Nanoflake NiMn Layered Double Hydroxide Coated on Porous Membrane-like Ni-Foam for Sustainable and Efficient Electrocatalytic Oxygen Evolution
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of NiMn LDH Thin-Film Electrode
2.2. Characterization and Electrochemical Measurements of NiMn LDH
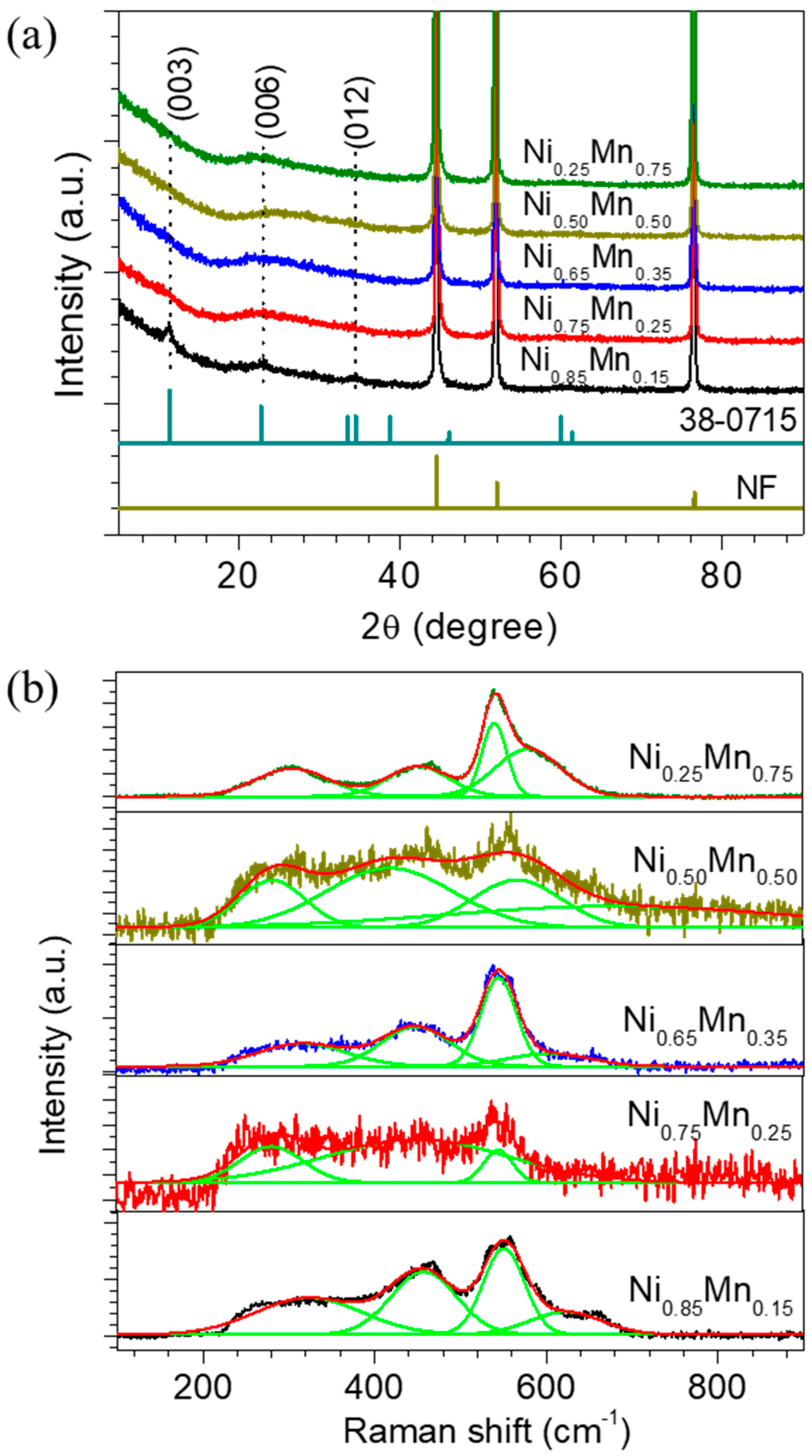
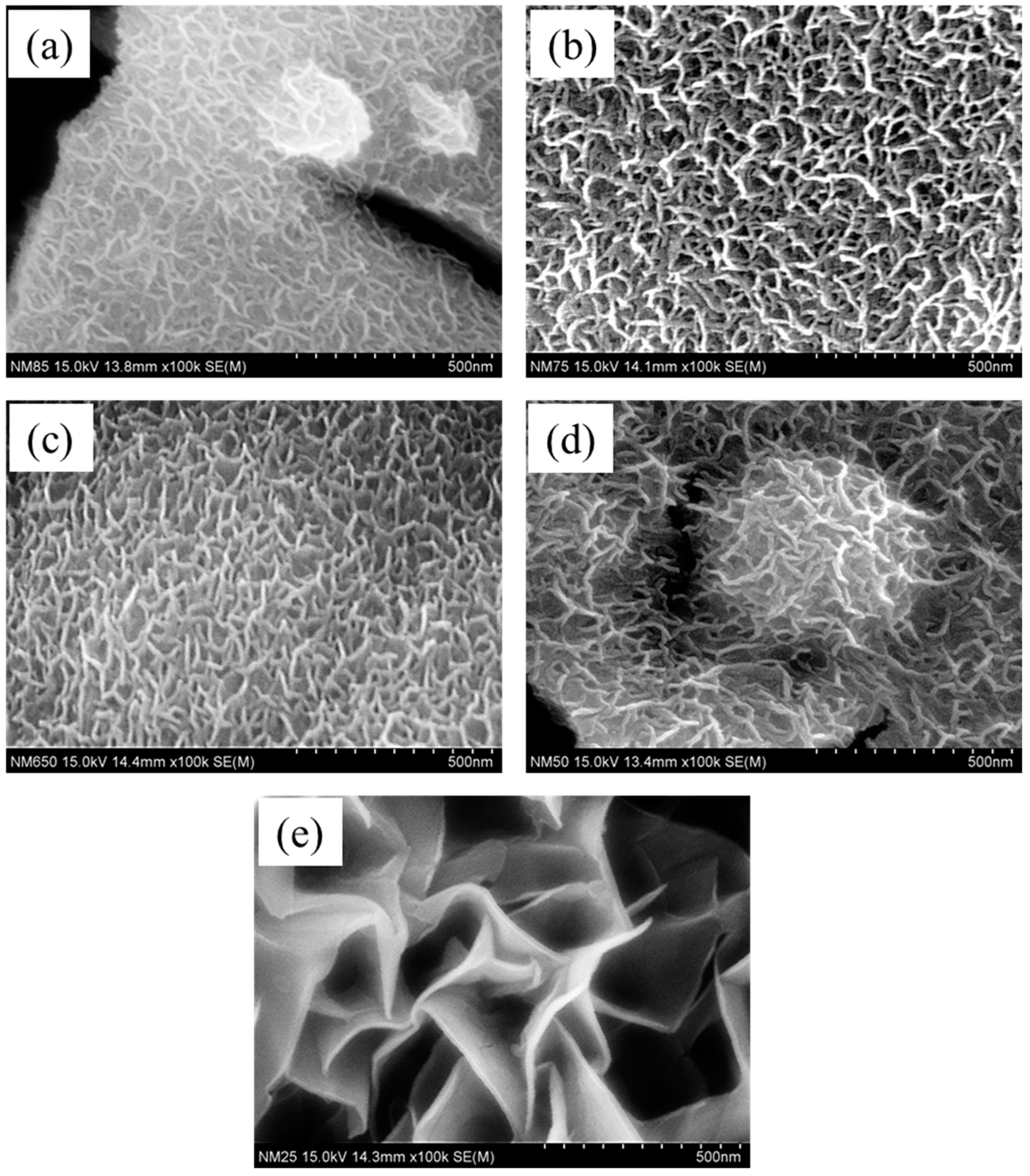
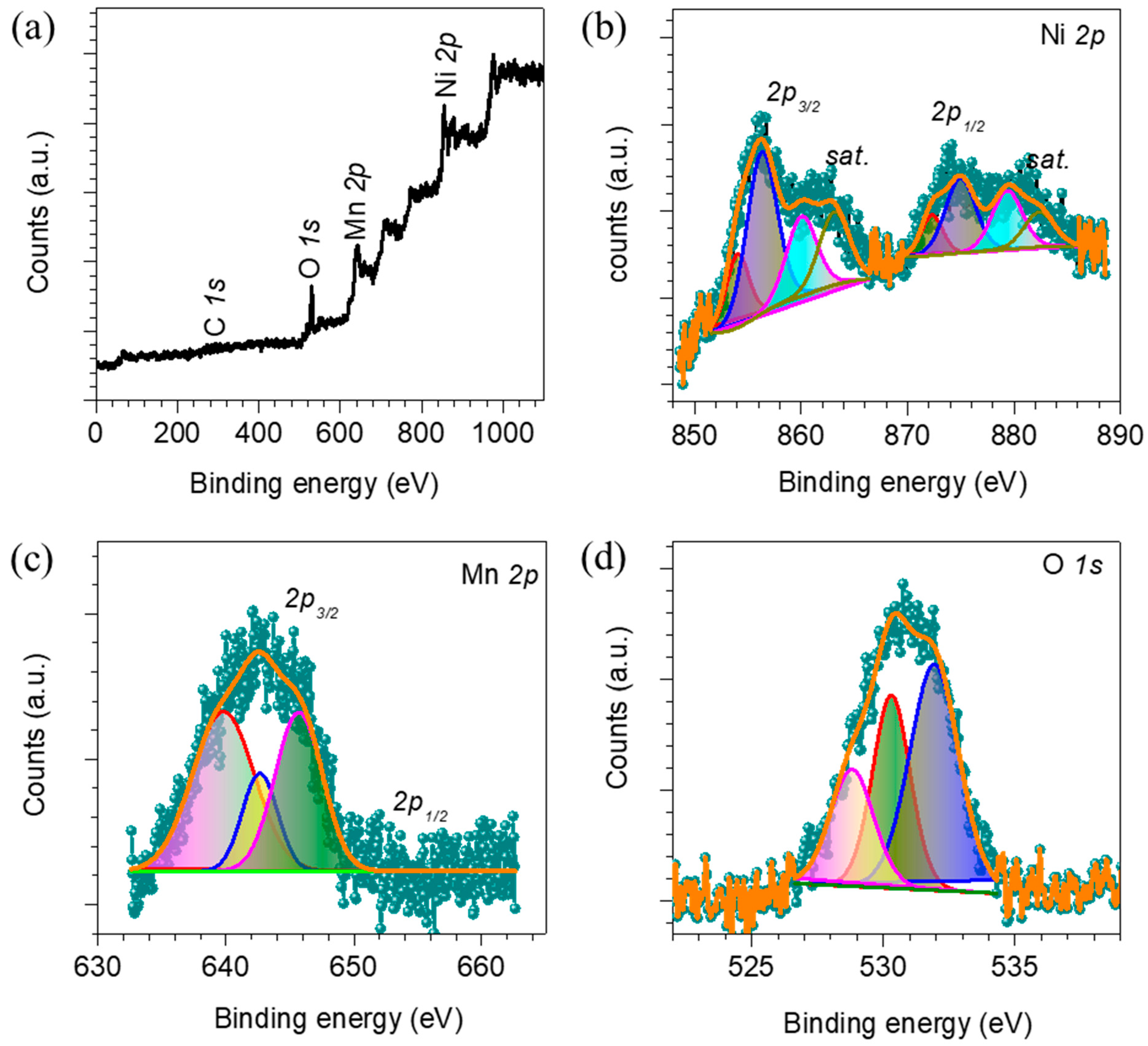
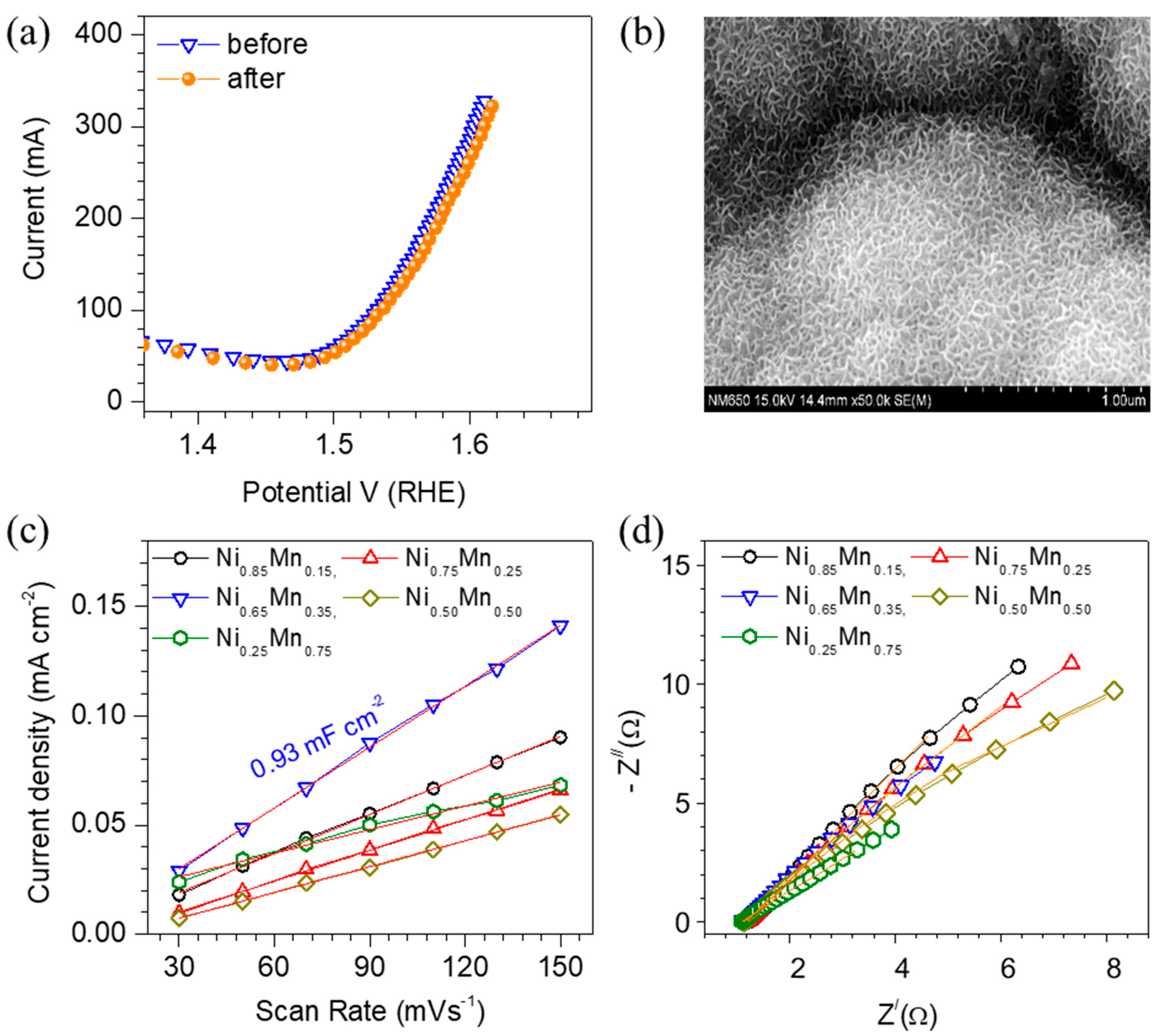
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.I.; Chavan, H.S.; Hou, B.; Lee, C.H.; Lee, S.U.; Cha, S.; Kim, H.; Im, H. A Robust Nonprecious CuFe Composite as a Highly Efficient Bifunctional Catalyst for Overall Electrochemical Water Splitting. Small 2020, 16, 1905884. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Li, H.; Sun, Z.; Cao, M.; Li, Z.; Fang, C.; Zhou, J.; Cao, C.; Dong, J.; et al. A semiconductor-electrocatalyst nano interface constructed for successive photoelectrochemical water oxidation. Nat. Commun. 2023, 14, 2574. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Wang, T.; Xiong, Q.; Wang, W.; Cao, L.; Dong, B. Zn Doped FeCo Layered Double Hydroxide Nanoneedle Arrays with Partial Amorphous Phase for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 13105–13114. [Google Scholar] [CrossRef]
- Asiri, A.M.; Akhtar, K.; Khan, S.B. Cobalt oxide nanocomposites and their electrocatalytic behavior for oxygen evolution reaction. Ceram. Int. 2019, 45, 13340–13346. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Dincer, I.; Rosen, M.A. Kinetic and electrochemical analyses of a CuCI/HCl electrolyzer. Int. J. Energy Res. 2019, 43, 6890–6906. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, C.; Yan, R.; Zou, X.; Hu, F.-L.; Mi, Y.; Yan, C.; Zhao, S. Constructing Built-in Electric Field in Heterogeneous Nanowire Arrays for Efficient Overall Water Electrolysis. Angew. Chem. Int. Ed. 2023, 62, e202302795. [Google Scholar] [CrossRef]
- Hu, G.; Xiang, J.; Li, J.; Liu, P.; Ali, R.N.; Xiang, B. Urchin-like ternary cobalt phosphosulfide as high-efficiency and stable bifunctional electrocatalyst for overall water splitting. J. Catal. 2019, 371, 126–134. [Google Scholar] [CrossRef]
- Li, K.; Qian, Y.; Zhang, H.; Zhang, L.; Chai, Q.; Wang, Q.; Du, J.; Han, Y.; Wang, W.; Kang, D.J. Highly efficient oxygen evolution electrocatalysts based on nanosheet-shaped CuS in situ grown on carbon cloth. Ceram. Int. 2019, 45, 10664–10671. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Hu, Y.; Liu, Y.; Chen, S. A potential-driven switch of activity promotion mode for the oxygen evolution reaction at Co3O4/NiOxHy interface. eScience 2022, 2, 438–444. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, Z.; Xu, H.; Liu, L.; Guan, Y.; Gu, X.; Wang, Y. Crystal-plane engineering of NiCo2O4 electrocatalysts towards efficient overall water splitting. J. Catal. 2018, 357, 238–246. [Google Scholar] [CrossRef]
- Chen, B.; Humayun, M.; Li, Y.; Zhang, H.; Sun, H.; Wu, y.; Wang, C. Constructing Hierarchical Fluffy CoO-Co4N@NiFe-LDH Nanorod Arrays for Highly Effective Overall Water Splitting and Urea Electrolysis. ACS Sustain. Chem. Eng. 2021, 9, 14180–14192. [Google Scholar] [CrossRef]
- Peng, L.; Shen, J.; Zheng, X.; Xiang, R.; Deng, M.; Mao, Z.; Feng, Z.; Zhang, L.; Li, L.; Wei, Z. Rationally design of monometallic NiO-Ni3S2/NF heteronanosheets as bifunctional electrocatalysts for overall water splitting. J. Catal. 2019, 369, 345–351. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Lu, X.F.; Xi, S.; Du, Y.; Lou, X.W. Metal Atom-Doped Co3O4 Hierarchical Nanoplates for Electrocatalytic Oxygen Evolution. Adv. Mater. 2020, 32, 2002235. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F.; Chen, J. Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef]
- Chavan, H.S.; Lee, C.H.; Inamdar, A.I.; Han, J.; Park, S.; Cho, S.; Shreshta, N.K.; Lee, S.U.; Hou, B.; Im, H.; et al. Designing and Tuning the Electronic Structure of Nickel–Vanadium Layered Double Hydroxides for Highly Efficient Oxygen Evolution Electrocatalysis. ACS Catal. 2022, 12, 3821–3831. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13, 2662. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2022, 18, 2104339. [Google Scholar] [CrossRef]
- Kumar, B.S.; Gudla, V.C.; Ambat, R.; Kalpathy, S.K.; Anandhan, S. Graphene nanoclusters embedded nickel cobaltite nanofibers as multifunctional electrocatalyst for glucose sensing and water-splitting applications. Ceram. Int. 2019, 45, 25078–25091. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Z.; Alam, M.K.; Ni, Y.; Robles-Hernandez, F.; Yu, L.; Chen, S.; Ren, Z.; Wang, Z.; Bao, J. Trimetallic NiFeMo for Overall Electrochemical Water Splitting with a Low Cell Voltage. ACS Energy Lett. 2018, 3, 546–554. [Google Scholar] [CrossRef]
- Fan, L.; Ji, Y.; Wang, G.; Chen, J.; Chen, K.; Liu, X.; Wen, Z. High Entropy Alloy Electrocatalytic Electrode toward Alkaline Glycerol Valorization Coupling with Acidic Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 7224–7235. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Seok, J.H.; Lee, C.H.; Shin, G.; Park, S.; Yeon, S.; Cho, S.; Park, Y.; Shrestha, N.K.; et al. Optimal rule-of-thumb design of NiFeMo layered double hydroxide nanoflakes for highly efficient and durable overall water-splitting at large currents. J. Mater. Chem. A 2022, 10, 20497–20508. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.; Xia, G.; Chen, J.; Zhang, R.; Chen, Q. Tuning Electronic Structures of Nonprecious Ternary Alloys Encapsulated in Graphene Layers for Optimizing Overall Water Splitting Activity. ACS Catal. 2017, 7, 469–479. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Muller, A.M. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Zhong, Y.; Chen, Y.; Ma, N.; Zhou, W.; Shao, Z. A surface-modified antiperovskite as an electrocatalyst for water oxidation. Nat. Commun. 2018, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, P.; Zhang, B.; Daniel, Q.; Timmer, B.J.; Zhang, F.; Sun, L. 3D Core–Shell NiFeCr Catalyst on a Cu Nanoarray for Water Oxidation: Synergy between Structural and Electronic Modulation. ACS Energy Lett. 2018, 3, 2865–2874. [Google Scholar] [CrossRef]
- Sultana, U.K.; Riches, J.D.; O’Mullane, A.P. Gold Doping in a Layered Co-Ni Hydroxide System via Galvanic Replacement for Overall Electrochemical Water Splitting. Adv. Funct. Mater. 2018, 28, 1804361. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Kumaravel, S.; Sankar, S.S.; Kundu, S. Enabling and Inducing Oxygen Vacancies in Cobalt Iron Layer Double Hydroxide via Selenization as Precatalysts for Electrocatalytic Hydrogen and Oxygen Evolution Reactions. Inorg. Chem. 2021, 60, 2023–2036. [Google Scholar] [CrossRef]
- Dong, C.; Han, L.; Zhang, C.; Zhang, Z. Scalable Dealloying Route to Mesoporous Ternary CoNiFe Layered Double Hydroxides for Efficient Oxygen Evolution. ACS Sustain Chem. Eng. 2018, 6, 16096–16104. [Google Scholar]
- Shen, X.; Li, H.; Zhang, Y.; Ma, T.; Li, Q.; Jiao, Q.; Zhao, Y.; Li, H.; Feng, C. Construction dual-regulated NiCo2S4 @Mo-doped CoFe-LDH for oxygen evolution reaction at large current density. Appl. Catal. B Environ. 2022, 319, 121917. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, M.; Liu, S.; Liu, X.; Hu, K.; Wang, L. Study of active sites on Se-MnS/NiS heterojunctions as highly efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2019, 7, 26975–26983. [Google Scholar] [CrossRef]
- Sumboja, A.; Chen, J.; Zong, Y.; Lee, P.S.; Liu, Z. NiMn layered double hydroxides as efficient electrocatalysts for the oxygen evolution reaction and their application in rechargeable Zn–air batteries. Nanoscale 2017, 9, 774. [Google Scholar] [CrossRef]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of Hierarchical Structure Composed of (Co/Ni)Mn-LDH Nanosheets on MWCNT Backbones for Efficient Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Li, H.; Zhang, M.; Lu, Y.; Zhang, L.; Xiao, J.; Boehm, F.; Yan, K. One-Step Synthesis of NiMn-Layered Double Hydroxide Nanosheets Efficient for Water Oxidation. Small Methods 2019, 3, 1800344. [Google Scholar] [CrossRef]
- Liu, G.; Huang, C.; Yang, Z.; Su, J.; Zhang, W. Ultrathin NiMn-LDH nanosheet structured electrocatalyst for enhanced electrocatalytic urea oxidation. Appl. Catal. A General 2021, 614, 118049.445. [Google Scholar] [CrossRef]
- Gao, G.; Wang, K.; Wang, X. Peony flower-like CuxS @ NiMn LDH heterostructure as an efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 1347–1359. [Google Scholar] [CrossRef]
- Dionigi, F.; Zhu, J.; Zeng, Z.; Merzdorf, T.; Sarodnik, H.; Gliech, M.; Pan, L.; Li, W.-X.; Greeley, J.; Strasser, P. Intrinsic Electrocatalytic Activity for Oxygen Evolution of Crystalline 3d-Transition Metal Layered Double Hydroxides. Angew. Chem. Int. Ed. 2021, 60, 14446–14457. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Li, F.; Zhang, L.; Chai, D.; Zhang, Z.; Xin, J. Bimetallic FeNi-MIL-88-derived NiFe2O4@Ni–Mn LDH composite electrode material for a high-performance asymmetric supercapacitor. Dalton Trans. 2020, 49, 10203–10211. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zou, Z.; Huang, J.; Gao, F. NiFe2O4 Nanoparticles/NiFe Layered Double-Hydroxide Nanosheet Heterostructure Array for Efficient Overall Water Splitting at large Current Densities. ACS Appl. Mater. Interfaces 2018, 10, 26283–26292. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Fe–Mn Mixed-Oxide Catalysts Containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Naeem, R.; Ehsan, M.A.; Yahya, R.; Sohail, M.; Khaledic, H.; Mazhar, M. Fabrication of pristine Mn2O3 and Ag–Mn2O3 composite thin films by AACVD for photoelectrochemical water splitting. Dalton Trans. 2016, 45, 14928–14939. [Google Scholar] [CrossRef]
- Xia, H.; Li, G.; Cai, H.; Li, X.; Sun, P.; Wang, P.; Huang, J.; Wang, L.; Zhang, D.; Yang, Y.; et al. Interlaced NiMn-LDH nanosheet decorated NiCo2O4 nanowire arrays on carbon cloth as advanced electrodes for high-performance flexible solid-state hybrid supercapacitors. Dalton Trans. 2019, 48, 12168–12176. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Zeng, T.; Zhang, Y.; Wan, Q.; Yang, N. A high-performance asymmetric supercapacitor using composite electrodes of layered double hydroxides and holey reduced graphene oxide. J. Energy Storage 2022, 52, 104899. [Google Scholar] [CrossRef]
- Liang, H.; Lin, J.; Jia, H.; Chen, S.; Qi, J.; Cao, J.; Lin, T.; Fei, W.; Feng, J. Hierarchical NiCo-LDH/NiCoP@NiMn-LDH hybrid electrodes on carbon cloth for excellent supercapacitors. J. Mater. Chem. A 2018, 6, 15040–15046. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, R.; Yu, M.; Zeng, Y.; Lu, F.; Kuang, X.; Lu, X. Bifunctional Iron–Nickel Nitride Nanoparticles as Flexible and Robust Electrode for Overall Water Splitting. Electrochim. Acta 2017, 247, 666. [Google Scholar] [CrossRef]
- Zhang, P.; Li, L.; Nordlund, D.; Chen, H.; Fan, L.; Zhang, B.; Sheng, X.; Daniel, Q.; Sun, L. Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 2018, 9, 381. [Google Scholar] [CrossRef]
- Fominykh, K.; Chernev, P.; Zaharieva, I.; Sicklinger, J.; Stefanic, G.; Doblinger, M.A.; Muller, A.; Pokharel, S.; Bocklein, C.; Scheu, T. Bein, Iron-Doped Nickel Oxide Nanocrystals as Highly Efficient Electrocatalysts for Alkaline Water Splitting. ACS Nano 2015, 9, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Lunkenbein, T.; Greiner, M.T.; Masliuk, L.; Salamon, S.; Landers, J.; Schlogl, R.; Wende, H.; et al. Behrens, The Role of Composition of Uniform and Highly Dispersed Cobalt Vanadium Iron Spinel Nanocrystals for Oxygen Electrocatalysis. ACS Catal. 2018, 8, 1259–1267. [Google Scholar] [CrossRef]
- Ye, W.; Fang, X.; Chen, X.; Yan, D. A three-dimensional nickel–chromium layered double hydroxide micro/nanosheet array as an efficient and stable bifunctional electrocatalyst for overall water splitting. Nanoscale 2018, 10, 19484–19491. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Guo, J.; Xu, J.; Qu, T.; Zhang, Y.; Chen, S.; Hao, P.; Li, M.; Xie, M.; Guo, X.; et al. Surface Sulfurization of NiCo-Layered Double Hydroxide Nanosheets Enable Superior and Durable Oxygen Evolution Electrocatalysis. ACS Appl. Energy Mater. 2018, 1, 4040–4049. [Google Scholar] [CrossRef]
- Kang, Q.; Lai, D.; Tang, W.; Lu, Q.; Gao, F. Intrinsic activity modulation and structural design of NiFe alloy catalysts for an efficient oxygen evolution reaction. Chem. Sci. 2021, 12, 3818. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Yao, H.; Bai, J.; Yue, S.; Guo, X. Interface Synergistic Effect from Hierarchically Porous Cu(OH)2@FCN MOF/CF Nanosheet Arrays Boosting Electrocatalytic Oxygen Evolution. Catalysts 2022, 12, 625. [Google Scholar] [CrossRef]
- Ye, S.-H.; Shi, Z.-X.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Activating CoOOH Porous Nanosheet Arrays by Partial Iron Substitution for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2018, 57, 2672–2676. [Google Scholar] [CrossRef]
- Deokate, R.J.; Chavan, H.S.; Bulakhe, S.C.; Tanwade, S.B.; Mujawar, S.H.; Mali, S.S.; Hong, C.K.; Im, H.; Inamdar, A.I. Electrodeposited bimetallic microporous MnCu oxide electrode as a highly stable electrocatalyst for oxygen evolution reaction. Int. J. Energy Res. 2022, 46, 5269–5279. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magotra, V.K.; Magotra, A.; Mali, S.S.; Jeon, H.C.; Kang, T.W.; Salunke, A.S.; Hong, C.K.; Shrestha, N.K.; Im, H.; Inamdar, A.I. Nanoflake NiMn Layered Double Hydroxide Coated on Porous Membrane-like Ni-Foam for Sustainable and Efficient Electrocatalytic Oxygen Evolution. Membranes 2023, 13, 748. https://doi.org/10.3390/membranes13090748
Magotra VK, Magotra A, Mali SS, Jeon HC, Kang TW, Salunke AS, Hong CK, Shrestha NK, Im H, Inamdar AI. Nanoflake NiMn Layered Double Hydroxide Coated on Porous Membrane-like Ni-Foam for Sustainable and Efficient Electrocatalytic Oxygen Evolution. Membranes. 2023; 13(9):748. https://doi.org/10.3390/membranes13090748
Chicago/Turabian StyleMagotra, Verjesh Kumar, Arjun Magotra, Sawanta S. Mali, Hee C. Jeon, Tae W. Kang, Amol S. Salunke, Chang Kook Hong, Nabeen K. Shrestha, Hyunsik Im, and Akbar I. Inamdar. 2023. "Nanoflake NiMn Layered Double Hydroxide Coated on Porous Membrane-like Ni-Foam for Sustainable and Efficient Electrocatalytic Oxygen Evolution" Membranes 13, no. 9: 748. https://doi.org/10.3390/membranes13090748
APA StyleMagotra, V. K., Magotra, A., Mali, S. S., Jeon, H. C., Kang, T. W., Salunke, A. S., Hong, C. K., Shrestha, N. K., Im, H., & Inamdar, A. I. (2023). Nanoflake NiMn Layered Double Hydroxide Coated on Porous Membrane-like Ni-Foam for Sustainable and Efficient Electrocatalytic Oxygen Evolution. Membranes, 13(9), 748. https://doi.org/10.3390/membranes13090748







