Tuning the Properties of Polyvinylidene Fluoride/Alkali Lignin Membranes to Develop a Biocatalytic Membrane Reactor for an Organophosphorus Pesticide Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation and Functionalization
2.2.1. Membrane Preparation
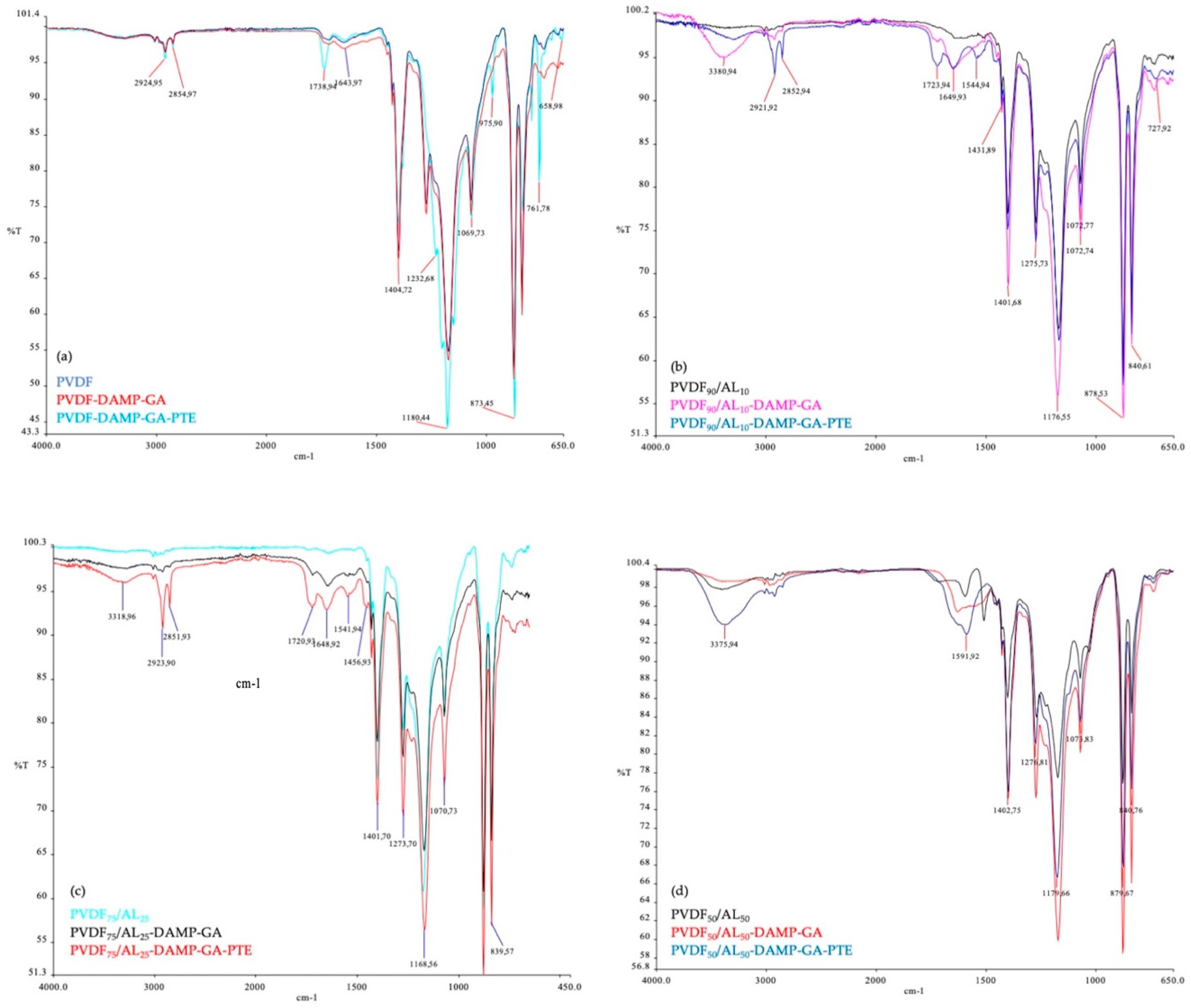
2.2.2. Membrane Chemical Functionalization and Enzyme Immobilization
2.3. Membrane Characterization
2.4. Activity Assays and Enzyme Stability Studies
2.5. Biocatalytic Membrane Reactor Set-Up
2.6. Data Reproducibility
3. Results and Discussion
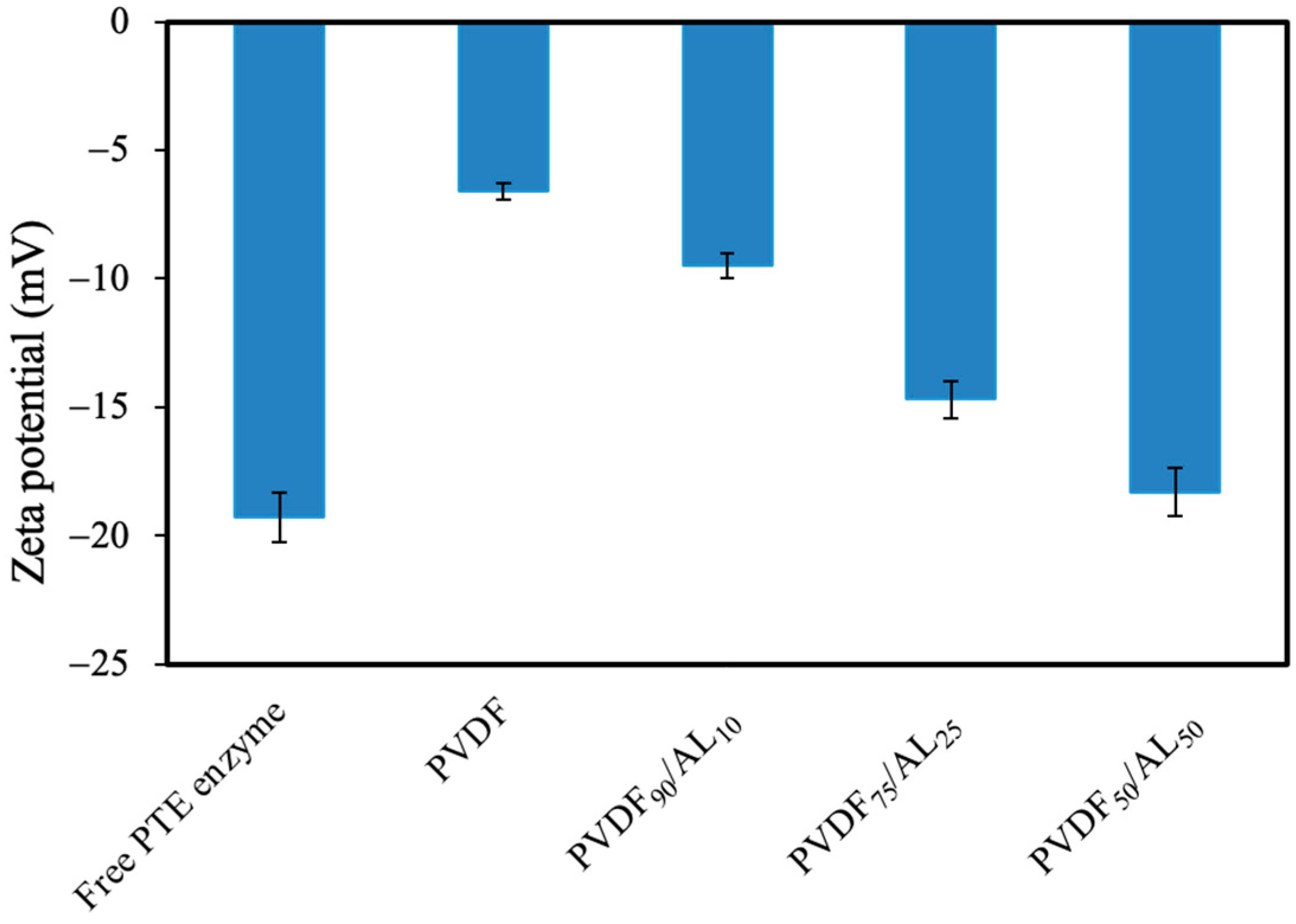
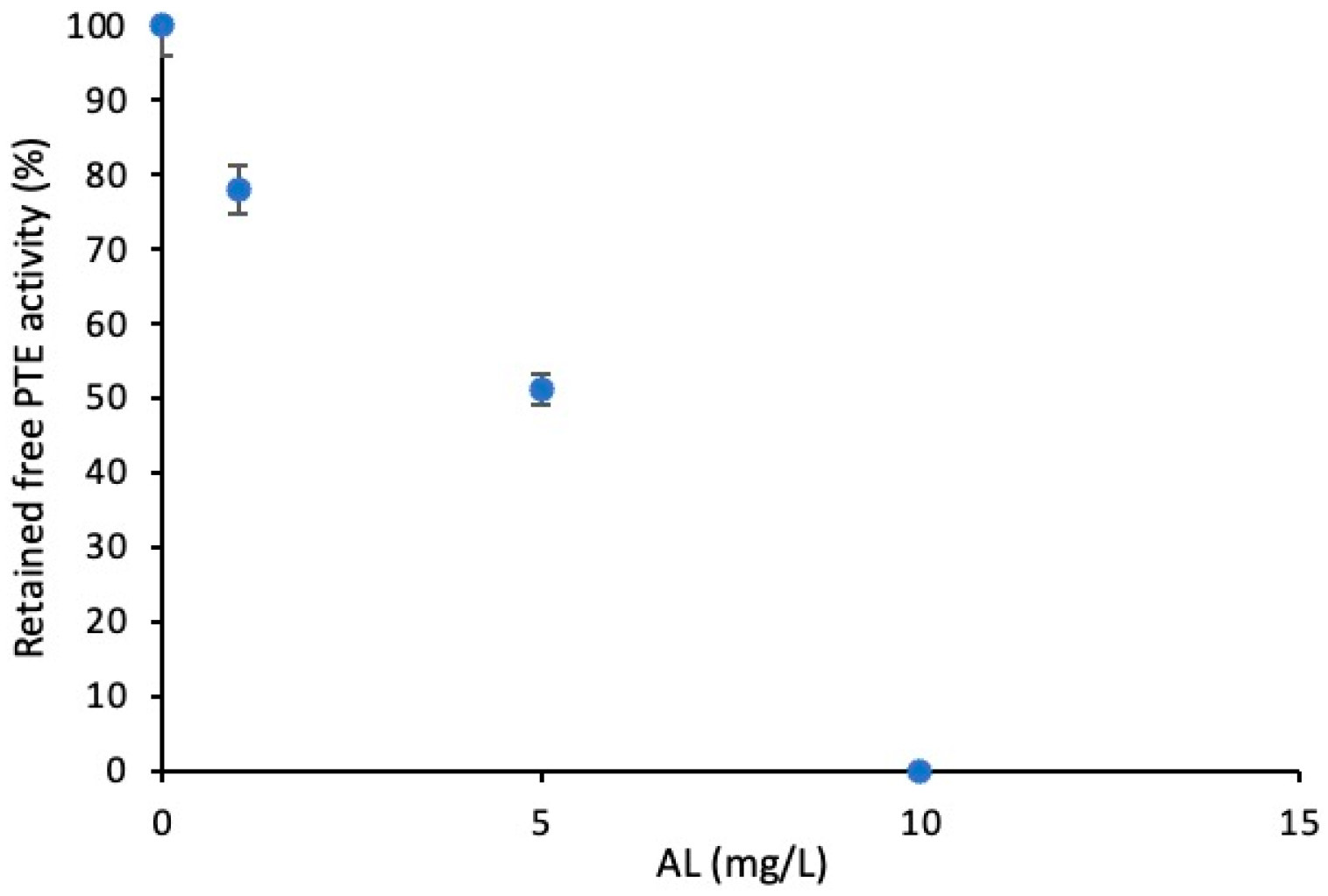
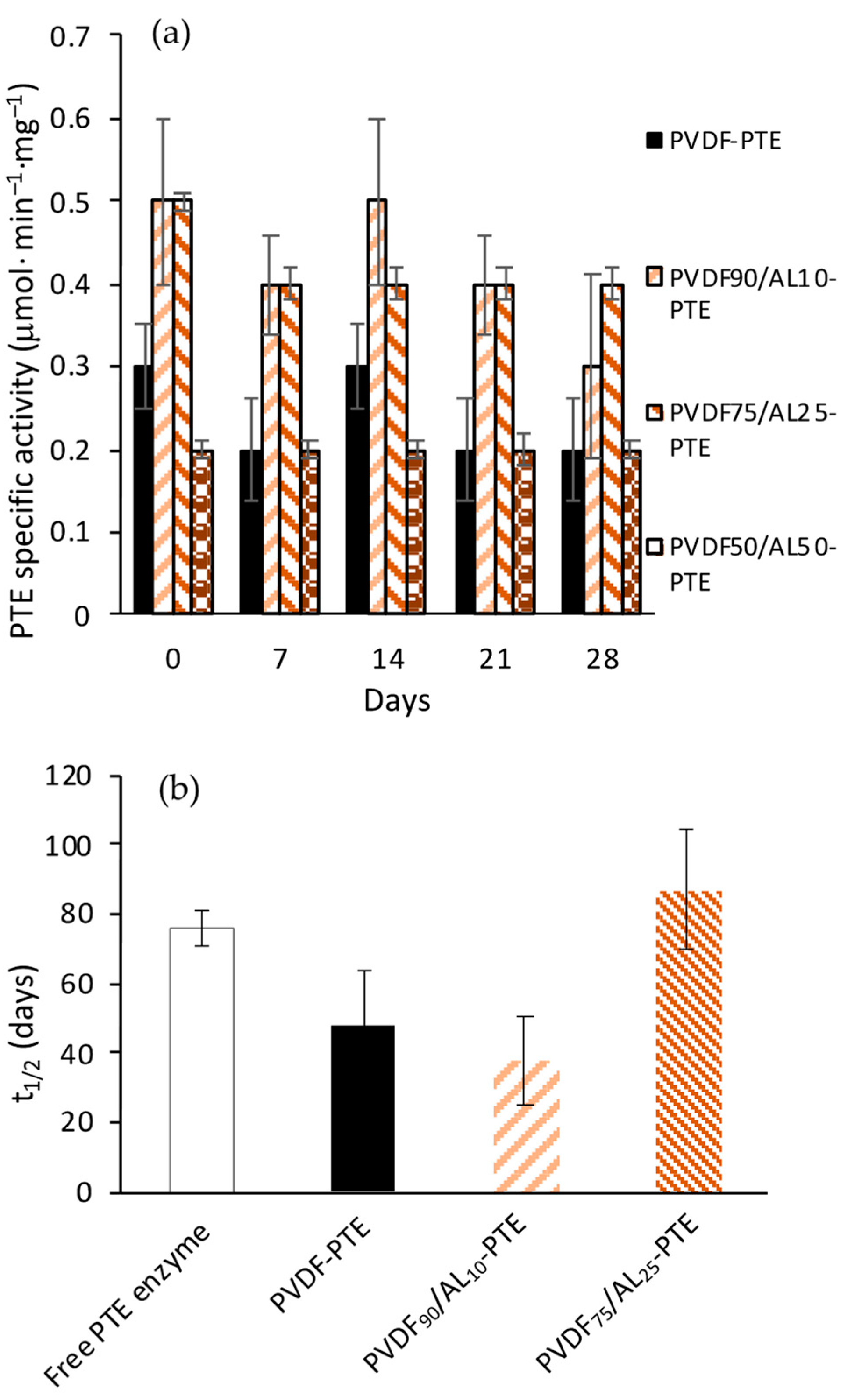
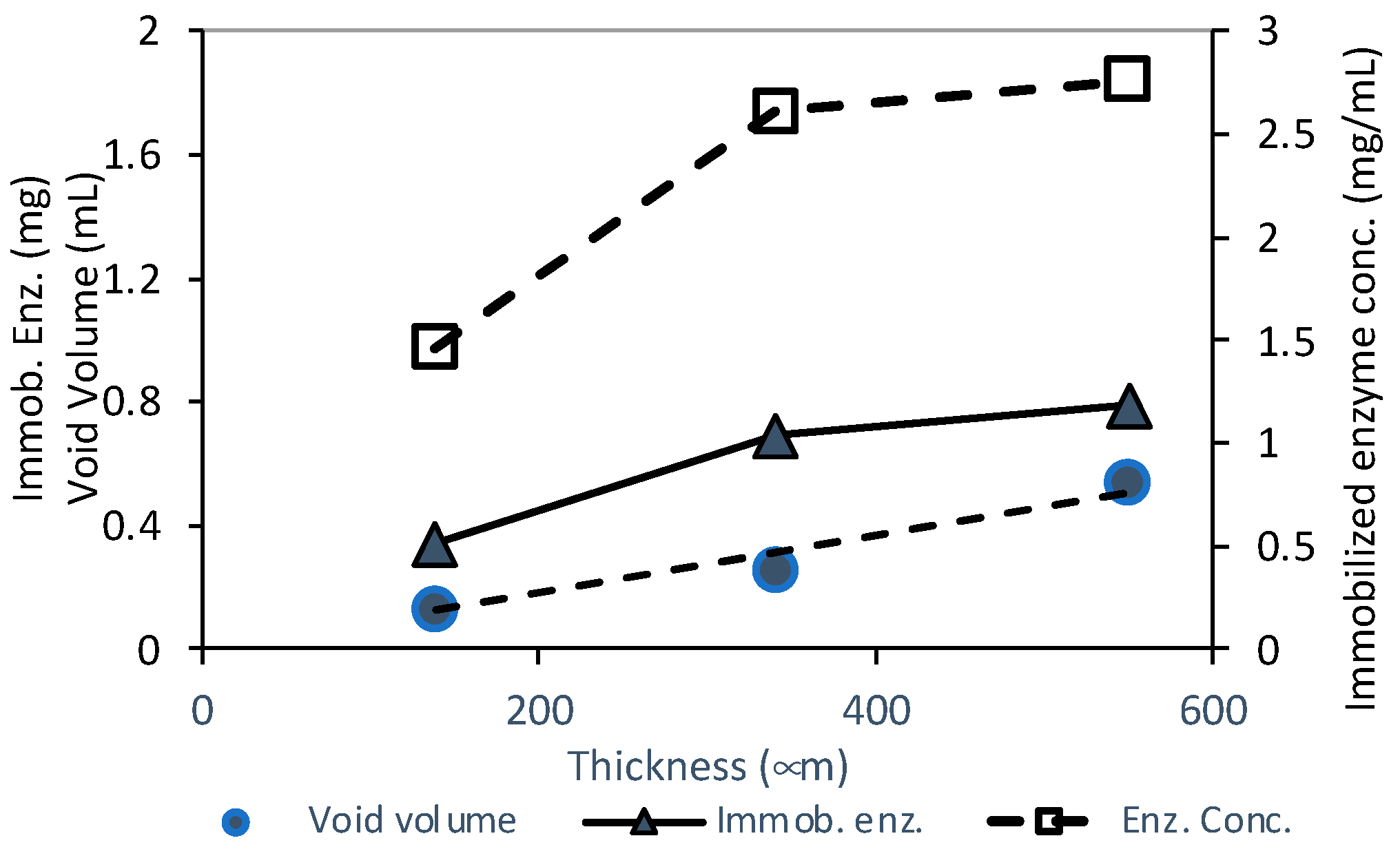
3.1. Screening of PVDF/AL Ratios on Enzyme-Loaded Membranes’ Performance
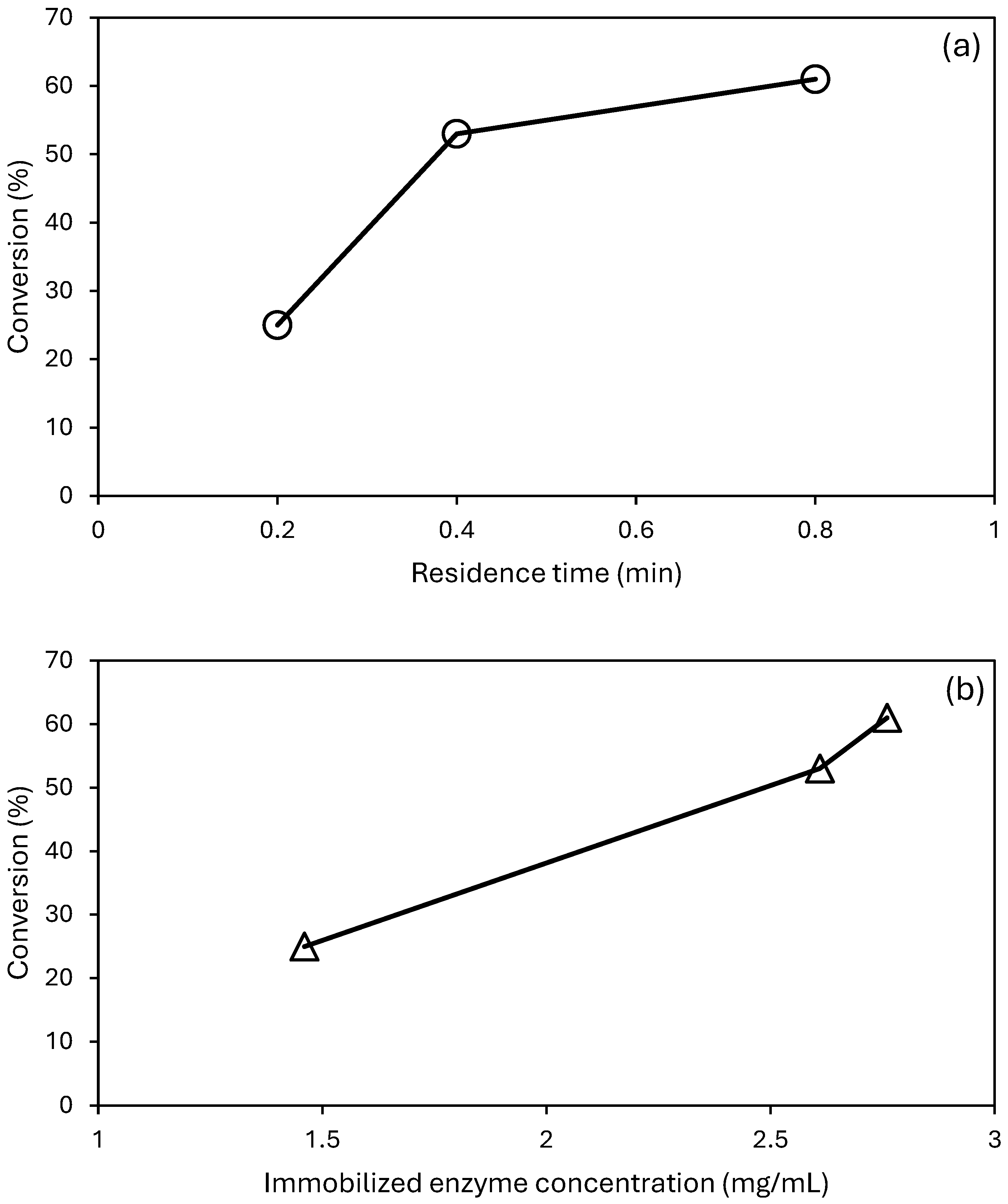
3.2. Paraoxon-Ethyl Degradation by BMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fane, A.G. Membrane bioreactors: Design & operational options. Filtr. Sep. 2002, 39, 26–29. [Google Scholar]
- Luo, J.; Song, S.; Zhang, H.; Zhang, H.; Zhang, J.; Wan, Y. Biocatalytic membrane: Go far beyond enzyme immobilization. Eng. Life Sci. 2020, 20, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.K.; Liu, Y.X.; Xue, Y.P.; Zheng, Y.G. Immobilization of enzymes in/on membranes and their applications. Adv. Synth. Catal. 2019, 361, 5500–5515. [Google Scholar] [CrossRef]
- Handayani, N.; Loos, K.; Wahyuningrum, D.; Buchari Zulfikar, M.A. Immobilization of Mucor miehei lipase onto macroporous aminated polyethersulfone membrane for enzymatic reactions. Membranes 2012, 2, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. 2011, 13, 1609–1623. [Google Scholar] [CrossRef]
- Chen-Goodspeed, M.; Sogorb, M.A.; Wu, F.; Hong, S.B.; Raushel, F.M. Structural determinants of the substrate and stereochemical specificity of phosphotriesterase. Biochemistry 2001, 40, 1325–1331. [Google Scholar] [CrossRef]
- Porzio, E.; Merone, L.; Mandrich, L.; Rossi, M.; Manco, G. A new phosphotriesterase from Sulfolobus acidocaldarius and its comparison with the homologue from Sulfolobus solfataricus. Biochimie 2007, 89, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Manco, G.; Porzio, E.; Suzumoto, Y. Enzymatic detoxification: A sustainable means of degrading toxic organophosphate pesticides and chemical warfare nerve agents. J. Chem. Technol. Biotechnol. 2018, 93, 2064–2082. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2013, 425, 30–39. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Poerio, T.; Porzio, E.; Manco, G.; Perrotta, I.; Militano, F.; Giorno, L. Biocatalytic membrane reactor development for organophosphates degradation. J. Hazard. Mater. 2019, 365, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Altintas, B.; Yilmaz, M.; Arica, M.Y. Immobilization of chloroperoxidase onto highly hydrophilic polyethylene chains via bio-conjugation: Catalytic properties and stabilities. Bioresour. Technol. 2011, 102, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef]
- Subasi, Y.; Cicek, B. Recent advances in hydrophilic modification of PVDF ultrafiltration membranes—A review: Part I. Membr. Technol. 2017, 2017, 7–12. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Zakaria, N.A.; Hussin, M.H.; Ahmad, A.L.; Leo, C.P.; Poh, P.E.; Behzadian, K.; Akinwumi, I.I.; Moghayedi, A.; Diazsolano, J. Lignin modified PVDF membrane with antifouling properties for oil filtration. J. Water Process Eng. 2021, 43, 102248. [Google Scholar] [CrossRef]
- Regina, S.; Poerio, T.; Mazzei, R.; Giorno, L. Polyvinylidene fluoride-alkali lignin blend: A new candidate for membranes development. J. Membr. Sci. Lett. 2024. accepted. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Aristizábal, S.L.; Silva, L.; Qasem, E.A.; Chisca, S.; Upadhyaya, L.; Althobaiti, D.; Coutinho, J.A.P.; Nunes, S.P. A lignin-based membrane fabricated with a deep eutectic solvent. Green Chem. 2023, 25, 4769–4780. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Poerio, T.; Barbieri, G.; Fontananova, E.; Büning, D.; Ulbricht, M.; Giorno, L. Influence of lipase immobilization mode on ethyl acetate hydrolysis in a continuous solid–gas biocatalytic membrane reactor. Bioconjugate Chem. 2019, 30, 2238–2246. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Giorno, L. Biohybrid membranes for organophosphate pesticides degradation: Hyperactivation of immobilized phosphotriesterase by surfactants. Environ. Technol. Innov. 2023, 30, 103053. [Google Scholar] [CrossRef]
- Bakeri, G.; Ismail, A.F.; Shariaty-Niassar, M.; Matsuura, T. Effect of polymer concentration on the structure and performance of polyetherimide hollow fiber membranes. J. Membr. Sci. 2010, 363, 103–111. [Google Scholar] [CrossRef]
- Ajdary, R.; Kretzschmar, N.; Baniasadi, H.; Trifol, J.; Seppälä, J.V.; Partanen, J.; Rojas, O.J. Selective Laser Sintering of Lignin-Based Composites. ACS Sustain. Chem. Eng. 2021, 9, 2727–2735. [Google Scholar] [CrossRef]
- Vicente, J.; Costa, P.; Lanceros-Mendez, S.; Abete, J.M.; Iturrospe, A. Electromechanical Properties of PVDF-Based Polymers Reinforced with Nanocarbonaceous Fillers for Pressure Sensing Applications. Materials 2019, 12, 3545. [Google Scholar] [CrossRef]
- Ignjatović, N.; Tomić, S.; Dakić, M.; Miljković, M.; Plavšić, M.; Uskoković, D. Synthesis and properties of hydroxyapatite/poly-L-lactide composite biomaterials. Biomaterials 1999, 20, 809–816. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Bernal, C.; Rodriguez, K.; Martinez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, J.; Salmon, S. Developing Enzyme Immobilization with Fibrous Membranes: Longevity and Characterization Considerations. Membranes 2023, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zhang, Z.; Lou, D.; Tan, J.; Zhu, L. The effects of macromolecular crowding and surface charge on the properties of an immobilized enzyme: Activity, thermal stability, catalytic efficiency and reusability. RSC Adv. 2017, 7, 38028–38036. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Hodnett, B.K.; Magner, E. Chloroperoxidase on periodic mesoporous organosilanes: Immobilization and reuse. Chem. Mater. 2007, 19, 2049–2055. [Google Scholar] [CrossRef]
- Lee, C.H.; Lang, J.; Yen, C.W.; Shih, P.C.; Lin, T.S.; Mou, C.Y. Enhancing stability and oxidation activity of cytochrome c by immobilization in the nanochannels of mesoporous aluminosilicates. J. Phys. Chem. B 2005, 109, 12277–12286. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Tangermann, O.; Hartmann, M. Adsorption of cytochrome c on mesoporous molecular sieves: Influence of pH, pore diameter, and aluminum incorporation. Chem. Mater. 2004, 16, 3056–3065. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xia, Y.; Sui, W.; Si, C. Lignin as a novel tyrosinase inhibitor: Effects of sources and isolation processes. ACS Sustain. Chem. Eng. 2018, 6, 9510–9518. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Giorno, L. Enzyme-loaded membrane reactor to degrade a pesticide in vegetative waters. J. Membr. Sci. 2021, 635, 119438. [Google Scholar] [CrossRef]


| Free Enzyme/Membrane | Free Enzyme (mg/mL) | Immob. Enzyme (mgE·gmem−1) | Specific Activity (µmol·min−1·mg−1) |
|---|---|---|---|
| Free enzyme | 9.9 × 10−4 | - | 3.6 ± 0.3 |
| PVDF-DAMP-GA | - | 6.2 (±0.4) | 0.3 (±0.05) |
| PVDF90/AL10-DAMP-GA | - | 7.5 (±0.6) | 0.5 (±0.1) |
| PVDF75/AL25-DAMP-GA | - | 6.5 (±0.3) | 0.5 (±0.01) |
| PVDF50/AL50-DAMP-GA | - | 8.5 (±0.5) | 0.2 (±0.01) |
| Membrane Type | Thickness (µm) | Porosity (%) | Pure Water Permeance (L·h−1·m−2·bar−1) | Void Volume (mL) |
|---|---|---|---|---|
| PVDF75/AL25-DAMP-GA | 138 (±2) * | 80 | 577 (±26) * | 0.13 |
| 340 (±3) | 66 | 560 (±30) | 0.25 | |
| 550 (±7) | 79 | 400 (±40) | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regina, S.; Vitola, G.; Mazzei, R.; Giorno, L. Tuning the Properties of Polyvinylidene Fluoride/Alkali Lignin Membranes to Develop a Biocatalytic Membrane Reactor for an Organophosphorus Pesticide Degradation. Membranes 2024, 14, 186. https://doi.org/10.3390/membranes14090186
Regina S, Vitola G, Mazzei R, Giorno L. Tuning the Properties of Polyvinylidene Fluoride/Alkali Lignin Membranes to Develop a Biocatalytic Membrane Reactor for an Organophosphorus Pesticide Degradation. Membranes. 2024; 14(9):186. https://doi.org/10.3390/membranes14090186
Chicago/Turabian StyleRegina, Serena, Giuseppe Vitola, Rosalinda Mazzei, and Lidietta Giorno. 2024. "Tuning the Properties of Polyvinylidene Fluoride/Alkali Lignin Membranes to Develop a Biocatalytic Membrane Reactor for an Organophosphorus Pesticide Degradation" Membranes 14, no. 9: 186. https://doi.org/10.3390/membranes14090186
APA StyleRegina, S., Vitola, G., Mazzei, R., & Giorno, L. (2024). Tuning the Properties of Polyvinylidene Fluoride/Alkali Lignin Membranes to Develop a Biocatalytic Membrane Reactor for an Organophosphorus Pesticide Degradation. Membranes, 14(9), 186. https://doi.org/10.3390/membranes14090186











