A Comparative Study of the Self-Cleaning and Filtration Performance of Suspension Plasma-Sprayed TiO2 Ultrafiltration and Microfiltration Membranes
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Membrane Preparation
2.2. Membrane Characterization
2.3. Membrane Performance
3. Results and Discussion
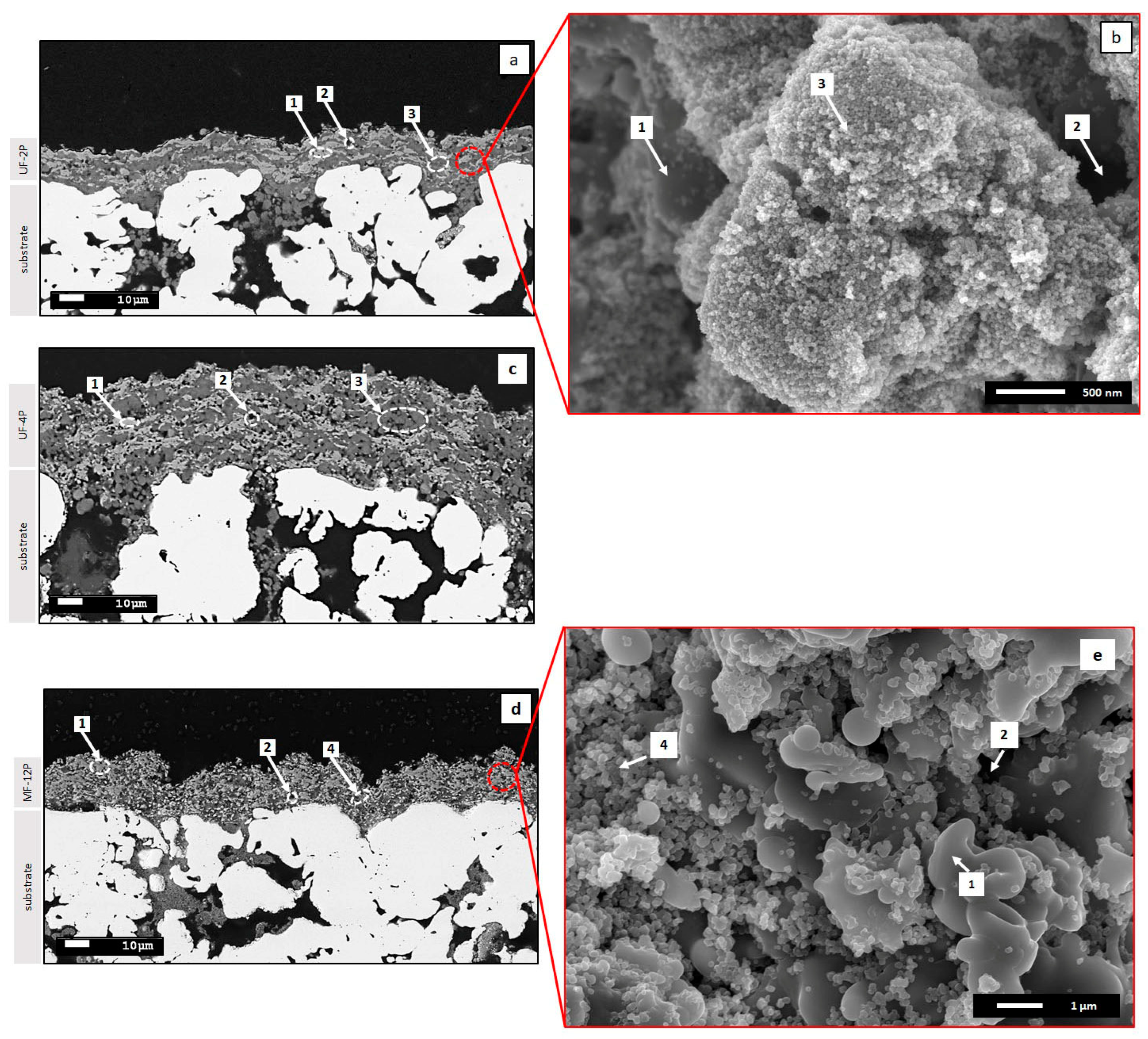
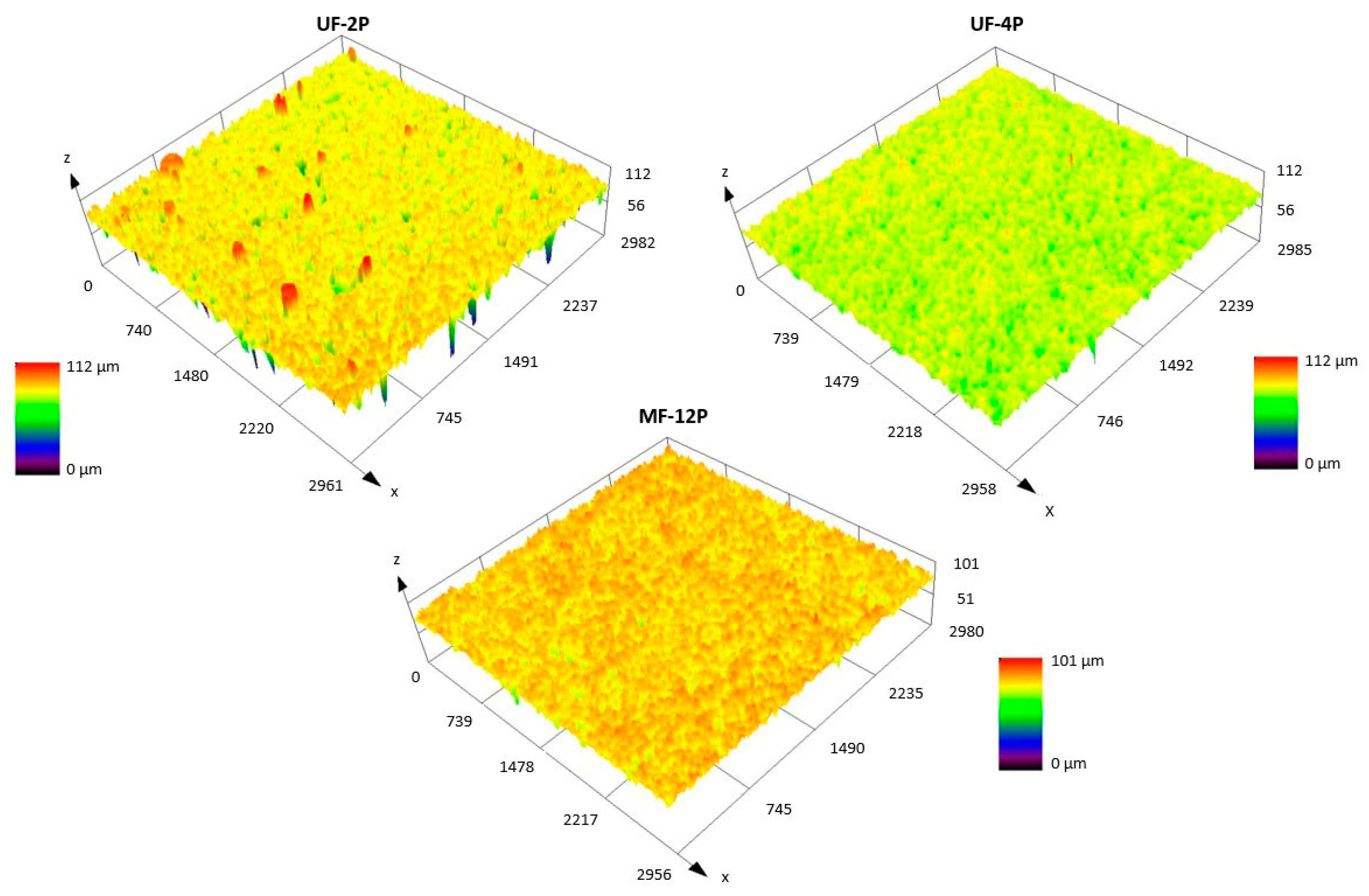
3.1. Membrane Microstructure and Roughness (As-Sprayed Membranes)
3.2. Membrane Performance (As-Sprayed Membranes)
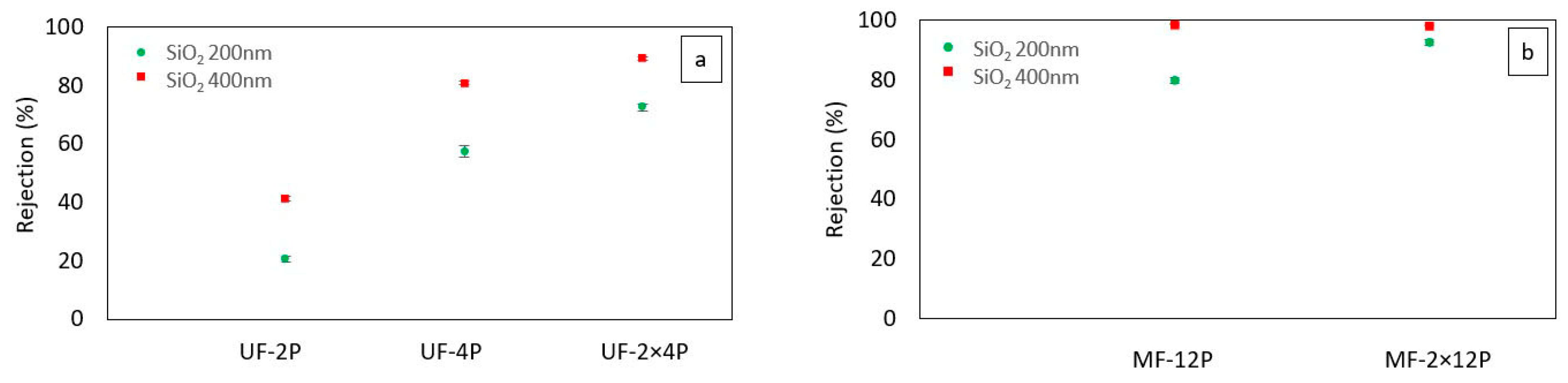
3.2.1. Separation Performance
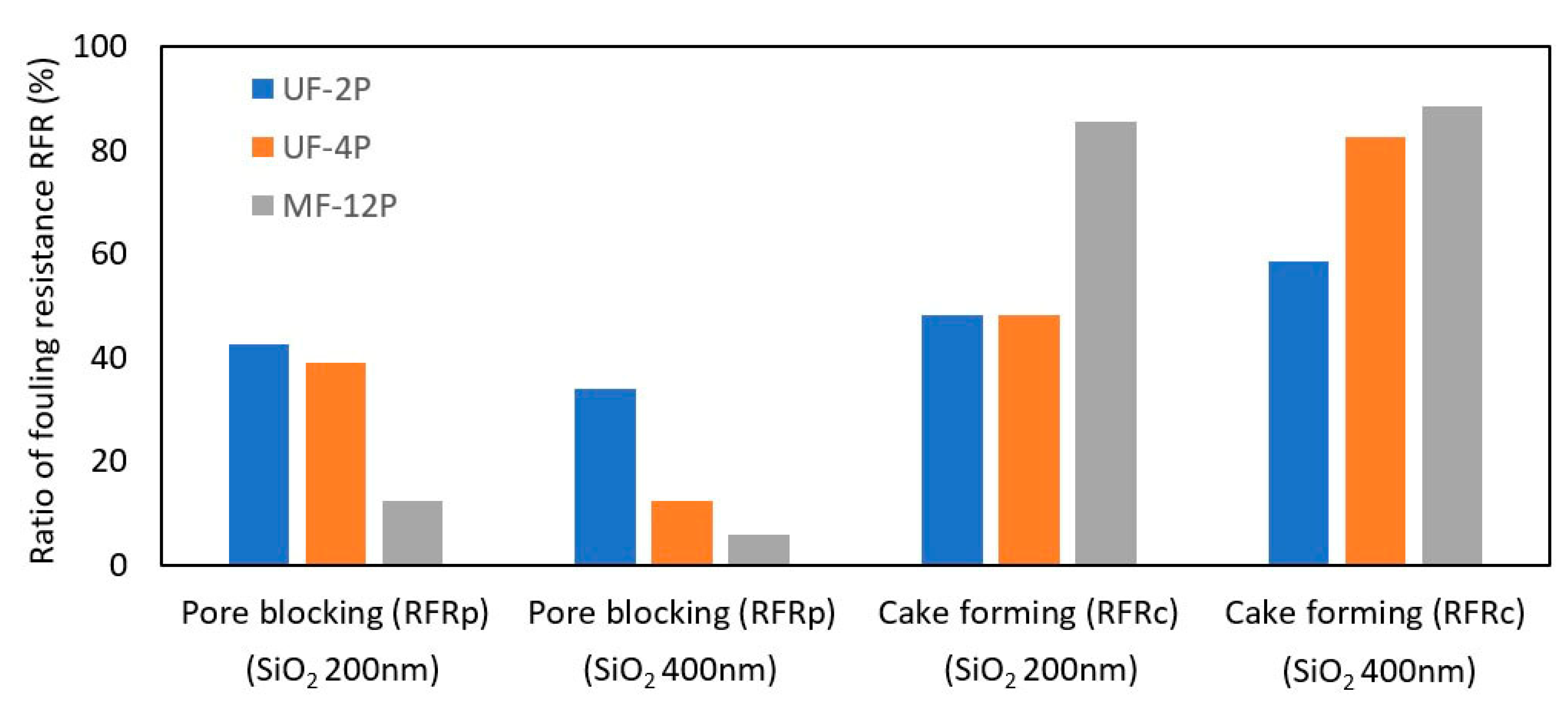
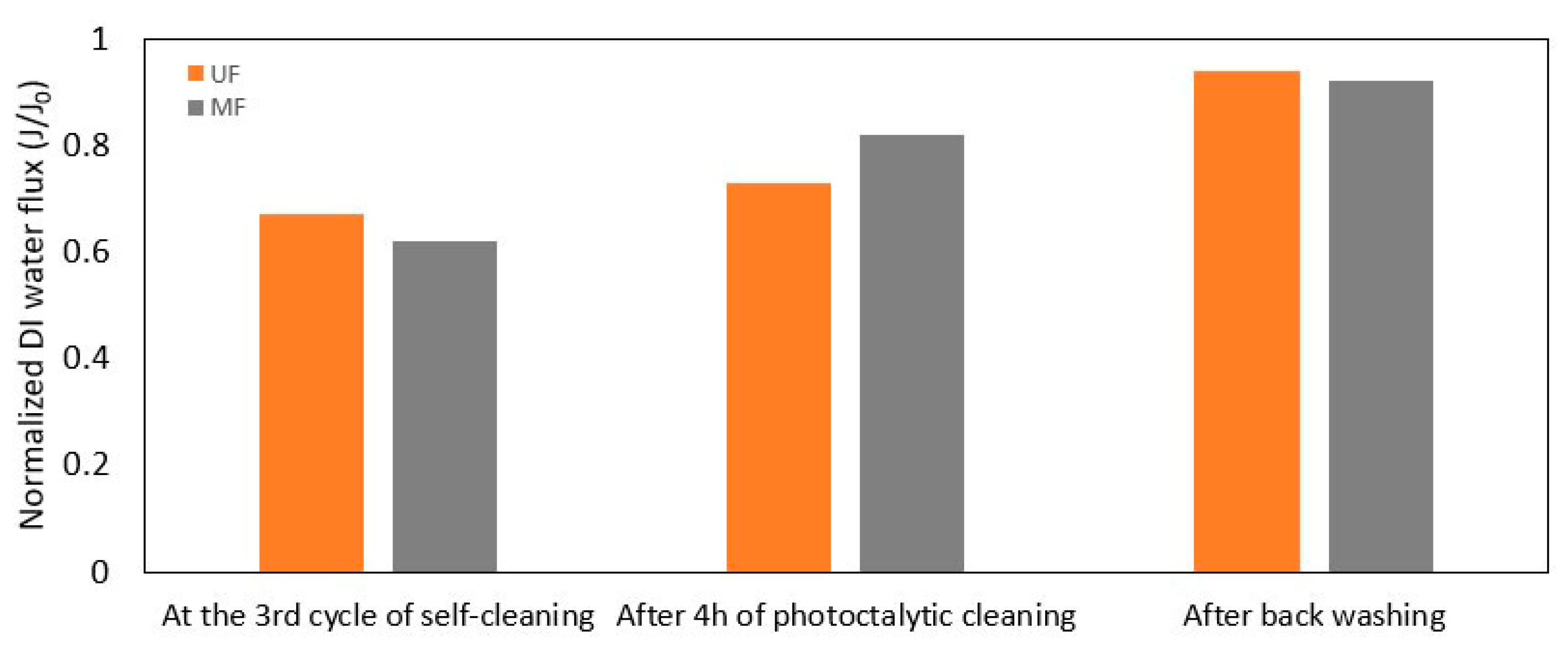
3.2.2. Self-Cleaning and Recyclability
3.2.3. Contact Angle Measurement
3.3. Infiltration of the UF Membranes with Agglomerates of TiO2 Nanoparticles (Filled Membranes)
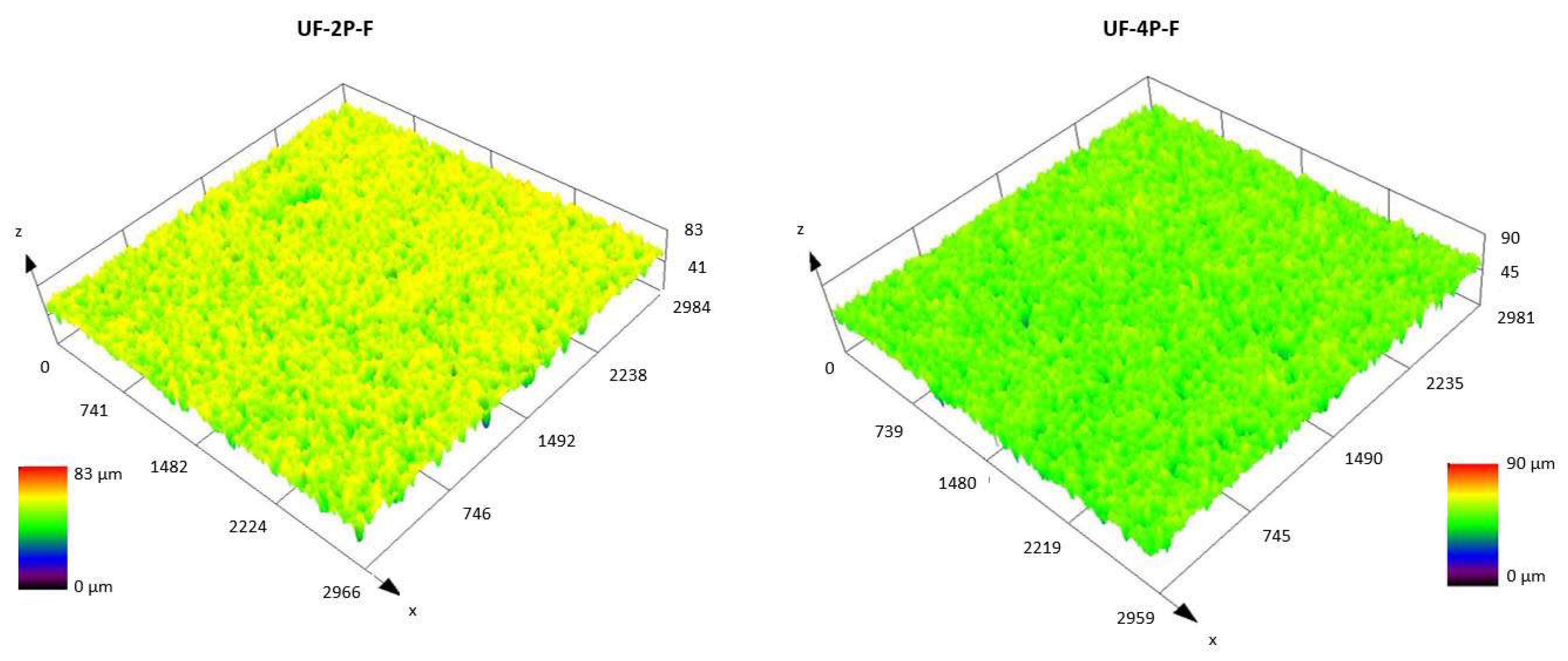
3.3.1. Surface Roughness
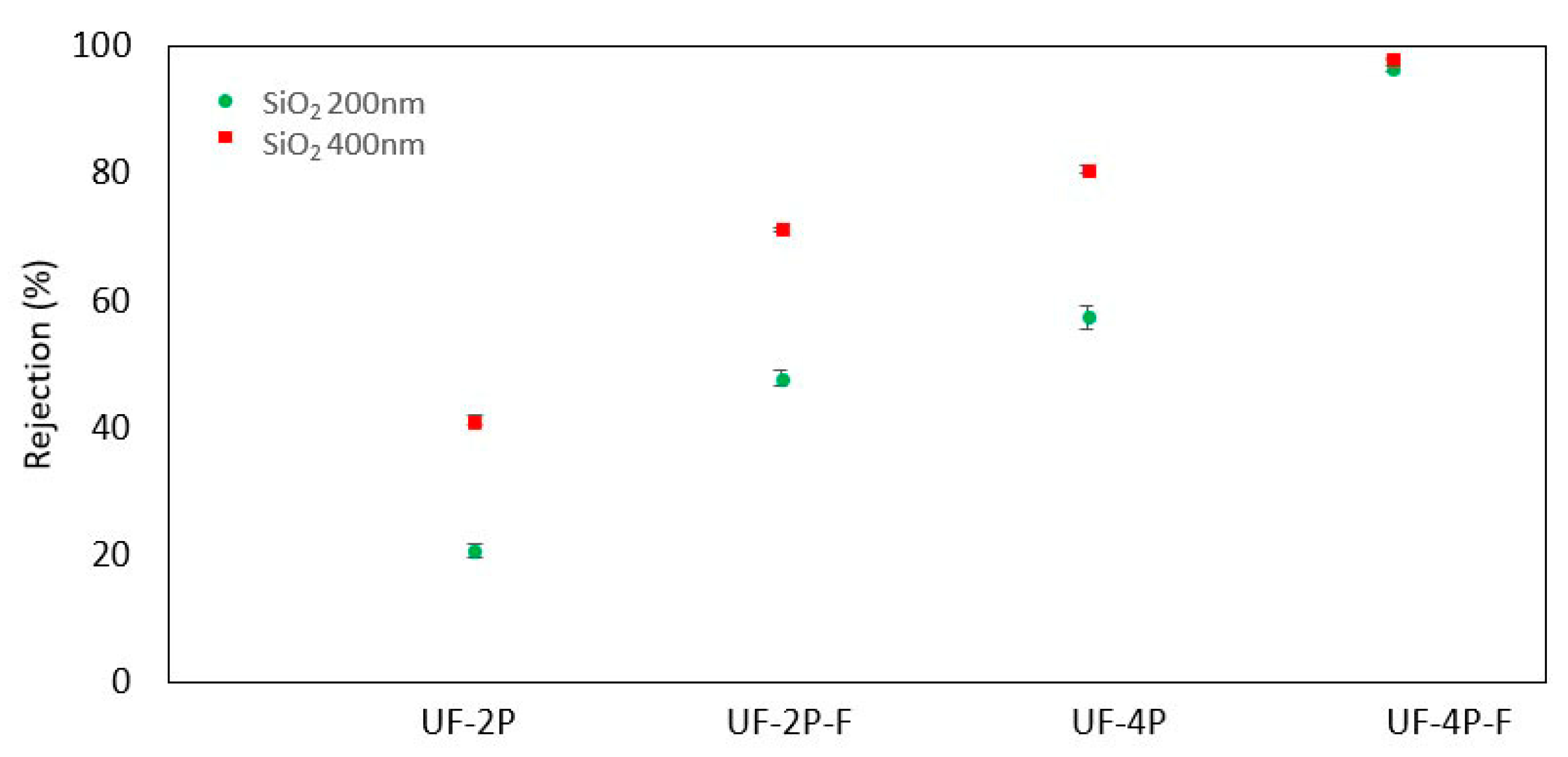
3.3.2. Separation Performance
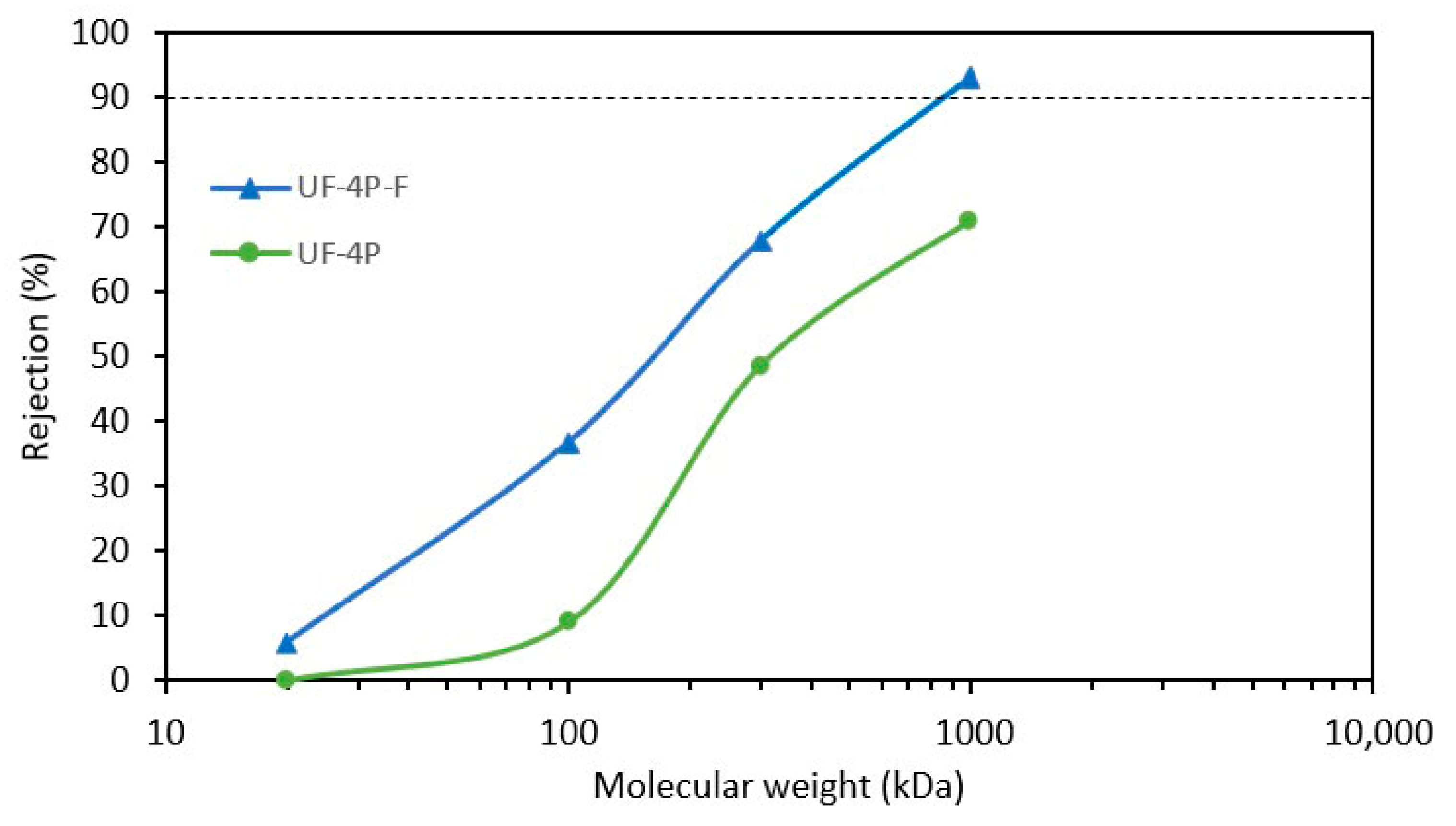
3.3.3. Molecular Weight Cut-Off (MWCO)
4. Conclusions
- The rejection efficiency of the MF and UF membranes can be tuned by adjusting the thickness of the membrane. Increasing the thickness of the membranes led to higher rejection rates, indicating the importance of membrane thickness in determining filtration performance.
- The SPS UF membrane exhibited enhanced rejection rates by effectively filling its larger pores with agglomerates of TiO2 nanoparticles. This modification improved the effective rejection of smaller particles, which could be attributed to the decrease in the average pore size and the total pore size distribution. This finding highlights the potential of incorporating nanoparticles to enhance the performance of ceramic membranes. Additionally, it suggests that the rejection efficiency of the membranes is influenced by both the average pore size and a uniform and narrow pore size distribution.
- Both SPS MF and UF membranes demonstrated recyclable self-cleaning properties under visible light, which is desirable for maintaining long-term filtration efficiency.
- Enhancing the performance of the UF membrane requires better optimization of its structure, focusing on achieving a more uniform membrane structure and narrow pore size distribution to enable more efficient filtration. The optimization can be carried out by adjusting the SPS process parameters.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Samadi, A.; Gao, L.; Kong, L.; Orooji, Y.; Zhao, S. Waste-derived low-cost ceramic membranes for water treatment: Opportunities, challenges and future directions. Resour. Conserv. Recycl. 2022, 185, 106497. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, L. Editorial: Advanced Membrane Science and Technology for Sustainable Environmental Applications. Front. Chem. 2020, 8, 609774. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, X.; Li, Y.; Yan, L.; Zhao, Y.; Shao, L. Progress reports of metal-phenolic network engineered membranes for water treatment. Sep. Purif. Technol. 2023, 320, 124225. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. Outlining the Roles of Membrane-Foulant and Foulant-Foulant Interactions in Organic Fouling During Microfiltration and Ultrafiltration: A Mini-Review. Front. Chem. 2020, 8, 538277. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.L.S.; Kumar, R.V. Insights on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef]
- Yang, H.; Yu, X.; Liu, J.; Tang, Z.; Huang, T.; Wang, Z.; Zhong, Y.; Long, Z.; Wang, L. A Concise Review of Theoretical Models and Numerical Simulations of Membrane Fouling. Water 2022, 14, 3537. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Yue, R.Y.; Yuan, P.C.; Zhang, C.M.; Wan, Z.H.; Wang, S.G.; Sun, X. Robust self-cleaning membrane with superhydrophilicity and underwater superoleophobicity for oil-in-water separation. Chemosphere 2023, 330, 138706. [Google Scholar] [CrossRef] [PubMed]
- Bekbolet, M.; Suphandag, A.S.; Uyguner, C.S. An investigation of the photocatalytic efficiencies of TiO2 powders on the decolourisation of humic acids. J. Photochem. Photobiol. A Chem. 2002, 148, 121–128. [Google Scholar] [CrossRef]
- Rawat, M.; Bulasara, V.K. Synthesis and characterization of low-cost ceramic membranes from fly ash and kaolin for humic acid separation. Korean J. Chem. Eng. 2018, 35, 725–733. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV-vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Qadafi, M.; Rosmalina, R.T.; Pitoi, M.M.; Wulan, D.R. Chlorination disinfection by-products in Southeast Asia: A review on potential precursor, formation, toxicity assessment, and removal technologies. Chemosphere 2023, 316, 137817. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L.; Lim, J.K. Investigation of anti-fouling and uv-cleaning properties of PVDF/TiO2 mixed-matrix membrane for humic acid removal. Membranes 2021, 11, 16. [Google Scholar] [CrossRef]
- Zhang, L.; Ng, T.C.A.; Liu, X.; Gu, Q.; Pang, Y.; Zhang, Z.; Lyu, Z.; He, Z.; Ng, H.Y.; Wang, J. Hydrogenated TiO2 membrane with photocatalytically enhanced anti-fouling for ultrafiltration of surface water. Appl. Catal. B 2020, 264, 118528. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Yang, C.; Li, S. Systematic evaluation of TiO2-GO-modified ceramic membranes for water treatment: Retention properties and fouling mechanisms. Chem. Eng. J. 2019, 378, 122138. [Google Scholar] [CrossRef]
- Lin, Y.F.; Tung, K.L.; Tzeng, Y.S.; Chen, J.H.; Chang, K.S. Rapid atmospheric plasma spray coating preparation and photocatalytic activity of macroporous titania nanocrystalline membranes. J. Memb. Sci. 2012, 389, 83–90. [Google Scholar] [CrossRef]
- Alebrahim, E.; Tarasi, F.; Rahaman, M.S.; Dolatabadi, A.; Moreau, C. Fabrication of titanium dioxide filtration membrane using suspension plasma spray process. Surf. Coat. Technol. 2019, 378, 124927. [Google Scholar] [CrossRef]
- Alebrahim, E.; Rahaman, M.S.; Moreau, C. TiO2 Photocatalytic Ultrafiltration Membrane Developed with Suspension Plasma Spray Process. Coatings 2022, 12, 1764. [Google Scholar] [CrossRef]
- Fauchais, P.; Rat, V.; Coudert, J.F.; Etchart-Salas, R.; Montavon, G. Operating parameters for suspension and solution plasma-spray coatings. Surf. Coat. Technol. 2008, 202, 4309–4317. [Google Scholar] [CrossRef]
- Delbos, C.; Fazilleau, J.; Rat, V.; Coudert, J.F.; Fauchais, P.; Pateyron, B. Phenomena involved in suspension plasma spraying part 2: Zirconia particle treatment and coating formation. Plasma Chem. Plasma Process. 2006, 26, 393–414. [Google Scholar] [CrossRef]
- Chen, Z.; Trice, R.W.; Besser, M.; Yang, X.; Sordelet, D. Air-plasma spraying colloidal solutions of nanosized ceramic powders. J. Mater Sci. 2004, 39, 4171–4178. [Google Scholar] [CrossRef]
- Tarasi, F.; Alebrahim, E.; Dolatabadi, A.; Moreau, C. A Comparative study of YSZ suspensions and coatings. Coatings 2019, 9, 188. [Google Scholar] [CrossRef]
- Rampon, R.; Marchand, O.; Filiatre, C.; Bertrand, G. Influence of suspension characteristics on coatings microstructure obtained by suspension plasma spraying. Surf. Coat. Technol. 2008, 202, 4337–4342. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Ambriz-Vargas, F.; Ettouil, F.B.; Moreau, C. An investigation on the photocatalytic activity of sub-stoichiometric TiO2-x coatings produced by suspension plasma spray. J. Eur. Ceram. Soc. 2021, 41, 544–556. [Google Scholar] [CrossRef]
- Vardelle, A.; Moreau, C.; Akedo, J.; Ashrafizadeh, H.; Berndt, C.C.; Berghaus, J.O.; Boulos, M.; Brogan, J.; Bourtsalas, A.C.; Dolatabadi, A.; et al. The 2016 Thermal Spray Roadmap. J. Therm. Spray Technol. 2016, 25, 1376–1440. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.Y.; Matsuura, T. Synthetic Polymeric Membranes; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Holliday, C.P.; Rasmussen, T.C.; Miller, W.P. Establishing the Relationship Between Turbidity and Total Suspended Sediment Concentration. In Proceedings of the 2003 Georgia Water Resources Conference, Athens, GA, USA, 23–24 April 2003. [Google Scholar]
- Hannouche, A.; Chebbo, G.; Ruban, G.; Tassin, B.; Lemaire, B.J.; Joannis, C. Relationship between turbidity and total suspended solids concentration within a combined sewer system. Water Sci. Technol. 2011, 64, 2445–2452. [Google Scholar] [CrossRef]
- Yue, R.; Raisi, B.; Rahmatinejad, J.; Ye, Z.; Barbeau, B.; Rahaman, M.S. A photo-Fenton nanocomposite ultrafiltration membrane for enhanced dye removal with self-cleaning properties. J. Colloid Interface Sci. 2021, 604, 458–468. [Google Scholar] [CrossRef]
- Kotp, Y.H. Enhancement of Industrial Effluents Quality by Using Nanocomposite Mg/Al LDH Ultrafiltration Membranes. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5244–5260. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.; Li, Y.; Song, R.; Lin, Y.; Huang, Q.; Shao, L. Acid-resistant supramolecular nanofibrous hydrogel membrane with core-shell structure for highly efficient oil/water separation. J. Membr. Sci. 2023, 679, 121705. [Google Scholar] [CrossRef]
- Sabde, A.D.; Trivedi, M.K.; Ramachandhran, V.; Hanra, M.S.; Misra, B.M. Casting and characterization of cellulose acetate butyrate based UF membranes. Desalination 1997, 114, 223–232. [Google Scholar] [CrossRef]
- Razzaghi, M.H.; Safekordi, A.; Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M. Morphological and separation performance study of PVDF/CA blend membranes. J. Membr. Sci. 2014, 470, 547–557. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Duek, A.; Gitis, V. Maximal pore size in UF membranes. J. Membr. Sci. 2012, 394–395, 89–97. [Google Scholar] [CrossRef]
- Singh, S.; Khulbe, K.C.; Matsuura, T.; Ramamurthy, P. Membrane characterization by solute transport and atomic force microscopy. J. Membr. Sci. 1998, 142, 111–127. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Dwivedi, G.; Sampath, S.; Orlov, A. Development and optimization of thermal sprayed ceramic microfiltration membranes. J. Membr. Sci. 2015, 489, 106–111. [Google Scholar] [CrossRef]
- Ding, X.; Fan, Y.; Xu, N. A new route for the fabrication of TiO2 ultrafiltration membranes with suspension derived from a wet chemical synthesis. J. Membr. Sci. 2006, 270, 179–186. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wei, Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H. Water Transport with Ultralow Friction through Partially Exfoliated g-C3N4 Nanosheet Membranes with Self-Supporting Spacers. Angew. Chem.-Int. Ed. 2017, 56, 8974–8980. [Google Scholar] [CrossRef]
- Urase, T.; Yamamoto, K.; Ohgaki, S. Effect of pore structure of membranes and module configuration on virus retention. J. Membr. Sci. 1996, 115, 21–29. [Google Scholar] [CrossRef]
- Tam, C.M.; Tremblay, A.Y. Membrane pore characterization-comparison between single and multicomponent solute probe techniques. J. Membr. Sci. 1991, 57, 271–287. [Google Scholar] [CrossRef]
- Bhave, R.R. Cross-Flow Filtration, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 1996. [Google Scholar] [CrossRef]
- Syafei, A.D.; Lin, C.F.; Wu, C.H. Removal of natural organic matter by ultrafiltration with TiO2-coated membrane under UV irradiation. J. Colloid Interface Sci. 2008, 323, 112–119. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behaviour of suspentions and macromolecular solutionsfiltration. J. Memb. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Chung, Y.; Park, D.; Kim, H.; Nam, S.E.; Kang, S. Novel method for the facile control of molecular weight cut-off (MWCO) of ceramic membranes. Water Res. 2022, 215, 118268. [Google Scholar] [CrossRef]
- Nyström, M.; Ruohomäki, K.; Kaipia, L. Humic acid as a fouling agent in filtration. Desalination 1996, 106, 79–87. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.V. Photocatalytic decomposition of humic acids on TiO2. Part I: Discussion of adsorption and mechanism. J. Photochem. Photobiol. A Chem. 2002, 152, 267–273. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Chiang, K.; Drikas, M.; Amal, R. Removal of humic acid using TiO2 photocatalytic process - Fractionation and molecular weight characterisation studies. Chemosphere 2008, 72, 263–271. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Malato, S.; Weber, J.V. Solar photocatalytic degradation of humic acids as a model of organic compounds of landfill leachate in pilot-plant experiments: Influence of inorganic salts. Appl. Catal. B 2004, 53, 127–137. [Google Scholar] [CrossRef]
- Corin, N.; Backhand, P.; Kulovaara, M. Degradation products formed during UV-irradiation of humic waters. Chemosphere 1996, 33, 245–255. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Chen, P.; Li, F.; Hu, X.; Hua, T. Photocatalytic and antifouling properties of TiO2-based photocatalytic membranes. Mater Today Chem. 2022, 23, 100650. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Memb. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Fan, E.S.C.; Kesler, O. Deposition of lanthanum strontium cobalt ferrite (LSCF) using suspension plasma spraying for oxygen transport membrane applications. J. Therm. Spray Technol. 2015, 24, 1081–1092. [Google Scholar] [CrossRef]
- Singh, R. Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–80. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Sefi, Y.; Hajaj, B.; Rothenberg, G.; Gitis, V. Kinetics and mechanism of plasmid DNA penetration through nanopores. J. Memb. Sci. 2011, 371, 45–51. [Google Scholar] [CrossRef]
- Cortalezzi, M.M.; Rose, J.; Barron, A.R.; Wiesner, M.R. Characteristics of ultrafiltration ceramic membranes derived from alumoxane nanoparticles. J. Memb. Sci. 2002, 205, 33–43. [Google Scholar] [CrossRef]
- Mauer, G.; Moreau, C. Process Diagnostics and Control in Thermal Spray. J. Therm. Spray Technol. 2022, 31, 818–828. [Google Scholar] [CrossRef]
- Mauer, G.; Vaßen, R. Coatings with Columnar Microstructures for Thermal Barrier Applications. Adv. Eng. Mater. 2020, 22, 1900988. [Google Scholar] [CrossRef]
- Berghaus, J.O.; Bouaricha, S.; Legoux, J.G.; Moreau, C. Injection conditions and in-flight particle states in suspension plasma spraying of alumina and zirconia nano-ceramics. In The International Thermal Spray Conference (ITSC); ASM International: Basel, Switzerland, 2005; pp. 1434–1440. [Google Scholar]

| Sample Name | No. of Spray Passes |
|---|---|
| UF-2P | 2 |
| UF-4P | 4 |
| UF-2×4P | stacking two UF-4P |
| MF-12P | 12 |
| MF-2×12P | stacking two MF-12P |
| Sample Name | Thickness (µm) |
|---|---|
| UF-2P | 17.2 ± 0.8 |
| UF-4P | 37 ± 1 |
| MF-12P | 20.5 ± 0.9 |
| Sample | Roughness (Ra) (µm) | Roughness (Rz) (µm) |
|---|---|---|
| UF-2P | 5.3 ± 0.3 | 41 ± 2.6 |
| UF-4P | 3.4 ± 0.1 | 25.3 ± 2.2 |
| MF-12P | 6.4 ± 0.1 | 43.6 ± 1.2 |
| Sample | FRR (%) | |
|---|---|---|
| SiO2 (200 nm) | SiO2 (400 nm) | |
| UF-2P | 96.8 | 98.1 |
| UF-4P | 96.5 | 95.1 |
| UF-2×4P | 76.9 | 79.7 |
| MF-12P | 94.5 | 98.1 |
| MF-2×12P | 80.9 | 82.7 |
| Sample | Roughness (Ra) (µm) | Roughness (Rz) (µm) |
|---|---|---|
| UF-2P-F | 3.5 ± 0.05 | 20.8 ± 0.4 |
| UF-4P-F | 2.9 ± 0.1 | 18.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alebrahim, E.; Moreau, C. A Comparative Study of the Self-Cleaning and Filtration Performance of Suspension Plasma-Sprayed TiO2 Ultrafiltration and Microfiltration Membranes. Membranes 2023, 13, 750. https://doi.org/10.3390/membranes13090750
Alebrahim E, Moreau C. A Comparative Study of the Self-Cleaning and Filtration Performance of Suspension Plasma-Sprayed TiO2 Ultrafiltration and Microfiltration Membranes. Membranes. 2023; 13(9):750. https://doi.org/10.3390/membranes13090750
Chicago/Turabian StyleAlebrahim, Elnaz, and Christian Moreau. 2023. "A Comparative Study of the Self-Cleaning and Filtration Performance of Suspension Plasma-Sprayed TiO2 Ultrafiltration and Microfiltration Membranes" Membranes 13, no. 9: 750. https://doi.org/10.3390/membranes13090750
APA StyleAlebrahim, E., & Moreau, C. (2023). A Comparative Study of the Self-Cleaning and Filtration Performance of Suspension Plasma-Sprayed TiO2 Ultrafiltration and Microfiltration Membranes. Membranes, 13(9), 750. https://doi.org/10.3390/membranes13090750








