Impacts of Organic Emerging Contaminants (Erythromycin, Ibuprofen, and Diclofenac) on the Performance of a Membrane Bioreactor Treating Urban Wastewater: A Heterotrophic Kinetic Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Laboratory Plant of the Membrane Bioreactor
2.2. Influent Characteristics
2.3. Operation Conditions
2.4. Analytical Determination
2.5. Kinetic Analysis
- -
- Oxygen consumption (OC) for each addition of sodium acetate:
- -
- Yield coefficient for heterotrophic biomass (YH):
- -
- Empirical specific growth rate for heterotrophic biomass (μemp):
- -
- Linearisation of the Monod model:
- -
- Decay coefficient of heterotrophic biomass (bH):
- -
- Substrate degradation rate of organic matter removal (rsu):
3. Results and Discussion
4. Conclusions
- -
- Heterotrophic biomass was affected and inhibited by the presence of pharmaceutical drugs in both phases of the operation. The system response to low concentrations of pharmaceutical compounds occurred in the initial phase. However, after 22 days of doping, the system response was inhibited. At the highest concentrations of pharmaceuticals, the system was not able to react, and the response was not activated; activity was completely inhibited after 11 days of doping.
- -
- Under the operating conditions of this study, there was a gradual decrease in the concentration of MLSS (from 5633 to 2133 mg L−1 for Phase 1 and from 2400 to 500 mg L−1 for Phase 2) and in the removal of COD (from 87% to 28% in Phase 1 and from 65% to 20% in Phase 2) in the system as it was not able to absorb the effect produced by the pharmaceutical compounds added in both phases.
- -
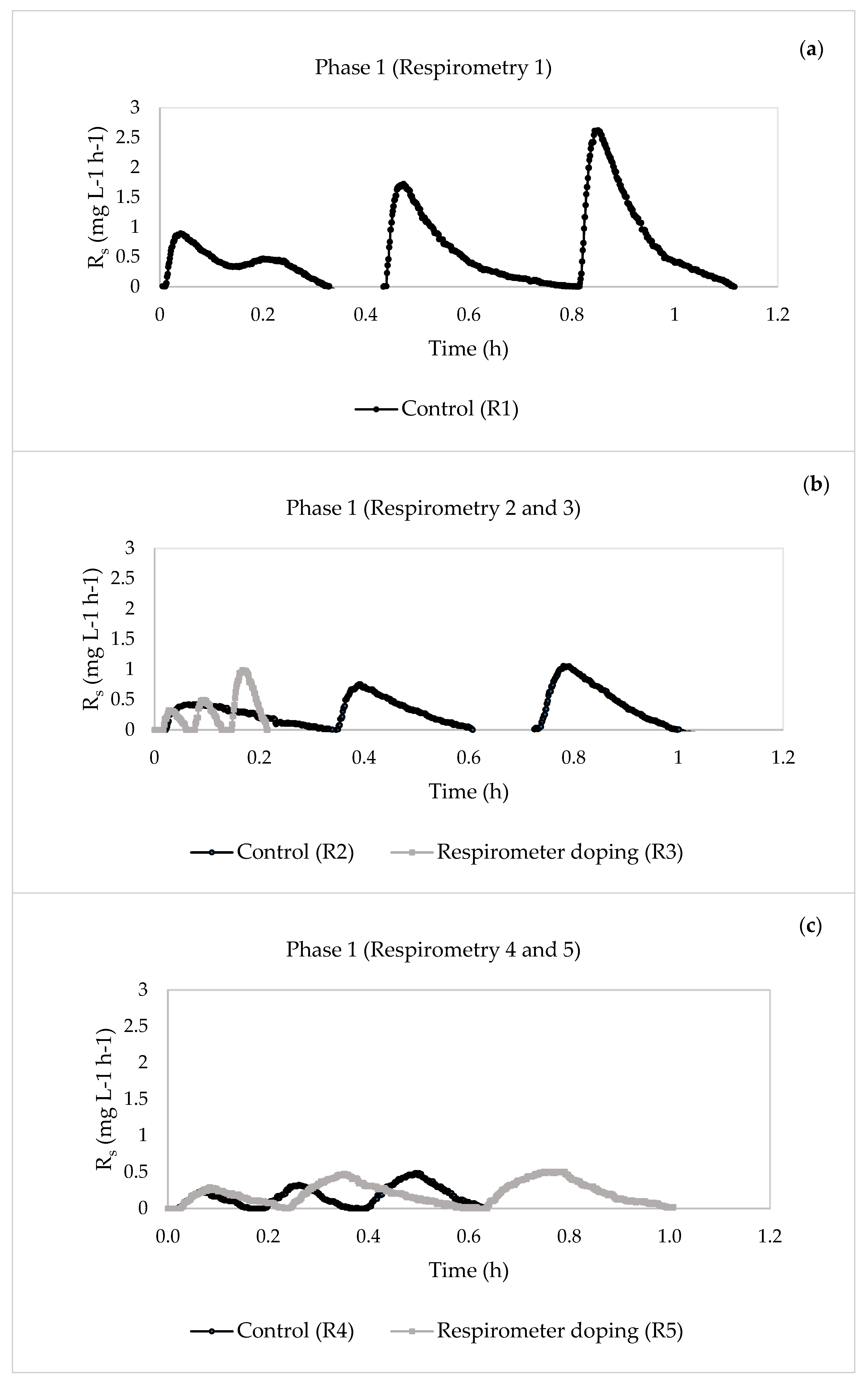
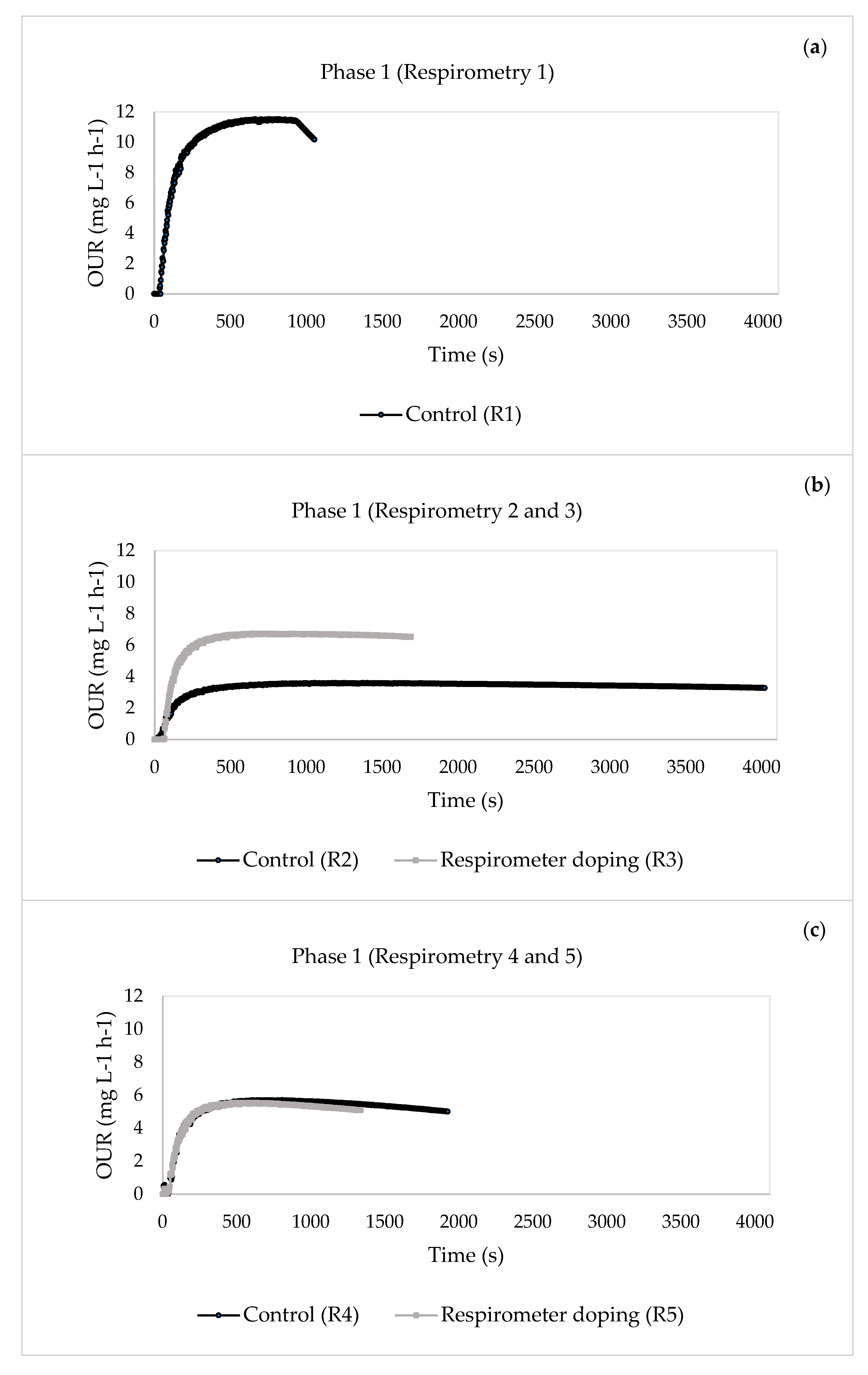
- For Phase 1, when the concentrations of the pharmaceuticals were lower, the substrate degradation rate of organic matter removal rsu,H increased from 11.4626 to 44.7977 mg O2 L−1 h−1 after four days of continuous doping, indicating that the system could actively mitigate the impact of the pharmaceuticals. However, the value decreased from day 5 of doping until the system was inhibited. This inactivation of the heterotrophic biomass was seen in the reduction of the percentage of COD elimination, which decreased from an initial 92% to 28%. The same behaviour was observed for Phase 2, where inhibition of the system occurred earlier than in Phase 1 because the pharmaceutical compounds had a greater effect on the heterotrophic biomass due to their higher concentrations.
- -
- For future investigations, it is advisable to incorporate the assessment of the removal performance of the dosed pharmaceuticals in the laboratory plant, along with the monitoring of phosphorus in its different forms and nitrogen removal, as well as the corresponding related compounds. Such comprehensive analyses would contribute to a more comprehensive understanding of the overall treatment efficiency and environmental impact of the pharmaceutical compounds in the system.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, D.; Zhou, C.; Bin Hashim, M.H.; Hang, T.M.; Gin, K.Y.-H.; Wang, C.-H.; Neoh, K.G. Emerging pharmaceutical and organic contaminants removal using carbonaceous waste from oil refineries. Chemosphere 2021, 271, 129542. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- Gros, M.; Petrovic, M.; Damia, B. Environmental Chemistry Wastewater Treatment Plants as a Pathway for Aquatic Contamination by Pharmaceuticals in the Ebro River Basin (Northeast Spain). Environ. Toxicol. Chem. 2007, 26, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Paz, A.; Tadmor, G.; Malchi, T.; Blotevogel, J.; Borch, T.; Polubesova, T.; Chefetz, B. Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: Sorption, leaching and plant uptake. Chemosphere 2016, 160, 22–29. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Antibiotics. In Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006; pp. 425–498. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM optimized adsorptive removal of erythromycin using magnetic activated carbon: Adsorption isotherm, kinetic modeling and thermodynamic studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, Z.; Ma, L.; Yan, T.; Wang, L.; Li, Y. The prevalence and removal of antibiotic resistance genes in full-scale wastewater treatment plants: Bacterial host, influencing factors and correlation with nitrogen metabolic pathway. Sci. Total Environ. 2022, 827, 154154. [Google Scholar] [CrossRef]
- Vieira, Y.; Pereira, H.A.; Leichtweis, J.; Mistura, C.M.; Foletto, E.L.; Oliveira, L.F.; Dotto, G.L. Effective treatment of hospital wastewater with high-concentration diclofenac and ibuprofen using a promising technology based on degradation reaction catalyzed by Fe0 under microwave irradiation. Sci. Total Environ. 2021, 783, 146991. [Google Scholar] [CrossRef] [PubMed]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Zhang, G.; Zhang, N.; Liu, Y.; Huang, T.; Chang, H. Diclofenac degradation in water by FeCeOx catalyzed H2O2: Influencing factors, mechanism and pathways. J. Hazard. Mater. 2017, 334, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A review on pharmaceuticals removal from waters by single and combined biological, membrane filtration and ultrasound systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Ayyoub, H.; Kitanou, S.; Bachiri, B.; Tahaikt, M.; Taky, M.; Elmidaoui, A. Membrane bioreactor (MBR) performance in fish canning industrial wastewater treatment. Water Pract. Technol. 2022, 17, 1358–1368. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Vo, T.-D.; Dat, N.D.; Nguyen, V.-T.; Tran, A.T.K.; Nguyen, P.-T.; Bui, X.-T. Performance of low flux sponge membrane bioreactor treating industrial wastewater for reuse purposes. Bioresour. Technol. Rep. 2023, 22, 101440. [Google Scholar] [CrossRef]
- Kim, M.J.; Sankararao, B.; Yoo, C.K. Determination of MBR fouling and chemical cleaning interval using statistical methods applied on dynamic index data. J. Membr. Sci. 2011, 375, 345–353. [Google Scholar] [CrossRef]
- Miura, Y.; Watanabe, Y.; Okabe, S. Membrane Biofouling in Pilot-Scale Membrane Bioreactors (MBRs) Treating Municipal Wastewater: Impact of Biofilm Formation. Environ. Sci. Technol. 2007, 41, 632–638. [Google Scholar] [CrossRef]
- Madan, R.; Madan, S.; Hussain, A. Kinetic Study for Startup of Aerobic Moving Bed Biofilm Reactor in Treatment of Textile Dye Wastewater. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Mannina, G.; Di Bella, G. Comparing two start-up strategies for MBRs: Experimental study and mathematical modelling. Biochem. Eng. J. 2012, 68, 91–103. [Google Scholar] [CrossRef]
- Cosenza, A.; Mannina, G.; Vanrolleghem, P.A.; Neumann, M.B. Global sensitivity analysis in wastewater applications: A comprehensive comparison of different methods. Environ. Model. Softw. 2013, 49, 40–52. [Google Scholar] [CrossRef]
- Trapani, D.D.; Mannina, G.; Viviani, G. Membrane Bioreactors for wastewater reuse: Respirometric assessment of biomass activity during a two year survey. J. Clean. Prod. 2018, 202, 311–320. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Xu, H.; Deng, Y.; Li, M.; Zhang, K.; Zou, J.; Yang, Y.; Shi, P.; Feng, Y.; Hu, C.; Wang, Z. Removal of tetracycline in nitrification membrane bioreactors with different ammonia loading rates: Performance, metabolic pathway, and key contributors. Environ. Pollut. 2023, 332, 121922. [Google Scholar] [CrossRef] [PubMed]
- Antiñolo Bermúdez, L.; Martín-Luis, A.; Leyva Díaz, J.C.; Muñío Martínez, M.d.M.; Poyatos Capilla, J.M. Kinetic Effects of Ciprofloxacin, Carbamazepine, and Bisphenol on Biomass in Membrane Bioreactor System at Low Temperatures to Treat Urban Wastewater. Membranes 2023, 13, 419. [Google Scholar] [CrossRef]
- Metcalf, L.; Eddy, H.P.; Tchobanoglous, G. Wastewater Energy: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2004; Volume 1819. [Google Scholar]
- Leyva-Díaz, J.C.; Calderón, K.; Rodríguez, F.A.; González-López, J.; Hontoria, E.; Poyatos, J.M. Comparative kinetic study between moving bed biofilm reactor-membrane bioreactor and membrane bioreactor systems and their influence on organic matter and nutrients removal. Biochem. Eng. J. 2013, 77, 28–40. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Martín-Pascual, J.; Poyatos, J.M. Moving bed biofilm reactor to treat wastewater. Int. J. Environ. Sci. Technol. 2016, 14, 881–910. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Calero-Díaz, G.; López-López, C.; Martín-Pascual, J.; Torres, J.C.; Poyatos, J.M. Kinetic study of the effect of bisphenol A on the rates of organic matter removal, decay and biomass generation in a membrane bioreactor. Biochem. Eng. J. 2017, 128, 45–53. [Google Scholar] [CrossRef]
- Ma, Q.; Qu, Y.; Shen, W.; Zhang, Z.; Wang, J.; Liu, Z.; Li, D.; Li, H.; Zhou, J. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef]
- Zhang, S.; Sheng, B.; Lin, W.; Meng, F. Day/night temperature differences (DNTD) trigger changes in nutrient removal and functional bacteria in membrane bioreactors. Sci. Total Environ. 2018, 636, 1202–1210. [Google Scholar] [CrossRef]
- Elnaker, N.A.; Elektorowicz, M.; Naddeo, V.; Hasan, S.W.; Yousef, A.F. Assessment of Microbial Community Structure and Function in Serially Passaged Wastewater Electro-Bioreactor Sludge: An Approach to Enhance Sludge Settleability. Sci. Rep. 2018, 8, 7013. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Shen, L.; Wang, X.; Ying, D.; Lin, H.; Li, R.; Xu, Y.; Hong, H. High propensity of membrane fouling and the underlying mechanisms in a membrane bioreactor during occurrence of sludge bulking. Water Res. 2023, 229, 119456. [Google Scholar] [CrossRef]
- Martins, A.M.P.; Pagilla, K.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Filamentous bulking sludge—A critical review. Water Res. 2004, 38, 793–817. [Google Scholar] [CrossRef]
- Aghili Dehnavi, H.; Amin, M.M.; Fatehizadeh, A.; Movahedian Attar, H.; Ebrahimpour, K.; Bina, B. Assessment of toxicity and kinetic effects of erythromycin on activated sludge consortium by fast respirometry method. Environ. Health Eng. Manag. J. 2021, 8, 205–214. [Google Scholar] [CrossRef]
- Xue, T.; Yu, K.-C.; Guan, J.; Huang, X.; Wen, X.-H.; Miao, X.-N.; Cui, Z.-G. Determination of kinetic parameters of activated sludge in an MBR wastewater treatment plant. Huanjing Kexue/Environ. Sci. 2011, 32, 1027–1033. [Google Scholar]
- Monteoliva-García, A.; Leyva-Díaz, J.C.; López-López, C.; Poyatos, J.M.; del Mar Muñío, M.; Martín-Pascual, J. Heterotrophic Kinetic Study and Nitrogen Removal of a Membrane Bioreactor System Treating Real Urban Wastewater under a Pharmaceutical Compounds Shock: Effect of the Operative Variables. Water 2019, 11, 1785. [Google Scholar] [CrossRef]
- Majewsky, M.; Gallé, T.; Yargeau, V.; Fischer, K. Active heterotrophic biomass and sludge retention time (SRT) as determining factors for biodegradation kinetics of pharmaceuticals in activated sludge. Bioresour. Technol. 2011, 102, 7415–7421. [Google Scholar] [CrossRef]
- Kraigher, B.; Kosjek, T.; Heath, E.; Kompare, B.; Mandic-Mulec, I. Influence of pharmaceutical residues on the structure of activated sludge bacterial communities in wastewater treatment bioreactors. Water Res. 2008, 42, 4578–4588. [Google Scholar] [CrossRef]


| Pharmaceutical | Phase 1 (mg L−1) | Phase 2 (mg L−1) |
|---|---|---|
| Erythromycin | 0.576 | 1.440 |
| Diclofenac | 0.948 | 2.370 |
| Ibuprofen | 0.056 | 0.140 |
| Pressure (bar) | ∆TMP | Permeability (m3/(m2 h bar)) | Permeability (L/(m2 h bar)) | |||
|---|---|---|---|---|---|---|
| Phase 1 | Day 0–15 | Suction | 0.08 | 0.93 | 0.002039 | 2.04 |
| Backwashing | 0.20 | 0.80 | 0.002358 | 2.36 | ||
| Day 16–35 | Suction | 0.28 | 0.73 | 0.002602 | 2.60 | |
| Backwashing | 0.20 | 0.80 | 0.002358 | 2.36 | ||
| Phase 2 | Day 0–15 | Suction | 0.10 | 0.90 | 0.001395 | 1.39 |
| Backwashing | 0.30 | 0.70 | 0.001793 | 1.79 | ||
| Day 16–35 | Suction | 0.05 | 0.95 | 0.001321 | 1.32 | |
| Backwashing | 0.25 | 0.75 | 0.001674 | 1.67 |
| Phase | Operation Time (Day) | Respirometric Test |
|---|---|---|
| 1 | 13 | 1—Control |
| 17 | 2—Control | |
| 3—Respirometer doping | ||
| 23 | 4—Control | |
| 5—Respirometer doping | ||
| 30 | 6—Control | |
| 7—Respirometer doping | ||
| 35 | 8—Control | |
| 9—Respirometer doping | ||
| 2 | 13 | 10—Control |
| 11—Respirometer doping | ||
| 17 | 12—Control | |
| 13—Respirometer doping | ||
| 24 | 14—Control | |
| 15—Respirometer doping | ||
| 35 | 16—Control | |
| 17—Respirometer doping |
| Phase | Day | MLSS (mg/L) | COD Influent (mgO2/L) | COD Removal (%) | BOD5 Removal (%) | pH Influent | pH Effluent | pH Bioreactor | Conductivity Influent (µS/cm) | Conductivity Effluent (µS/cm) | Conductivity Bioreactor (µS/cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0–12 | 5394 ± 421 | 483 ± 22 | 92 ± 2 | 91 ± 1 | 7.86 ± 0.08 | 7.72 ± 0.32 | 7.63 ± 0.41 | 1306 ± 50 | 1223 ± 78 | 1295 ± 73 |
| 13 | 5633 | 549 | 87 | 82 | 8.02 | 8.01 | 7.78 | 1485 | 1138 | 1172 | |
| 17 | 3200 | 306 | 81 | 79 | 8.01 | 8.24 | 7.91 | 1503 | 1370 | 1445 | |
| 23 | 3900 | 100 | 80 | 65 | 7.54 | 8.53 | 6.96 | 1517 | 1601 | 1292 | |
| 30 | 3933 | 329 | 66 | 56 | 7.74 | 8.38 | 6.49 | 1378 | 1364 | 1200 | |
| 35 | 2133 | 323 | 28 | 21 | 8.00 | 6.63 | 6.97 | 1310 | 1287 | 1291 | |
| 2 | 0–12 | 5145 ± 1286 | 434 ± 8 | 72 ± 3 | 65 ± 4 | 7.70 ± 0.20 | 7.82 ± 0.69 | 7.7 ± 0.75 | 791 ± 52 | 662 ± 57 | 705 ± 47 |
| 13 | 2400 | 524 | 65 | 57 | 7.84 | 7.26 | 7.00 | 1071 | 732 | 826 | |
| 17 | 2433 | 534 | 52 | 44 | 8.06 | 7.90 | 8.15 | 1410 | 1242 | 1268 | |
| 20 | 1233 | 349 | 36 | 29 | 8.31 | 6.78 | 6.93 | 1239 | 1002 | 1016 | |
| 22 | 1967 | 309 | 21 | 15 | 8.21 | 5.88 | 6.31 | 1225 | 966 | 977 | |
| 24 | 1433 | 496 | 22 | 14 | 8.11 | 6.00 | 6.74 | 1174 | 936 | 968 | |
| 35 | 500 | 420 | 20 | 14 | 7.38 | 7.25 | 8.26 | 1408 | 1085 | 1220 |
| Phase | Operation Time (Day) | Respirometric Test | YH (mgVSS/mgO2) | μmax (h−1) | KM (mgO2/L) | bH (day−1) | rsu,H (mgO2/Lh) |
|---|---|---|---|---|---|---|---|
| 1 | 13 | 1—Control | 0.5907 | 0.0324 | 20.5560 | 0.0725 | 11.4626 |
| 17 | 2—Control | 0.6353 | 0.0272 | 11.6292 | 0.0418 | 10.8555 | |
| 3—Respirometer doping | 0.6683 | 0.1167 | 11.4102 | 0.0825 | 44.7977 | ||
| 23 | 4—Control | 0.6585 | 0.0090 | 1.5089 | 0.0475 | 12.7022 | |
| 5—Respirometer doping | 0.6415 | 0.0052 | 1.3799 | 0.0453 | 7.9161 | ||
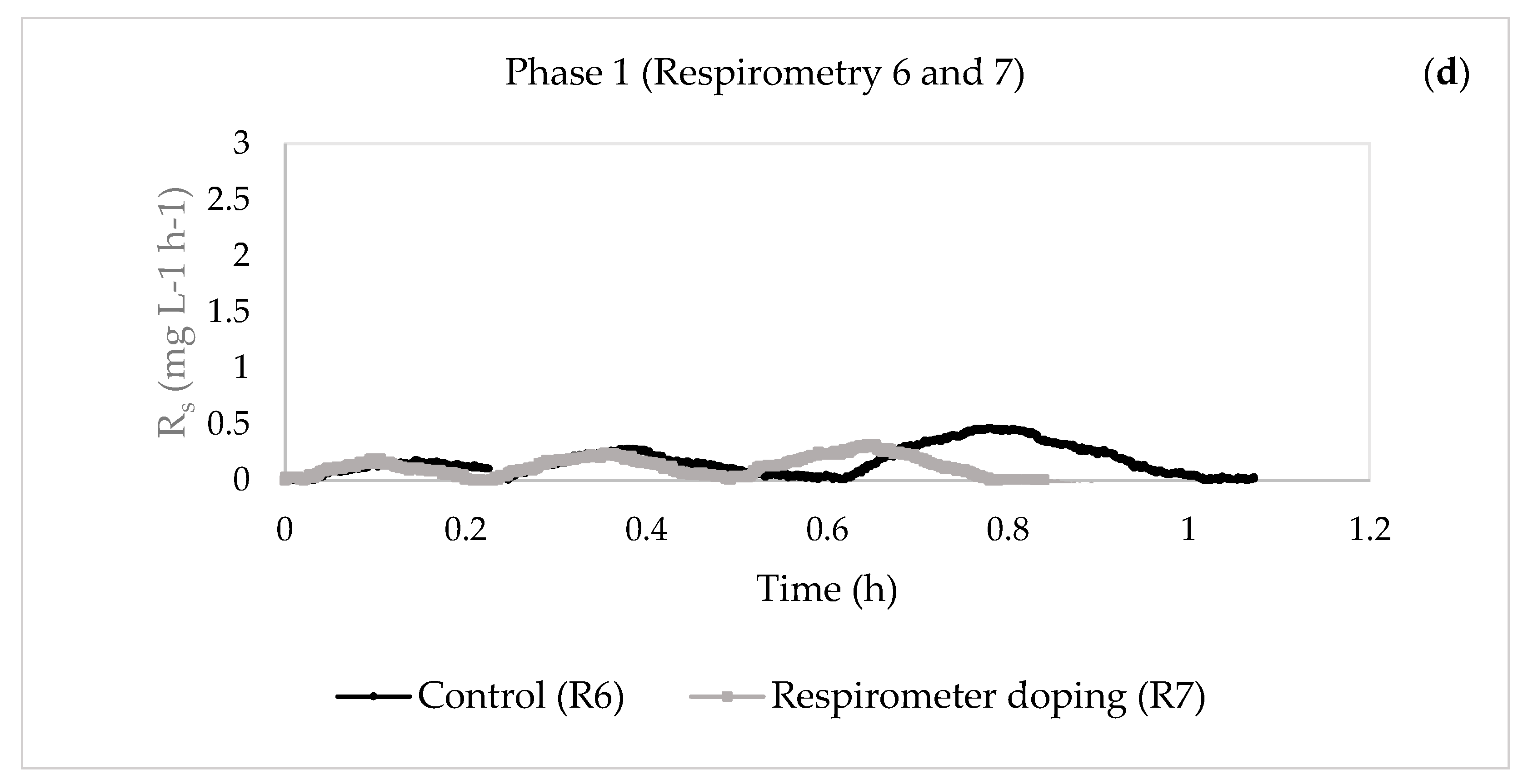
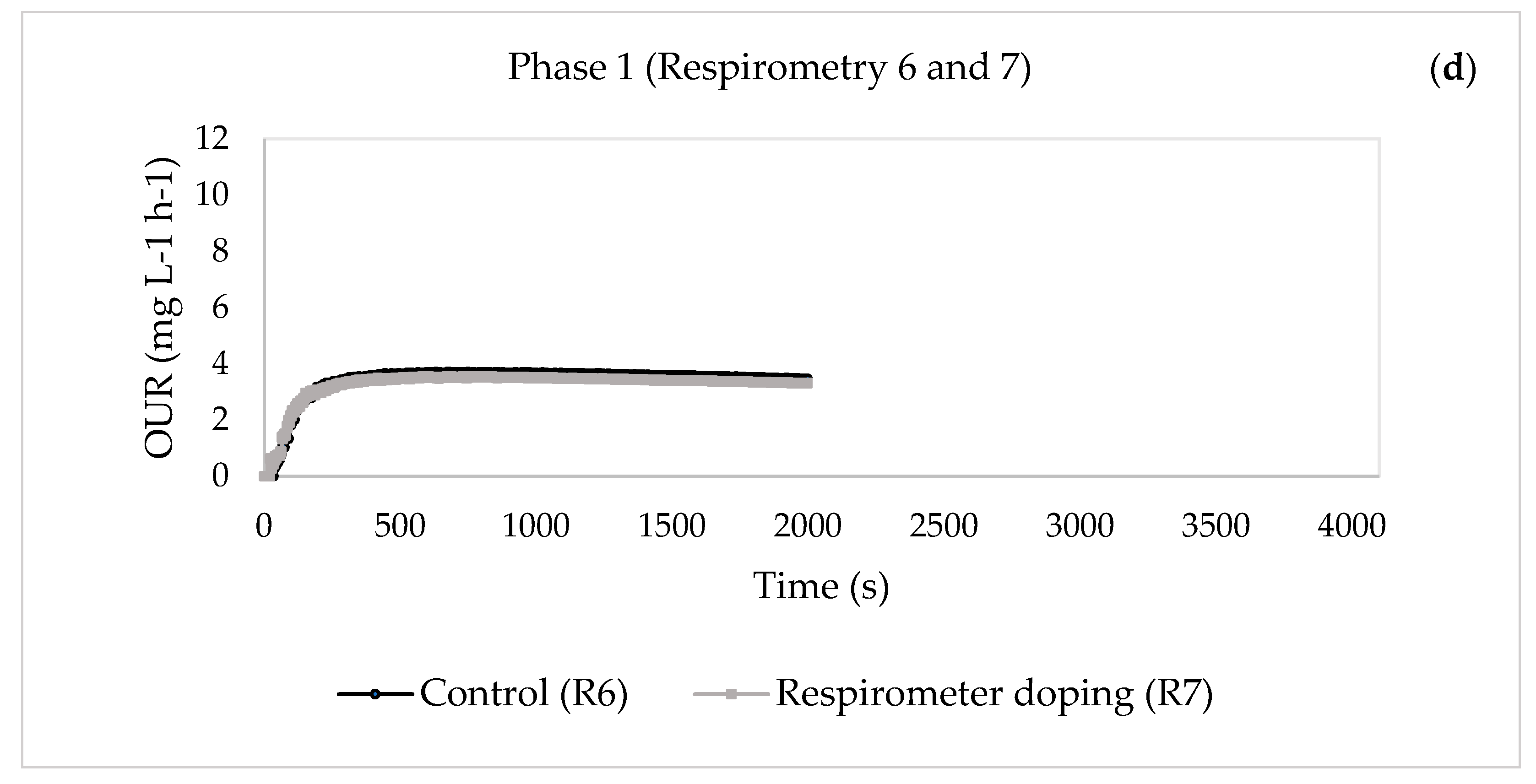
| 30 | 6—Control | 0.6541 | 0.0101 | 5.6285 | 0.0357 | 8.3688 | |
| 7—Respirometer doping | 0.6624 | 0.0054 | 1.1072 | 0.0346 | 12.2834 | ||
| 35 | 8—Control | ND | ND | ND | ND | ND | |
| 9—Respirometer doping | ND | ND | ND | ND | ND | ||
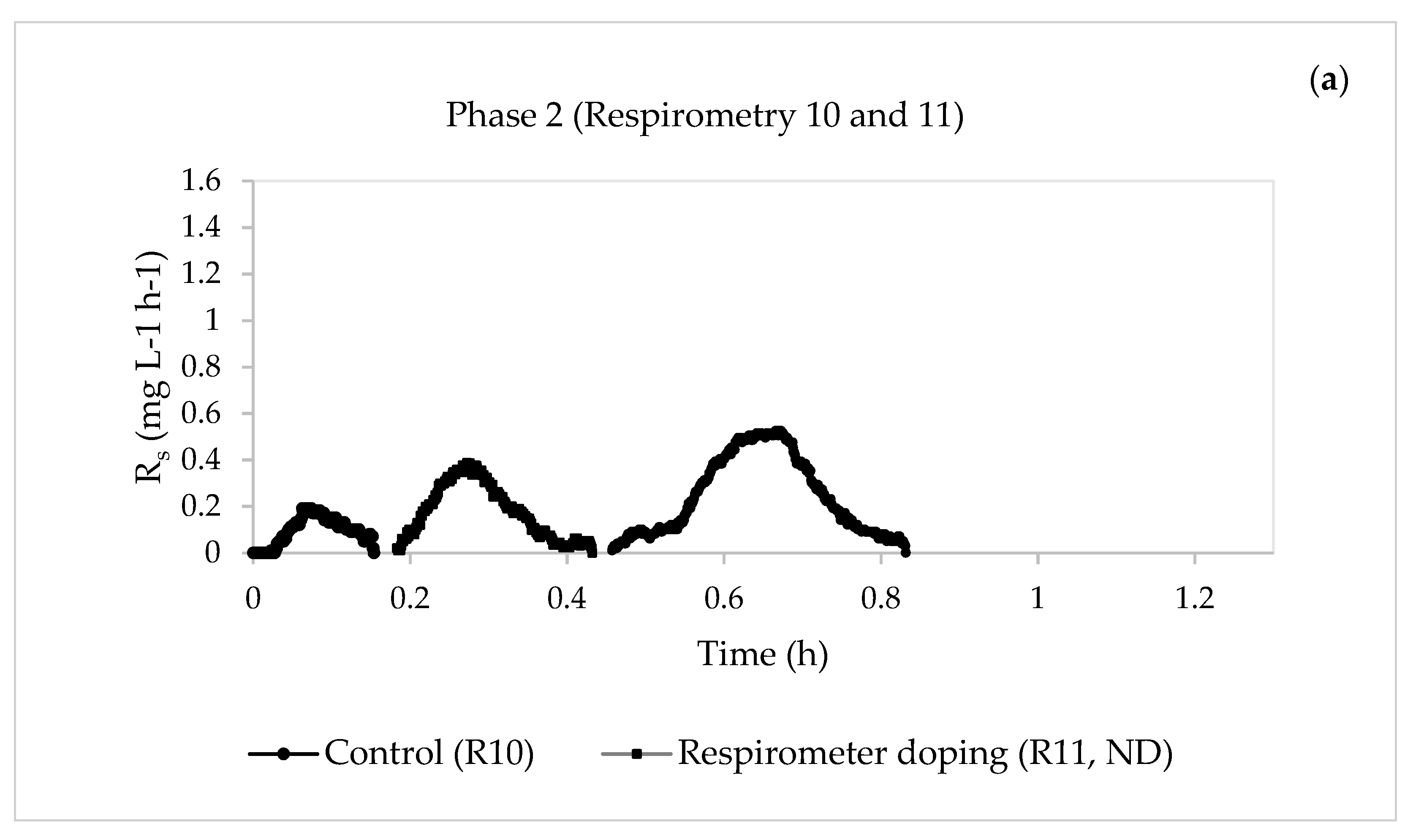
| 2 | 13 | 10—Control | 0.6620 | 0.2492 | 38.9945 | 0.1701 | 15.3234 |
| 11—Respirometer doping | ND | ND | ND | ND | ND | ||
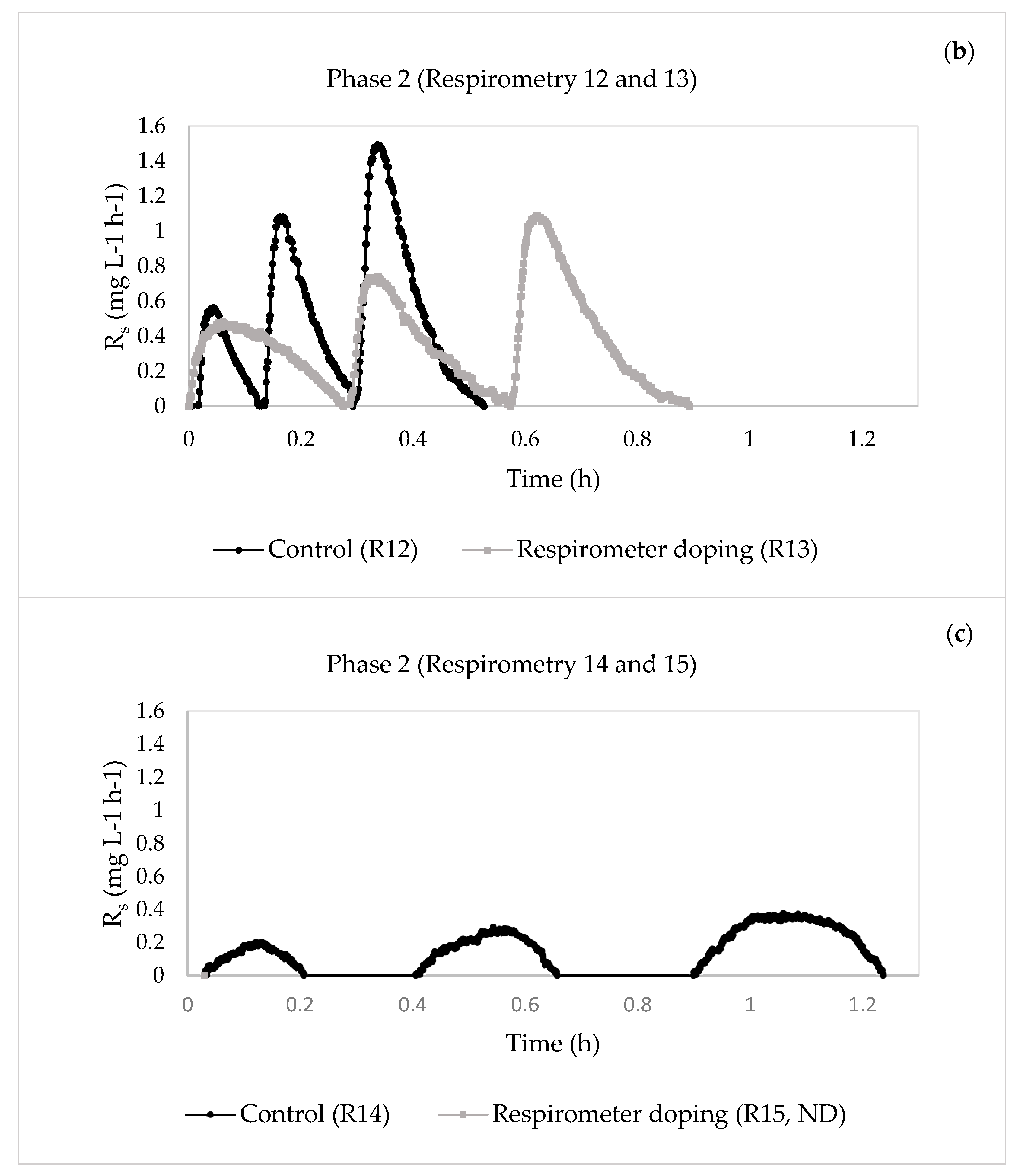
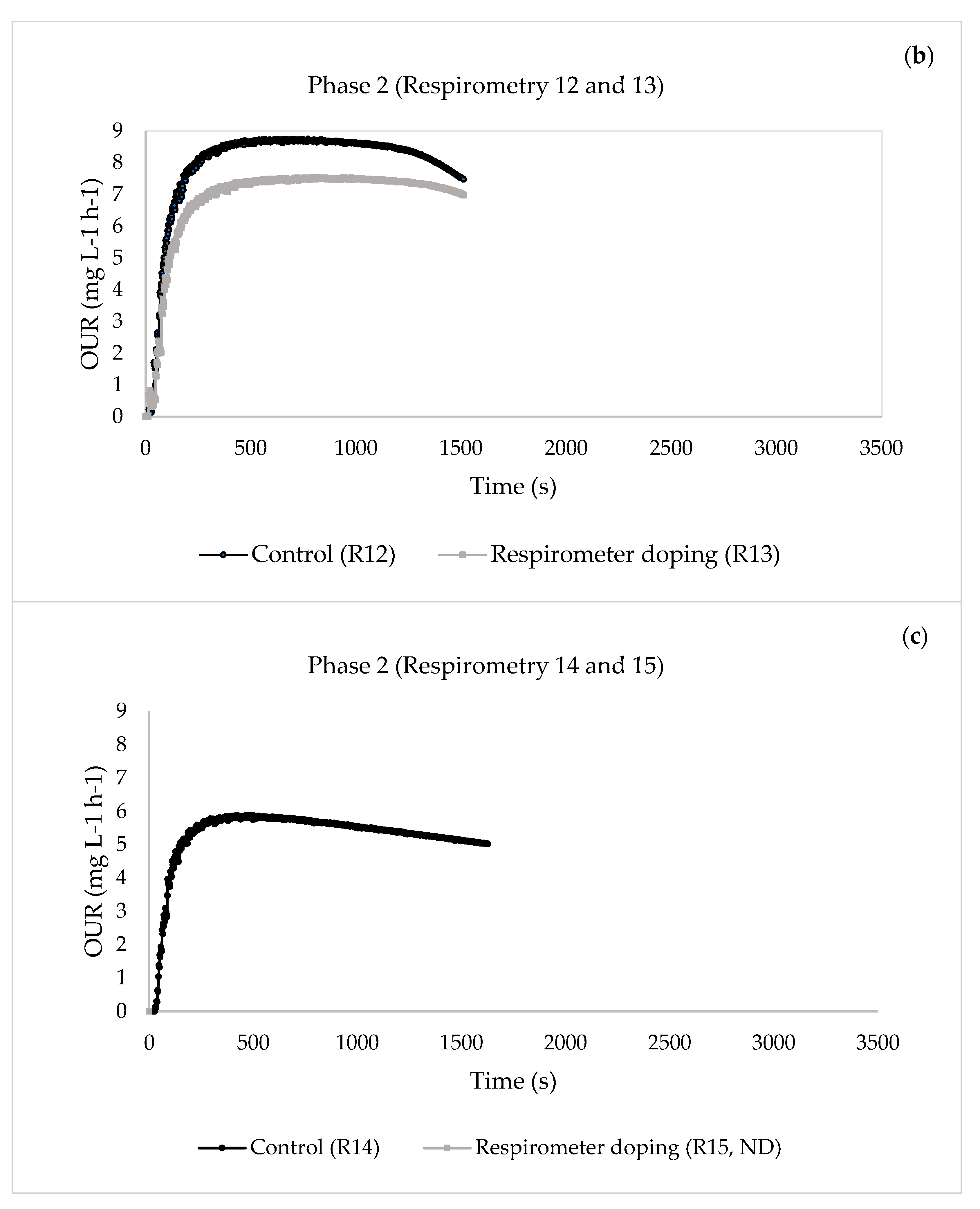
| 17 | 12—Control | 0.6413 | 0.0732 | 13.9664 | 0.1389 | 17.4938 | |
| 13—Respirometer doping | 0.6270 | 0.0152 | 4.6379 | 0.1170 | 9.5998 | ||
| 24 | 14—Control | 0.6423 | 0.0090 | 1.0064 | 0.1411 | 5.5038 | |
| 15—Respirometer doping | ND | ND | ND | ND | ND | ||
| 35 | 16—Control | ND | ND | ND | ND | ND | |
| 17—Respirometer doping | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antiñolo Bermúdez, L.; Martínez Sánchez, E.M.; Leyva Díaz, J.C.; Muñio Martínez, M.d.M.; Poyatos Capilla, J.M.; Martín Pascual, J. Impacts of Organic Emerging Contaminants (Erythromycin, Ibuprofen, and Diclofenac) on the Performance of a Membrane Bioreactor Treating Urban Wastewater: A Heterotrophic Kinetic Investigation. Membranes 2023, 13, 697. https://doi.org/10.3390/membranes13080697
Antiñolo Bermúdez L, Martínez Sánchez EM, Leyva Díaz JC, Muñio Martínez MdM, Poyatos Capilla JM, Martín Pascual J. Impacts of Organic Emerging Contaminants (Erythromycin, Ibuprofen, and Diclofenac) on the Performance of a Membrane Bioreactor Treating Urban Wastewater: A Heterotrophic Kinetic Investigation. Membranes. 2023; 13(8):697. https://doi.org/10.3390/membranes13080697
Chicago/Turabian StyleAntiñolo Bermúdez, Laura, Elena María Martínez Sánchez, Juan Carlos Leyva Díaz, María del Mar Muñio Martínez, Jose Manuel Poyatos Capilla, and Jaime Martín Pascual. 2023. "Impacts of Organic Emerging Contaminants (Erythromycin, Ibuprofen, and Diclofenac) on the Performance of a Membrane Bioreactor Treating Urban Wastewater: A Heterotrophic Kinetic Investigation" Membranes 13, no. 8: 697. https://doi.org/10.3390/membranes13080697
APA StyleAntiñolo Bermúdez, L., Martínez Sánchez, E. M., Leyva Díaz, J. C., Muñio Martínez, M. d. M., Poyatos Capilla, J. M., & Martín Pascual, J. (2023). Impacts of Organic Emerging Contaminants (Erythromycin, Ibuprofen, and Diclofenac) on the Performance of a Membrane Bioreactor Treating Urban Wastewater: A Heterotrophic Kinetic Investigation. Membranes, 13(8), 697. https://doi.org/10.3390/membranes13080697







