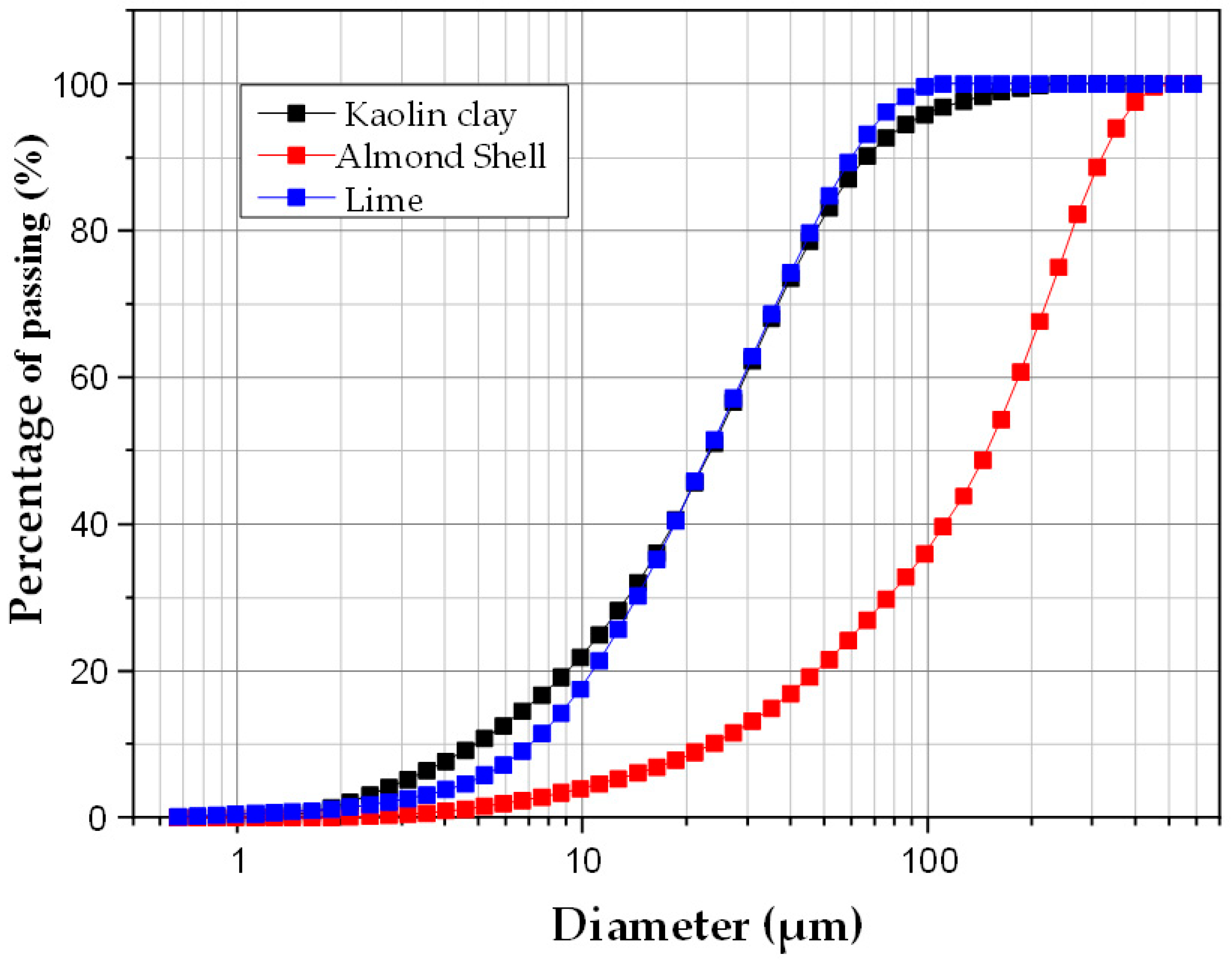
3.1.1. Particle Size Distribution
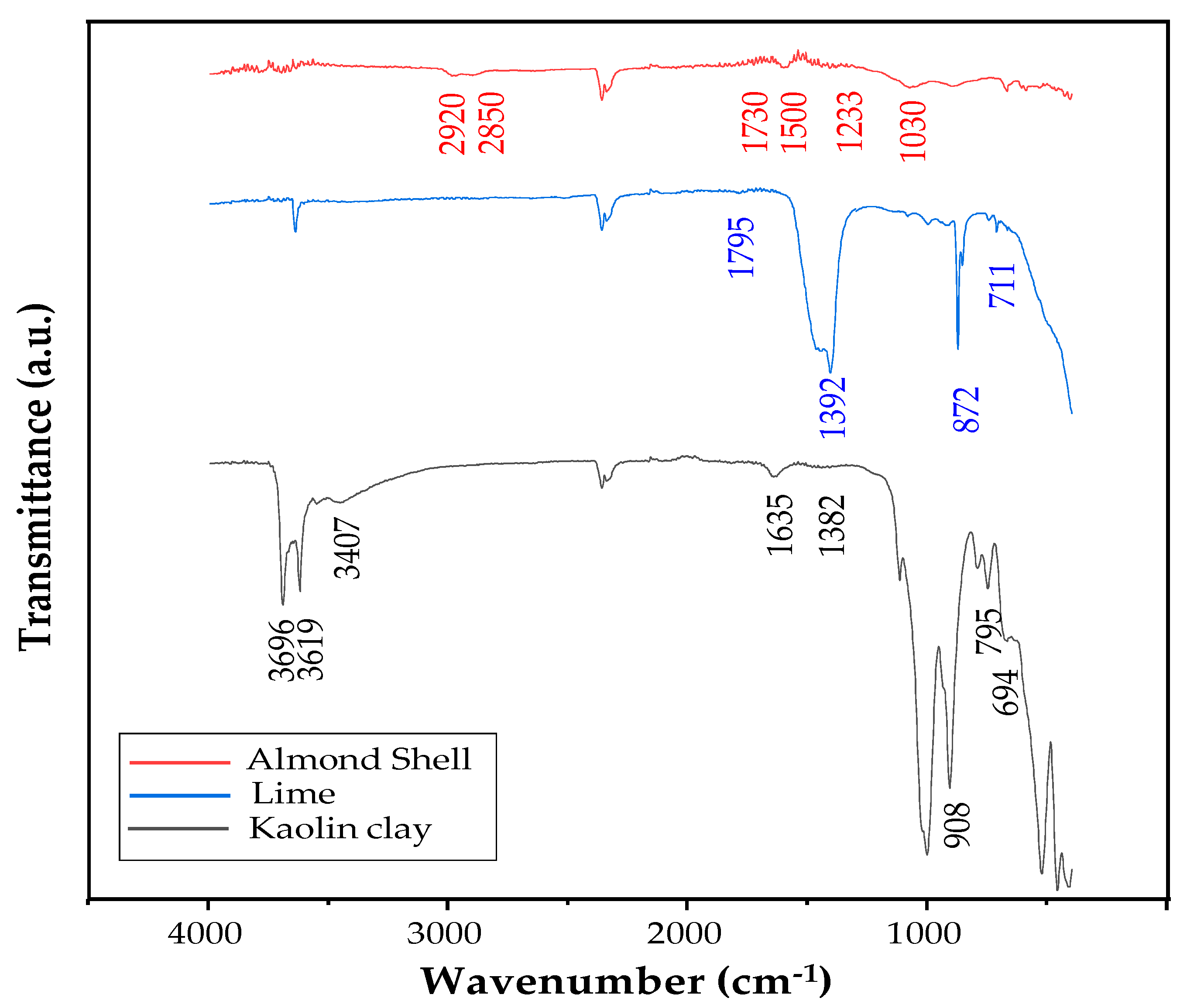
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
3.1.4. Thermogravimetric Analysis
3.2. Characterizations of the MK Membranes Prepared
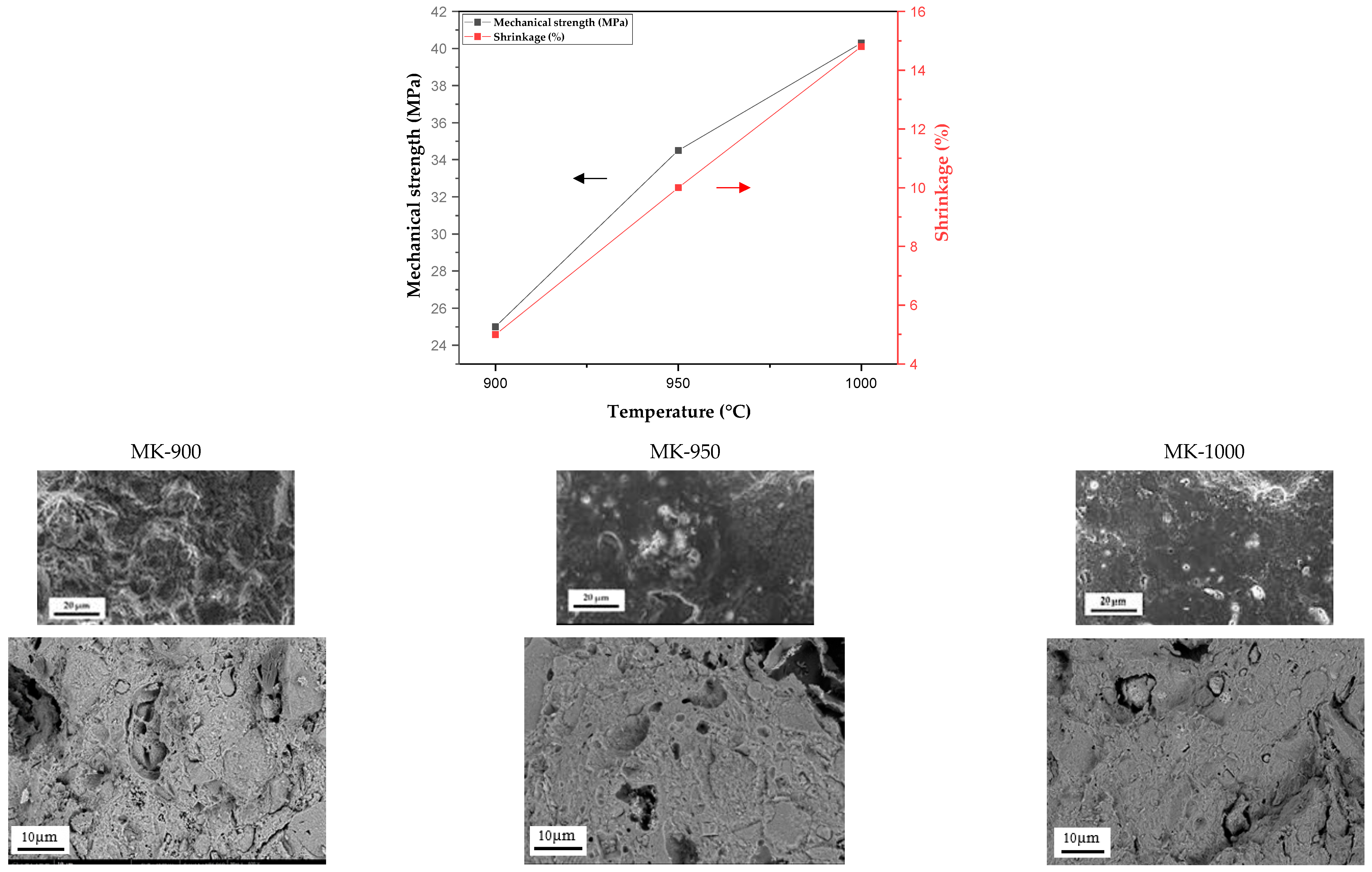
3.2.1. Shrinkage and Mechanical Strength
3.2.2. Chemical Stability
3.2.3. Porosity
3.2.4. Water Permeability and Pore Size Determination
3.3. Application to the Removal of Indigo Blue (IB) Dye
3.4. Fouling Study and Regeneration of Membrane Performance
3.4.1. Fouling Study
3.4.2. Regeneration of Membrane Performance After Successive Filtration of the Dye Solution
3.4.3. Cost Analysis
- Raw material procurement and modification,
- Shaping processes,
- Sintering energy requirements.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane Technology for Sustainable Water Resources Management: Challenges and Future Projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef]
- Favre, E. The Future of Membrane Separation Processes: A Prospective Analysis. Front. Chem. Eng. 2022, 4, 916054. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2019, 598, 117761. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Al-Juboori, R.A.; Khatri, M.; Ahmed, F.E.; Ibrahim, Y.; Hilal, N. Sustainability in Membrane Technology: Membrane Recycling and Fabrication Using Recycled Waste. Membranes 2024, 14, 52. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Al-Juboori, R.A.; Al Aani, S.; Ladewig, B.P.; Hilal, N. Natural and recycled materials for sustainable membrane modification: Recent trends and prospects. Sci. Total Environ. 2022, 838, 153014. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A 2019, 578, 12351. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef]
- Charcosset, C. Classical and Recent Applications of Membrane Processes in the Food Industry. Food Eng. Rev. 2021, 13, 322–343. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tian, T.F.; Liu, X.Q.; Meng, G.Y. Titania membrane preparation with chemical stability for very harsh environments applications. J. Membr. Sci. 2006, 280, 261–269. [Google Scholar] [CrossRef]
- Yoshino, Y.; Suzuki, T.; Nair, B.N.; Taguchi, H.; Itoh, N. Development of tubular substrates, silica based membranes and membrane modules for hydrogen separation at high temperature. J. Membr. Sci. 2005, 267, 8–17. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Zhang, G.; Yang, F.; Lin, S.; Dong, Y. Robust Zirconia Ceramic Membrane with Exceptional Performance for Purifying Nano-Emulsion Oily Wastewater. Water Res. 2022, 208, 117859. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Silva, K.R.; Menezes, R.R.; Campos, L.F.A.; Santana, L.N.L. A review on the production of porous ceramics using organic and inorganic industrial waste. Cerâmica 2022, 68, 270–284. [Google Scholar] [CrossRef]
- Jafari, B.; Rezaei, E.; Dianat, M.J.; Abbasi, M.; Hashemifard, S.A.; Khosravi, A.; Sillanpää, M. Development of a new composite ceramic membrane from mullite, silicon carbide and activated carbon for treating greywater. Ceram. Int. 2021, 47, 34667–34675. [Google Scholar] [CrossRef]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.; Ouammou, M.; Smith, A.; et al. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Harrati, A.; Arkame, Y.; Manni, A.; Aqdim, S.; Zmemla, R.; Chari, A.; El Bouari, A.; El Hassani, I.E.; Sdiri, A.; Hassani, F.O.; et al. Akermanite-based ceramics from Moroccan dolomite and perlite: Characterization and in vitro bioactivity assessment. Open Ceram. 2022, 10, 100276. [Google Scholar] [CrossRef]
- Samadi, A.; Gao, L.; Kong, L.; Orooji, Y.; Zhao, S. Waste-Derived Low-Cost Ceramic Membranes for Water Treatment: Opportunities, Challenges and Future Directions. Resour. Conserv. Recycl. 2022, 185, 106497. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Mohd, H.D.O.; Ismail, A.F.; Mukhlis, A.R.; Juhana, J.; Yuji, I.; Sawao, H.; Mohd, I.H.M.D.; Mohd, Z.M.Y. Fabrication of low cost, green silica based ceramic hollow fibre membrane prepared from waste rice husk for water filtration application. Ceram. Int. 2018, 44, 10498–10509. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Zawati, H.; Mohd, H.D.O.; Hubadillah, S.K.; Muhamad, Z.Y.; Ahmad, F.I. Morphology and property study of green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash (WSBA). Ceram. Int. 2018, 44, 18450–18461. [Google Scholar] [CrossRef]
- Mouiya, M.; Bouazizi, A.; Abourriche, A.; El Khessaimi, Y.; Benhammou, A.E.Y.; Taha, Y. Results in materials effect of sintering temperature on the microstructure and mechanical behavior of porous ceramics made from clay and banana peel powder. Results Mater. 2019, 4, 100028. [Google Scholar] [CrossRef]
- Kamgang-Syapnjeu, P.; Njoya, D.; Kamseu, E.; Cornette De Saint Cyr, L.; Marcano-Zerpa, A.; Balme, S.; Bechelany, M.; Soussan, L. Elaboration of a new ceramic membrane support from Cameroonian clays, coconut husks and eggshells: Application for Escherichia Coli bacteria retention. Appl. Clay Sci. 2020, 198, 105836. [Google Scholar] [CrossRef]
- Bisht, V.; Das, C. Fabrication and characterization of the novel tubular ceramic membrane using walnut shells and its application in TiO2 nanoparticles separation from suspension. Ceram. Int. 2024, 50, 8706–8717. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Achour, S.; Larbot, A. Porousceramic supports for membranes prepared from kaolinand doloma mixtures. J. Eur. Ceram. Soc. 2006, 26, 1663–1671. [Google Scholar] [CrossRef]
- Ahmed, N.; Mir, F.Q. Preparation and characterization of ceramic membrane using waste almond shells as pore forming agent. Mater. Today Proc. 2021, 47, 1485–1489. [Google Scholar] [CrossRef]
- Rahim, F.A.M.; Noh, M.Z.; Rashid, M.W.A.; Mohamed, J.J.; Nor, M.A.A.M. Preparation and characterization of ceramic membrane by using palm fibers as pore forming agent. In Materials Characterization Using X-Rays and Related Techniques, Proceedings of the AIP Conference Proceedings, Kelantan, Malaysia, 18–19 August 2018; AIP Publishing: Melville, NY, USA, 2019; Volume 2068. [Google Scholar] [CrossRef]
- Guechi, A.; Harabi, A.; Condom, S.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Foughali, L. Elaboration and characterization of tubular supports for membranes filtration. Desalin. Water Treat. 2016, 57, 5246–5252. [Google Scholar] [CrossRef]
- Bouzid Rekik, S.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development and characterization of porous membranes based on kaolin/chitosan composite. Appl. Clay Sci. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Bousbih, S.; Errais, E.; Darragi, F.; Duplay, J.; Trabelsi-Ayadi, M.; Daramola, M.O.; Ben Amar, R. Treatment of Textile Wastewater using monolayered Ultrafiltration Ceramic Membrane Fabricated from Natural Kaolin Clay. Environ. Technol. 2020, 42, 3348–3359. [Google Scholar] [CrossRef]
- Mouratib, R.; Achiou, B.; El Krati, M.; AlimiYounssi, S.; Tahiri, S. Low-cost ceramic membrane made from alumina- and silica-rich water treatment sludge and its application to wastewater filtration. J. Eur. Ceram. Soc. 2020, 40, 5942–5950. [Google Scholar] [CrossRef]
- Halliday, D.; Resnick, R.; Walker, J. Principe d’Archimède, chapitre sur la statique des fluides. In Fundamentals of Physics, 6th ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Huang, H.; Yu, J.; Guo, H.; Shen, Y.; Yang, F.; Wang, H.; Liu, R.; Liu, Y. Improved antifouling performance of ultrafiltration membrane via preparing novel zwitterionic polyimide. Appl. Surf. Sci. 2018, 427, 38–47. [Google Scholar] [CrossRef]
- Ouallal, H.; Azrour, M.; Messaoudi, M.; Moussout, H.; Messaoudi, L.; Tijani, N. Incorporation effect of olive pomace on the properties of tubular membranes. J. Environ. Chem. Eng. 2020, 8, 103668. [Google Scholar] [CrossRef]
- Shen, X.; Tiande, X.; Jiangang, W.; Fan, W. Improved fouling resistance of poly (vinylidene fluoride) membrane modified with poly (acryloylmorpholine)-based amphiphilic copolymer. Colloid Polym. Sci. 2017, 295, 1211–1221. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Jadda, A.; Chakraborty, S.; Amar, B.R. Characterization of an Asymmetric Ultrafiltration Membrane Prepared from TiO2-Smectite Nanocomposites Doped with Commercial TiO2 and Its Application to the Treatment of Textile Wastewater. Euro-Mediterr. J. Environ. Integr. 2020, 5, 10. [Google Scholar] [CrossRef]
- Mountoumnjou, O.; Szymczyk, A.; Lyonga Mbambyah, E.E.; Njoya, D.; Elimbi, A. New Low-Cost Ceramic Microfiltration Membranes for Bacteria Removal. Membranes 2022, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Effect of carbonates composition on the permeation characteristics of low-cost ceramic membrane supports. J. Ind. Eng. Chem. 2016, 44, 185–194. [Google Scholar] [CrossRef]
- Malathy, R.; Shanmugam, R.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Mechanical and Microstructural Properties of Composite Mortars with Lime, Silica Fume and Rice Husk Ash. Processes 2022, 10, 1424. [Google Scholar] [CrossRef]
- Mäkelä, M.; Paananen, T.; Kokkonen, T.; Makkonen, H.; Heino, J.; Dahl, O. Preliminary Evaluation of Fly Ash and Lime for Use as Supplementary Cementing Materials in Cold-Agglomerated Blast Furnace Briquetting. ISIJ Int. 2011, 51, 776–781. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Azenha, M.; Lourenço, P.B.; Meneghini, A.; Guimarães, E.T.; Castro, F.; Soares, D. Experimental analysis of the carbonation and humidity diffusion processes in aerial lime mortar. Constr. Build. Mater. 2017, 148, 38–48. [Google Scholar] [CrossRef]
- El-Habacha, M.; Dabagh, A.; Lagdali, S.; Miyah, Y.; Mahmoudy, G.; Sinan, F.; Chiban, M.; Iaich, S.; Zerbet, M. An efficient and adsorption of methylene blue dye on a natural clay surface: Modeling and equilibrium studies. Environ. Sci. Pollut. Res. 2023, 31, 62065–62079. [Google Scholar] [CrossRef] [PubMed]
- Elomari, H.; Achiou, B.; Beqqour, D.; Khaless, K.; Beniazza, R.; Ouammou, M.; Aaddane, A.; Younssi, S.A.; Benhida, R. Preparation and characterization of low-cost zirconia/clay membrane for removal of acid orange 74 dye. Mater. Today Proc. 2022, 51, 1948–1956. [Google Scholar] [CrossRef]
- Saffaj, N.; Persin, M.; Alami Younssi, S.; Albizane, A.; Cretin, M.; Larbot, A. Elaboration and characterization of microfiltration and ultrafiltration membranes deposited on raw support prepared from natural Moroccan clay: Application to filtration of solution containing dyes and salts. Appl. Clay Sci. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Beicha, A.; Zaamouche, R.; Sulaiman, N.M. Dynamic ultrafiltration model based on concentration polarization-cake layer interplay. Desalination 2009, 242, 138–148. [Google Scholar] [CrossRef]
- Khmiri, Y.; Attia, A.; Elboughdiri, N.; Ghernaout, D.; Charcosset, C.; Dammak, L.; Ben Amar, R. Preparing Sustainable Membranes Made From Zeolite–Smectite for Treating Textile Wastewater and Pulp Industry Wastewater. ChemistrySelect 2024, 9, e202404190. [Google Scholar] [CrossRef]
- Aloulou, H.; Aloulou, W.; Duplay, J.; Baklouti, L.; Dammak, L.; Ben Amar, R. Development of ultrafiltration kaolin membranes over sand and zeolite supports for the treatment of electroplating wastewater. Membranes 2022, 12, 1066. [Google Scholar] [CrossRef]
- Bahrouni, J.; Aloulou, H.; Attia, A.; Dammak, L.; Ben Amar, R. Surface modification of a zeolite microfiltration membrane: Characterization and application to the treatment of colored and oily wastewaters. Chem. Afr. 2024, 7, 4513–4527. [Google Scholar] [CrossRef]


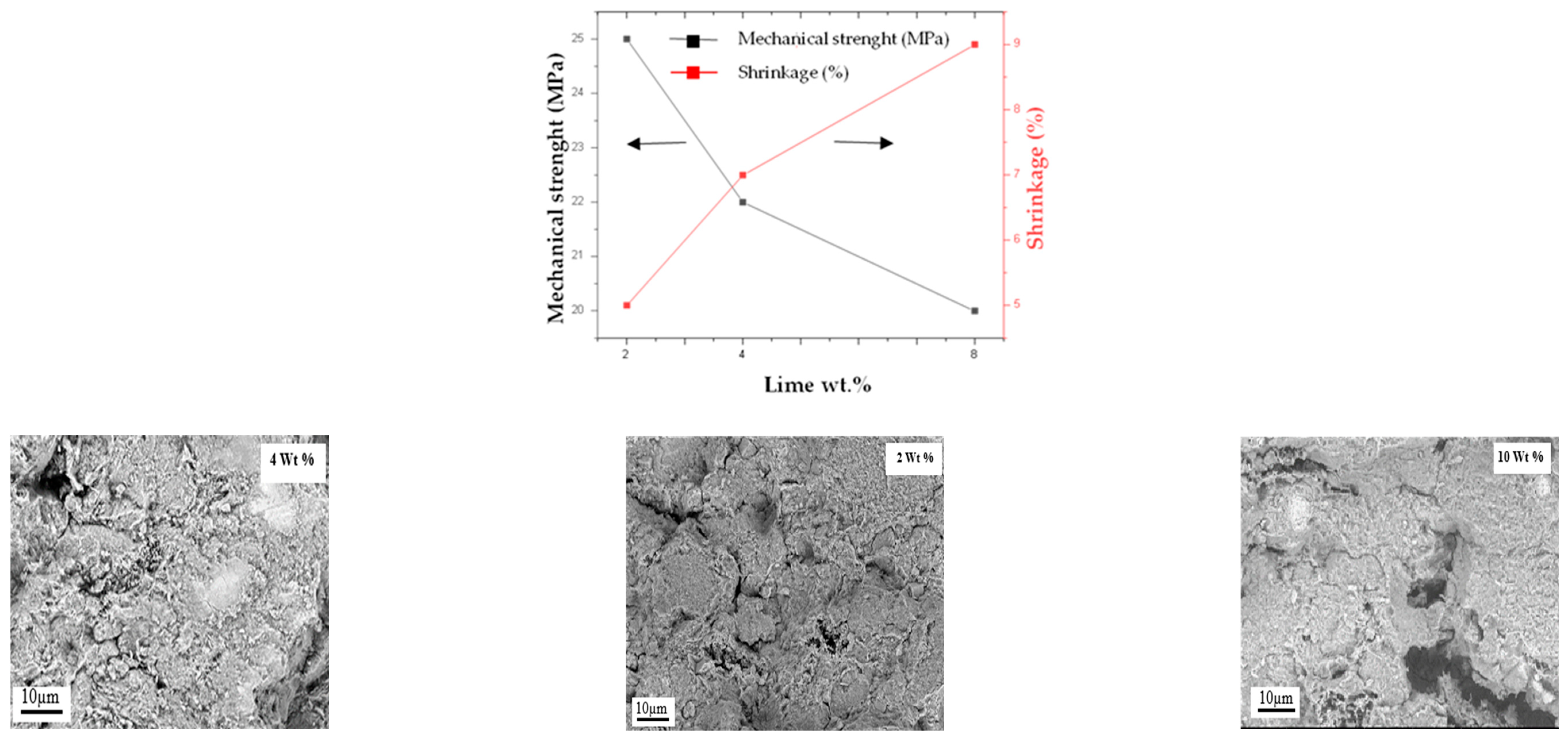
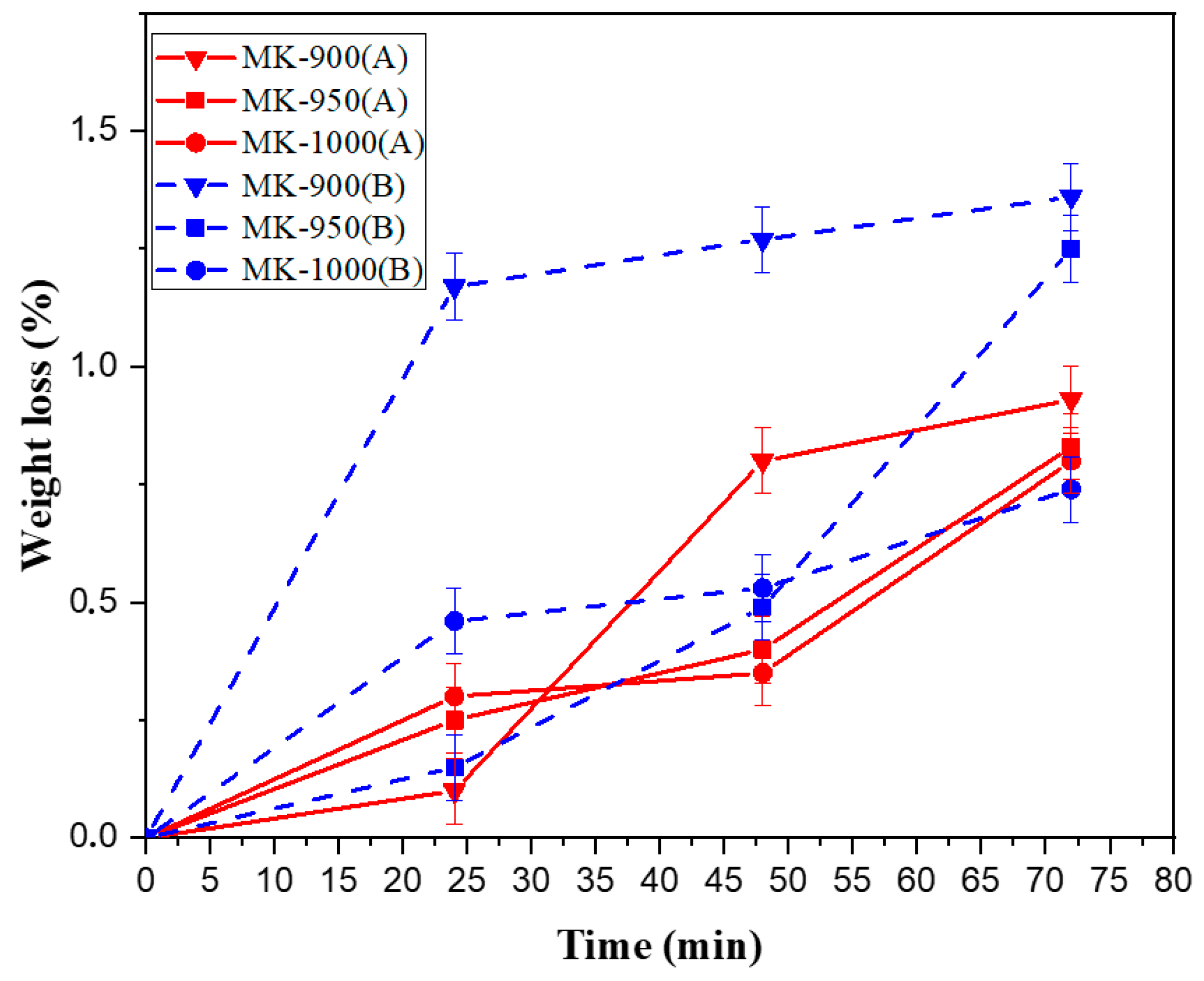


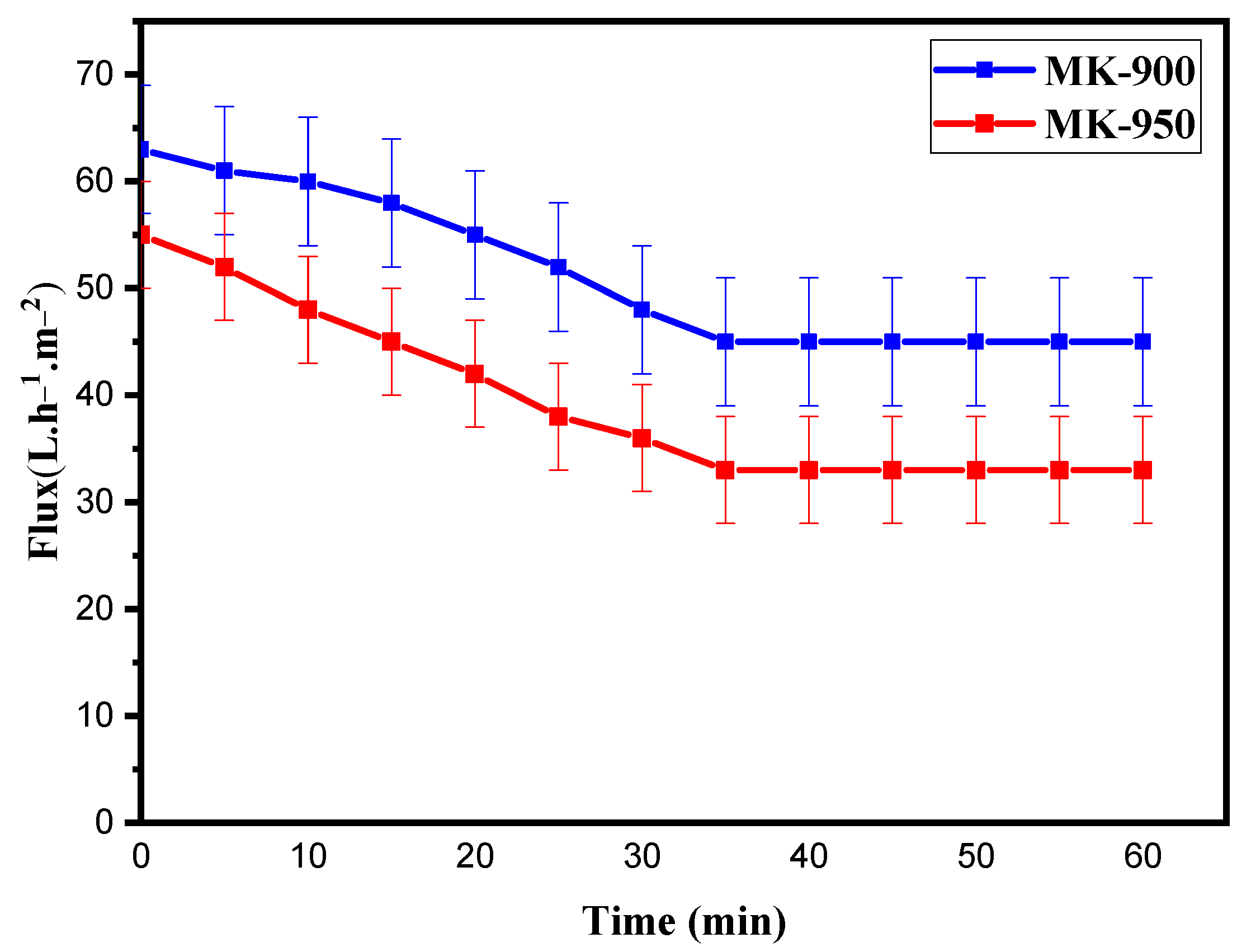
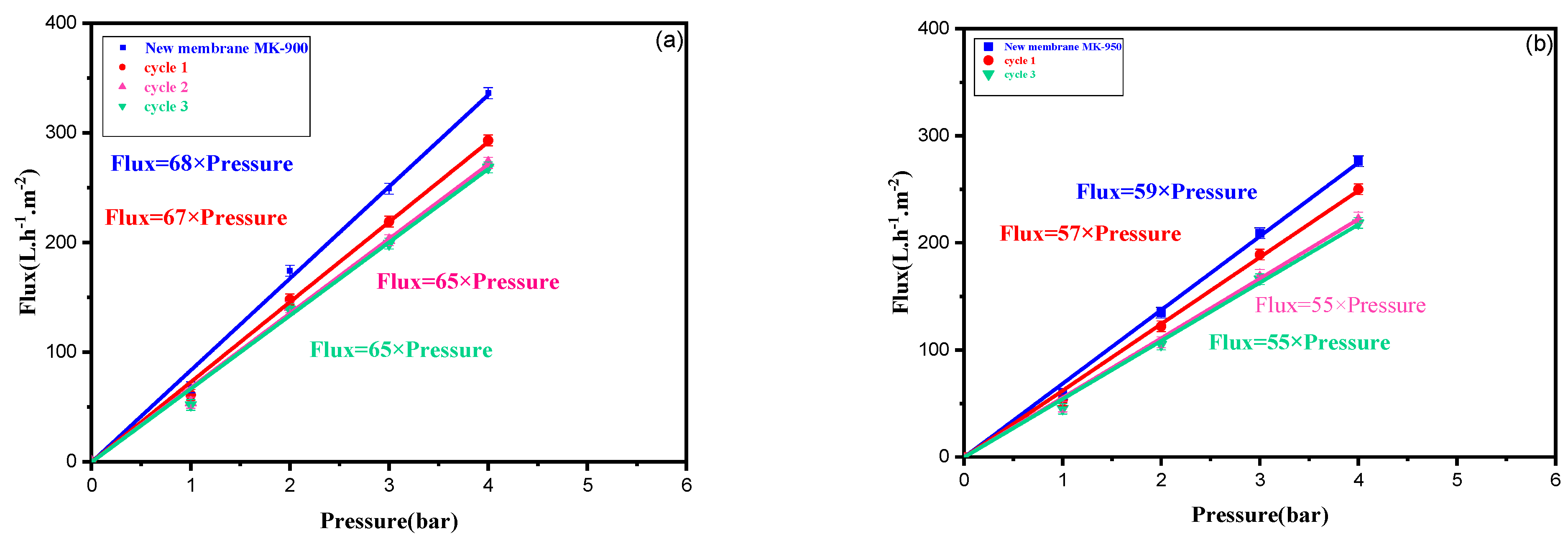
| SiO2 | Al2O3 | K2O | CaO | Fe2O3 | MgO | TiO2 | LOI 1 |
|---|---|---|---|---|---|---|---|
| 42.96 | 37.70 | 0.94 | 0.74 | 0.32 | 0.23 | 0.03 | 16.50 |
| Tsintering (°C) | 900 | 950 | 1000 |
|---|---|---|---|
| Shrinkage (%) | 5 | 10 | 15 |
| Mechanical strength (MPa) | 25 | 35 | 40 |
| Thickness (mm) | 4.2 | 4.0 | 3.6 |
| Porosity (%) | 30 | 27 | 26 |
| Water permeability (Lh−1m−2) | 68 | 59 | - |
| Average pore size (nm) | 42 | 44 | - |
| Turbidity (NTU) | R (%) | Color (Abs610) | R (%) | COD (mg/L) | R (%) | ||
|---|---|---|---|---|---|---|---|
| Feed | 187 | - | 1.132 | - | 147 | - | |
| Permeate | MK-900 | 4 | 98 | 0.248 | 78 | 35 | 76 |
| MK-950 | 2 | 99 | 0.023 | 92 | 15 | 90 | |
| Membrane | Jw1 (Lm−2h−1) | Jwf (Lm−2h−1) | Jw2 (Lm−2h−1) | FRR (%) | Rt (%) | Rr (%) | Rir (%) |
|---|---|---|---|---|---|---|---|
| MK-900 | 68 | 46 | 59 | 86 | 32 | 19 | 13 |
| MK-950 | 59 | 33 | 46 | 79 | 44 | 22 | 22 |
| Lp (L m−2 h−1 bar−1) | Virgin | After Cycle 1 | Change (%) | After Cycle 2 | Change (%) | After Cycle 3 | Change (%) | Total Change (%) |
|---|---|---|---|---|---|---|---|---|
| MK-900 | 68 | 67 | 1.5 | 65 | 3.0 | 65 | <0.5 | <5 |
| MK-950 | 59 | 57 | 3.3 | 55 | 3.6 | 55 | <0.5 | <7.5 |
| Price of Raw Materials | |||
|---|---|---|---|
| Material | Unit per Kg ($) | Amount of Raw Material (g) | Price ($) |
| kaolin powder | 0.16 | 384 | 0.0614 |
| Lime | 1 | 8 | 0.008 |
| Distilled water | 0.28 | 200 | 0.056 |
| Almond shells | - | 8 | - |
| Total raw materials cost for the fabrication of 15 membranes | 0.1254 | ||
| Energy cost (Based on the power consumption) | |||
| Mixer | 0.031 | ||
| Dry oven | 0.027 | ||
| Extruder | 0.138 | ||
| Furnace | 0.086 | ||
| Total production cost for the fabrication of 15 membranes ($) | 0.4074 | ||
| (Surface of membrane = 1.7 × 10−3 m2) | |||
| Total production cost of the (MK) Kaolin membrane ($m−2) | 15.976 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrouni, J.; Attia, A.; Elberrichi, F.Z.; Dammak, L.; Baklouti, L.; Ben Aissa, M.-A.; Ben Amar, R.; Deratani, A. Green and Sustainable Clay Ceramic Membrane Preparation and Application to Textile Wastewater Treatment for Color Removal. Membranes 2025, 15, 292. https://doi.org/10.3390/membranes15100292
Bahrouni J, Attia A, Elberrichi FZ, Dammak L, Baklouti L, Ben Aissa M-A, Ben Amar R, Deratani A. Green and Sustainable Clay Ceramic Membrane Preparation and Application to Textile Wastewater Treatment for Color Removal. Membranes. 2025; 15(10):292. https://doi.org/10.3390/membranes15100292
Chicago/Turabian StyleBahrouni, Jamila, Afef Attia, Fatima Zohra Elberrichi, Lasâad Dammak, Lassaad Baklouti, Mohamed-Ali Ben Aissa, Raja Ben Amar, and Andre Deratani. 2025. "Green and Sustainable Clay Ceramic Membrane Preparation and Application to Textile Wastewater Treatment for Color Removal" Membranes 15, no. 10: 292. https://doi.org/10.3390/membranes15100292
APA StyleBahrouni, J., Attia, A., Elberrichi, F. Z., Dammak, L., Baklouti, L., Ben Aissa, M.-A., Ben Amar, R., & Deratani, A. (2025). Green and Sustainable Clay Ceramic Membrane Preparation and Application to Textile Wastewater Treatment for Color Removal. Membranes, 15(10), 292. https://doi.org/10.3390/membranes15100292







