Improved Flux Performance in Brackish Water Reverse Osmosis Membranes by Modification with ZnO Nanoparticles and Interphase Polymerization
Abstract
1. Introduction
2. Materials and Methods
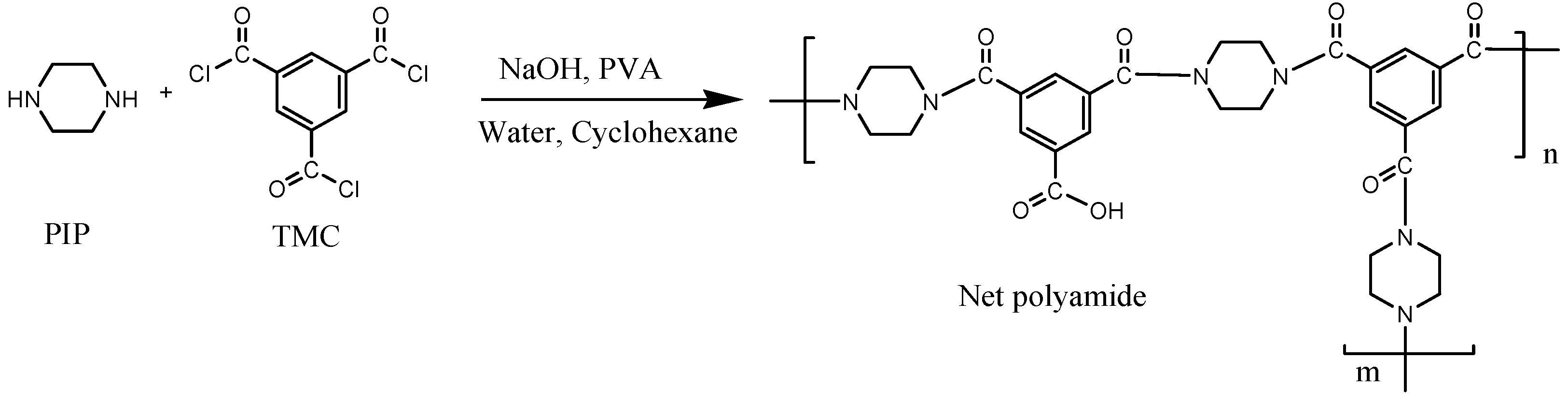
2.1. Modification of BW30 Membranes with ZnO NP
2.2. Characterization of Modified Membranes
2.3. Membrane Performance Test
3. Results and Discussion
3.1. Characterization of BW30 Membrane, Control Membrane, and Membranes Modified with ZnO NP
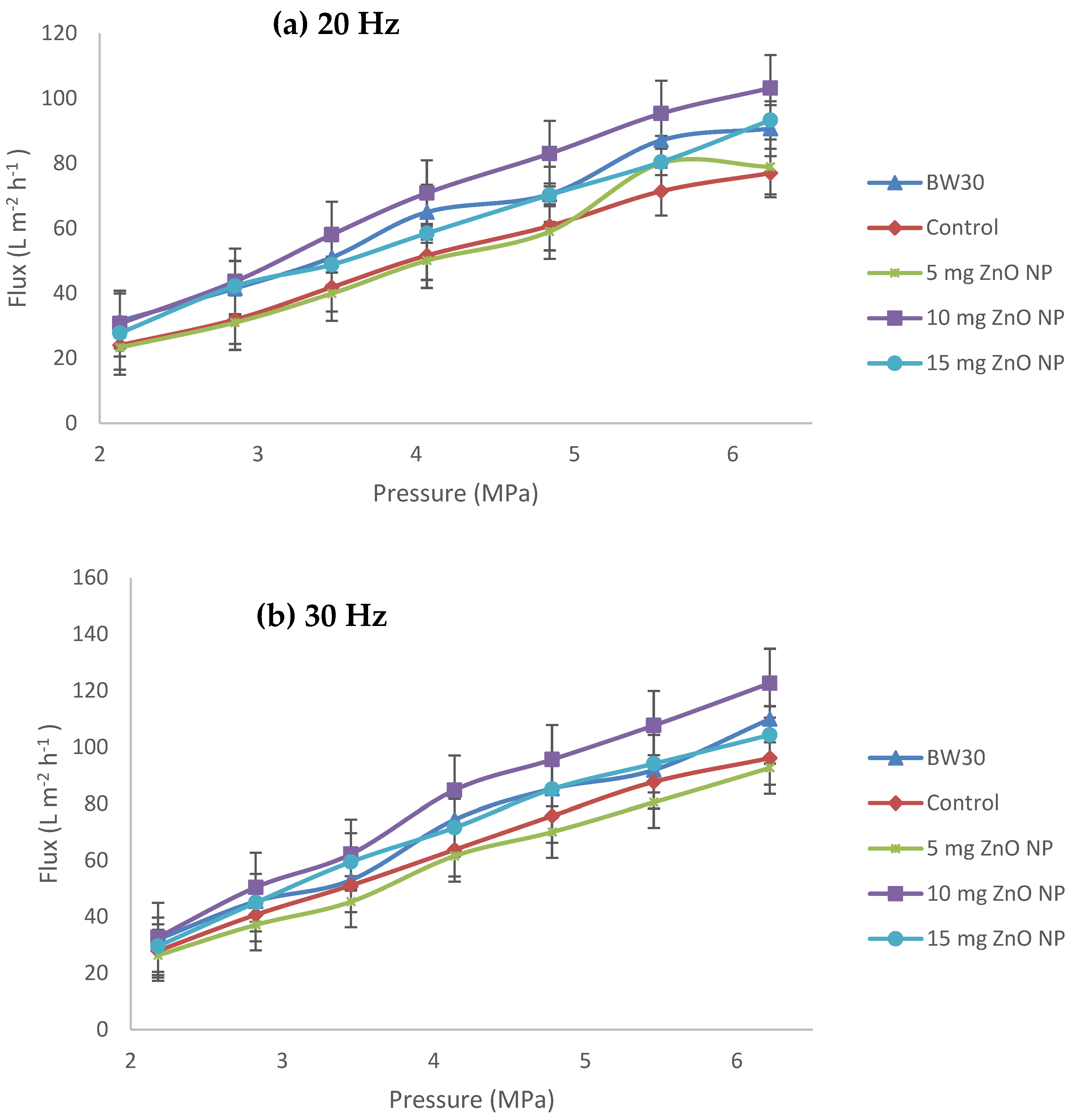
3.2. Performance Test in Reverse Osmosis Membranes in an Equipment Cross Flow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Variables | Description | Units |
| C | Electrical conductivity of water at the measured temperature. In conclusion, mean conductivity of synthetic seawater | (μS cm−1) |
| z | Adjustment interactions between the conductivity of synthetic seawater and seawater | (mg L−1) |
| T | The temperature at which conductivity was measured in the seawater. | (K) |
| Qp | Permeate flow rate | (m3 s−1) |
| Am | Membrane area | (m2) |
| Jv | Flux | (m s−1) |
| μw | Viscosity | (Pa s) |
| %Robs | Salt rejection observed | (%) |
| Ca | Feed water concentration | (mg L−1) |
| Cp | Permeate water concentration | (mg L−1) |
| Rm | Membrane resistance | (1 m−1) |
| β | Experimental constant with a value of 4.10 × 10−7 for salt from instant ocean sea salt. | |
| α₀ | Constant for instant ocean sea salt of 0.0209026. | |
| α₁ | Constant for instant ocean sea salt of 0.0347997. | |
| ω | Mass fraction of salt water in process. | |
| b₁, b₂, b₃, b₄ y b₅ | Constants for solution viscosity calculation: −1.07266, 1.2722 × 10−7, −56.2241, 1.3332 × 10−6, 1.2053 × 10−5 respectively and were obtained experimentally with instant ocean sea salt. | |
| TMC | Synonyms: 1,3,5-Benzenetricarbonyl trichloride, Benzene-1,3,5-tricarbonyl chloride, Trimesic acid trichloride, and Trimesoyl chloride. | |
| Concentration polarization | ||
| ΔP | Pressure change | |
| %Rint | Intrinsic salt rejection |
References
- ONU. Available online: https://www.un.org/es/global-issues/water#:~:text=Todav%C3%ADa%20hay%20alrededor%20de%202.000,potable%20(Banco%20Mundial%202023) (accessed on 27 May 2024).
- Dévora Isiordia, G.E.; Robles Lizárraga, A.; Fimbres Weihs, G.A.; Álvarez Sánchez, J. Comparación de métodos de descarga para vertidos de salmueras, provenientes de una planta desalinizadora en Sonora, México. Rev. Int. De Contam. Ambient. 2017, 33, 45–54. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Z. Spatiotemporal heterogeneity of urban and rural water scarcity and its influencing factors across the world. Ecol. Indic. 2023, 153, 110386. [Google Scholar] [CrossRef]
- CONAGUA (Comisión Nacional del Agua, Parte de la Secretaría de Medio Ambiente y Recursos Naturales [SEMARNAT]), Gobierno de México. Available online: https://smn.conagua.gob.mx/es/climatologia/monitor-de-sequia/monitor-de-sequia-en-mexico (accessed on 18 July 2024).
- Almacenamiento de Presas. Aportaciones de la Cuenca del Río Yaqui, de Distrito de Riego del Río Yaqui. Sitio Web. 2024. Available online: http://www.drryaqui.org.mx/sistemas_Presas.html#gallerysitio2 (accessed on 13 May 2024).
- Ismail, A.F.; Matsuura, T. Membrane Separation Processes: Theories, Problems, and Solutions; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Torres-Valenzuela, P.G.; Álvarez-Sánchez, J.; Dévora-Isiordia, G.E.; Armendáriz-Ontiveros, M.M.; del Rosario Martínez-Macias, M.; Pérez-Sicairos, S.; Fimbres Weihs, G.A. Modification and characterization of TFC membranes with Ag nanoparticles: Application in seawater desalination. Polym. Bull. 2023, 80, 6285–6306. [Google Scholar] [CrossRef]
- Pérez-Sicairos, S.; Miranda-Ibarra, S.A.; Lin-Ho, S.W.; Álvarez-Sánchez, J.; Pérez-Reyes, J.C.; Corrales-López, K.A.; Morales-Cuevas, J.B. Membranas de nanofiltración, preparadas vía polimerización en interfase, dopadas con nanopartículas de ZnO: Efecto en su desempeño. Rev. Mex. De Ing. Química 2016, 15, 961–975. Available online: https://www.redalyc.org/articulo.oa?id=62048168025 (accessed on 1 August 2024). [CrossRef]
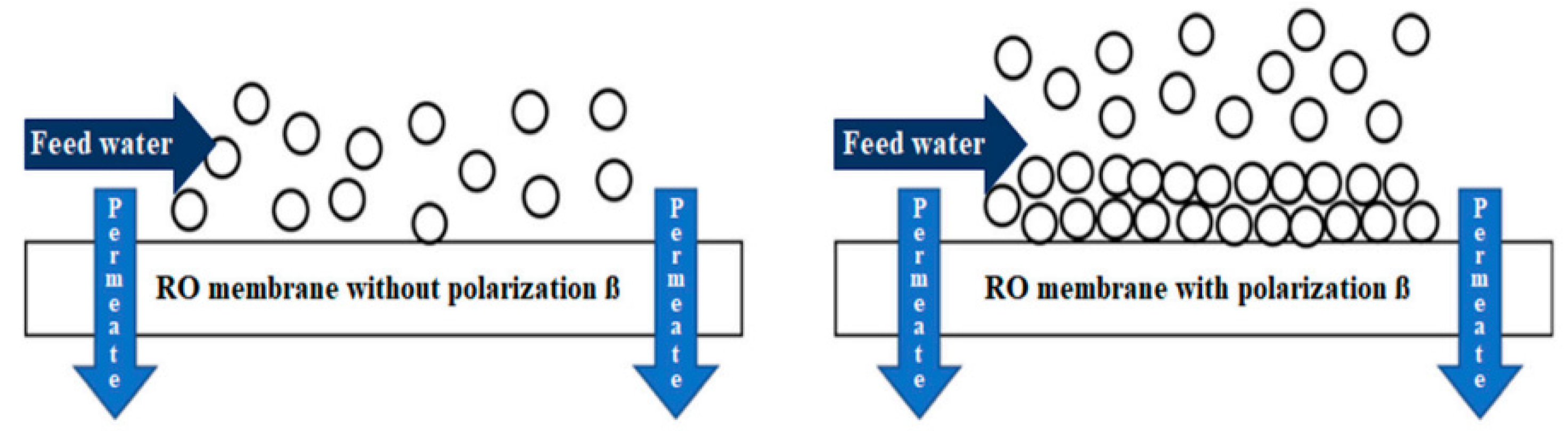
- Dévora-Isiordia, G.E.; Cásares-De la Torre, C.A.; Morales-Mendívil, D.P.; Montoya-Pizeno, R.; Velázquez-Limón, N.; Aguilar-Jiménez, J.A.; Ríos-Arriola, J. Evaluation of Concentration Polarization Due to the Effect of Feed Water Temperature Change on Reverse Osmosis Membranes. Membranes 2023, 13, 3. [Google Scholar] [CrossRef]
- Kucera, J. Reverse Osmosis: Industrial Processes and Applications, 2nd ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2015. [Google Scholar] [CrossRef]
- Dévora-Isiordia, G.E.; Villegas-Peralta, Y.; Piña-Martinez, H.A.; Sánchez-Duarte, R.G.; Álvarez-Sánchez, J. Determination of the concentration polarization in a reverse osmosis plant to desalinate sea water Determinación de polarización de la concentración en una planta de ósmosis inversa para desalinizar agua de mar. Rev. Mex. De Ing. Química 2023, 22, 2349. [Google Scholar] [CrossRef]
- Ríos-Arriola, J.; Velázquez, N.; Aguilar-Jiménez, J.A.; Dévora-Isiordia, G.E.; Cásares-de la Torre, C.A.; Corona-Sánchez, J.A.; Islas, S. State of the Art of Desalination in Mexico. Energies 2022, 15, 8434. [Google Scholar] [CrossRef]
- Rodríguez-López, J.; Robles-Lizárraga, A.; Encinas-Guzmán, M.I.; Correa-Díaz, F.; Dévora-Isiordia, G.E. Assessment of fixed, single-axis, and dual-axis photovoltaic systems applied to a reverse osmosis desalination process in northwest Mexico. Desalination Water Treat. 2021, 2, 1. [Google Scholar] [CrossRef]
- Istirokhatun, T.; Lin, Y.; Shen, Q.; Guan, K.; Wang, S.; Matsuyama, H. Ag-based nanocapsuleregulated interfacial polymerization Enables synchronous nanostructure towards high-performance nanofiltration membrane for sustainable water remediation. J. Membr. Sci. 2022, 645, 120196–120206. [Google Scholar] [CrossRef]
- Rabiee, H.; Vatanpour, V.; Farahani, M.H.D.A.; Zarrabi, H. Improvement in flux and antifouling properties of PVC ultrafiltration membranes by incorporation of zinc oxide (ZnO) nanoparticles. Sep. Purif. Technol. 2015, 156, 299–310. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2020, 241, 125068. [Google Scholar] [CrossRef] [PubMed]
- Isawi, H.; El-Sayed, M.H.; Feng, X.; Shawky, H.; Abdel Mottaleb, M.S. Surface nanostructuring of thin film composite membranes via grafting polymerization and incorporation of ZnO nanoparticles. Appl. Surf. Sci. 2016, 385, 268–281. [Google Scholar] [CrossRef]
- NIST. Thermophysical Properties of Fluid Systems. 2018. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 1 August 2024).
- Jiang, J.; Sandler, S.I. A New Model for the Viscosity of Electrolyte Solutions. Ind. Eng. Chem. Res. 2003, 42, 6267–6272. [Google Scholar] [CrossRef]
- Armendáriz-Ontiveros, M.M.; Álvarez-Sánchez, J.; Dévora-Isiordia, G.E.; García, A.; Fimbres Weihs, G.A. Effect of seawater variability on endemic bacterial biofouling of a reverse osmosis membrane coated with iron nanoparticles (FeNPs). Chem. Eng. Sci. 2020, 223, 115753. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, J.; Encinas-Meneses, E.; Pérez-Sicairos, S.; Ríos-Vazquez, N.J.; Dévora-Isiordia, G.E.; González-Enríquez, R. Preparación y caracterización de membranas compuestas elaboradas a partir de 2, 4, 6 Trimetil m-Fenilendiamina y Cloruro de Trimesoílo. Rev. Iberoam. De Cienc. 2014, 1, 123–136. [Google Scholar]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation performance of thin-film composite nanofiltration membrane through interfacial polymerization using different amine monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Guo, H.; Ma, X.-H.; Lin, C.-E.; Zhou, Y.; Tang, C.Y.; Cao, B.; Zhu, B.; Shih, K. A novel thin-film nano-templated composite membrane with in situ silver nanoparticles loading: Separation performance enhancement and implications. J. Membr. Sci. 2017, 544, 351–358. [Google Scholar] [CrossRef]
- Vue Oh, N.-W.; Jonggeon, J.; Lee, K.-H. Preparation and characterization of nanofiltration composite membranes using polyacrylonitrile (PAN). II. Preparation and characterization of polyamide composite membranes. J. Appl. Polym. Sci. 2001, 80, 2729–2736. [Google Scholar] [CrossRef]
- Carey, F.A.; Giuliano, R.M. Organic Chemistry, 9th ed.; Mc Graw Hill: New York, NY, USA, 2016; Volume 1. [Google Scholar]
- Chung, Y.T.; Ba-Abbad, M.M.; Mohammad, A.W.; Benamor, A. Functionalization of zinc oxide (ZnO) nanoparticles and its effects on polysulfone-ZnO membranes. Desalination Water Treat. 2016, 57, 7801–7811. [Google Scholar] [CrossRef]
- Dávila, J.L.; Galeas, S.; Guerrero, V.H.; Pontón, P.; Rosas, N.M.; Sotomayor, V.; Valdivieso, C. Nuevos Materiales: Aplicaciones Estructurales e Industriales; Escuela Politécnica Nacional: Quito, Ecuador, 2011; Available online: https://bibdigital.epn.edu.ec/handle/15000/4532 (accessed on 1 August 2024).
- Mocanu, A.; Rusen, E.; Diacon, A.; Isopencu, G.; Mustățea, G.; Şomoghi, R.; Dinescu, A. Antimicrobial properties of polysulfone membranes modified with carbon nanofibers and silver nanoparticles. Mater. Chem. Phys. 2018, 223, 39–45. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanoparticle Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Jafarzadeh, Y.; Masoumi, S.; Rostamizadeh, M. Oil-in-water emulsion separation by PVC membranes embedded with GO-ZnO nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104992. [Google Scholar] [CrossRef]
- van den Berg, T.; Ulbricht, M. Polymer nanocomposite ultrafiltration membranes: The influence of polymeric additive, dispersion quality and particle modification on the integration of zinc oxide nanoparticles into polyvinylidene difluoride membranes. Membranes 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Leo, C.P.; Cathie Lee, W.P.; Ahmad, A.L.; Mohammad, A.W. Polysulfone membranes blended with ZnO nanoparticles for reducing fouling by oleic acid. Sep. Purif. Technol. 2012, 89, 51–56. [Google Scholar] [CrossRef]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO/polyethersulfone (PES) hybrid membranes. Desalination 2012, 293, 21–29. [Google Scholar] [CrossRef]
- Martínez-Pérez, J.A.; Martin, P.P. Coeficiente de correlación intraclase. Medicina de Familia. SEMERGEN 2023, 49, 101907. [Google Scholar] [CrossRef]
- Nova Martínez, M.A.; Sorza Álvarez, E.G.; Zabala Arango, L.M. Adecuación de Modelos de Regresión Lineal Simple en R-Studio. 2023. Available online: https://repository.ucc.edu.co/server/api/core/bitstreams/60bd12ac-ac7c-4753-bbe6-484bfb2e0776/content (accessed on 1 August 2024).
- Secretaría de Salud. Norma Oficial Mexicana NOM-127-SSA1-2021. Salud Ambiental, Agua Para Consumo Humano-Límites Permisibles de Calidad y Tratamiento Que Debe Someterse el Agua Para su Potabilización. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5650705&02/05/2022#gsc.tab=0 (accessed on 9 August 2024).
- Armendáriz-Ontiveros, M.M.; Dévora-Isiordia, G.E.; Rodríguez-López, J.; Sánchez-Duarte, R.G.; Álvarez-Sánchez, J.; Villegas-Peralta, Y.; Martínez-Macias, M.D. Effect of Temperature on Energy Consumption and Polarization in Reverse Osmosis. Desalination Using a Spray-Cooled Photovoltaic System. Energies 2022, 15, 7787. [Google Scholar] [CrossRef]
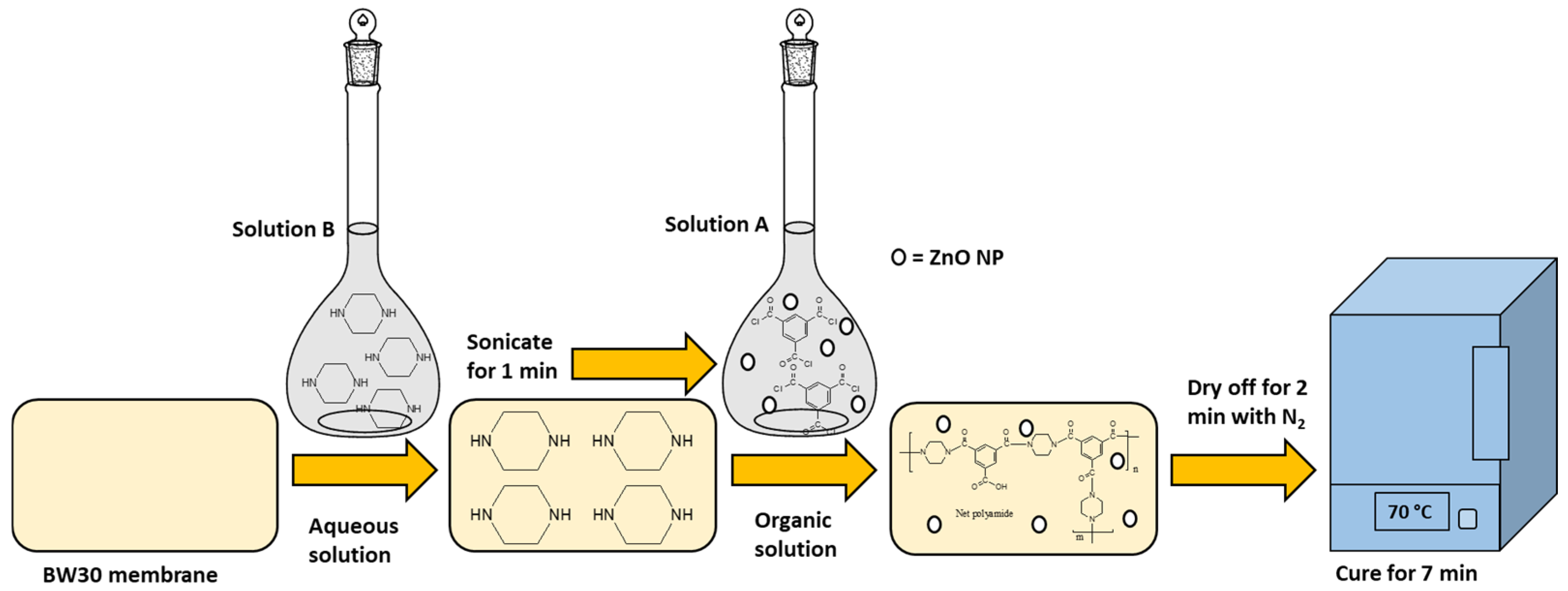


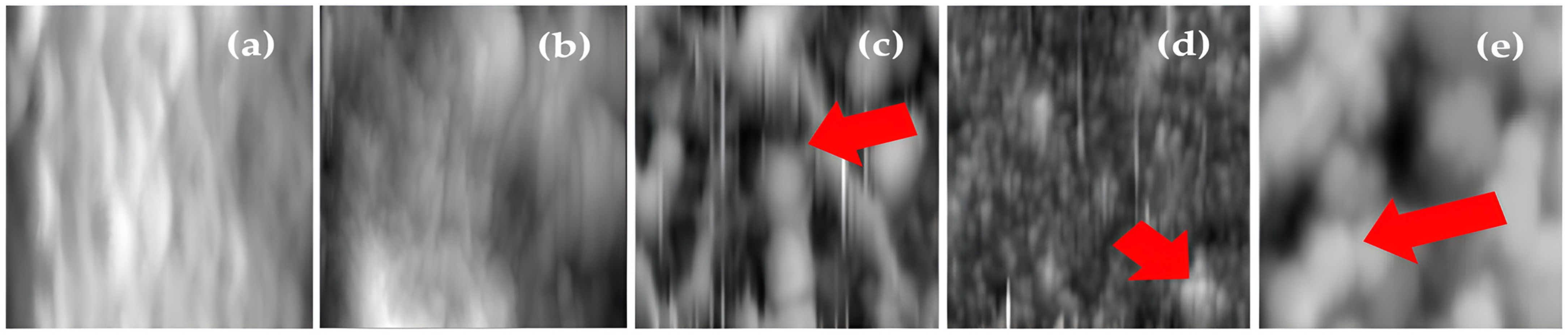
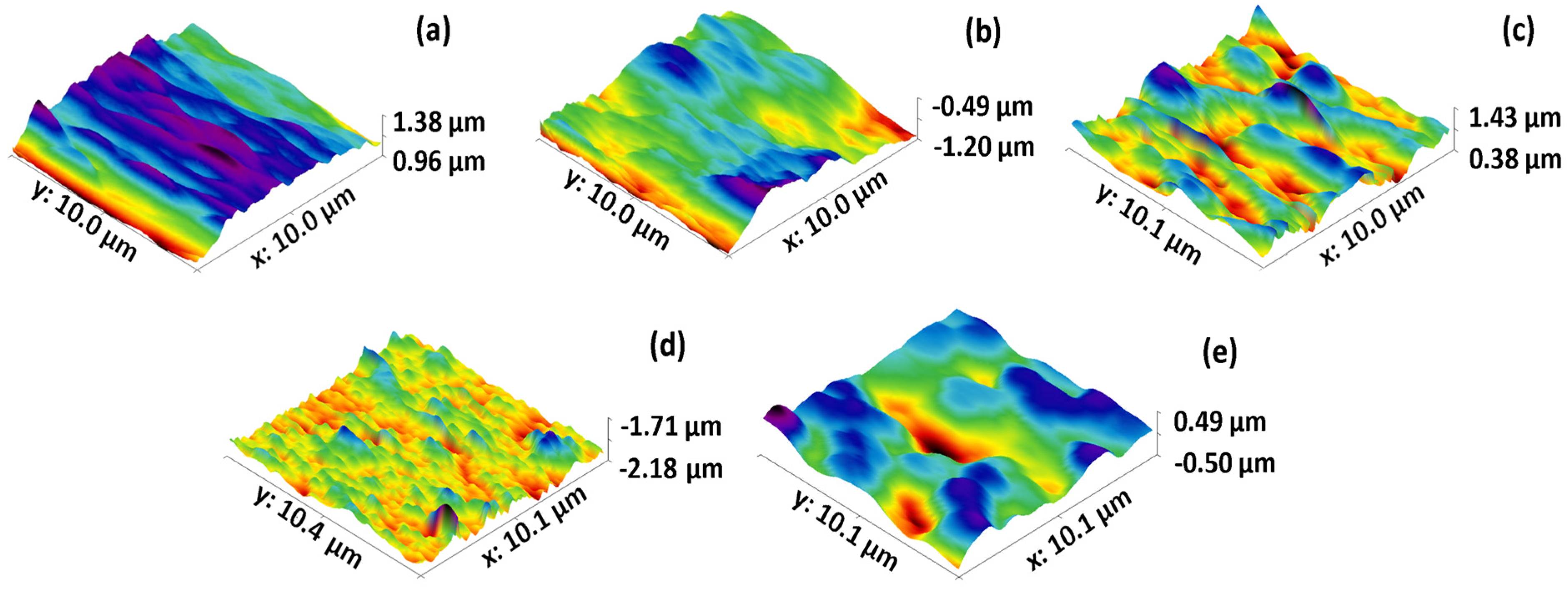
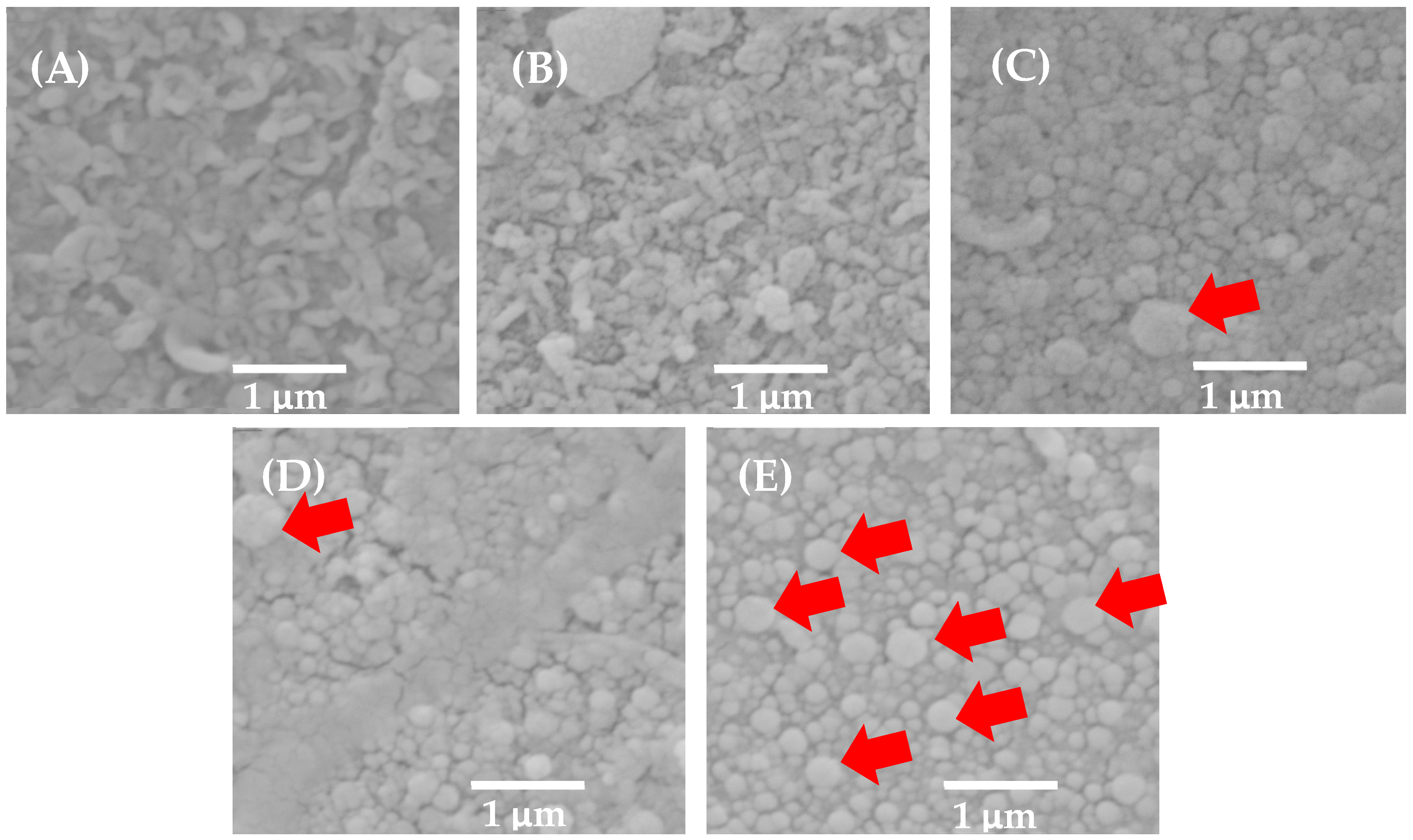
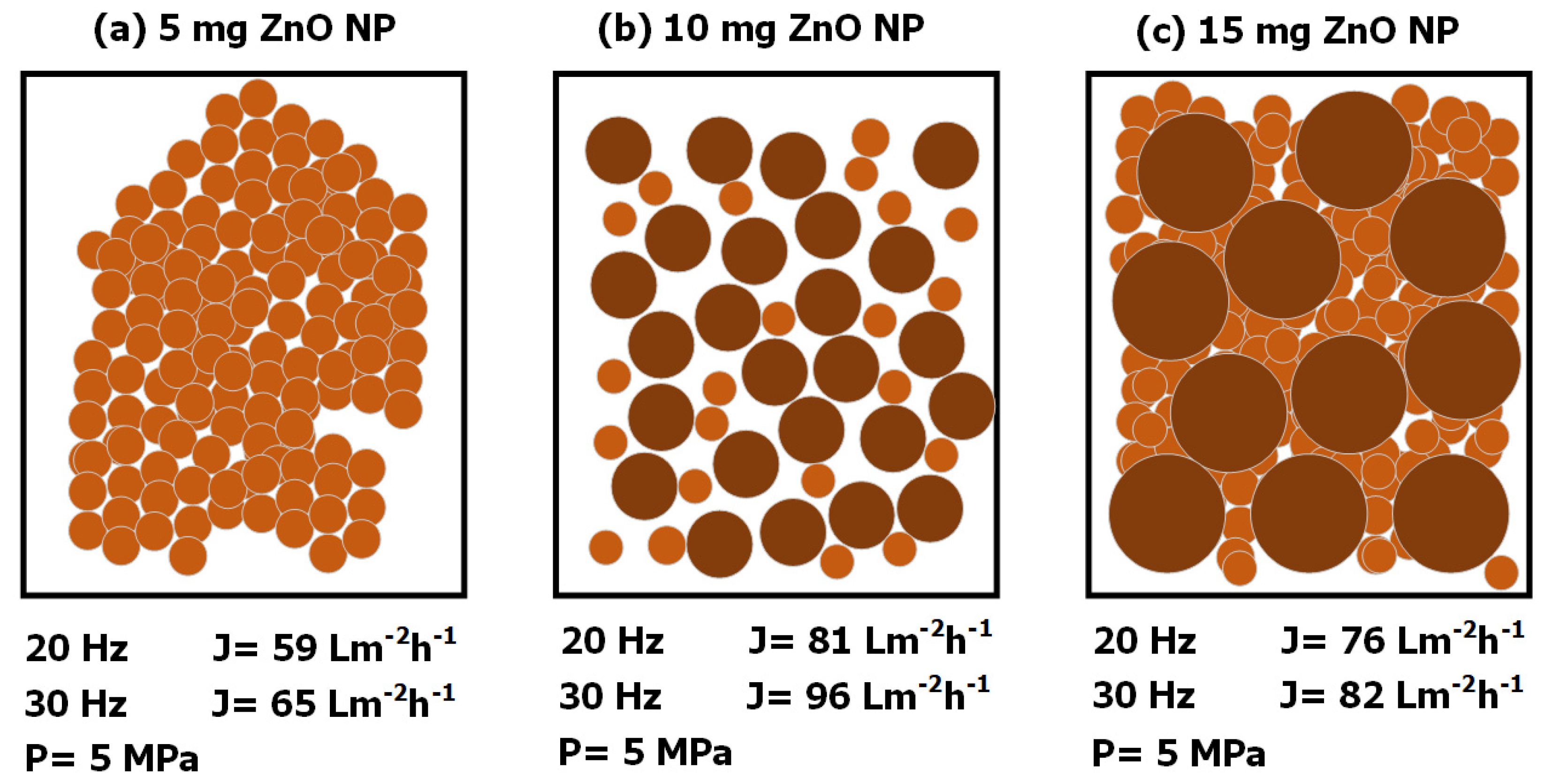
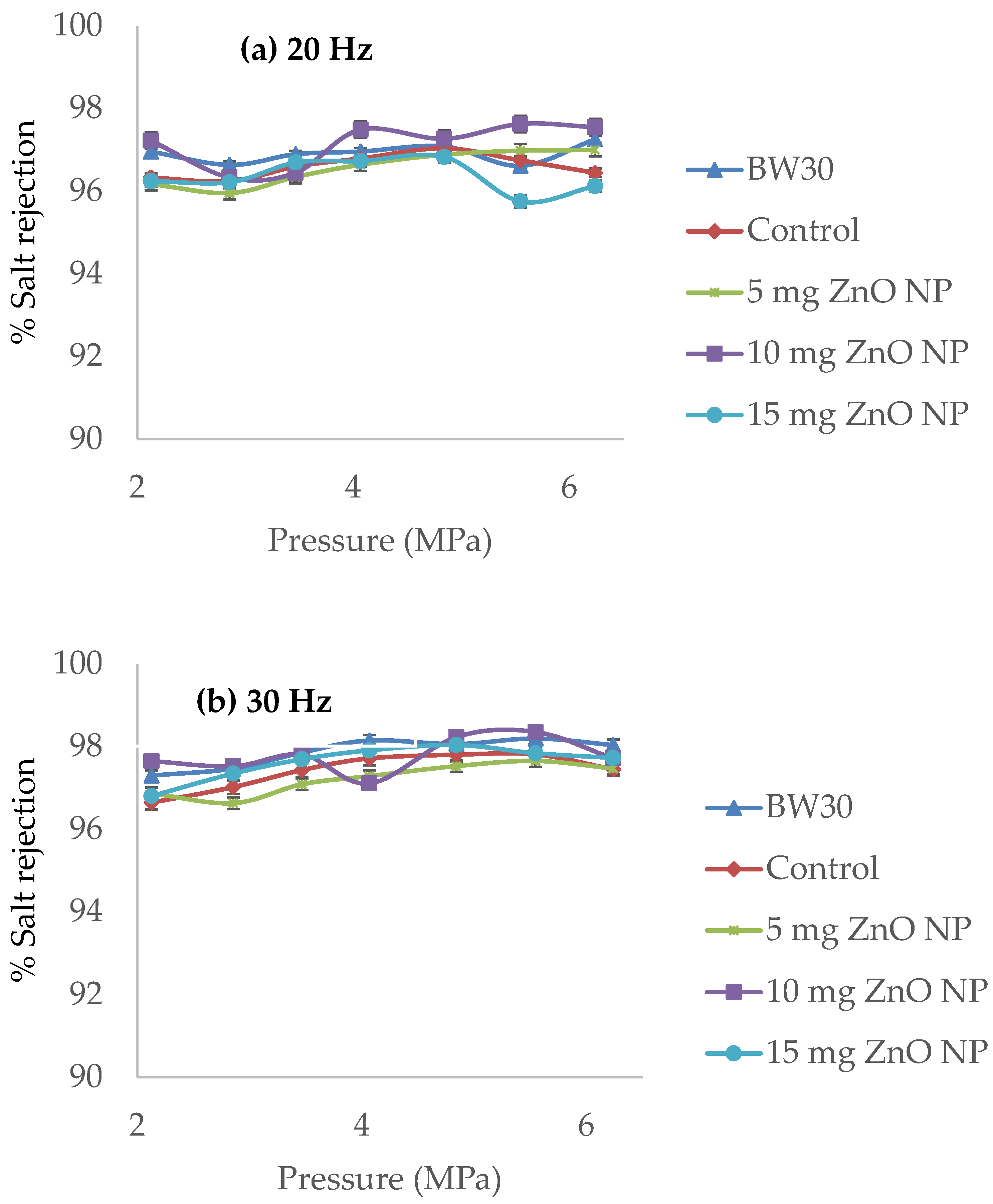
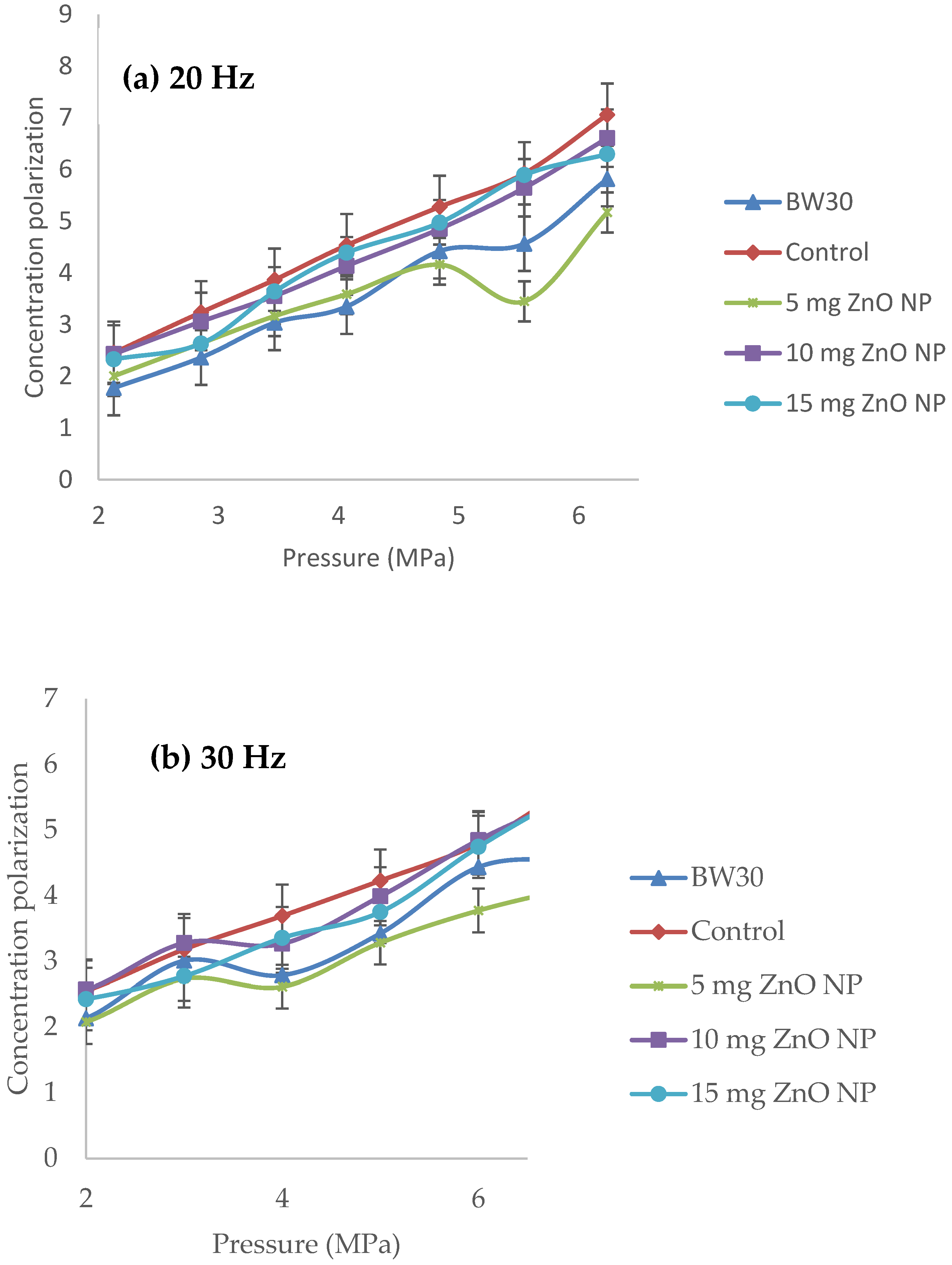
| Membranes | Solution B (Aqueous) (ZnO NP) |
|---|---|
| BW30 | - |
| Control | - |
| 5 mg ZnO NP | 5 mg |
| 10 mg ZnO NP | 10 mg |
| 15 mg ZnO NP | 15 mg |
| Equation | Equation Number | Description and Reference |
|---|---|---|
| (1) | Adjustment interactions between the conductivity of synthetic seawater and seawater [12] | |
| (2) | Viscosity of distillated water [19] | |
| (3) | Permeate flux [6,7] | |
| (4) | Viscosity of the salt water. [20] | |
| (5) | Observed salt rejection [6,7,12] | |
| (6) | Polarization factor [12,21] | |
| (7) | Intrinsic rejection of salts [12,21] |
| Functional Groups | Range 1/λ (cm−1) | 1/λ (cm−1) BW30 | 1/λ (cm−1) BW30 Control | 1/λ (cm−1) 5 mg ZnO | 1/λ (cm−1) 10 mg ZnO | 1/λ (cm−1) 15 mg ZnO |
|---|---|---|---|---|---|---|
| Carboxylic group R-COOH | 1725–1700 | - | 1718.80 | 1718.18 | 1716.26 | 1716.15 |
| Stretching –C=O (Amide III) | 1680–1630 | 1659.90 | - | - | - | - |
| Bending Vibration CO-NH (Amide I and II) | 1640–1550 | 1609.44 | 1617.30 | 1616.87 | 1617.08 | 1619.88 |
| Bending Vibration –CH3 | 1475–1365 | 1488.27 1458.96 & 417.25 | & 1479.83 1438.08 & 1419.62 | & 1483.15 1443.62 & 1419.20 | &1473.39 1442.99 & 1416.33 | &1471.88 1443.46 & 1418.01 |
| Asymmetric stretching C-O-C of the aryl ether group | 1300–1000 | 1244.60 | 1246.84 | 1245.16 | 1248.01 | 1247.25 |
| Symmetric stretching of the sulfone functional group attached to aromatic rings (Ar-SO2-Ar) | 1300–1000 | 1169.59 & 1151.22 | 1172.09 & 1157.16 | 1171.17 & 1152.93 | 1187.14 & 1151.59 | 1185.75 & 1152.34 |
| Membrane | Average Roughness (nm) with Different Scanning Areas | Thickness (µm) | ||
|---|---|---|---|---|
| 10 µm | 30 µm | 50 µm | ||
| BW 30 | 79.65 | 59.30 | 60.60 | 133.53 ± 3.84 |
| Control | 131.55 | 222.50 | 323.00 | 131.35 ± 1.24 |
| 5 mg ZnO NP | 170.03 | 263.00 | 434.50 | 134.98 ± 3.42 |
| 10 mg ZnO NP | 140.20 | 338.50 | 354.00 | 133.53 ± 2.48 |
| 15 mg ZnO NP | 266.50 | 430.00 | 619.50 | 133.89 ± 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Sánchez, J.; Dévora-Isiordia, G.E.; Muro, C.; Villegas-Peralta, Y.; Sánchez-Duarte, R.G.; Torres-Valenzuela, P.G.; Pérez-Sicairos, S. Improved Flux Performance in Brackish Water Reverse Osmosis Membranes by Modification with ZnO Nanoparticles and Interphase Polymerization. Membranes 2024, 14, 207. https://doi.org/10.3390/membranes14100207
Álvarez-Sánchez J, Dévora-Isiordia GE, Muro C, Villegas-Peralta Y, Sánchez-Duarte RG, Torres-Valenzuela PG, Pérez-Sicairos S. Improved Flux Performance in Brackish Water Reverse Osmosis Membranes by Modification with ZnO Nanoparticles and Interphase Polymerization. Membranes. 2024; 14(10):207. https://doi.org/10.3390/membranes14100207
Chicago/Turabian StyleÁlvarez-Sánchez, Jesús, Germán Eduardo Dévora-Isiordia, Claudia Muro, Yedidia Villegas-Peralta, Reyna Guadalupe Sánchez-Duarte, Patricia Guadalupe Torres-Valenzuela, and Sergio Pérez-Sicairos. 2024. "Improved Flux Performance in Brackish Water Reverse Osmosis Membranes by Modification with ZnO Nanoparticles and Interphase Polymerization" Membranes 14, no. 10: 207. https://doi.org/10.3390/membranes14100207
APA StyleÁlvarez-Sánchez, J., Dévora-Isiordia, G. E., Muro, C., Villegas-Peralta, Y., Sánchez-Duarte, R. G., Torres-Valenzuela, P. G., & Pérez-Sicairos, S. (2024). Improved Flux Performance in Brackish Water Reverse Osmosis Membranes by Modification with ZnO Nanoparticles and Interphase Polymerization. Membranes, 14(10), 207. https://doi.org/10.3390/membranes14100207








