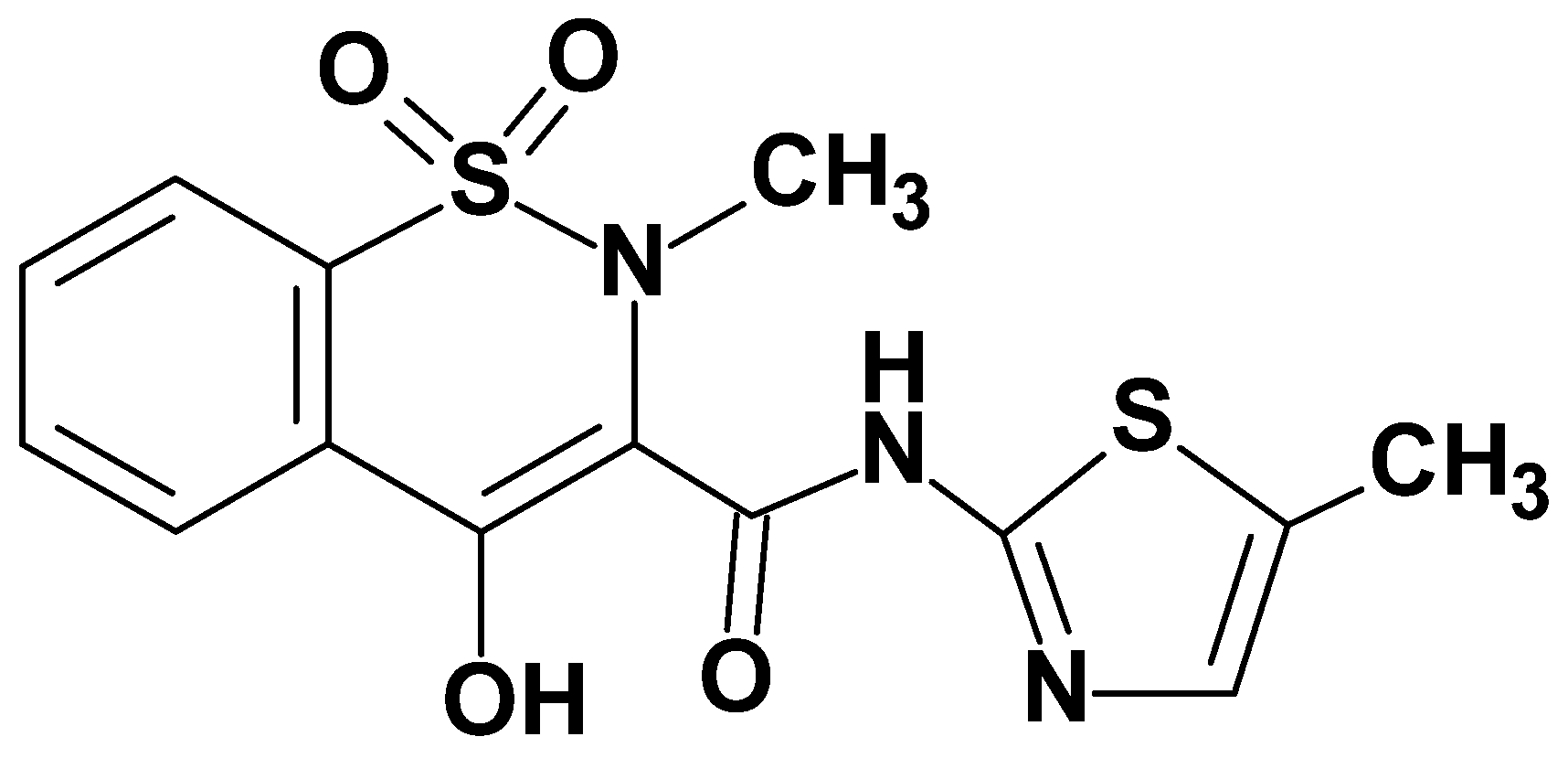
Anti-Inflammatory Properties of Novel 1,2-Benzothiazine Derivatives and Their Interaction with Phospholipid Model Membranes
Abstract
1. Introduction
2. Materials and Methods
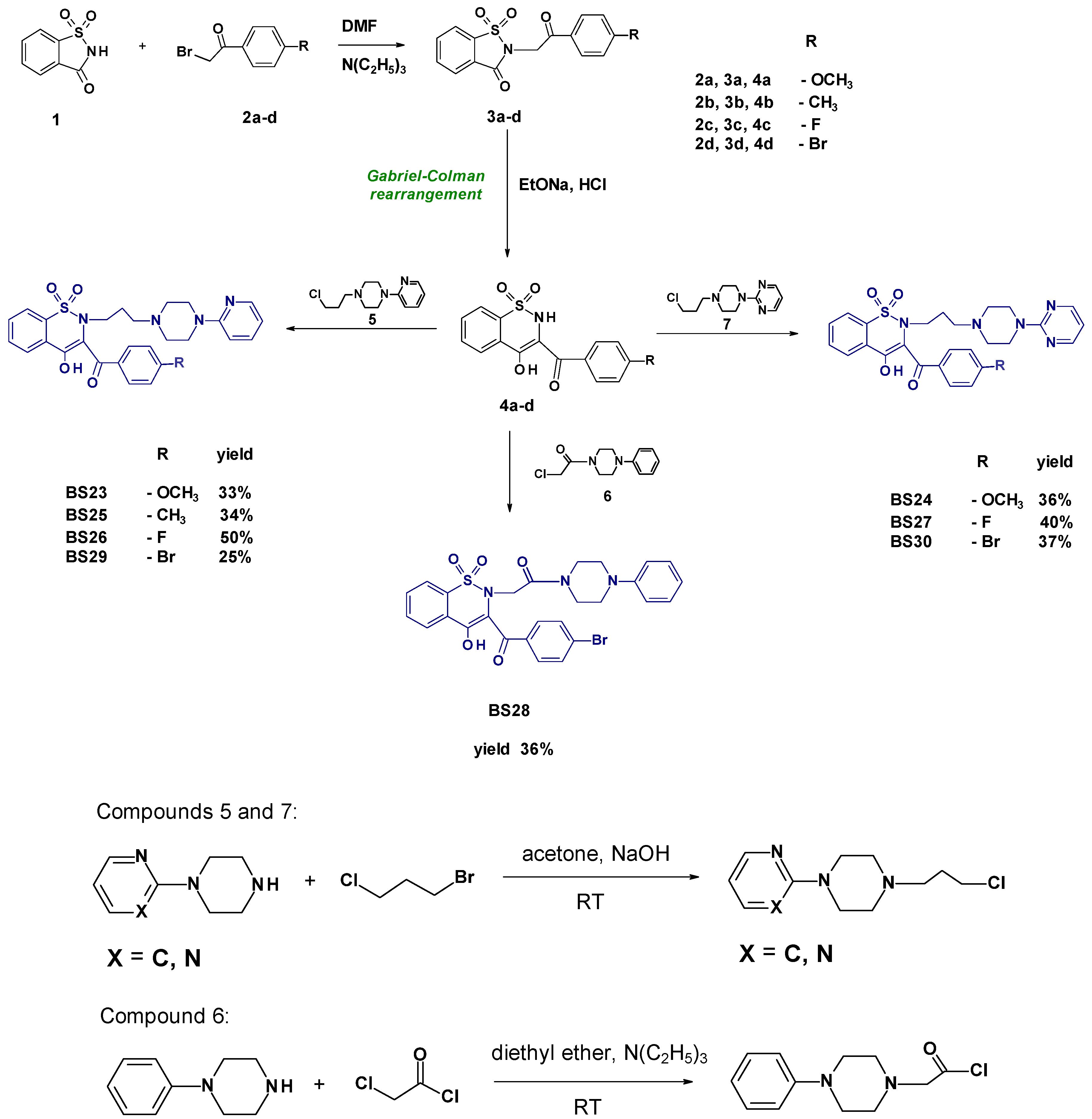
2.1. Chemistry
- Synthesis and Experimental Data of Compounds BS23–BS30
- 3-(4-Methoxybenzoyl)-4-hydroxy-2-{3-[4-(2-pyridyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS23
- 3-(4-Methoxybenzoyl)-4-hydroxy-2-{3-[4-(2-pyrimidyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS24
- 3-(4-Methylbenzoyl)-4-hydroxy-2-{3-[4-(2-pyridyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS25
- 3-(4-Fluorobenzoyl)-4-hydroxy-2-{3-[4-(2-pyridyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS26
- 3-(4-Fluorobenzoyl)-4-hydroxy-2-{3-[4-(2-pyrimidyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS27
- 3-(4-Bromobenzoyl)-2-[2-(4-phenylpiperazin-1-yl)-2-oxoethyl]-4-hydroxy-2H-1,2-benzothiazine 1,1-dioxide BS28
- 3-(4-Bromobenzoyl)-4-hydroxy-2-{3-[4-(2-pyridyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS29
- 3-(4-Bromobenzoyl)-4-hydroxy-2-{3-[4-(2-pyrimidyl)-1-piperazinyl)propyl]}-2H-1,2-benzothiazine 1,1-dioxide BS30
2.2. In Vitro Study
2.2.1. Cyclooxygenase Inhibition Assay
2.2.2. Biological Evaluation
Cell Line and Culture Conditions
Tested Compounds
MTT Assay
Inflammation Model
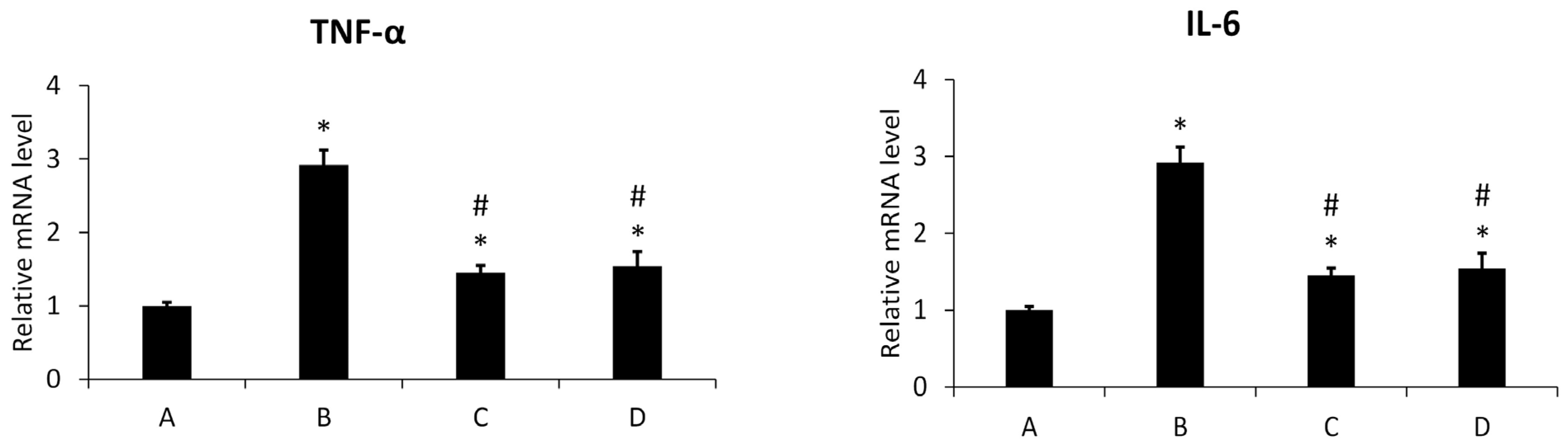
Gene Expression of TNF-α and IL-6 Detected by Real-Time RT-PCR
In Vitro Antioxidant Assays
- 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) as an Antioxidant Capacity
- ABTS Assay
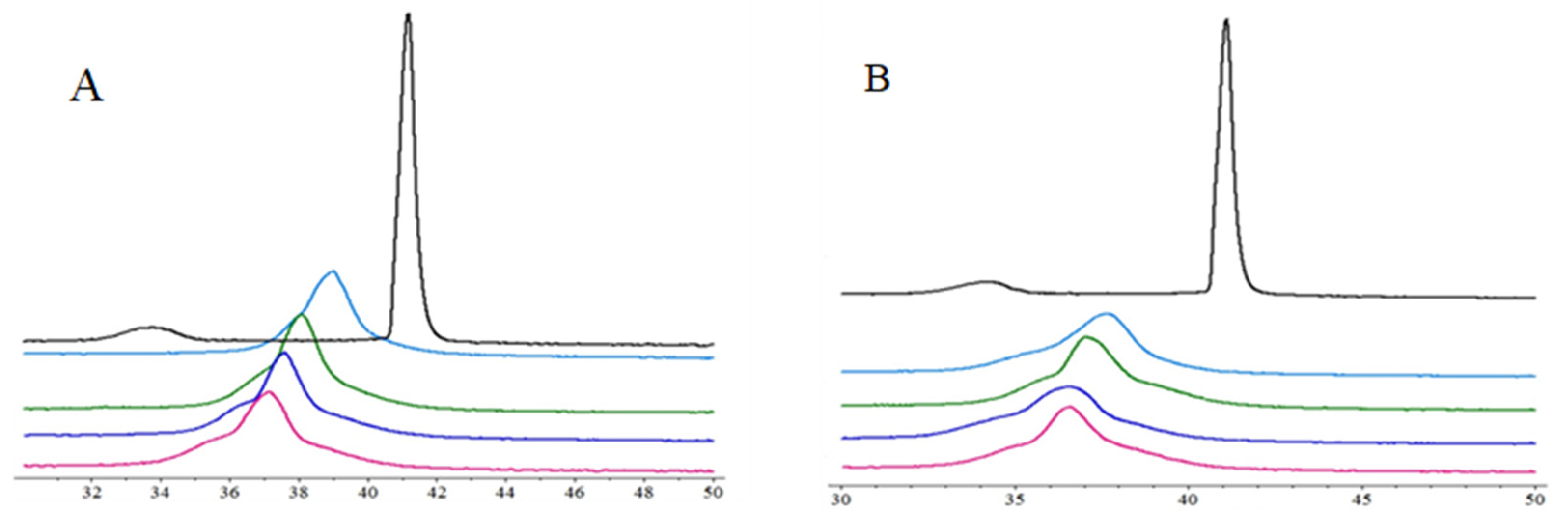
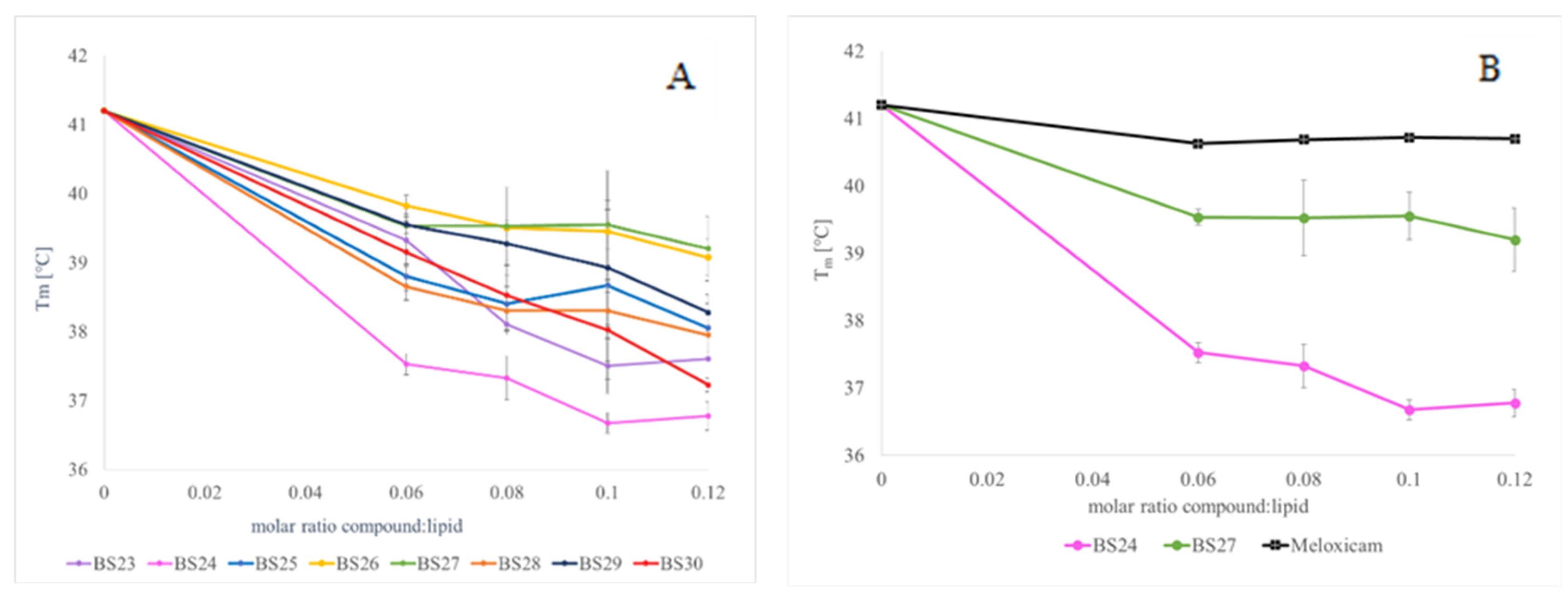
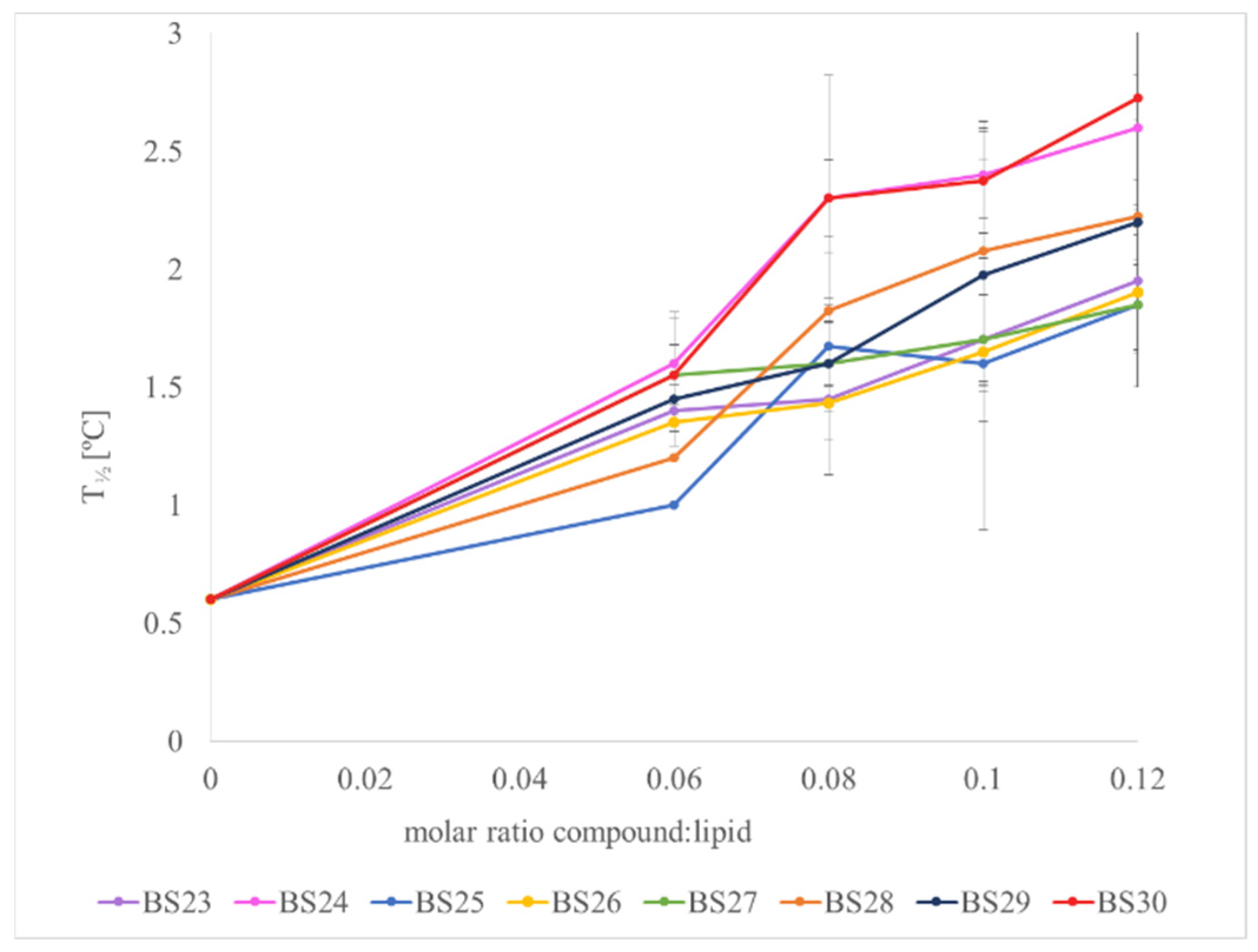
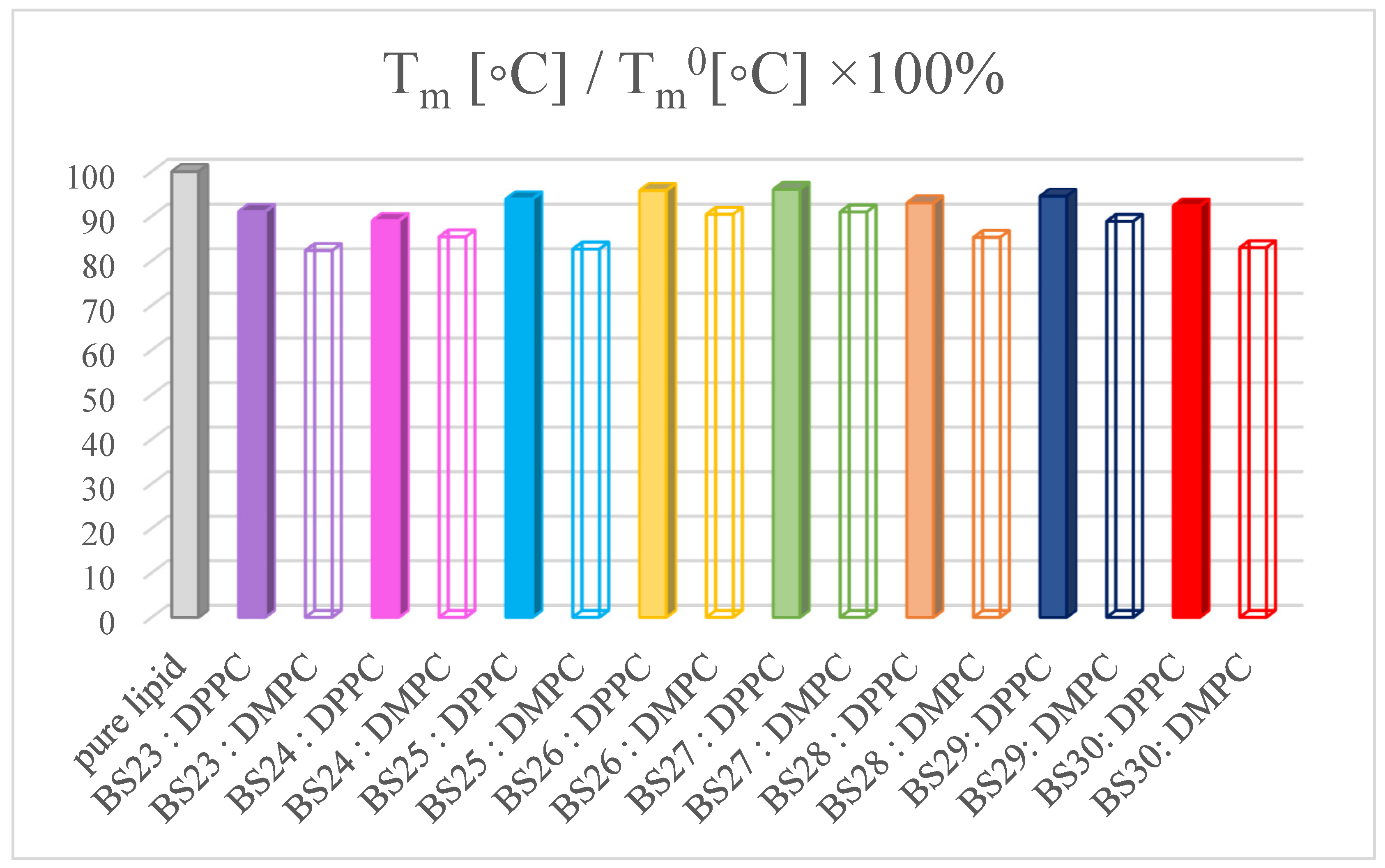
2.3. Differential Scanning Calorimetry (DSC)
2.4. In Silico Study
2.4.1. The Reactivity of Designed Compounds
2.4.2. Molecular Docking
3. Results
3.1. Chemistry
3.2. Evaluation of COX-1/COX-2 Selectivity and Potency
3.2.1. In Vitro Cyclooxygenase Inhibition Assay
3.2.2. Cytotoxicity Assay
3.3. The Interactions with Phospholipid Bilayers
3.4. Anti-Inflammatory Properties of Designed Compounds
3.4.1. Inflammation Assay
3.4.2. Effect of New Meloxicam Derivatives on the Cytokine mRNAs Level in Inflamed Cells
3.4.3. Antioxidant Assays
3.5. The Reactivity of Designed Compounds
3.6. Molecular Docking Studies
4. Discussion
- The substitution of Ile523 in COX-1 with Val523 in COX-2 creates space for selective inhibitors with bulky hydrophobic groups; therefore, compounds with the specific side chains that exploit this pocket should be designed (e.g., 1-(2-pyrido)piperazine fragment of our compounds);
- The sulfonamide group able to interact with polar amino acid residues as arginine or serine via hydrogen bonds (BS23, BS24, BS26, BS27, and BS30);
- Halogen atoms enhance binding affinity and selectivity by improving hydrophobic and dipole interactions within the COX-2 pocket;
- Aromatic scaffolds enhance π–π stacking interactions with hydrophobic residues in COX-2;
- Methoxy substituent, which contributes to increased hydrophobic interactions within the binding pocket and enhances π–π stacking with aromatic residues in the COX-2 active site (compound BS23 and BS24).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romanelli, M.N. Cyclooxygenase. In Metalloenzymes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 431–447. [Google Scholar]
- Dogné, J.-M.; Supuran, C.T.; Pratico, D. Adverse Cardiovascular Effects of the Coxibs. J. Med. Chem. 2005, 48, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk Between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A. Interplay between Inflammation and Cancer. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 119, pp. 199–245. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Khatami, M. Chronic Inflammation: Synergistic Interactions of Recruiting Macrophages (TAMs) and Eosinophils (Eos) with Host Mast Cells (MCs) and Tumorigenesis in CALTs. M-CSF, Suitable Biomarker for Cancer Diagnosis! Cancers 2014, 6, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic Inflammation, Cancer Development and Immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.; Liu, H.-X. The Double-Edged Roles of ROS in Cancer Prevention and Therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Mogilski, S.; Wiglusz, R.J.; Janczak, J.; Maniewska, J.; Malinka, W.; Filipek, B. Synthesis and Pharmacological Evaluation of Novel Arylpiperazine Oxicams Derivatives as Potent Analgesics without Ulcerogenicity. Bioorg. Med. Chem. 2019, 27, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Hatnapure, G.D.; Keche, A.P.; Rodge, A.H.; Birajdar, S.S.; Tale, R.H.; Kamble, V.M. Synthesis and Biological Evaluation of Novel Piperazine Derivatives of Flavone as Potent Anti-Inflammatory and Antimicrobial Agent. Bioorg. Med. Chem. Lett. 2012, 22, 6385–6390. [Google Scholar] [CrossRef]
- Boido, A.; Canu Boido, C.; Sparatore, F. Synthesis and Pharmacological Evaluation of Aryl/Heteroaryl Piperazinyl Alkyl Benzotriazoles as Ligands for Some Serotonin and Dopamine Receptor Subtypes. Il Farm. 2001, 56, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.M.; Khalil, N.A.; Ahmed, E.M.; Eissa, K.I. Synthesis and Anti-Inflammatory Activity of Novel Pyridazine and Pyridazinone Derivatives as Non-Ulcerogenic Agents. Arch. Pharm. Res. 2012, 35, 2077–2092. [Google Scholar] [CrossRef]
- Kono, M.; Matsumoto, T.; Imaeda, T.; Kawamura, T.; Fujimoto, S.; Kosugi, Y.; Odani, T.; Shimizu, Y.; Matsui, H.; Shimojo, M.; et al. Design, Synthesis, and Biological Evaluation of a Series of Piperazine Ureas as Fatty Acid Amide Hydrolase Inhibitors. Bioorg. Med. Chem. 2014, 22, 1468–1478. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Jastrzębska-Więsek, M.; Filipek, B.; Dybała, M.; Karczmarzyk, Z.; Urbańczyk-Lipkowska, Z.; Kalicki, P. Derivatives of Pyrrolo[3,4-d]Pyridazinone, a New Class of Analgesic Agents. Eur. J. Med. Chem. 2011, 46, 4992–4999. [Google Scholar] [CrossRef]
- Kono, M.; Matsumoto, T.; Kawamura, T.; Nishimura, A.; Kiyota, Y.; Oki, H.; Miyazaki, J.; Igaki, S.; Behnke, C.A.; Shimojo, M.; et al. Synthesis, SAR Study, and Biological Evaluation of a Series of Piperazine Ureas as Fatty Acid Amide Hydrolase (FAAH) Inhibitors. Bioorg. Med. Chem. 2013, 21, 28–41. [Google Scholar] [CrossRef]
- Krzyżak, E.; Szczęśniak-Sięga, B.; Malinka, W. Synthesis and Thermal Behaviour of New Benzo-1,2-Thiazine Long-Chain Aryl-Piperazine Derivatives. J. Therm. Anal. Calorim. 2014, 115, 793–802. [Google Scholar] [CrossRef][Green Version]
- Szczęśniak-Sięga, B.M.; Gębczak, K.; Gębarowski, T.; Maniewska, J. Synthesis, COX-1/2 Inhibition and Antioxidant Activities of New Oxicam Analogues Designed as Potential Chemopreventive Agents. Acta Biochim. Pol. 2018, 65, 199–207. [Google Scholar] [CrossRef]
- Maniewska, J.; Gąsiorowska, J.; Szczęśniak-Sięga, B.M.; Michalak, K. The Interaction of New Oxicam Derivatives with Lipid Bilayers as Measured by Calorimetry and Fluorescence Spectroscopy. Acta Biochim. Pol. 2018, 65, 185–191. [Google Scholar] [CrossRef]
- Murty, M.S.R.; Ram, K.R.; Venkateswara Rao, R.; Yadav, J.S.; Venkateswara Rao, J.; R. Velatooru, L. Synthesis of New S-Alkylated-3-Mercapto-1,2,4-Triazole Derivatives Bearing Cyclic Amine Moiety as Potent Anticancer Agents. Lett. Drug Des. Discov. 2012, 9, 276–281. [Google Scholar] [CrossRef]
- Wiatrak, B.; Balon, K. Protective Activity of Aβ on Cell Cultures (PC12 and THP-1 after Differentiation) Preincubated with Lipopolysaccharide (LPS). Mol. Neurobiol. 2021, 58, 1453–1464. [Google Scholar] [CrossRef]
- Gaussian 16, Revision C.01. 2016. Available online: https://gaussian.com/relnotes/ (accessed on 12 November 2024).
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Padmaja, L.; Ravikumar, C.; Sajan, D.; Hubert Joe, I.; Jayakumar, V.S.; Pettit, G.R.; Faurskov Nielsen, O. Density Functional Study on the Structural Conformations and Intramolecular Charge Transfer from the Vibrational Spectra of the Anticancer Drug Combretastatin-A2. J. Raman Spectrosc. 2009, 40, 419–428. [Google Scholar] [CrossRef]
- Koopmans, T. Über Die Zuordnung von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hermanson, D.J.; Banerjee, S.; Ghebreselasie, K.; Clayton, G.M.; Garavito, R.M.; Marnett, L.J. Oxicams Bind in a Novel Mode to the Cyclooxygenase Active Site via a Two-Water-Mediated H-Bonding Network. J. Biol. Chem. 2014, 289, 6799–6808. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A Web Server for Automatic Structure Matching and RMSD Calculations among Identical and Similar Compounds in Protein-Ligand Docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Peregrym, K.; Szczukowski, Ł.; Wiatrak, B.; Potyrak, K.; Czyżnikowska, Ż.; Świątek, P. In Vitro and In Silico Evaluation of New 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]Pyridazinone as Promising Cyclooxygenase Inhibitors. Int. J. Mol. Sci. 2021, 22, 9130. [Google Scholar] [CrossRef]
- Glomb, T.; Wiatrak, B.; Gębczak, K.; Gębarowski, T.; Bodetko, D.; Czyżnikowska, Ż.; Świątek, P. New 1,3,4-Oxadiazole Derivatives of Pyridothiazine-1,1-Dioxide with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2020, 21, 9122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Menche, G.; Power, T.D.; Sower, L.; Peterson, J.W.; Schein, C.H. Accounting for Ligand-bound Metal Ions in Docking Small Molecules on Adenylyl Cyclase Toxins. Proteins Struct. Funct. Bioinform. 2007, 67, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA. Dassault Systèmes. Biovia Diccovery Studio Vizualizer, V21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2020.
- Wiatrak, B.; Krzyżak, E.; Szczęśniak-Sięga, B.; Szandruk-Bender, M.; Szeląg, A.; Nowak, B. Effect of Tricyclic 1,2-Thiazine Derivatives in Neuroinflammation Induced by Preincubation with Lipopolysaccharide or Coculturing with Microglia-like Cells. Pharmacol. Rep. 2022, 74, 890–908. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-Inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-Inflammatory Drugs and Their Mechanism of Action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ciurko, D.; Łaba, W.; Żarowska, B.; Janek, T. Enzymatic Hydrolysis Using Bacterial Cultures as a Novel Method for Obtaining Antioxidant Peptides from Brewers’ Spent Grain. RSC Adv. 2021, 11, 4688–4700. [Google Scholar] [CrossRef] [PubMed]
- Ciurko, D.; Neuvéglise, C.; Szwechłowicz, M.; Lazar, Z.; Janek, T. Comparative Analysis of the Alkaline Proteolytic Enzymes of Yarrowia Clade Species and Their Putative Applications. Int. J. Mol. Sci. 2023, 24, 6514. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Ertürk, Ö.; Değirmenci, A.; Yurdakul Ertürk, E.; Atlı Şekeroğlu, Z.; Çol Ayvaz, M.; Kontaş Yedier, S. Antimicrobial, Antioxidant and Cytotoxic Activities of Some Analgesic or Anti-inflammatory Drugs. Biologia 2021, 76, 2365–2379. [Google Scholar] [CrossRef]
- Janak, J.F. Proof That ∂E∂ni = εin in Density-Functional Theory. Phys. Rev. B 1978, 18, 7165–7168. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Roy, D.R.; Chattaraj, P.K. Electrophilicity Index as a Possible Descriptor of Biological Activity. Bioorg. Med. Chem. 2004, 12, 5533–5543. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M.; Kumar, V.; Mohan, C.G. Synthesis, Biological Evaluation and Molecular Docking Studies of Stellatin Derivatives as Cyclooxygenase (COX-1, COX-2) Inhibitors and Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2011, 21, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Borer, J.S.; Simon, L.S. Cardiovascular and Gastrointestinal Effects of COX-2 Inhibitors and NSAIDs: Achieving a Balance. Arthritis Res. Ther. 2005, 7, S14. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef] [PubMed]
- Rayar, A.-M.; Lagarde, N.; Ferroud, C.; Zagury, J.-F.; Montes, M.; Sylla-Iyarreta Veitia, M. Update on COX-2 Selective Inhibitors: Chemical Classification, Side Effects and Their Use in Cancers and Neuronal Diseases. Curr. Top. Med. Chem. 2017, 17, 2935–2956. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.R.; Pawlitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Gierse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J.; et al. Structural Insights into the Stereochemistry of the Cyclooxygenase Reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef]
- Zambrano, P.; Manrique-Moreno, M.; Petit, K.; Colina, J.R.; Jemiola-Rzeminska, M.; Suwalsky, M.; Strzalka, K. Differential Scanning Calorimetry in Drug-Membrane Interactions. Biochem. Biophys. Res. Commun. 2024, 709, 149806. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Wang, Z.-M.; Romero, J.J.; Ulloa, C.; Perez, J.C.; Giraud, M.-N.; Barreto, J.C. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Associate with Zwitterionic Phospholipids: Insight into the Mechanism and Reversal of NSAID-Induced Gastrointestinal Injury. Nat. Med. 1995, 1, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Bringezu, F.; Reis, S.; Lima, J.L.F.C.; Brezesinski, G. Binding of Nonsteroidal Anti-Inflammatory Drugs to DPPC: Structure and Thermodynamic Aspects. Langmuir 2008, 24, 4132–4139. [Google Scholar] [CrossRef]
- Kyrikou, I.; Hadjikakou, S.K.; Kovala-Demertzi, D.; Viras, K.; Mavromoustakos, T. Effects of Non-Steroid Anti-Inflammatory Drugs in Membrane Bilayers. Chem. Phys. Lipids 2004, 132, 157–169. [Google Scholar] [CrossRef]
- Shabbir, A.; Shahzad, M.; Ali, A.; Zia-ur-Rehman, M. Discovery of New Benzothiazine Derivative as Modulator of Pro- and Anti-Inflammatory Cytokines in Rheumatoid Arthritis. Inflammation 2016, 39, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Al-Fayez, N.; Elsawy, H.; Mansour, M.A.; Akbar Ali, M.; Elghamry, I. Synthesis, Anticancer, Antioxidant, Anti-Inflammatory, Antimicrobial Activities, Molecular Docking, and DFT Studies of Sultams Derived from Saccharin. Molecules 2022, 27, 7104. [Google Scholar] [CrossRef] [PubMed]



| Compound | Cyclooxygenase Inhibition Assay | Selectivity Ratio: (COX-2/COX-1) | MTT Assay IC50 (µM)] | |||
|---|---|---|---|---|---|---|
| COX-1 | COX-2 | |||||
| IC50 [µM] | SD | IC50 [µM] | SD | |||
| BS23 | 241.64 ± 4.2 | 13.19 ± 2.1 | 0.05 | 228.40 ± 28.1 | ||
| BS24 | 126.54 ± 3.6 | 26.34 ± 3.4 | 0.21 | 163.46 ± 15.7 | ||
| BS25 | 130.96 ± 7.5 | 62.63 ± 1.7 | 0.48 | 200.10 ± 20.9 | ||
| BS26 | 128.73 ± 9.1 | 18.20 ± 4.3 | 0.14 | 203.28 ± 22.9 | ||
| BS27 | 116.89 ± 4.3 | 25.55 ± 2.0 | 0.22 | 190.60 ± 12.5 | ||
| BS28 | 95.88 ± 5.7 | 12.46 ± 1.9 | 0.13 | 179.74 ± 13.6 | ||
| BS29 | 124.81 ± 6.7 | 17.80 ± 2.1 | 0.14 | 232.51 ± 11.8 | ||
| BS30 | 121.51 ± 3.4 | 59.22 ± 3.7 | 0.49 | 168.50 ± 30.4 | ||
| meloxicam | 267.71 ± 8.1 | 112.67 ± 3.3 | 0.42 | 174.23 ± 20.3 | ||
| Compound | ABTS Assay IC50 [µM] | DPPH Assay IC50 [µM] |
|---|---|---|
| BS23 | 54.21 ± 2.2 | >500 |
| BS24 | 63.22 ± 2.8 | >500 |
| BS25 | 75.26 ± 1.4 | >500 |
| BS26 | 83.72 ± 3.2 | >500 |
| BS27 | 83.34 ± 2.5 | >500 |
| BS28 | 88.44 ± 1.9 | >500 |
| BS29 | 53.74 ± 1.7 | >500 |
| BS30 | 88.28 ± 1.6 | >500 |
| meloxicam | 89.45 ± 1.5 | >500 |
| ascorbic acid | 124.27 ± 2.3 | 218.31 ± 1.8 |
| HOMO | Δε | LUMO | |
|---|---|---|---|
| BS23 | 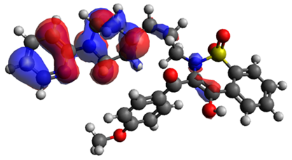 | 3.523 eV |  |
| BS24 |  | 3.600 eV |  |
| BS28 |  | 2.997 eV |  |
| HOMO | LUMO | GAP | I | A | η | s | μ | χ | ω | |
|---|---|---|---|---|---|---|---|---|---|---|
| BS23 | −5.764 | −2.241 | 3.523 | 5.764 | 2.241 | 4.643 | 0.107 | −3.993 | 3.993 | 1.717 |
| BS24 | −5.853 | −2.253 | 3.600 | 5.853 | 2.253 | 4.726 | 0.105 | −4.053 | 4.053 | 1.737 |
| BS25 | −5.762 | −2.370 | 3.392 | 5.762 | 2.370 | 4.570 | 0.109 | −4.066 | 4.066 | 1.806 |
| BS26 | −5.769 | −2.437 | 3.332 | 5.769 | 2.437 | 4.550 | 0.109 | −4.103 | 4.103 | 1.849 |
| BS27 | −5.886 | −2.449 | 3.437 | 5.886 | 2.449 | 4.661 | 0.107 | −4.167 | 4.167 | 1.862 |
| BS28 | −5.576 | −2.579 | 2.997 | 5.576 | 2.579 | 4.286 | 0.116 | −4.077 | 4.077 | 1.939 |
| BS29 | −5.764 | −2.591 | 3.173 | 5.764 | 2.591 | 4.468 | 0.111 | −4.177 | 4.177 | 1.952 |
| BS30 | −5.908 | −2.591 | 3.317 | 5.908 | 2.591 | 4.612 | 0.108 | −4.249 | 4.249 | 1.957 |
| ΔGbinding [kcal/mol] | Van Der Waals | Hydrogen Bonds | π-Type Interactions | |
|---|---|---|---|---|
| BS23 | −15.6 | Leu93, Met113, Leu117, Ser119, Ser353, Tyr355, Leu359, Phe381, Tyr385, Trp387, Leu534 | Ser530 | Val116, Arg120, Ile345, Leu384, Gly526, Leu531, Met535 |
| BS24 | −15.3 | Leu93, Met113, Leu117, Ser119, Phe205, Phe209, Tyr348, Leu359, Phe381, Trp387, Val523, Gly526 | Ala527, Ser530, Leu531 | Val116, Ile345, Val349, Leu352, Tyr366, Leu534, Met535 |
| BS25 | −15.2 | Leu93, Met113, Ser119, Ser353, Tyr355, Leu359, Phe381, Leu384, Trp387, Phe518, Gly526, Ser530, Leu534 | Ala527 | Val116, Arg120, Ile345, Leu352, Val523, Met535 |
| BS26 | −15.1 | Leu117, Ser353, Phe357, Phe381, Leu384, Tro387, Gly526, Phe518, Ser530, Leu531, Leu534, | Arg120, Tyr355 | Val116, Leu359, Val349, Leu352, Met522, Ala527 |
| BS27 | −15.4 | Met113, Leu117, Ser353, Phe381, Tyr385, Trp387 | Arg120, Ser530 | Val116, Leu351, Leu384, Leu534, Met535, Met522, Gly526 |
| BS28 | −15.4 | Val89, Leu93, Met113, Leu117, Ser119, Ser353, Leu534, Tyr355, Leu359, Phe381, Tyr385, Trp387, Phe518, Ser530 | Ala527 | Val116, Ile345, Gly526, Leu531 |
| BS29 | −15.5 | Leu93, Met113, Leu117, Ser119, Ser353, Leu534, Tyr355, Leu359, Phe381, Trp387, Phe518, Ser530 | Ala527 | Val116, Ile345, Leu352, Leu531 |
| BS30 | −15.6 | Leu117, Ser353, Phe381, Leu384, Tyr385 | Arg120, Ser530 | Met113, Val116, Ile345, Trp387, Met522, Gly526, Leu531, Met535, Leu543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczęśniak-Sięga, B.M.; Maniewska, J.; Wiatrak, B.; Janek, T.; Nowotarska, P.; Czyżnikowska, Ż. Anti-Inflammatory Properties of Novel 1,2-Benzothiazine Derivatives and Their Interaction with Phospholipid Model Membranes. Membranes 2024, 14, 274. https://doi.org/10.3390/membranes14120274
Szczęśniak-Sięga BM, Maniewska J, Wiatrak B, Janek T, Nowotarska P, Czyżnikowska Ż. Anti-Inflammatory Properties of Novel 1,2-Benzothiazine Derivatives and Their Interaction with Phospholipid Model Membranes. Membranes. 2024; 14(12):274. https://doi.org/10.3390/membranes14120274
Chicago/Turabian StyleSzczęśniak-Sięga, Berenika M., Jadwiga Maniewska, Benita Wiatrak, Tomasz Janek, Paulina Nowotarska, and Żaneta Czyżnikowska. 2024. "Anti-Inflammatory Properties of Novel 1,2-Benzothiazine Derivatives and Their Interaction with Phospholipid Model Membranes" Membranes 14, no. 12: 274. https://doi.org/10.3390/membranes14120274
APA StyleSzczęśniak-Sięga, B. M., Maniewska, J., Wiatrak, B., Janek, T., Nowotarska, P., & Czyżnikowska, Ż. (2024). Anti-Inflammatory Properties of Novel 1,2-Benzothiazine Derivatives and Their Interaction with Phospholipid Model Membranes. Membranes, 14(12), 274. https://doi.org/10.3390/membranes14120274










