Effect of Thermally Reduced Graphene on the Characteristics and Performance of Polysulfone Mixed Matrix Ultrafiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TRG Synthesis and Characterization
2.3. Membrane Fabrication
2.4. Membrane Characterization
2.5. Oil Separation Membrane Performance Testing
2.6. Water Vapor Permeation Performance Analysis
3. Results and Discussions
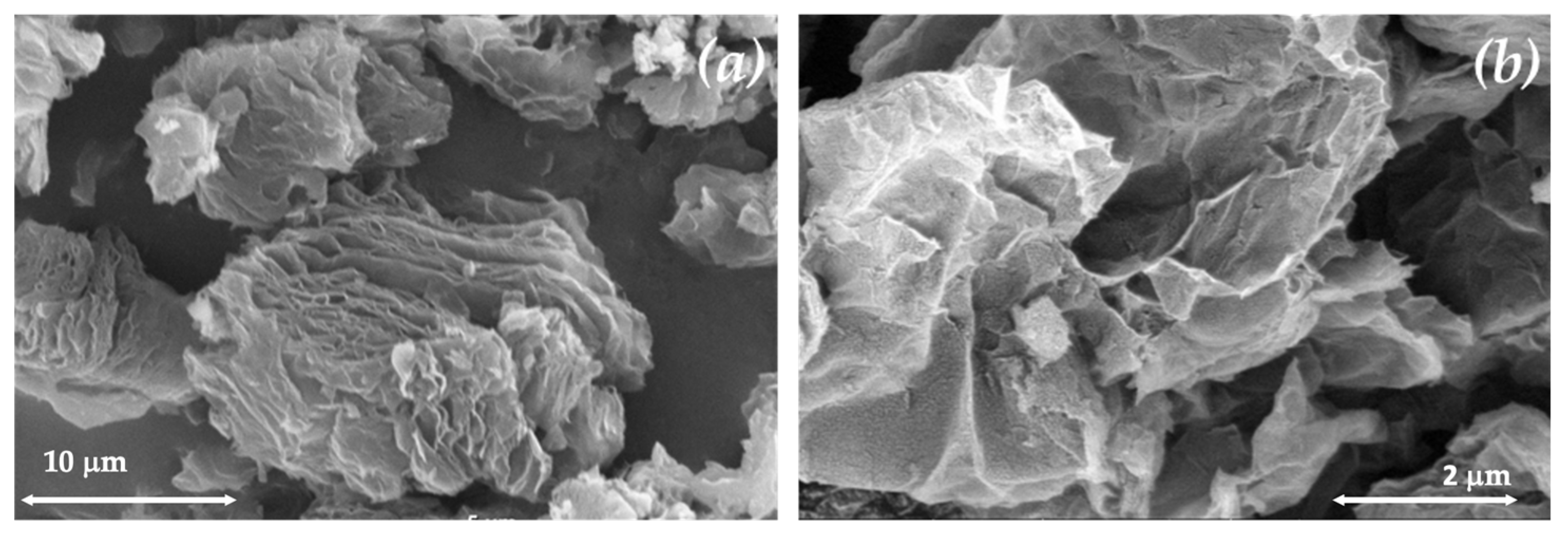
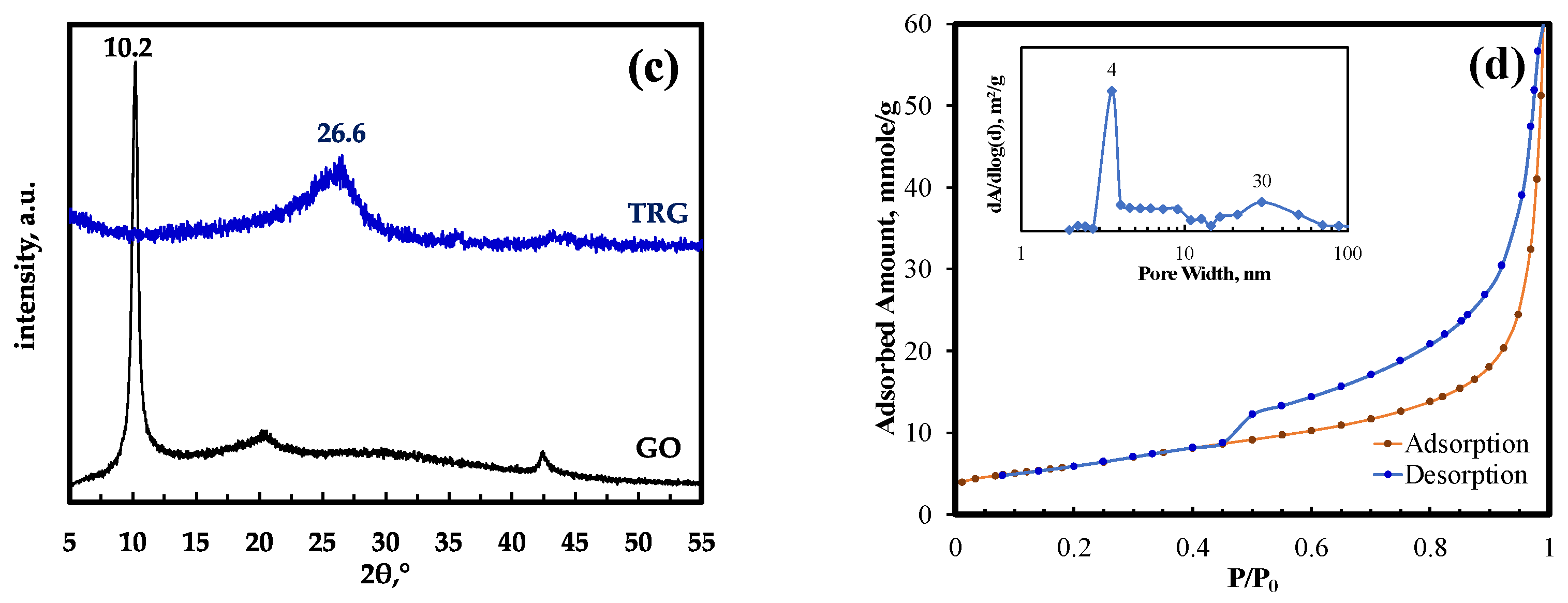
3.1. TRG Characterization
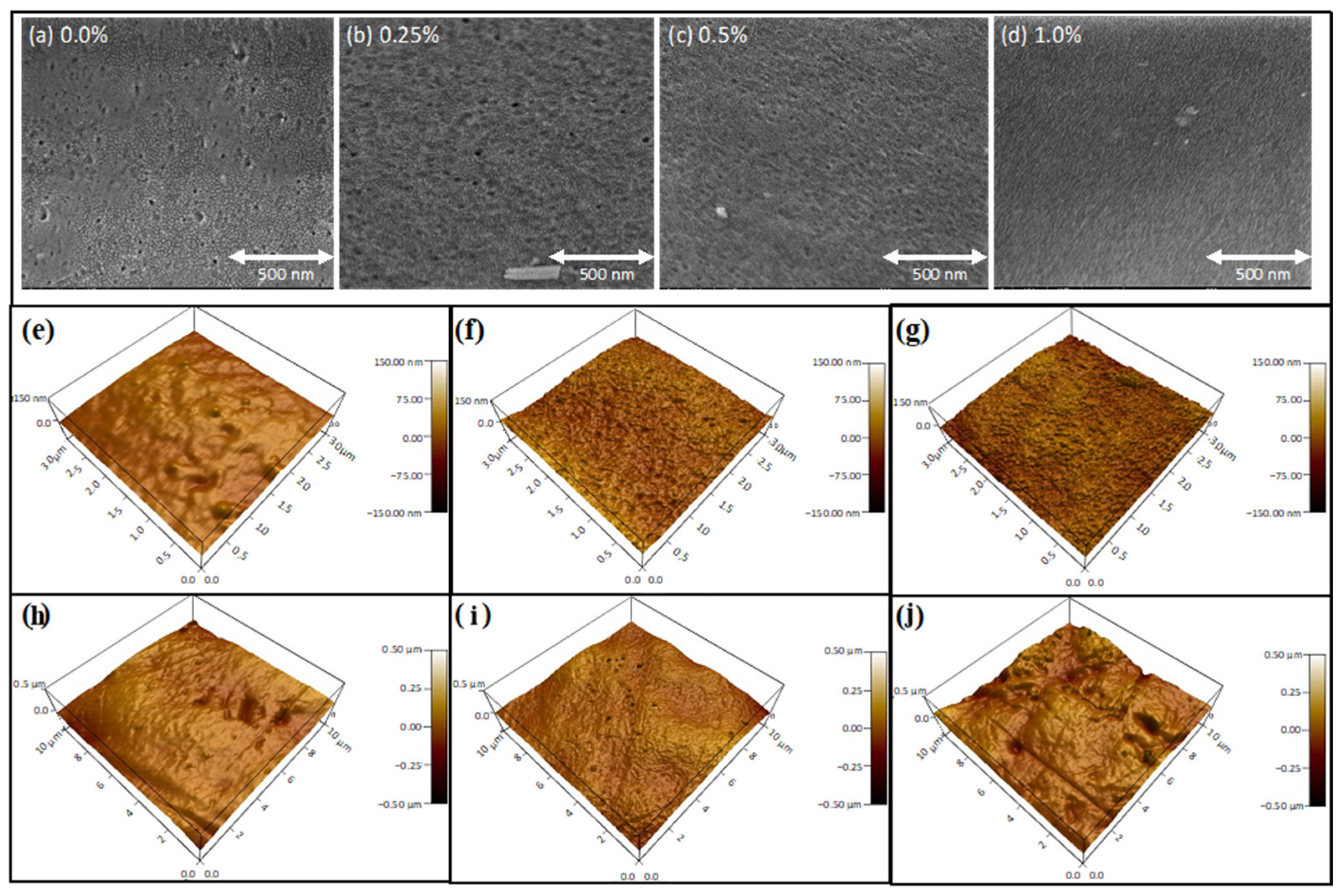
3.2. Membrane Characterizations
3.3. Effect of TRG Concentration on the Permeance and Selectivity of Air-Dehumidification Membrane
4. Conclusions
- Synthesized TRG via a simple thermal exfoliation method and successfully incorporated it into PSF to fabricate an MMM.
- Investigated the effect of TRG loading on membrane morphology, hydrophilicity, porosity, roughness, and mechanical properties.
- Demonstrated that adding TRG led to increased membrane porosity and hydrophilicity compared to the control PSF membrane.
- Showed that TRG incorporation improved water flux by up to 70% and oil rejection by up to 98.9% for the PSF-TRG MMMs compared to the control PSF membrane.
- PSF-TRG MMM with 0.25 wt.% TRG exhibited the optimal combination of enhanced permeability (70% higher) and selectivity (higher oil rejection) for oil/water separation.
- PSF-TRG MMMs demonstrated over 20% increased WVP compared to the control PSF membrane, indicating promise as air-dehumidification membrane supports.
- Provided a simple and effective method to synthesize and incorporate TRG to engineer high-performance polymeric MMMs suitable for oil/water separation and air-dehumidification applications.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolto, B.; Zhang, J.; Wu, X.; Xie, Z. A Review on Current Development of Membranes for Oil Removal from Wastewaters. Membranes 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, B.; Al-Shaeli, M.N.Z. Fundamentals of Membrane Bioreactors: Materials, Systems and Membrane Fouling. By Bradley Ladewig, Muayad Nadhim Zemam Al-Shaeli; Springer: Singapore, 2017. [Google Scholar]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Othman, M.H.D.; Tai, Z.S.; Usman, J.; Ismail, N.J.; Rahman, M.A.; Jaafar, J. Oily Wastewater Treatment. In Water Pollution and Remediation: Organic Pollutants; Ahamed, M.I., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 353–385. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research Progress and Prospects of Marine Oily Wastewater Treatment: A Review. Water 2019, 11, 2517. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Silva Junior, C.J.; Amorim, J.D.; Durval, I.J.; Costa, A.F.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Nascimbén Santos, É.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Micari, M.; Duan, X.; Agrawal, K.V. Atmospheric water harvesting in semi-arid regions by membranes: A techno-economic assessment. J. Membr. Sci. 2023, 672, 121437. [Google Scholar] [CrossRef]
- Bui, D.T.; Vivekh, P.; Islam, M.R.; Chua, K.J. Studying the characteristics and energy performance of a composite hollow membrane for air dehumidification. Appl. Energy 2022, 306, 118161. [Google Scholar] [CrossRef]
- Fix, A.J.; Braun, J.E.; Warsinger, D.M. Vapor-selective active membrane energy exchanger for high efficiency outdoor air treatment. Appl. Energy 2021, 295, 116950. [Google Scholar] [CrossRef]
- Cho, H.-J.; Cheon, S.-Y.; Jeong, J.-W. Energy impact of vacuum-based membrane dehumidification in building air-conditioning applications. Appl. Therm. Eng. 2021, 182, 116094. [Google Scholar] [CrossRef]
- Lekshminarayanan, G.; Croal, M.; Maisonneuve, J. Recovering latent and sensible energy from building exhaust with membrane-based energy recovery ventilation. Sci. Technol. Built Environ. 2020, 26, 1000–1012. [Google Scholar] [CrossRef]
- IEA. Space Cooling; IEA: Paris, France, 2022. [Google Scholar]
- Liu, X.; Ren, Z.; Ngo, H.H.; He, X.; Desmond, P.; Ding, A. Membrane technology for rainwater treatment and reuse: A mini review. Water Cycle 2021, 2, 51–63. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H.; Huang, J.; Fu, H. Water vapor capture using microporous ceramic membrane. Desalination 2020, 482, 114405. [Google Scholar] [CrossRef]
- Xie, W.; Geise, G.; Freeman, B.; Lee, H.-S.; Byun, G.; McGrath, J. Polyamide interfacial composite membranes prepared from m-phenylene diamine, trimesoyl chloride and a new disulfonated diamine. J. Membr. Sci. 2012, 403, 152–161. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Choi, W.K.; Park, S.R.; Kang, E.C.; Lee, H.K. Development of carboxylated TiO2 incorporated thin film nanocomposite hollow fiber membranes for flue gas dehydration. J. Membr. Sci. 2016, 514, 622–635. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H.; Li, C. Composite hollow fiber membrane dehumidification: A review on membrane module, moisture permeability and self-cleaning performance. Int. J. Heat Mass Transf. 2021, 181, 121832. [Google Scholar] [CrossRef]
- Ingole, P.G.; Choi, W.K.; Lee, G.B.; Lee, H.K. Thin-film-composite hollow-fiber membranes for water vapor separation. Desalination 2017, 403, 12–23. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W. A Review on Carbon Nanotubes and Graphene as Fillers in Reinforced Polymer Nanocomposites. J. Ind. Eng. Chem. 2014, 21, 11–25. [Google Scholar] [CrossRef]
- Paul, R.; Vincent, M.; Etacheri, V.; Roy, A.K. Chapter 1—Carbon nanotubes, graphene, porous carbon, and hybrid carbon-based materials: Synthesis, properties, and functionalization for efficient energy storage. In Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion; Paul, R., Etacheri, V., Wang, Y., Lin, C.-T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Lai, G.S.; Yusob, M.H.M.; Lau, W.J.; Gohari, R.J.; Emadzadeh, D.; Ismail, A.F.; Goh, P.S.; Isloor, A.M.; Arzhandi, M.R.-D. Novel mixed matrix membranes incorporated with dual-nanofillers for enhanced oil-water separation. Sep. Purif. Rev. 2017, 178, 113–121. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Devanadera, K.P.O.; Duena, A.N.R.; Luo, Z.-Y.; Chiao, Y.-H.; Millare, J.C.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R. Modifying Cellulose Acetate Mixed-Matrix Membranes for Improved Oil–Water Separation: Comparison between Sodium and Organo-Montmorillonite as Particle Additives. Membranes 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Pagliero, C.; Mattea, M.; Ochoa, N.; Marchese, J. Fouling of polymeric membranes during degumming of crude sunflower and soybean oil. J. Food Eng. 2007, 78, 194–197. [Google Scholar] [CrossRef]
- Abdalla, O.; Wahab, M.A.; Abdala, A. Mixed matrix membranes containing aspartic acid functionalized graphene oxide for enhanced oil-water emulsion separation. J. Environ. Chem. Eng. 2020, 8, 104269. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Z.; Li, F.; Chen, Q.; Yin, D.; Min, X. A novel reduced graphene oxide-based composite membrane prepared via a facile deposition method for multifunctional applications: Oil/water separation and cationic dyes removal. Sep. Purif. Rev. 2018, 200, 130–140. [Google Scholar] [CrossRef]
- Chen, C.; Chen, B. Graphene oxide coated meshes with stable underwater superoleophobicity and anti-oil-fouling property for highly efficient oil/water separation. Sci. Total Environ. 2019, 696, 133777. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Liu, Y.; Li, Y.; Jiang, Z. Free-Standing Graphene Oxide-Palygorskite Nanohybrid Membrane for Oil/Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 8247–8256. [Google Scholar] [CrossRef]
- Qian, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. TiO2/sulfonated graphene oxide/Ag nanoparticle membrane: In situ separation and photodegradation of oil/water emulsions. J. Membr. Sci. 2018, 554, 16–25. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud'homme, R.K. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Claridge, D.E.; Culp, C.; Liu, W.; Pate, M.; Haberl, J.; Bynum, J.; Tanskyi, O.; Schaff, F. A new approach for drying moist air: The ideal Claridge-Culp-Liu dehumidification process with membrane separation, vacuum compression and sub-atmospheric condensation. Int. J. Refrig. 2019, 101, 211–217. [Google Scholar] [CrossRef]
- Min, Y.; Zhang, K.; Zhao, W.; Zheng, F.; Chen, Y.; Zhang, Y. Enhanced chemical interaction between TiO2 and graphene oxide for photocatalytic decolorization of methylene blue. Chem. Eng. J. 2012, 193, 203–210. [Google Scholar] [CrossRef]
- Rahimpour, A.; Siavash, S.; Ghorbani, S.; Shockravi, A. The influence of sulfonated polyethersulfone ( SPES ) on surface nano-morphology and performance of polyethersulfone (PES) membrane Applied Surface Science The influence of sulfonated polyethersulfone (SPES) on surface nano-morphology and performance. Appl. Surf. Sci. 2010, 256, 1825–1831. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Pouresmaeel-Selakjani, P.; Jahanshahi, M.; Peyravi, M. Synthesis of cellulose/silica nanocomposite through electrostatic interaction to reinforce polysulfone membranes. Cellulose 2017, 24, 1333–1353. [Google Scholar] [CrossRef]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyżewski, A. Investigations on the Properties and Performance of Mixed-Matrix Polyethersulfone Membranes Modified with Halloysite Nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Shi, J.-Y.; Wang, J.-L.; Li, Y.-L.; Gao, N.-N.; Liu, Z.-X.; Lian, W.-T. Preparation and characterization of PEG-g-MWCNTs/PSf nano-hybrid membranes with hydrophilicity and antifouling properties. RSC Adv. 2015, 5, 84746–84753. [Google Scholar] [CrossRef]
- Nguyen, H.T.V.; Ngo, T.H.A.; Do, K.D.; Nguyen, M.N.; Dang, N.T.T.; Nguyen, T.T.H.; Vien, V.; Vu, T.A. Preparation and Characterization of a Hydrophilic Polysulfone Membrane Using Graphene Oxide. J. Chem. 2019, 2019, 3164373. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Wang, K.; Abdalla, A.A.; Khaleel, M.A.; Hilal, N.; Khraisheh, M.K. Mechanical properties of water desalination and wastewater treatment membranes. Desalination 2017, 401, 190–205. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Li, Y.; Bao, M.; Zhang, D.; Wang, Z. Integrated asymmetric superwetting Janus membrane for the efficient separation of various surfactant-stabilized oil–water emulsions. Environ. Sci. Nano 2021, 8, 2235–2248. [Google Scholar] [CrossRef]
- Shi, G.; Shen, Y.; Mu, P.; Wang, Q.; Yang, Y.; Ma, S.; Li, J. Effective separation of surfactant-stabilized crude oil-in-water emulsions by using waste brick powder-coated membranes under corrosive conditions. Green Chem. 2020, 22, 1345–1352. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Sun, D.D.; Ng, W.J. The efficient separation of surfactant-stabilized oil–water emulsions with a flexible and superhydrophilic graphene–TiO2 composite membrane. J. Mater. Chem. A 2014, 2, 14082–14088. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Membr. Sci. 2021, 620, 118900. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration Membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Halakoo, E.; Lau, W.J.; Kassim, M.A.; Matsuura, T.; Ismail, A.F. Novel polyethersulfone (PES)/hydrous manganese dioxide (HMO) mixed matrix membranes with improved anti-fouling properties for oily wastewater treatment process. RSC Adv. 2014, 4, 17587–17596. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Abdel-Aty, A.A.R.; Aziz, Y.S.A.; Ahmed, R.M.G.; ElSherbiny, I.M.A.; Panglisch, S.; Ulbricht, M.; Khalil, A.S.G. High performance isotropic polyethersulfone membranes for heavy oil-in-water emulsion separation. Sep. Purif. Rev. 2020, 253, 117467. [Google Scholar] [CrossRef]




| Membrane Type | Absolute Humidity (kg/m3) | Humidity Removed | Water Vapor Permeance (GPU) | H2O/Air Selectivity | ||
|---|---|---|---|---|---|---|
| Feed | Retentate | % | Water | Air | ||
| PSF (Control) | 0.0271 | 0.0191 | 29.4 | 13,710 | 2010 | 7 |
| PSF 0.25% TRG | 0.0255 | 0.0174 | 31.8 | 16,650 | 3520 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdala, O.; Nabeeh, A.; Rehman, A.; Abdel-Wahab, A.; Hassan, M.K.; Abdala, A. Effect of Thermally Reduced Graphene on the Characteristics and Performance of Polysulfone Mixed Matrix Ultrafiltration Membranes. Membranes 2023, 13, 747. https://doi.org/10.3390/membranes13080747
Abdala O, Nabeeh A, Rehman A, Abdel-Wahab A, Hassan MK, Abdala A. Effect of Thermally Reduced Graphene on the Characteristics and Performance of Polysulfone Mixed Matrix Ultrafiltration Membranes. Membranes. 2023; 13(8):747. https://doi.org/10.3390/membranes13080747
Chicago/Turabian StyleAbdala, Omnya, Ahmed Nabeeh, Abdul Rehman, Ahmed Abdel-Wahab, Mohammad K. Hassan, and Ahmed Abdala. 2023. "Effect of Thermally Reduced Graphene on the Characteristics and Performance of Polysulfone Mixed Matrix Ultrafiltration Membranes" Membranes 13, no. 8: 747. https://doi.org/10.3390/membranes13080747
APA StyleAbdala, O., Nabeeh, A., Rehman, A., Abdel-Wahab, A., Hassan, M. K., & Abdala, A. (2023). Effect of Thermally Reduced Graphene on the Characteristics and Performance of Polysulfone Mixed Matrix Ultrafiltration Membranes. Membranes, 13(8), 747. https://doi.org/10.3390/membranes13080747








