Low-Resistance Membrane vs. High-Resistance Membrane Performance Utilizing Electrodialysis–Evaporator Hybrid System in Treating Reject Brine from Kuwait Desalination Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
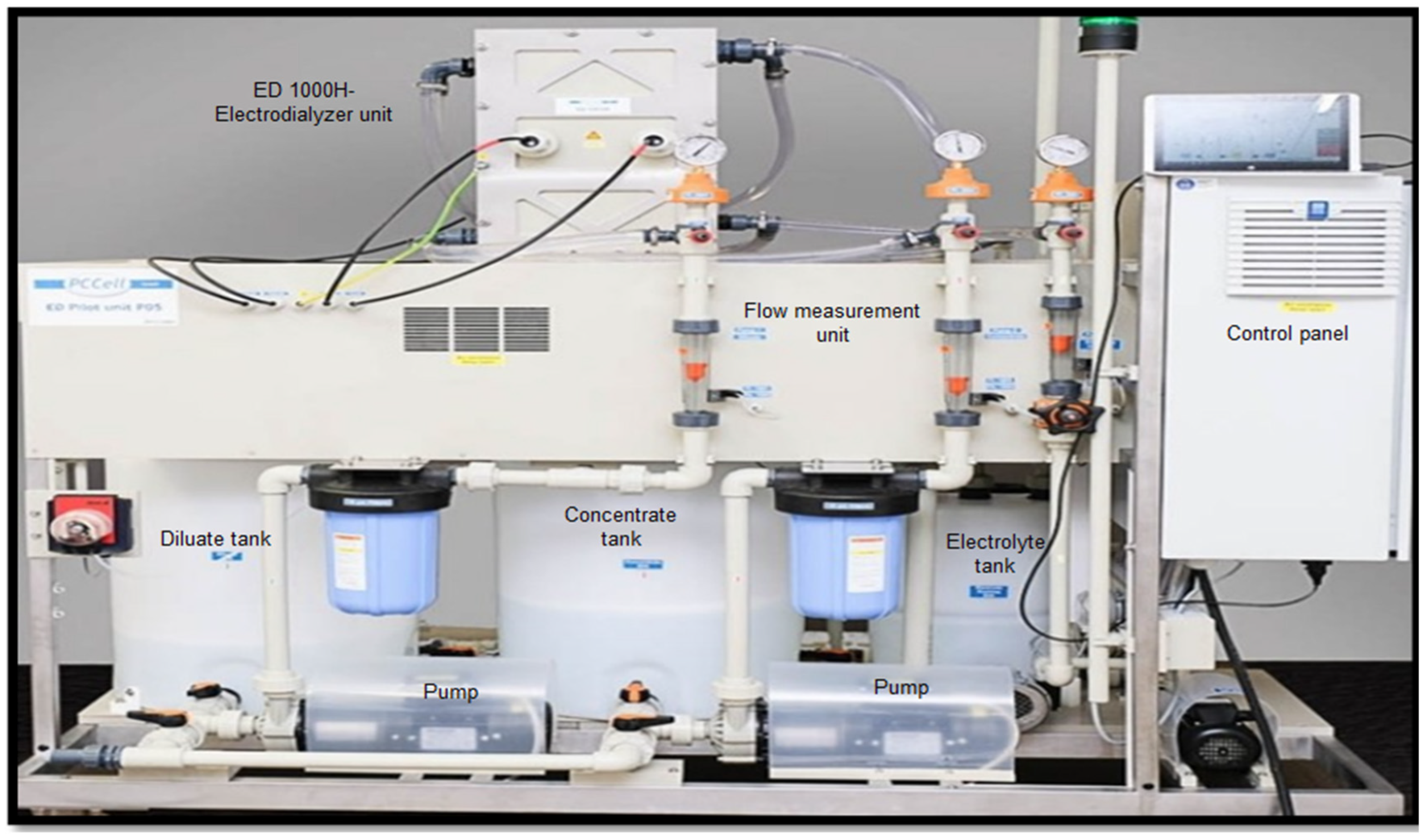
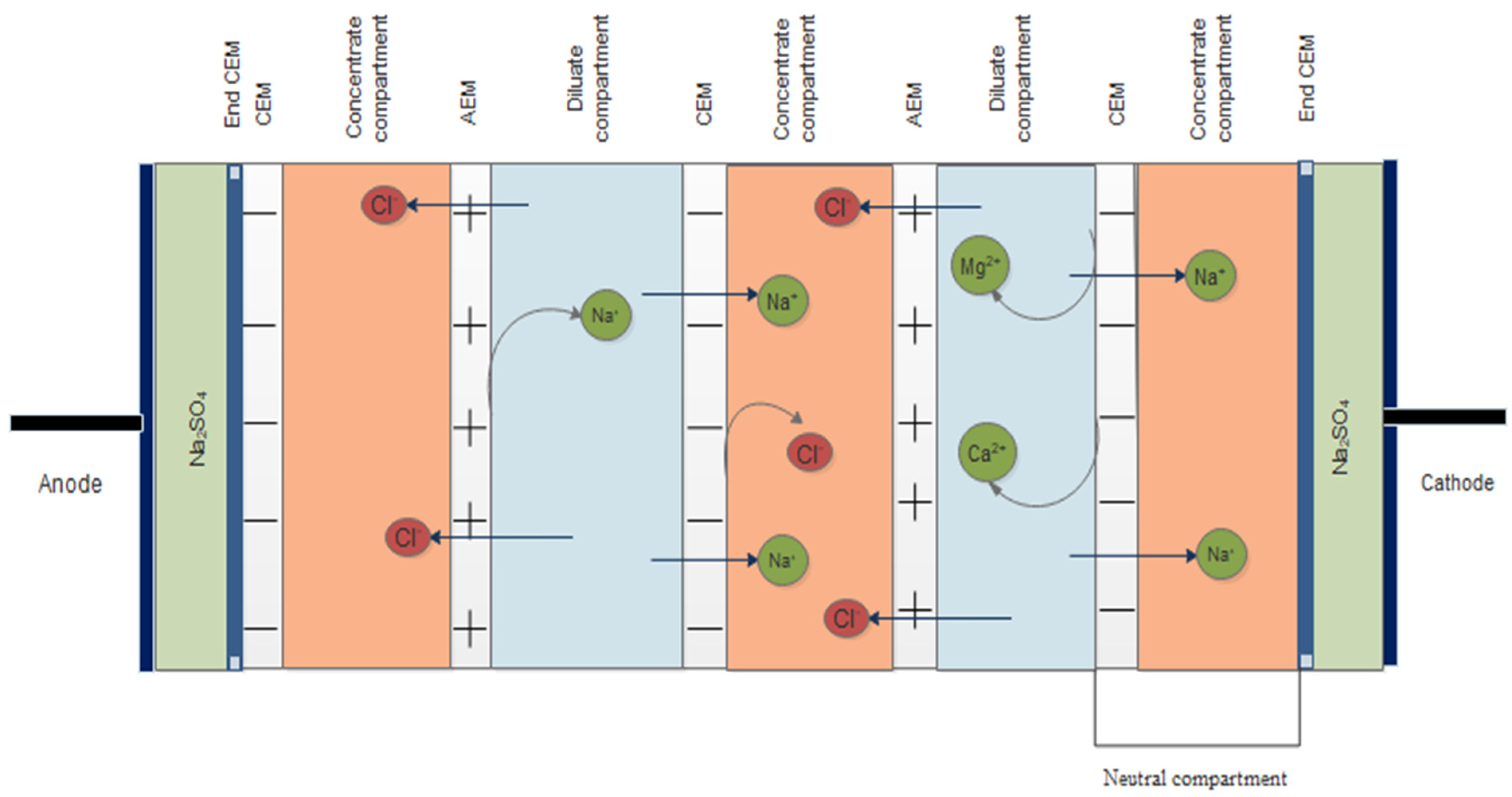
2.2. Electrodialysis Cell, Equipment Set Up, and Operation Procedure
2.3. Sampling and Analytical Methods
2.4. Data Analysis and Calculation
2.4.1. Langelier Saturation Index (LSI)
2.4.2. Desalination Rate (D)
2.4.3. Ion Removal Rate (R)
2.4.4. ED Membrane Permselectivity
2.4.5. Water Recovery (W)
2.4.6. Current Efficiency (CE)
2.4.7. Energy Consumption (E)
2.4.8. Purity of Salt (P)
3. Results and Discussion
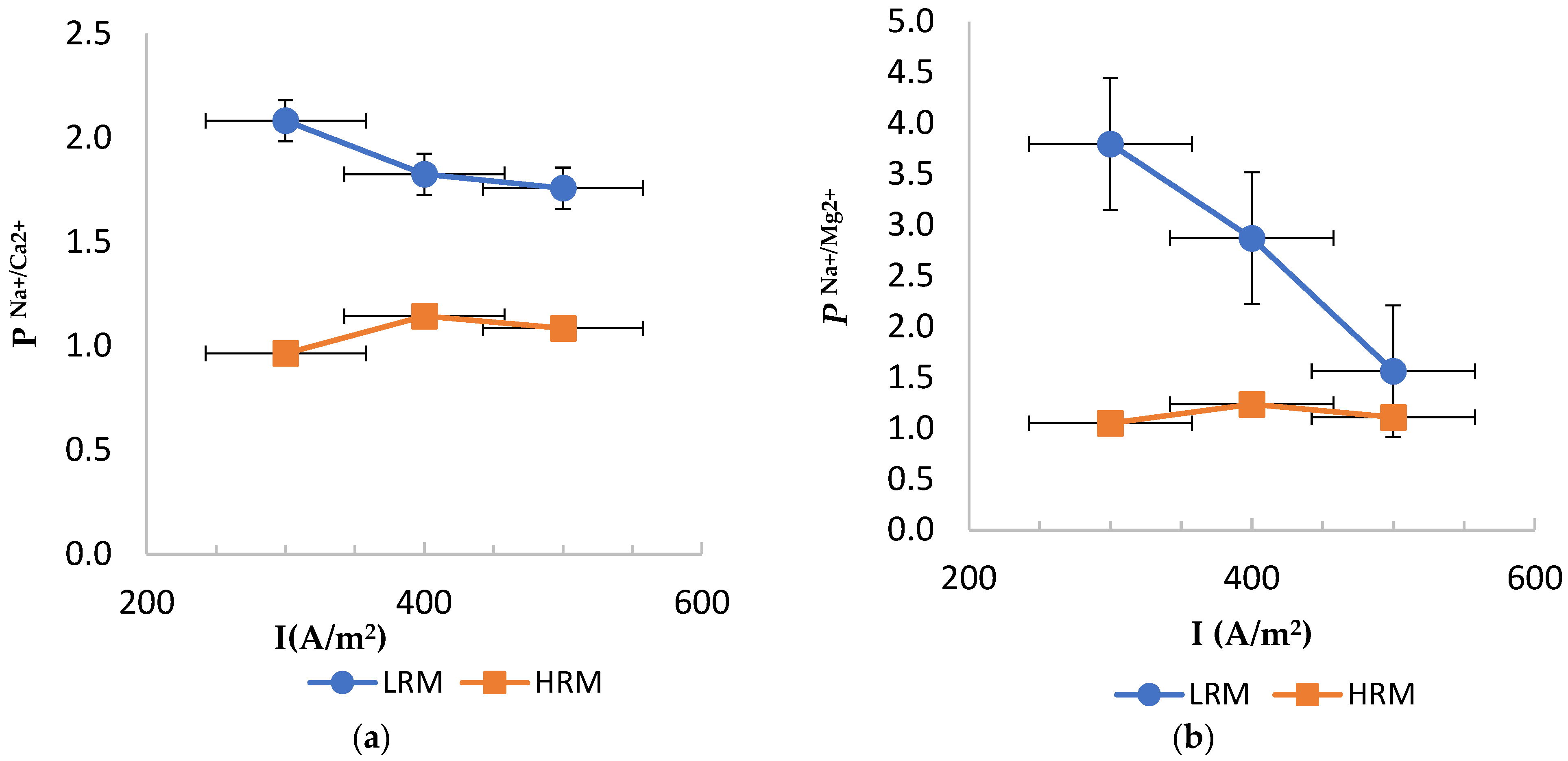
3.1. Permselectivity of LRM vs. HRM
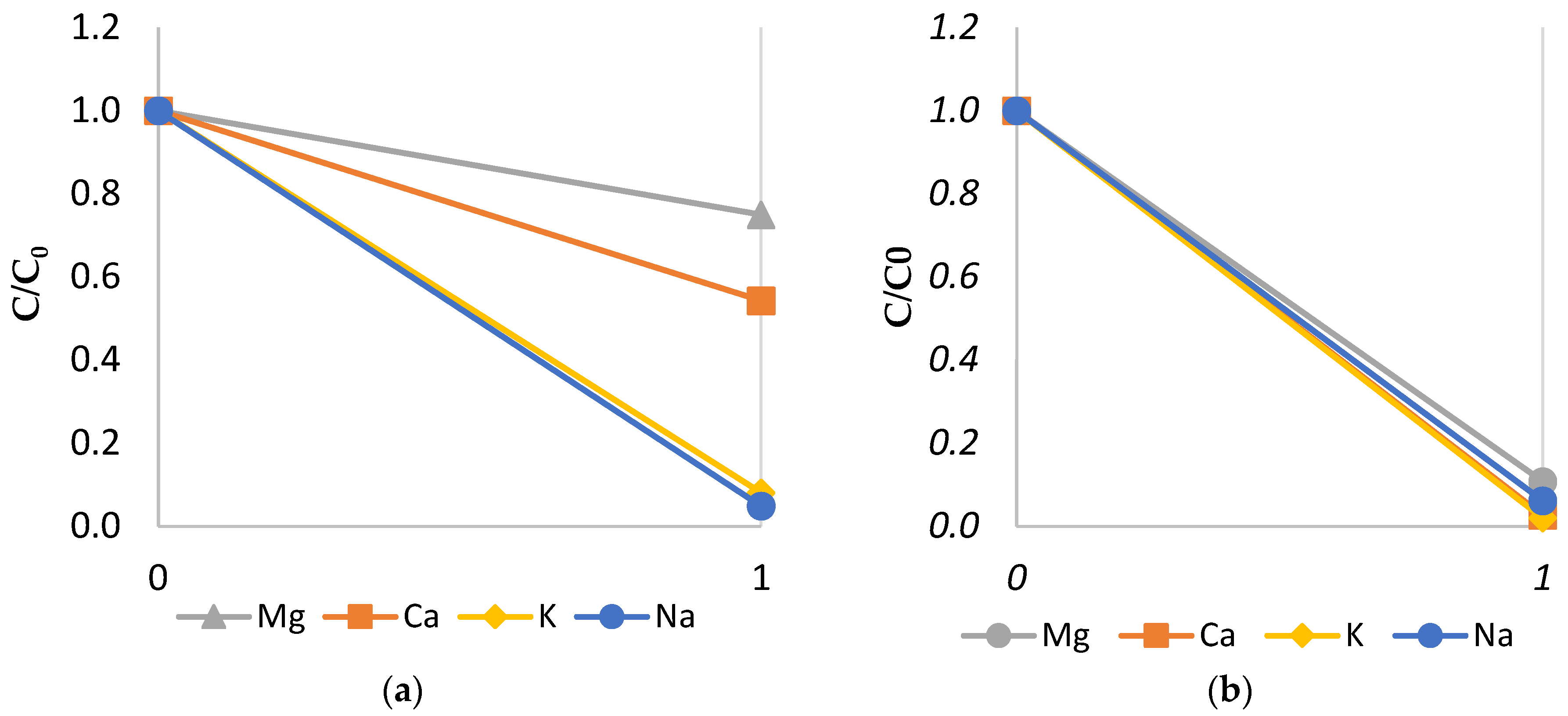
3.1.1. LRM and HRM Cation Monovalent Selectivity
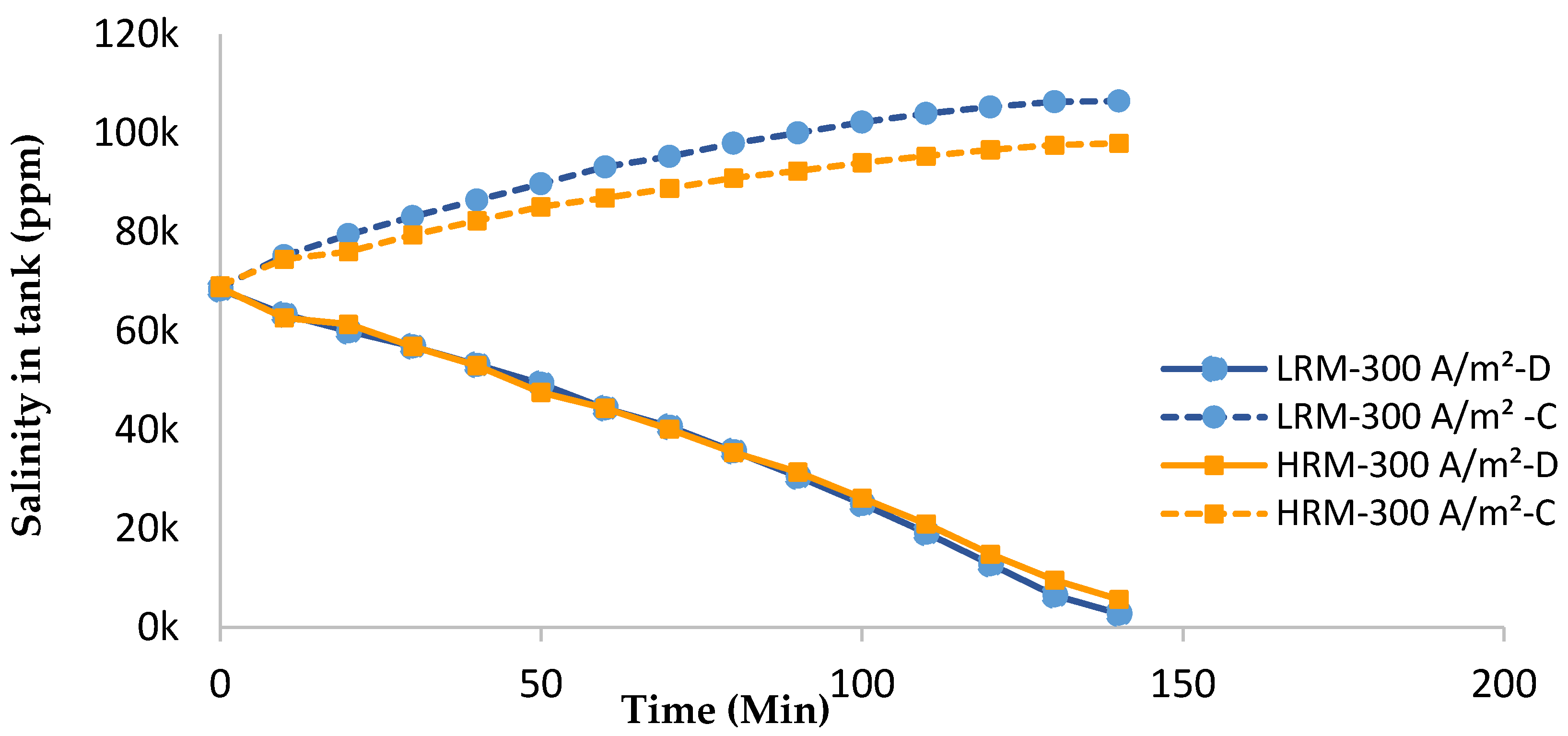
3.1.2. Overall Salinity Profile
3.1.3. Effect of Monovalent Selectivity of LRM and HRM on Cations in the Diluate
3.2. Performance of LRM and HRM under the Same Operating Conditions
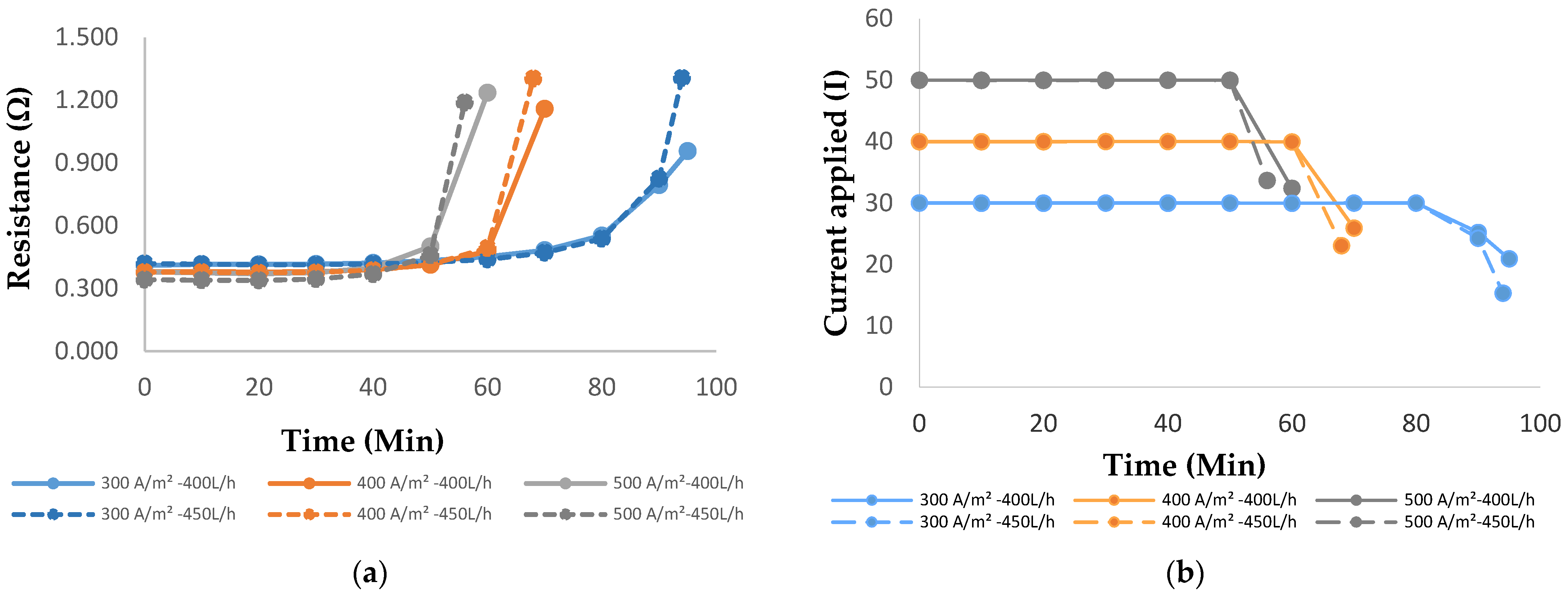
3.2.1. Influence of Current Densities and Feed Flowrates
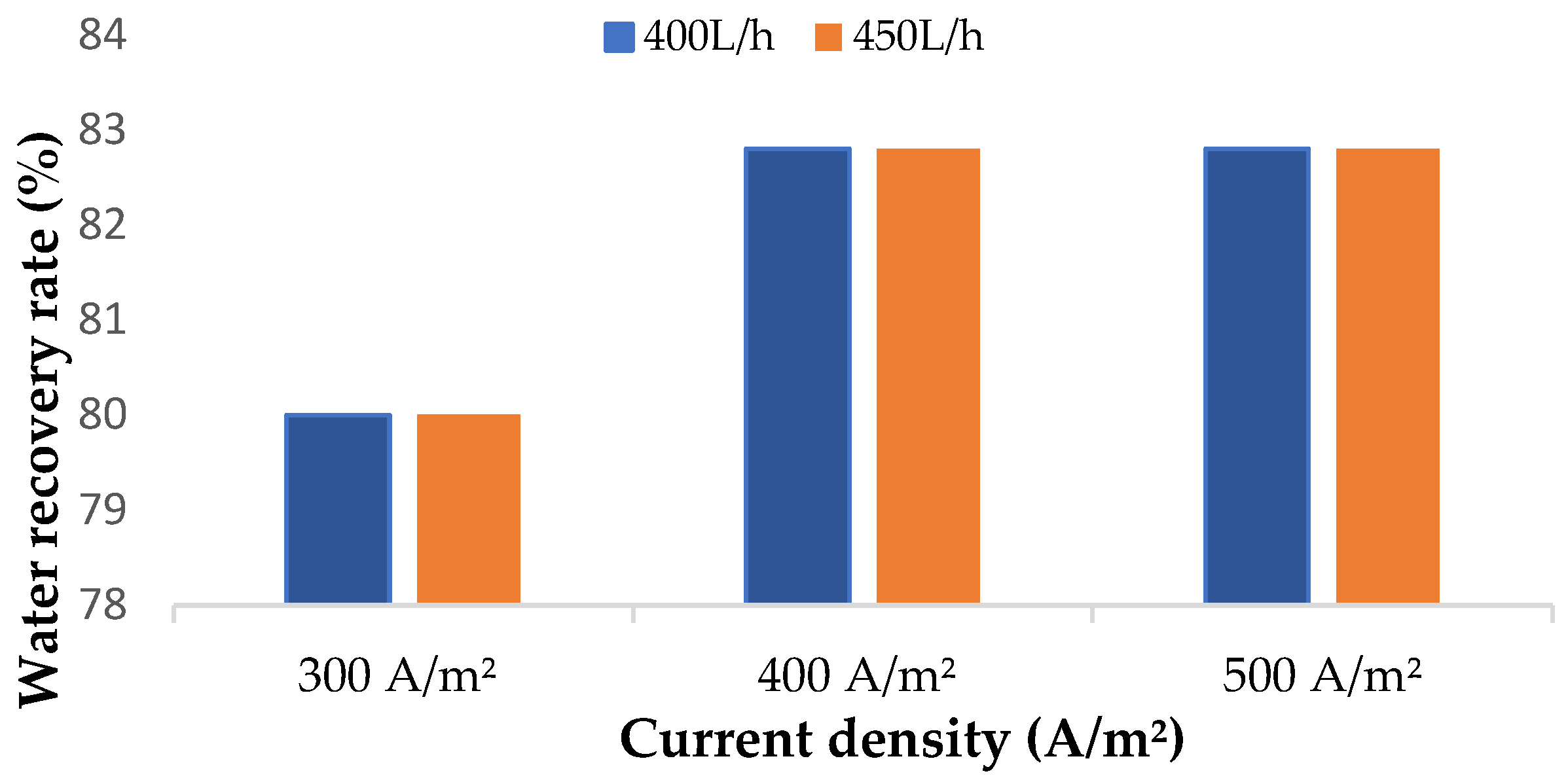
3.2.2. Water Recovery
3.2.3. Energy Consumption
3.3. Performance Comparison between HRM and LRM
3.4. Scale Formation and Prevention
3.4.1. Langlier Saturation Index (LSI) of the Feed Water
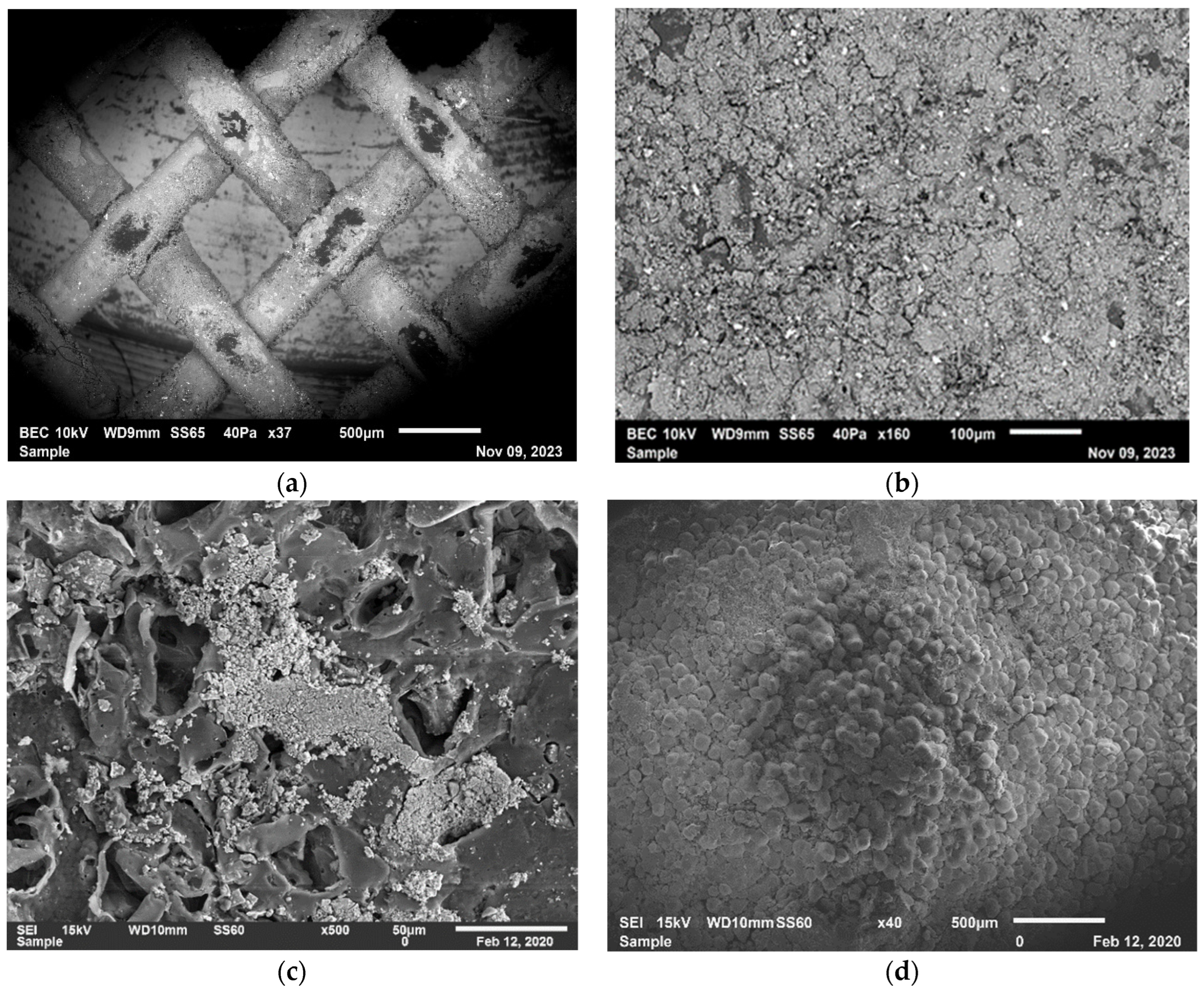
3.4.2. Assessment of the Addition of the Neural Cell to the ED System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef] [PubMed]
- Voutchkov, N. Desalination—Past, Present and Future. International Water Association. 2016. Available online: https://iwa-network.org/Desalination,-past-present-future (accessed on 13 October 2020).
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayji, K.; Aleisa, E. Characterizing the fossil fuel impacts in water desalination plants in Kuwait: A Life Cycle Assessment approach. Energy 2018, 158, 681–692. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Ahmed, M.; Anwar, R. An Assessment of the Environmental Impact of Brine Disposal in Marine Environment. Int. J. Mod. Eng. Res. (IJMER) 2012, 2, 2756–2761. [Google Scholar]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Baroutaji, A.; Olabi, A.G. Environmental impact of desalination processes: Mitigation and control strategies. Sci. Total Environ. 2020, 740, 140125. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.F.; Chung, T.S. Maximize the operating profit of a SWRO-PRO integrated process for optimal water production and energy recovery. Renew. Energy 2016, 94, 304–313. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef] [PubMed]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V. A review of the management and treatment of brine solutions. Environ. Sci. Water Res. Technol. 2017, 3, 625–658. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Thomas, A.; Fernandes, J. Lab scale assessment of power generation using pressure retarded osmosis from wastewater treatment plants in the state of Kuwait. Desalination 2016, 396, 57–69. [Google Scholar] [CrossRef]
- Al-Anzi, B.S.; Thomas, A. One-dimensional analytical modeling of pressure- retarded osmosis in a parallel flow configuration for the desalination industry in the State of Kuwait. Sustainability 2018, 10, 1288. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Van der Bruggen, B. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, X.; Fan, A.; Fu, L.; Gao, C. An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J. Memb. Sci. 2014, 449, 119–126. [Google Scholar] [CrossRef]
- Casas, S.; Aladjem, C.; Cortina, J.L.; Larrotcha, E.; Cremades, L.V. Seawater Reverse Osmosis Brines as a New Salt Source for the Chlor-Alkali Industry: Integration of NaCl Concentration by Electrodialysis. Solvent Extr. Ion Exch. 2012, 30, 322–332. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ehara, R.; Itoi, S.; Goto, T. Ion-exchange membrane electrodialytic salt production using brine discharged from a reverse osmosis seawater desalination plant. J. Memb. Sci. 2003, 222, 71–86. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology: In the Chemical Industry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Al-Anzi, B.S.; Lienhard, J.H. Cost and energy needs of RO-ED-crystallizer systems for zero brine discharge seawater desalination. Desalination 2019, 457, 115–132. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of concentrated brine from RO plant to produce coarse salt and freshwater. J. Memb. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Memb. Sci. 2019, 570–571, 245–257. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Memb. Sci. 2009, 326, 392–399. [Google Scholar] [CrossRef]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Dominguez, K.P.; McCance, A.; Al-Anzi, B.S.; Lienhard, J.H. Cost and energy requirements of hybrid RO and ED brine concentration systems for salt production. Desalination 2019, 456, 97–120. [Google Scholar] [CrossRef]
- Medina, V.F.; Johnson, J.L.; Waisner, S.A.; Wade, R.; Mattei-Sosa, J. Development of a Treatment Process for Electrodialysis Reversal Concentrate with Intermediate Softening and Secondary Reverse Osmosis to Approach 98-Percent Water Recovery. J. Environ. Eng. 2015, 141, 04015002. [Google Scholar] [CrossRef]
- He, C.; Carpenter, G.; Westerhoff, P. Demonstrating and Innovative Combination of Ion Exchange Pretreatment and Electrodialysis Reversal for Reclaimed Water Reverse Osmosis Concentrate Minimization; Water Reuse Research Foundation: Alexandria, VA, USA, 2013. [Google Scholar]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of brine solutions discharged from an RO plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- Korngold, E.; Aronov, L.; Belayev, N.; Kock, K. Electrodialysis with brine solutions oversaturated with calcium sulfate. Desalination 2005, 172, 63–75. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; Jullok, N.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. RO concentrate treatment by a hybrid system consisting of a pellet reactor and electrodialysis. Chem. Eng. Sci. 2012, 79, 228–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Vanherpe, R.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. RO concentrate minimization by electrodialysis: Techno-economic analysis and environmental concerns. J. Environ. Manag. 2012, 107, 28–36. [Google Scholar] [CrossRef]
- Karimi, L.; Ghassemi, A.; Sabzi, H.Z. Quantitative studies of electrodialysis performance. Desalination 2018, 445, 159–169. [Google Scholar] [CrossRef]
- Choi, S.; Kim, B.; Nayar, K.G.; Yoon, J.; Al-Hammadi, S.; Lienhard, V.J.H.; Han, J.; Al-Anzi, B. Techno-economic analysis of ion concentration polarization desalination for high salinity desalination applications. Water Res. 2019, 155, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Anzi, B.S.; Al-Rashidi, A.; Abraham, L.; Fernandes, J.; Al-Sheikh, A.; Alhazza, A. Brine management from desalination plants for salt production utilizing high current density electrodialysis-evaporator hybrid system: A case study in Kuwait. Desalination 2021, 498, 114760. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Electrodialysis on RO concentrate to improve water recovery in wastewater reclamation. J. Memb. Sci. 2011, 378, 101–110. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Martí-Calatayud, M.; Garthe, M.; Tiwari, R.; Walther, A.; Wessling, M. On the permselectivity of cation-exchange membranes bearing an ion selective coating. J. Memb. Sci. 2020, 600, 117854. [Google Scholar] [CrossRef]
- Sata, T. Studies on ion exchange membranes with permselectivity for specific ions in electrodialysis. J. Memb. Sci. 1994, 93, 117–135. [Google Scholar] [CrossRef]
- Xu, X.; He, Q.; Ma, G.; Wang, H.; Nirmalakhandan, N.; Xu, P. Selective separation of mono- and di-valent cations in electrodialysis during brackish water desalination: Bench and pilot-scale studies. Desalination 2018, 428, 146–160. [Google Scholar] [CrossRef]
- Kamcev, J.; Sujanani, R.; Jang, E.-S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Memb. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Galama, A.H.; Saakes, M.; Bruning, H.; Rijnaarts, H.H.M.; Post, J.W. Seawater predesalination with electrodialysis. Desalination 2014, 342, 61–69. [Google Scholar] [CrossRef]
- Takagi, R.; Vaselbehagh, M.; Matsuyama, H. Theoretical study of the permselectivity of an anion exchange membrane in electrodialysis. J. Memb. Sci. 2014, 470, 486–493. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Memb. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Uddin, M.R.; Hossain, M.M.; Akter, S.; Ali, M.E.; Ahsan, M.A. Assessment of some physicochemical parameters and determining the corrosive characteristics of the Karnaphuli estuarine water, Chittagong, Bangladesh. Water Sci. 2020, 34, 164–180. [Google Scholar] [CrossRef]





| Properties | Concentration (S) | Concentration (T) |
|---|---|---|
| Conductivity (µs/cm) | 95,790 | 220 |
| pH | 7.5 | 10.2 |
| TDS (ppm) | 67,053 | 154 |
| Alkalinity (ppm) | 61 | 51 |
| Sodium (ppm) | 21,300 | 14.6 |
| Magnesium (ppm) | 11 | 6.5 |
| Calcium (ppm) | 42 | 1.9 |
| Potassium (ppm) | 120 | 1.3 |
| Chloride (ppm) | 36,354 | 18.1 |
| Sulphate (ppm) | 1866 | 8.9 |
| Parameter | Value |
|---|---|
| ED stack model | PC Cell ED 1000H |
| Number of cell pairs (ncp) | 25 |
| Active membrane area (Am,active) | 1000 cm2 |
| Channel height (h) | 0.4 mm |
| Channel flow width (wm,flow) | 27 cm |
| Capacity of diluate tank (Vd,cap) | 70 L |
| Capacity of concentrate tank (Vc,cap) | 70 L |
| Membrane | Thickness (μm) | pH Stability | Area Resistance (Ω.cm2) | Burst Strength (kg.cm−2) | Max. Temperature (°C) | Reinforcement | |
|---|---|---|---|---|---|---|---|
| LRM | PC-MVA | 110 | 0–9 | 5 | 4–5 | 60 | Polyester |
| PC-MVK | 100–120 | 0–10 | 6 | 4–5 | 45 | Polyester | |
| HRM | PC-MVA | 110 | 0–9 | 20 | 2 | 60 | PVC |
| PC-MVK | 100 | 0–10 | - | 3 | 40 | PVC |
| Membrane Type | Current (A/m2) | Flowrate L/h | Volume (L) | Operational Time (min) | WRR (%) | EC (kwh) | CE (%) |
|---|---|---|---|---|---|---|---|
| LRM | 300 | 400 | 50 | 80 | 79.17 | 0.546 | 52.30 |
| HRM | 300 | 400 | 50 | 150 | 70.83 | 1.15 | 30.80 |
| HRM | LRM | |||||
|---|---|---|---|---|---|---|
| Ion | 300 A/m2 | 400 A/m2 | 500 A/m2 | 300 A/m2 | 400 A/m2 | 500 A/m2 |
| Ca+2 | 97% | 86% | 91% | 45.7% | 51% | 53% |
| K+ | 98% | 96% | 90% | 91.95% | 92% | 92% |
| Na+ | 94% | 98% | 98% | 95% | 92% | 92% |
| Mg+2 | 89% | 79% | 89% | 25.31% | 32% | 59% |
| Cl− | 99.8% | 95% | 94% | 95.70% | 95.20% | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anzi, B.S.; Awadh, M.K. Low-Resistance Membrane vs. High-Resistance Membrane Performance Utilizing Electrodialysis–Evaporator Hybrid System in Treating Reject Brine from Kuwait Desalination Plants. Membranes 2024, 14, 163. https://doi.org/10.3390/membranes14080163
Al-Anzi BS, Awadh MK. Low-Resistance Membrane vs. High-Resistance Membrane Performance Utilizing Electrodialysis–Evaporator Hybrid System in Treating Reject Brine from Kuwait Desalination Plants. Membranes. 2024; 14(8):163. https://doi.org/10.3390/membranes14080163
Chicago/Turabian StyleAl-Anzi, Bader S., and Maryam K. Awadh. 2024. "Low-Resistance Membrane vs. High-Resistance Membrane Performance Utilizing Electrodialysis–Evaporator Hybrid System in Treating Reject Brine from Kuwait Desalination Plants" Membranes 14, no. 8: 163. https://doi.org/10.3390/membranes14080163
APA StyleAl-Anzi, B. S., & Awadh, M. K. (2024). Low-Resistance Membrane vs. High-Resistance Membrane Performance Utilizing Electrodialysis–Evaporator Hybrid System in Treating Reject Brine from Kuwait Desalination Plants. Membranes, 14(8), 163. https://doi.org/10.3390/membranes14080163







