NMR Investigation of Water Molecular Dynamics in Sulfonated Polysulfone/Layered Double Hydroxide Composite Membranes for Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Sulfonated Polysulfone
2.3. Synthesis of Layered Double Hydroxides
2.4. Preparation of Sulfonated Polysulfone Membranes
2.5. Membranes’ Characterization
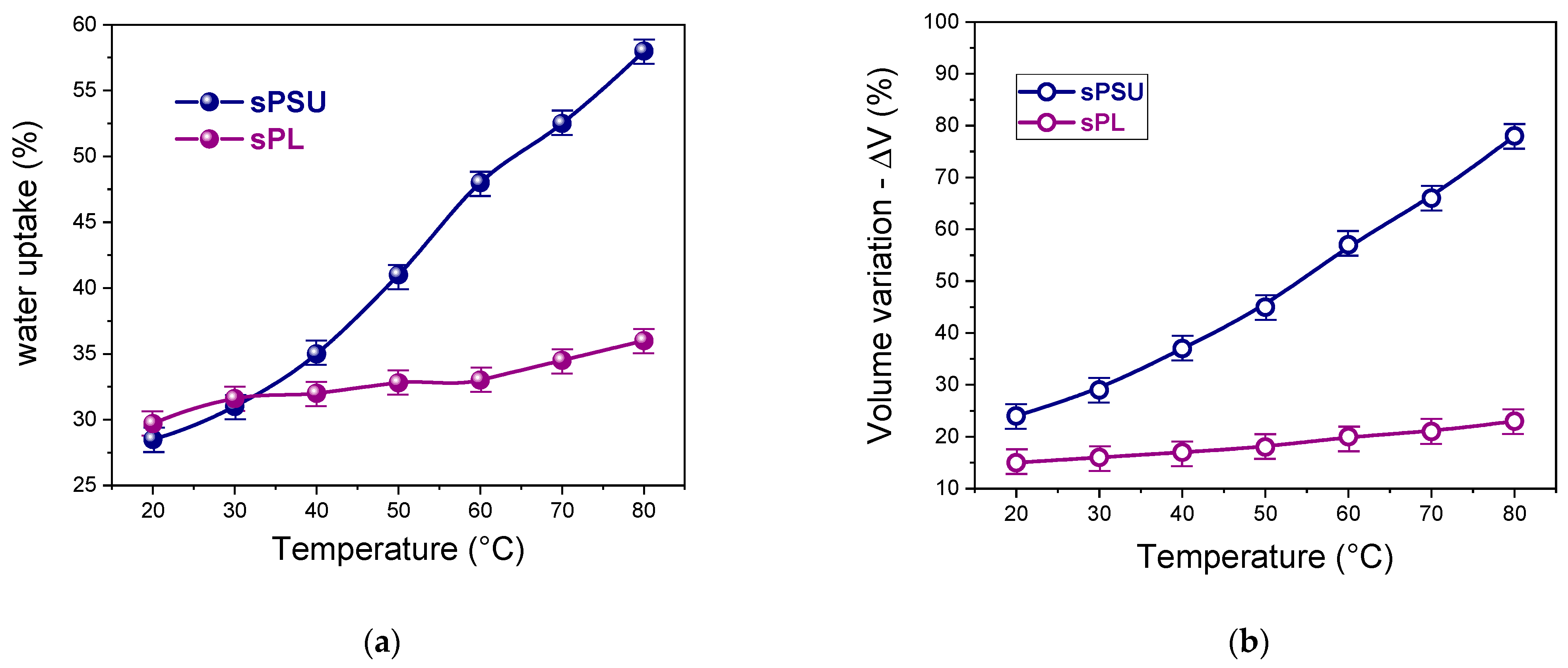
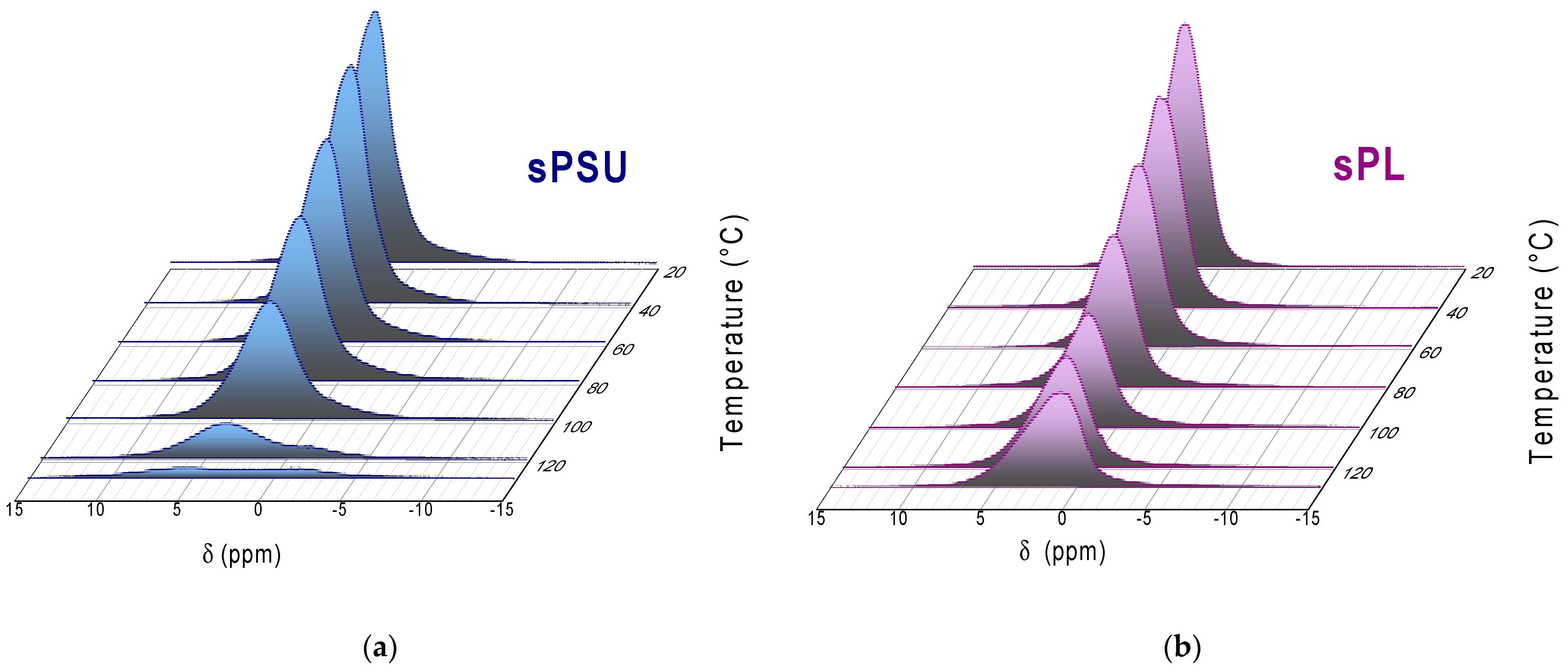
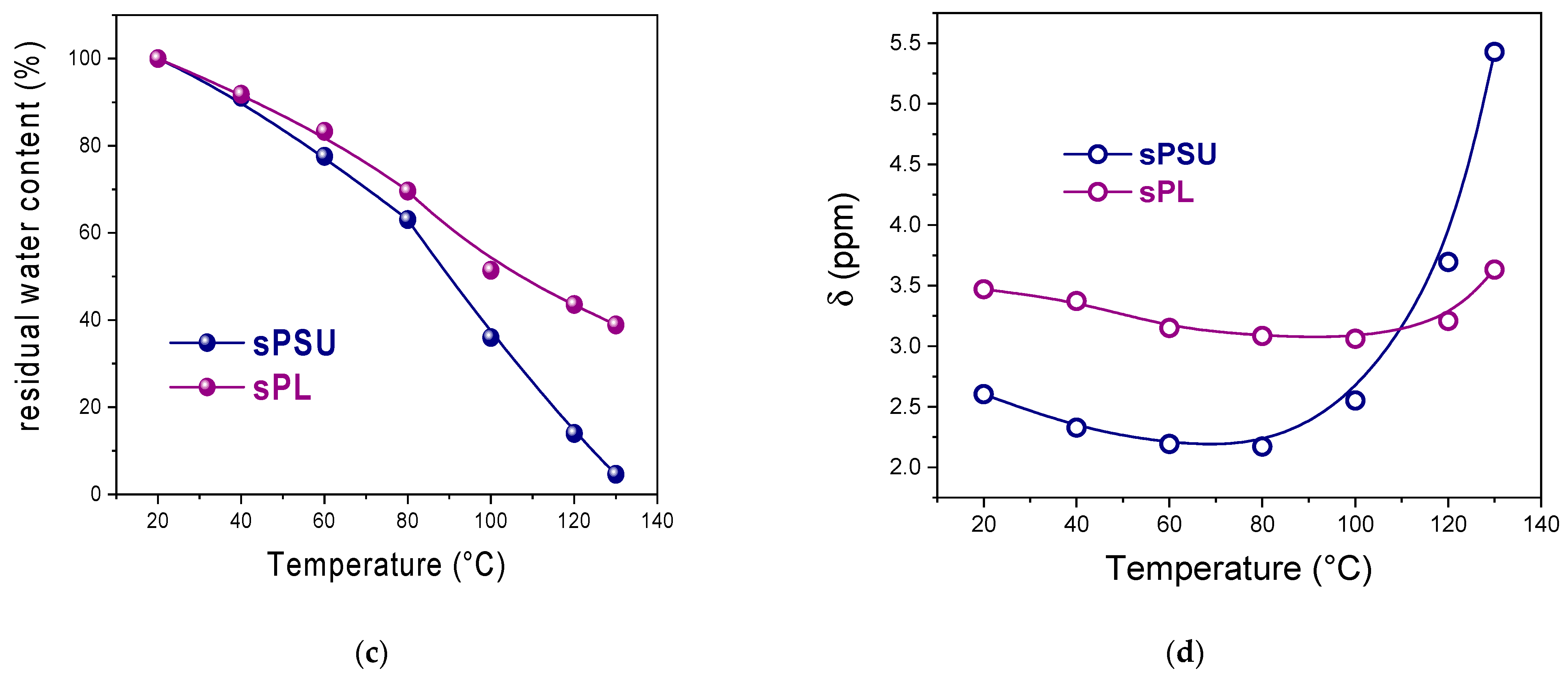
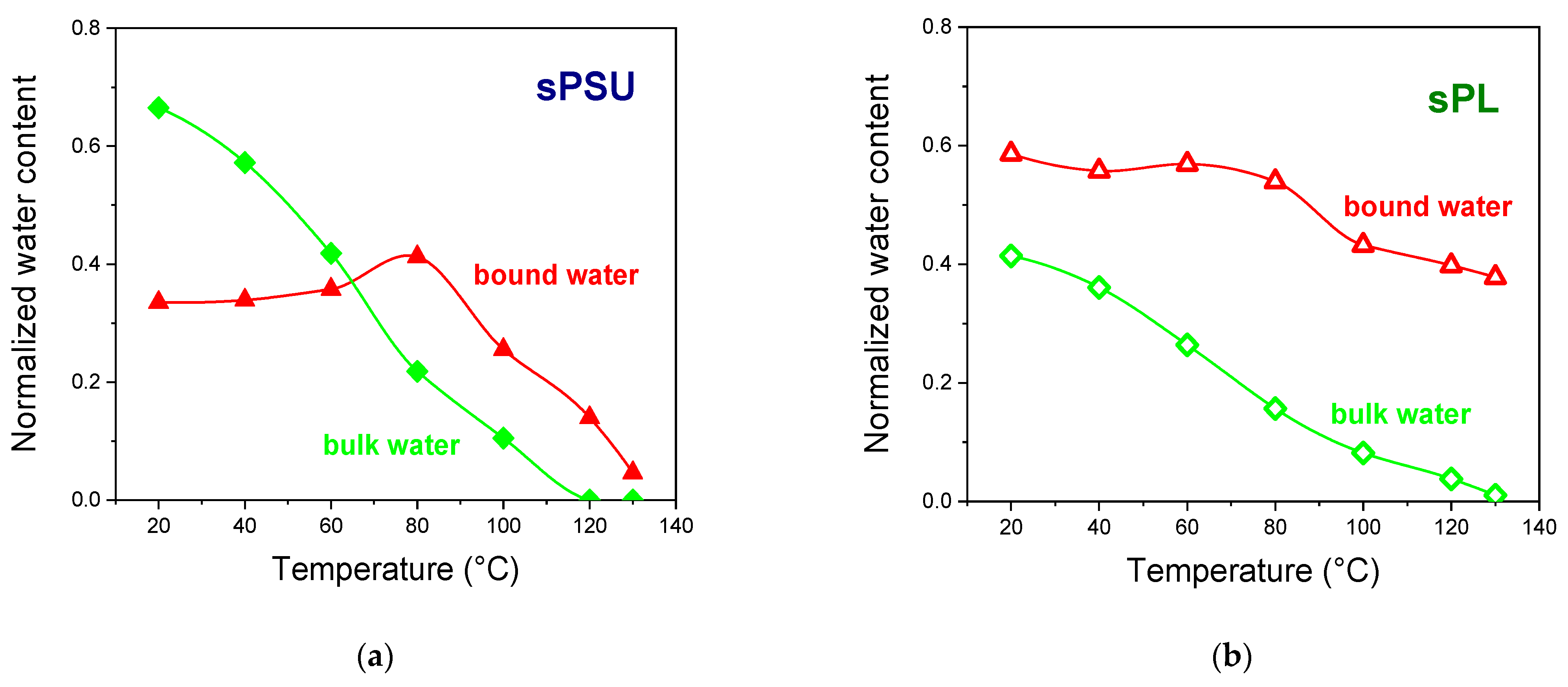
3. Results and Discussion
- (i)
- At room temperature, the amount of bulk water in the completely swollen sPSU is surely predominant, whereas the introduction of LDH nanoplatelets induces an impressive increase in the amount of bulk water. This proves that the filler particles only affect the water distribution rather than altering the microstructure of the hydrophilic clusters.
- (ii)
- Water evaporation mostly involves the bulk water, which is more mobile and thus easily evaporates during heating. However, considering the water loss at 100 °C, sPSU loses 64% wt. of the total amount initially absorbed (−55% wt. arising from peak-2; −8% wt. from peak-1). Contrariwise, the water loss for sPL only amounts to 40% wt. From this amount, 25% wt. arises from the bulk population, and 15.2% wt. arises from the solvation shells.
- (iii)
- While the bare polymer reaches 130 °C in an almost dehydrated state, the sPL nanocomposite still contains a considerable amount of water, i.e., ca. 40% wt., which exclusively arises from the water population in the bound state. This suggests water molecules experience strong electrostatic interactions with the LDH platelets, thereby preventing a considerable degree of water evaporation.
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dijoux, E.; Steiner, N.Y.; Benne, M.; Péra, M.C.; Pérez, B.G. A Review of Fault Tolerant Control Strategies Applied to Proton Exchange Membrane Fuel Cell Systems. J. Power Sources 2017, 359, 119–133. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A Review of Polymer-Nanocomposite Electrolyte Membranes for Fuel Cell Application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Raja Rafidah, R.S.; Rashmi, W.; Khalid, M.; Wong, W.Y.; Priyanka, J. Recent Progress in the Development of Aromatic Polymer-Based Proton Exchange Membranes for Fuel Cell Applications. Polymers 2020, 12, 1061. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (Pemfcs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, O.Z.; Orhan, M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 35, ISBN 2177491223. [Google Scholar]
- Cleghorn, S.J.C.; Ren, X.; Springer, T.E.; Wilson, M.S.; Zawodzinski, C.; Zawodzinski, T.A.; Gottesfeld, S. PEM Fuel Cells for Transportation and Stationary Power Generation Applications. Int. J. Hydrogen Energy 1997, 22, 1137–1144. [Google Scholar] [CrossRef]
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and Technical Challenges to High Temperature Operation of Proton Exchange Membrane Fuel Cells. J. Power Sources 2001, 103, 1–9. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric Proton Conducting Membranes for Medium Temperature Fuel Cells (110–160°C). J. Memb. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, S.H.; Lee, H.C.; Hong, M.Z.; Kim, K.; Lee, H.I. Organic-Inorganic Composite Membranes as Addition of SiO2 for High Temperature-Operation in Polymer Electrolyte Membrane Fuel Cells (PEMFCs). Electrochim. Acta 2004, 49, 4787–4796. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ben Belgacem, I.; Emori, W.; Uzoma, P.C. Nafion Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Sopian, K.; Wan Daud, W.R. Challenges and Future Developments in Proton Exchange Membrane Fuel Cells. Renew. Energy 2006, 31, 719–727. [Google Scholar] [CrossRef]
- Zare, A.; Montané, X.; Reina, J.A.; Giamberini, M. Applications of Membranes in Sustainable Energy Systems: Energy Production and Storage. In Polymer Engineering, 2nd ed.; Tylkowski, B., Wieszczycka, K., Jastrząb, R., Montane, X., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2023; pp. 219–248. [Google Scholar]
- Antonucci, P.L.; Aricò, A.S.; Cretì, P.; Ramunni, E.; Antonucci, V. Investigation of a Direct Methanol Fuel Cell Based on a Composite Nafion-Silica Electrolyte for High Temperature Operation. Solid State Ionics 1999, 125, 431–437. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and Characterization of Metal Oxide−Nafion Composite Membranes for Elevated-Temperature H2/O2 PEM Fuel Cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar] [CrossRef]
- Mishra, A.K.; Bose, S.; Kuila, T.; Kim, N.H.; Lee, J.H. Silicate-Based Polymer-Nanocomposite Membranes for Polymer Electrolyte Membrane Fuel Cells. Prog. Polym. Sci. 2012, 37, 842–869. [Google Scholar] [CrossRef]
- Del Río, C.; Morales, E.; Escribano, P.G. Nafion/SPOSS Hybrid Membranes for PEMFC. Single Cell Performance and Electrochemical Characterization at Different Humidity Conditions. Int. J. Hydrogen Energy 2014, 39, 5326–5337. [Google Scholar] [CrossRef]
- D’Epifanio, A.; Navarra, M.A.; Weise, F.C.; Mecheri, B.; Farrington, J.; Licoccia, S.; Greenbaum, S. Composite Nafion/Sulfated Zirconia Membranes: Effect of the Filler Surface Properties on Proton Transport Characteristics. Chem. Mater. 2010, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, I.; Simari, C.; Boutsika, L.G.; Coppola, L.; Spyrou, K.; Enotiadis, A. NMR Investigation on Nanocomposite Membranes Based on Organosilica Layered Materials Bearing Different Functional Groups for PEMFCs. Int. J. Hydrogen Energy 2017, 42, 27940–27949. [Google Scholar] [CrossRef]
- Enotiadis, A.; Boutsika, L.G.; Spyrou, K.; Simari, C.; Nicotera, I. A Facile Approach to Fabricating Organosilica Layered Material with Sulfonic Groups as an Efficient Filler for Polymer Electrolyte Nanocomposites. New J. Chem. 2017, 41, 9489–9496. [Google Scholar] [CrossRef]
- Branchi, M.; Sgambetterra, M.; Pettiti, I.; Panero, S.; Navarra, M.A. Functionalized Al2O3 Particles as Additives in Proton-Conducting Polymer Electrolyte Membranes for Fuel Cell Applications. Int. J. Hydrogen Energy 2015, 40, 14757–14767. [Google Scholar] [CrossRef]
- Chien, H.C.; Tsai, L.D.; Huang, C.P.; Kang, C.Y.; Lin, J.N.; Chang, F.C. Sulfonated Graphene Oxide/Nafion Composite Membranes for High-Performance Direct Methanol Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Kumar, R.; Xu, C.; Scott, K. Graphite Oxide/Nafion Composite Membranes for Polymer Electrolyte Fuel Cells. RSC Adv. 2012, 2, 8777–8782. [Google Scholar] [CrossRef]
- Shao, Z.G.; Xu, H.; Li, M.; Hsing, I.M. Hybrid Nafion-Inorganic Oxides Membrane Doped with Heteropolyacids for High Temperature Operation of Proton Exchange Membrane Fuel Cell. Solid State Ionics 2006, 177, 779–785. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, X.; Zhang, G.; Li, H.; Shao, K.; Zhu, J.; Zhao, C.; Liu, Z.; Han, M.; Na, H. Preparation and Properties of Epoxy-Based Cross-Linked Sulfonated Poly(Arylene Ether Ketone) Proton Exchange Membrane for Direct Methanol Fuel Cell Applications. Int. J. Hydrogen Energy 2010, 35, 6409–6417. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Na, H. Novel Cross-Linked Sulfonated Poly (Arylene Ether Ketone) Membranes for Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2010, 35, 2176–2182. [Google Scholar] [CrossRef]
- Kim, N.H.; Mishra, A.K.; Kim, D.Y.; Lee, J.H. Synthesis of Sulfonated Poly(Ether Ether Ketone)/Layered Double Hydroxide Nanocomposite Membranes for Fuel Cell Applications. Chem. Eng. J. 2015, 272, 119–127. [Google Scholar] [CrossRef]
- Sonpingkam, S.; Pattavarakorn, D. Mechanical Properties of Sulfonated Poly (Ether Ether Ketone) Nanocomposite Membranes. Int. J. Chem. Eng. Appl. 2014, 5, 181–185. [Google Scholar] [CrossRef]
- Kawaguti, C.A.; Dahmouche, K.; Gomes, A.d.S. Nanostructure and Properties of Proton-Conducting Sulfonated Poly(Ether Ether Ketone) (SPEEK) and Zirconia-SPEEK Hybrid Membranes for Direct Alcohol Fuel Cells: Effect of the Nature of Swelling Solvent and Incorporation of Heteropolyacid. Polym. Int. 2012, 61, 82–92. [Google Scholar] [CrossRef]
- You, P.Y.; Kamarudin, S.K.; Masdar, M.S. Improved Performance of Sulfonated Polyimide Composite Membranes with Rice Husk Ash as a Bio-Filler for Application in Direct Methanol Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 1857–1866. [Google Scholar] [CrossRef]
- Miyatake, K.; Furuya, H.; Tanaka, M.; Watanabe, M. Durability of Sulfonated Polyimide Membrane in Humidity Cycling for Fuel Cell Applications. J. Power Sources 2012, 204, 74–78. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Cao, L.; He, X.; Shi, B.; Li, Y.; Xu, M.; Jiang, Z. Enhanced Proton Conductivity of Sulfonated Polysulfone Membranes under Low Humidity via the Incorporation of Multifunctional Graphene Oxide. ACS Appl. Nano Mater. 2019, 2, 4734–4743. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Brunetti, A.; Barbieri, G.; Nicotera, I. Polysulfone and Organo-Modified Graphene Oxide for New Hybrid Proton Exchange Membranes: A Green Alternative for High-Efficiency PEMFCs. Electrochim. Acta 2021, 380, 138214. [Google Scholar] [CrossRef]
- Maier, G.; Meier-Haack, J. Sulfonated Aromatic Polymers for Fuel Cell Membranes. Adv. Polym. Sci. 2008, 216, 1–62. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Devrim, Y.; Colpan, C.O.; Hamdullahpur, F. Evaluation of Sulfonated Polysulfone/Zirconium Hydrogen Phosphate Composite Membranes for Direct Methanol Fuel Cells. Electrochim. Acta 2017, 256, 196–210. [Google Scholar] [CrossRef]
- Lufrano, F.; Squadrito, G.; Patti, A.; Passalacqua, E. Sulfonated Polysulfone as Promising Membranes for Polymer Electrolyte Fuel Cells. J. Appl. Polym. Sci. 2000, 77, 1250–1256. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroǧlu, I. Preparation and Characterization of Sulfonated Polysulfone/Titanium Dioxide Composite Membranes for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Simari, C.; Prejanò, M.; Lufrano, E.; Sicilia, E.; Nicotera, I. Exploring the Structure–Performance Relationship of Sulfonated Polysulfone Proton Exchange Membrane by a Combined Computational and Experimental Approach. Polymers 2021, 13, 959. [Google Scholar] [CrossRef] [PubMed]
- Rives, V. Layered Double Hydroxides: Present and Future; Rives, V., Ed.; Nova Science Publishers, Inc.: New York, NJ, USA, 2001; ISBN 1-59033-060-9. [Google Scholar]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Catalytic Transfer Hydrogenation of Citral on Calcined Layered Double Hydroxides. Appl. Catal. A Gen. 2001, 206, 95–101. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and Catalytic Properties of Cationic and Anionic Clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like Anionic Clays in Catalytic Organic Reactions. Catal. Rev.-Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Primo, J. Base Catalysis for Fine Chemical Production: Claisen-Schmidt Condensation on Zeolites and Hydrocalcites for the Production of Chalcones and Flavanones of Pharmaceutical Interes. J. Catal. 1995, 151, 60–66. [Google Scholar] [CrossRef]
- Oestreicher, V.; Jobbágy, M.; Regazzoni, A.E. Halide Exchange on Mg(II)-Al(III) Layered Double Hydroxides: Exploring Affinities and Electrostatic Predictive Models. Langmuir 2014, 30, 8408–8415. [Google Scholar] [CrossRef]
- Herrero, M.; Martos, A.M.; Varez, A.; Galván, J.C.; Levenfeld, B. Synthesis and Characterization of Polysulfone/Layered Double Hydroxides Nanocomposite Membranes for Fuel Cell Application. Int. J. Hydrogen Energy 2014, 39, 4016–4022. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Brunetti, A.; Barbieri, G.; Nicotera, I. Highly-Performing and Low-Cost Nanostructured Membranes Based on Polysulfone and Layered Doubled Hydroxide for High-Temperature Proton Exchange Membrane Fuel Cells. J. Power Sources 2020, 471, 228440. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V.; Nicotera, I. Barrier Properties of Sulfonated Polysulfone/Layered Double Hydroxides Nanocomposite Membrane for Direct Methanol Fuel Cell Operating at High Methanol Concentrations. Int. J. Hydrogen Energy 2020, 45, 20647–20658. [Google Scholar] [CrossRef]
- Lufrano, F.; Gatto, I.; Staiti, P.; Antonucci, V.; Passalacqua, E. Sulfonated Polysulfone Ionomer Membranes for Fuel Cells. Solid State Ionics 2001, 145, 47–51. [Google Scholar] [CrossRef]
- Gayathri, R.; Prabhu, M.R. Protonated State and Synergistic Role of Nd3+ doped Barium Cerate Perovskite for the Enhancement of Ionic Pathways in Novel Sulfonated Polyethersulfone for H2/O2 fuel Cells. Soft Matter 2020, 16, 4220–4233. [Google Scholar] [CrossRef] [PubMed]
- Simari, C.; Lo Vecchio, C.; Baglio, V.; Nicotera, I. Sulfonated Polyethersulfone/Polyetheretherketone Blend as High Performing and Cost-Effective Electrolyte Membrane for Direct Methanol Fuel Cells. Renew. Energy 2020, 159, 336–345. [Google Scholar] [CrossRef]
- Nicotera, I.; Policicchio, A.; Conte, G.; Giuseppe, R.; Habib, M.; Rehman, U.; Lufrano, E.; Simari, C. Quaternized Polyepichlorohydrin-Based Membrane as High-Selective CO2 Sorbent for Cost-Effective Carbon Capture. J. CO2 Util. 2022, 63, 102135. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Enotiadis, A.; Nicotera, I. Sulfonated Polyether Ether Ketone and Organosilica Layered Nanofiller for Sustainable Proton Exchange Membranes Fuel Cells (PEMFCs). Appl. Sci. 2022, 12, 963. [Google Scholar] [CrossRef]
- Lufrano, E.; Nicotera, I.; Enotiadis, A.; Rehman, M.H.U.; Simari, C. Elucidating the Water and Methanol Dynamics in Sulfonated Polyether Ether Ketone Nanocomposite Membranes Bearing Layered Double Hydroxides. Membranes 2022, 12, 419. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Nicotera, I. Transport Properties and Mechanical Features of Sulfonated Polyether Ether Ketone/Organosilica Layered Materials Nanocomposite Membranes for Fuel Cell Applications. Membranes 2020, 10, 87. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Godbert, N.; Gournis, D.; Coppola, L.; Nicotera, I. Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes. Nanomaterials 2020, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Zhou, H.; Matsuo, T.; Uchida, H.; Watanabe, M. Proton Conductive Polyimide Electrolytes Containing Trifluoromethyl Groups: Synthesis, Properties, and DMFC Performance. Macromolecules 2004, 37, 4961–4966. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly(Ether Ether Ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The Effect of Sulfonated Graphene Oxide on Sulfonated Poly (Ether Ether Ketone) Membrane for Direct Methanol Fuel Cells. J. Memb. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Li, Y.; Li, J.; Xu, M.; Jiang, Z. Preparation of Anion Exchange Membrane with Enhanced Conductivity and Alkaline Stability by Incorporating Ionic Liquid Modi Fi Ed Carbon Nanotubes. J. Memb. Sci. 2019, 573, 1–10. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.S.; Sebastian, D.; Baglio, V. Sulfonated Graphene Oxide Platelets in Nafion Nanocomposite Membrane: Advantages for Application in Direct Methanol Fuel Cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; D’Urso, C.; Aricò, A.S.; Baglio, V. Methanol and Proton Transport in Layered Double Hydroxide and Smectite Clay-Based Composites: Influence on the Electrochemical Behavior of Direct Methanol Fuel Cells at Intermediate Temperatures. J. Solid State Electrochem. 2015, 19, 2053–2061. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; Ranieri, G.A.; Sgambetterra, M.; Panero, S.; Navarra, M.A. An NMR Study on the Molecular Dynamic and Exchange Effects in Composite Nafion/Sulfated Titania Membranes for PEMFCs. Int. J. Hydrogen Energy 2015, 40, 14651–14660. [Google Scholar] [CrossRef]
- Simari, C.; Nicotera, I.; Perrotta, I.D.; Clarizia, G.; Bernardo, P. Microscopic and Macroscopic Investigation on the Gas Diffusion in Poly(Ether-Block-Amide) Membranes Doped with Polysorbate Nonionic Surfactants. Polymer 2020, 209, 122949. [Google Scholar] [CrossRef]
- Simari, C.; Tuccillo, M.; Brutti, S.; Nicotera, I. Sodiated Nafion Membranes for Sodium Metal Aprotic Batteries. Electrochim. Acta 2022, 410, 139936. [Google Scholar] [CrossRef]
- Policicchio, A.; Conte, G.; Agostino, R.G.; Caputo, P.; Oliviero Rossi, C.; Godbert, N.; Nicotera, I.; Simari, C. Hexagonal Mesoporous Silica for Carbon Capture: Unrevealing CO2 Microscopic Dynamics by Nuclear Magnetic Resonance. J. CO2 Util. 2022, 55, 101809. [Google Scholar] [CrossRef]
- Slichter, C. Principles of Magnetic Resonance, 3rd ed.; Springer Science & Business Media: New York, NJ, USA, 1990. [Google Scholar]
- Cossari, P.; Pugliese, M.; Simari, C.; Mezzi, A.; Maiorano, V.; Nicotera, I.; Gigli, G. Simplified All-Solid-State WO3 Based Electrochromic Devices on Single Substrate: Toward Large Area, Low Voltage, High Contrast, and Fast Switching Dynamics. Adv. Mater. Interfaces 2020, 7, 1901663. [Google Scholar] [CrossRef]
- Naim, R.; Ismail, A.F.; Saidi, H.; Saion, E. Development of Sulfonated Polysulfone Membranes as a Material for Proton Exchange Membrane (PEM). Proc. Reg. Symp. Membr. Sci. Technol. 2004, 1–17. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simari, C. NMR Investigation of Water Molecular Dynamics in Sulfonated Polysulfone/Layered Double Hydroxide Composite Membranes for Proton Exchange Membrane Fuel Cells. Membranes 2023, 13, 684. https://doi.org/10.3390/membranes13070684
Simari C. NMR Investigation of Water Molecular Dynamics in Sulfonated Polysulfone/Layered Double Hydroxide Composite Membranes for Proton Exchange Membrane Fuel Cells. Membranes. 2023; 13(7):684. https://doi.org/10.3390/membranes13070684
Chicago/Turabian StyleSimari, Cataldo. 2023. "NMR Investigation of Water Molecular Dynamics in Sulfonated Polysulfone/Layered Double Hydroxide Composite Membranes for Proton Exchange Membrane Fuel Cells" Membranes 13, no. 7: 684. https://doi.org/10.3390/membranes13070684
APA StyleSimari, C. (2023). NMR Investigation of Water Molecular Dynamics in Sulfonated Polysulfone/Layered Double Hydroxide Composite Membranes for Proton Exchange Membrane Fuel Cells. Membranes, 13(7), 684. https://doi.org/10.3390/membranes13070684






